Abstract
Mycobacterium tuberculosis lung infection in mice was controlled at an approximately stationary level after 20 days of log linear growth. Onset of stationary level infection was associated with the generation by the host of T helper type 1 (Th1) immunity, as evidenced by the accumulation of CD4 Th1 cells specific for the early secretory antigen (ESAT-6) of M. tuberculsosis encoded by esat6, and for a mycolyl transferase (Ag85B) encoded by fbpB. CD4 T cells specific for these antigens were maintained at relatively high numbers throughout the course of infection. The number of CD4 T cells generated against ESAT-6 was larger than the number generated against Ag85B, and this was associated with a higher transcription level of esat6. The total number of transcripts of esat6 increased during the first 15 days of infection, after which it decreased and then approximately stabilized at 106#x00B7;5 per lung. The total number of fbpB transcripts increased for 20 days of infection before decreasing and then approximately stabilizing at 104·8 per lung. The number of transcripts of esat6 per colony-forming unit of M. tuberculosis fell from 8·6 to 0·8 after day 15, and of fbpB from 0·3 to less than 0·02 after day 10, suggesting that at any given time during stationary level infection the latter gene was expressed by a very small percentage of bacilli. Expressed at an even lower level was an M. tuberculosis replication gene involved in septum formation (ftsZ), indicating that there was no significant turnover of the M. tuberculosis population during stationary level infection.
Keywords: antigen-encoding genes, Mycobacterium tuberculosis, T cells
Introduction
Tuberculosis is a major world disease that kills over 2 million people annually.1 Although the disease can be successfully treated with chemotherapy, the increasing incidence of cases caused by multidrug-resistant strains of Mycobacterium tuberculosis underscores the need for an effective vaccine. Therefore, numerous laboratories are currently engaged in developing a vaccine that is more effective than the currently available vaccine, bacillus Calmette–Guérin (BCG), an attenuated strain of Mycobacterium bovis. Needless to say, the rational design of a more efficacious vaccine will be based on knowledge of the M. tuberculosis antigens against which immunity needs to be generated and of the level of immunity that is required to cause M. tuberculosis infection to resolve. It is known that immunity to tuberculosis is mediated by CD4 T helper type 1 (Th1) cells with the aid of CD8 T cells.2,3 It is also known that Th1 CD4 cells are generated against a number of different M. tuberculosis antigens of which the early secretory antigen target protein (ESAT-6) encoded by esat6 (Rv3875), and a mycolyl transferase (Ag85B) encoded by fbpB (Rv1886c) are conspicuous examples.4,5 It has been shown4,5 that mice vaccinated with plasmid DNA expressing ESAT-6 or Ag85B show increased resistance to an M. tuberculosis challenge infection. ESAT-6 is considered a dominant antigen because a large proportion of memory T cells acquired by mice cured of M. tuberculosis infection6 are specific for a major epitope of this antigen. However, the extent to which ESAT-6-specific T cells might dominate over those specific for Ag85B during the primary immune response to infection appears not to have been formally investigated. Neither has the reason for the antigenic dominance of ESAT-6.
This paper shows that during the generation and maintenance of immunity to airborne M. tuberculosis infection in mice, Th1 cells specific for ESAT-6 accumulate and persist in the lungs in larger number than Th1 cells specific for Ag85B. It shows, in addition, that the antigenic dominance of ESAT-6 over Ag85B is associated with a higher level of esat6 transcription. It also shows that stationary level infection is associated with a very low level of transcription of an M. tuberculosis replication gene, ftsZ,7 indicating that stationary level infection is not associated with a significant turnover of the M. tuberculosis population.
Materials and methods
Mice
Male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and were used in experiments at 12 weeks of age. Infected mice were housed under barrier conditions in a Level III Biosafety Animal Facility, according to guidelines formulated by the Trudeau Institute Animal Care and Use Committee.
Infection
The H37Rv strain of M. tuberculosis (Trudeau Mycobacterial Culture Collection no.102) was grown as a suspension culture in Proskauer and Beck medium containing 0·01% Tween-80, and harvested while in log-phase growth, as described previously.8 The culture was subjected to two 5-second bursts of ultrasound to break up clumps and diluted appropriately in phosphate-buffered saline (PBS) containing 0·01% Tween-80 for infection via the respiratory route in an aerosol infection chamber, as described previously.8 Infection was monitored by measuring changes against time in the total number of M. tuberculosis colony-forming units (CFU) in the lungs. This involved killing five mice at the times indicated, homogenizing their lungs, plating serial dilutions of lung homogenates on enriched Middlebrook 7H11 agar, and counting colonies after incubating the plates for 21 days at 37°.
Mycobacterium tuberculosis antigens
The M. tuberculosis antigens used to stimulate lung cells to produce interferon-γ (IFN-γ) in the Elispot assay were ESAT-6 (1–20) peptide6 and Ag85B (240–260) peptide9. The peptides were purchased from New England Peptide, Fitchburg, MA. ESAT-6 is an early-secreted M. tuberculosis protein whereas Ag85B is a member of a family of mycolyl transferases involved in cell wall lipid biosynthesis.10
Lung cell preparation
Lung cells were harvested as previously described.8 Briefly, mice were killed by cervical dislocation, and their lungs were perfused via the right ventricle with PBS containing 10 U/ml of heparin to remove most intravascular leucocytes. The lungs were then perfused with an enzyme cocktail consisting of 150 U/ml of collagenase, 0·2 U/ml of elastase (Roche Applied Science, Indianapolis, IN) and 1 mg/ml of DNAse (Sigma, St Louis, MO) in RPMI-1640. The lungs were removed, placed in a dish, diced into small fragments, and the fragments were incubated in the enzyme mixture at 37° for 1 hr. The preparation was then passed through a 60-mesh per square inch stainless screen, and the resulting suspension was triturated with a pipette to break up aggregates. The cells were pelleted and resuspended in red cell lysis buffer (Sigma). They were then washed, passed through a 70-μm nylon cell strainer (BD Falcon, San Jose, CA), pelleted and resuspended in RPMI-1640 containing 10% fetal calf serum (RPMI-FCS) for counting and analysis.
Elispot
Changes in numbers of M. tuberculosis-specific cells in the lungs against time of infection were determined by enumerating changes in the numbers of cells capable of making IFN-γ in response to M. tuberculosis antigens in an 18-hr Elispot assay. This was performed with a commercially available Elispot kit (Mouse IFN-γ ELISPOT Set, BD Biosciences, San Jose, CA) according to the manufacturer's instructions using pooled cells from four mice, as described previously.8 To supplement the antigen-presenting cells (APC) already present in the lung cell suspension bone marrow-derived dendritic cells prepared as previously described8 were added to the cell suspension at a ratio of 5 : 1 lung cells to dendritic cells. Two-fold serial dilutions of the admixture (100 μl) were added in triplicate to the wells starting at 105 lung cells per well. The wells then received 100 μl of RPMI-FCS containing no antigen, 2 μg of ESAT-6 (1–20) peptide, or 2 μg of Ag85B (240–260) peptide. The number of cells specific for each antigen preparation was calculated by subtracting the number of spots that formed in the absence of added antigen from the number that formed in its presence. The experiment was repeated three times. To determine whether cells that made IFN-γ in the assay were CD4+, the lung cell suspension was selectively depleted of CD4 cells magnetically after reacting it with biotin-conjugated anti-CD4 rat anti-mouse monoclonal antibody (BD Biosciences) and then with streptavidin-coupled magnetic nanoparticles (IMag Streptavidin Particles, BD BioSciences) according to the manufacturer's instructions, as described previously.8
Quantification of bacterial gene expression in infected lungs by real-time reverse transcription–polymerase chain reaction (RT-PCR)
Lungs from four or five mice were harvested at progressive times of infection, and snap frozen in liquid nitrogen. To extract total RNA, M. tuberculosis -infected lungs were homogenized in Trizol reagent (Invitrogen, Carlsbad, CA) using glass tubes and close-fitting pestles. The homogenized tissue was centrifuged for 5 min at 2000 g and supernatant and pellet were separated. Host lung RNA was extracted from the supernatant, whereas M. tuberculosis RNA was extracted from the pellet. The pellet from each whole lung was resuspended in 2 ml Tri reagent (MRC, Cincinnati, OH) in the presence of 20 μl polyacryl carrier molecules (MRC, Cincinnati, OH). The resuspended pellet was subjected to 30–40 s of ultrasound generated by a Braun-Sonic 1510 sonicator set at 400 watts, followed by three cycles of freezing and thawing (liquid nitrogen/warm water) to break open the bacterial cell wall. Following the addition of 0·1 ml of 1-bromo-3-chloropropane (MRC, Cincinnati, OH) samples were centrifuged at 10 000 g at 4° for 15 min for phase separation. The RNA partitioned into the upper aqueous phase and was transferred to a new tube where it was mixed with an equal volume of isopropanol. Following centrifugation the M. tuberculosis RNA pellet was washed with 75% ethanol and dissolved in diethylpyrocarbonate (DEPC)-treated distilled water. To remove any contaminating bacterial genomic DNA, RNA samples were digested with RNase-free DNase I (Ambion, Austin, TX) for 1 hr at 37°. RNA samples were then purified on RNeasy mini columns (Qiagen, Valencia, CA), and treated further with DNase I using the Ambion DNA-free kit reagents (Ambion, Austin, TX) to remove any remaining traces of DNA. RNA samples were stored at −70°.
TaqMan primers and probes for esat6, fbpB and ftsZ, were designed with Primer Express Software (PE Biosystems, Foster City, CA). The primers were purchased from Integrated DNA Technologies (Coralville, IA), and probes containing a fluorescent reporter dye (6-carboxy-fluorescein at the 5′ end) and a quencher (Black Hole Quencher 1, at the 3′ end) were purchased from Biosearch Technologies (Novato, CA). The oligonucleotide sequences for the forward primer, reverse primer, and probe, respectively, were as follows: esat6: GTACCAGGGTGTCCAGCAAAA, CTGCAGCGCGTTGTTCAG, and GGGACGCCACGGCTACCG; fbpB: CCTGCGGTTTATCTGCTCGA, TGTAGAAGCTGGACTGCCCG, and AACACCCCGGCGTTCGAGTGGTACT; and ftsZ: GGTTGCTGCAGATGGGAGAT, GGCGAAGTCGACGTTGATTAG, and CTGATGGATGCTTTCCGTAGCGCC.
Amplicons for each gene were generated by PCR from M. tuberculosis RNA using the gene-specific primers described above and were initially analysed by agarose gel electrophoresis to confirm their size. Amplicons were then purified using the WIZARD SV Gel and PCR Clean-up System (Promega, Madison, WI), cloned into the pPCR-Script Amp vector (Stratagene, La Jolla, CA) and their sequences were verified by thermocycler sequencing using a Beckman Coulter CEQ 8000 Genetic Analysis System.
To generate RNA standards for Real-Time RT-PCR analysis, a separate set of purified amplicons containing the T7 phage promoter sequence (incorporated into the forward primer) were used as templates for in vitro transcription using the T7-MEGAshortscript kit (Ambion, Austin, TX). The PCR transcription templates were then removed by digestion with DNase I, and the RNA was purified using RNeasy mini columns followed by further treatment with the DNA-free kit (Ambion, Austin TX) and quantified with the RiboGreen (Molecular Probes, Eugene, OR) assay or by absorbance measurements at 260 nm. To obtain a standard curve, serial dilutions of each transcript were performed to give dilutions ranging from 108 to 101 molecules. The dilutions were then subjected to real-time RT-PCR analysis as described below.
For real-time RT-PCR, 0·5–1·0 μg of M. tuberculosis RNA was reverse transcribed by using the gene-specific reverse primers described above, and a C. therm. (Carboxydothermus hydrogenoformans) polymerase two step RT-PCR kit (Roche, Mannheim, Germany), or the Thermoscript kit (Invitrogen) according to the manufacturer's instructions. Real-time PCR to enumerate M. tuberculosis esat6, fbpB and ftsZ amplicons was performed in an ABI-prism 7700 instrument. PCR amplification was performed in a total of 25 μl containing 10 μl cDNA sample, 2·5 μl 10× Taqman Buffer A, 3–9 mm MgCl2, 200 μm each of dATP, dCTP and dGTP, 400 μm dUTP, 0·1–0·3 μm of each primer, 0·625 U AmpliTaq Gold and 0·25 U AmpErase Uracil N-glycosylase (Perkin Elmer/Applied Biosystems, Foster City, CA). The reaction also contained 0·2 μm of detection probe. Amplification was performed under the following conditions: 2 min at 50° and 10 min at 94° followed by a total of 40 two-temperature cycles (15 s at 94° and 1 min at 60°). The copy number in each sample was calculated according to the formula N = (Ct − b)/m, where N is copy number, Ct is the threshold cycle, b is the y-intercept and m is the slope of the standard curve line. Copy number data for esat6 and ftsZ were obtained following analysis of replicate samples from four separate experiments, while fbpB data were collected from two of these experiments.
Results
Course of lung infection and generation of ESAT-6- and Ag85B-specific Th1 cells
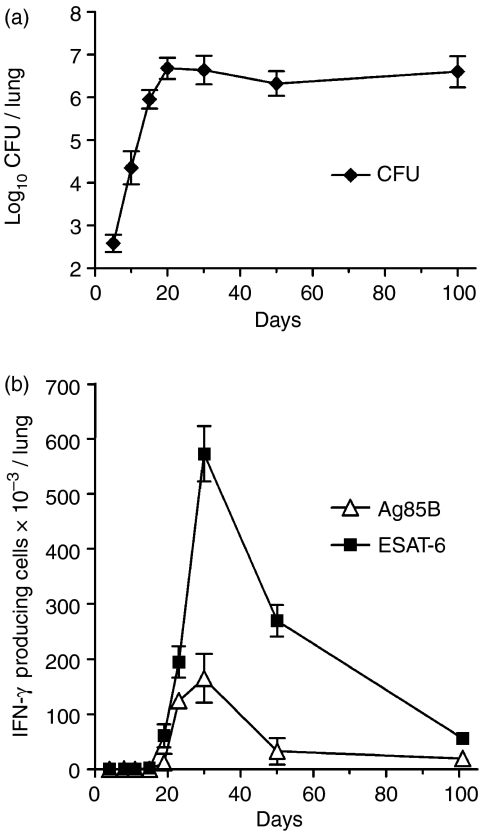
The progression of lung infection was monitored by enumerating changes against time in the number of M. tuberculosis CFU, whereas the host response to lung infection was monitored in a separate group of mice by enumerating changes in the number of M. tuberculosis-specific T cells. Figure 1 shows, in agreement with previously published results,8 that infection with approximately 102M. tuberculosis CFU via the respiratory route resulted in 20 days of log linear M. tuberculosis growth in the lungs, after which further growth was inhibited and infection was held at an approximately stationary level of 5 × 106 CFU. Figure 1 shows, in addition, that inhibition of M. tuberculosis growth was associated with the accumulation in the lungs of M. tuberculosis-specific T cells, enumerated according to their ability to secrete IFN-γ in response to exposure to ESAT-6 (1–20) peptide, or Ag85B (240–260) peptide in the Elispot assay. Antigen-specific cells began to accumulate in the lungs between days 15 and 18 of infection, reached peak numbers on day 30, and then gradually declined in number until termination of the experiment. At peak accumulation there were 5·7 × 105 ESAT-6-specific cells, compared to 1·65 × 105 Ag85B-specific T cells. Although antigen-specific T cells declined in number after day 30, they remained present in appreciable numbers until day 100 when the experiment was terminated. These results were obtained in two separate experiments and are in close agreement with results that were published earlier.8
Figure 1.
Course of M. tuberculosis infection (a) in the lungs of mice infected with approximately 102 CFU of M. tuberculosis via the airborne route. The M. tuberculosis grew progressively for approximately 20 days before further growth was inhibited and the level of infection was stabilized by acquired immunity. The means ± SD of five mice are shown per time-point. Kinetics of development of acquired immunity (b) in terms of the accumulation in the lungs of T cells capable of making IFN-γ in response to ESAT-6 (1–20) peptide, or Ag85B (240–260) peptide in an 18-hr Elispot assay. The means ± SD of the number of spots in triplicate wells using pooled cells of four mice are shown per time-point. Identical results were obtained in two separate experiments.
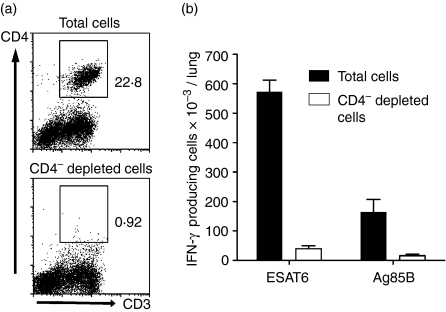
Most antigen-specific T cells that made IFN-γ in the Elispot assay were CD4-positive, as evidenced by more than a 90% reduction in their number (Fig. 2) after selectively depleting the lung cell suspension of CD4 cells.
Figure 2.
Evidence that cells that made IFN-γ in response to ESAT-6 (1–20) or Ag85B (240–260) peptide as measured with the Elispot assay were CD4 T cells. Flow cytometric analysis (a) showing that magnetic depletion of lung cells treated with biotin-conjugated, anti-CD4 monoclonal antibody and then with streptavidin-coupled magnetic nanoparticles was approximately 95% successful at removing CD4 cells. Depletion of CD4 T cells (b) resulted in loss (open bars) of most cells capable of making IFN-γ (closed bars) in response to ESAT-6 and Ag85B peptide.
Levels of transcription of esat6, fbpB and ftsZ during the course of infection
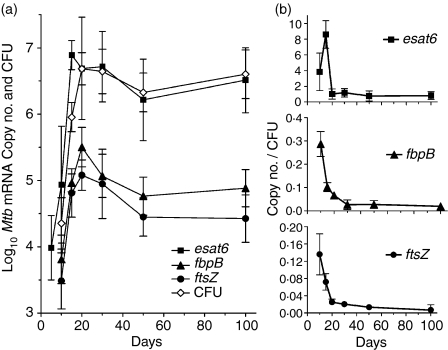
The foregoing results show that according to the number of ESAT-6- and Ag85B-specific T cells that accumulated in the lungs, ESAT-6 was the more dominant antigen. To determine whether antigenic dominance was associated with a higher level of esat6 transcription, real-time RT-PCR was employed to determine changes in the copy number of transcripts of each antigen-encoding gene during the course of infection. In addition, changes in the number of transcripts of ftsZ, a gene required for M. tuberculosis division, were monitored. The results show (Fig. 3a) that the transcription levels per lung of fbpB and ftsZ increased in concert with growth of M. tuberculosis during the first 20 days or so of infection, after which transcription levels declined before approximately stabilizing at constant levels of 104·8 and 104·4, respectively. The transcription level of esat6 peaked on day 15 before declining and then stabilizing at a constant level of 106·5. Therefore, number of transcripts of esat6 per lung was 50-fold higher than the number of transcripts of fbpB during the course of stationary level infection.
Figure 3.
Changes against time of infection (a) in the log10 copy number per lung of mRNA for esat6, fbpB and ftsZ, as quantified by real-time RT-PCR. Changes in the log10 CFU of M. tuberculosis are also shown. The number of esat6 transcripts increased for the first 15 days of infection, and of fbpB and ftsZ for the first 20 days, after which transcript numbers declined briefly and then approximately stabilized. When transcripts of esat6, fbpB and ftsZ were expressed as copies per CFU of M. tuberculosis (b) the number of esat6 transcripts declined from 8·6 to 0·8 per CFU, of fbpB from 0·3 to 0·02 per CFU, and of ftsZ from 0·14 to 0·007. Therefore, most M. tuberculosis were not transcribing the latter two genes at any one time of infection, particularly after infection became stationary. Means ± SD for M. tuberculosis RNA from lungs of four or five mice per time-point.
Reduction and stabilization of the number of transcripts of each gene per lung was associated with a drop (Fig. 3b) in the number of transcripts per CFU of M. tuberculosis. Thus after day 15 the number of transcripts of esat6 per CFU fell from 8·6 to 0·8, while after day 10 the number of transcripts of fbpB fell from 0·3 to 0·02, and of ftsZ from 0·14 to 0·007. Thus the number of transcripts of fbpB declined to approximately 1 per 50 CFU, and of ftsZ to approximately 1 per 150 CFU, meaning that at any one time during stationary level infection these genes were not being transcribed by most bacilli.
Discussion
The results of this study show that inhibition of Mtb growth in the lungs of mice at day 20 of an airborne infection was associated with the accumulation in the lungs of CD4 T cells specific for M. tuberculosis antigens, as enumerated with the IFN-γ Elispot assay. CD4 T cells began accumulating on days 15–18 of infection, and reached peak number on day 30, at which time the number of T cells specific for ESAT-6 (5·7 × 105) was three- or fourfold more than the number specific for Ag85B (1·65 × 105). Therefore, according to the number of T cells generated, ESAT-6 was dominant as an antigen over Ag85B, a finding in keeping with the conclusion of Andersen and colleagues6 that ESAT-6-specific memory T cells are present in dominant numbers in the spleens of mice cured of a primary M. tuberculosis infection. After day 30 the number of T cells specific for each antigen slowly declined, but nevertheless remained relatively high until the experiment was terminated at day 100. Because it is known that antigen-specific CD4 cells in the lungs during stationary level infection belong to a replicating population,12 are produced continuously in absence of the thymus12 and have an activation phenotype,8,12 it is reasonable to assume that the majority of them were generated continuously in the lungs in response to M. tuberculosis antigens at sites of infection. It is almost certain, however, that the antecedents of these T cells were generated in draining lymph nodes, and that they reach the lungs after entering the blood via efferent lymph. The interpretation that activated, M. tuberculosis-specific CD4 Th1 cells are responsible for inhibiting the growth of M. tuberculosis and for stabilizing infection at a stationary level is based on the knowledge that M. tuberculosis growth is not inhibited in mice incapable of generating CD4-mediated Th1 immunity13,14 and that depleting mice of CD4 T cells during stationary level infection results in a resumption of M. tuberculosis growth.15 Given the evidence that the retention of activated, antigen-specific CD4+ T cells requires the continuous presence of antigen,16 the maintenance of Th1 immunity in an active state almost certainly required the continuous presentation of M. tuberculosis antigens during stationary level infection.
According to this study, ESAT-6- and Ag85B-encoding genes were each transcribed at an approximately constant level during the course of stationary level infection. Therefore, it seems reasonable to conclude that these antigens were synthesized at a constant level by the M. tuberculosis lung population, and were continuously processed and presented to CD4 T cells by infected APCs. It also seems likely that macrophages were the key APCs involved, because it is within these cells that M. tuberculosis almost exclusively resides during infection. The finding that esat6 was transcribed at a 50-fold higher level than fbpB would serve to explain why ESAT-6 is a more dominant antigen than Ag85B. However, the reason for the antigenic dominance of ESAT-6 is more complex than this, because the much smaller number of transcripts of fbpB than of esat6 per CFU of M. tuberculosis means that a much smaller number of macrophages may have been processing and presenting Ag85B. Thus during stationary level infection the number of transcripts of fbpB was less than 1 per 50 bacilli, whereas the number of transcripts of esat6 was approximately one per bacillus. This assumes that mRNA for each gene was present at similar steady-state levels in all bacilli that made up the M. tuberculosis population in the lung. Low average steady-state levels of mRNA for protein-encoding genes are a common finding in bacteria.17–20 If it is assumed that during stationary infection each macrophage contained 50 bacilli, then, at any one time only one bacillus per macrophage would have been transcribing fbpB, whereas essentially all bacilli per macrophage would have been transcribing esat6. Under these conditions ESAT-6 would probably be able to outcompete Ag85B for processing and presentation within the same APC. On the other hand, if Mtb gene expression follows stochastic mechanisms 21–24 then transcripts of both antigen-encoding genes could have been present at more than one copy per bacillus. In this case, all infected macrophages would still have contained some ESAT-6-transcribing bacilli, whereas only a small percentage would have contained bacilli transcribing fbpB. Under these conditions ESAT-6 would dominate as an antigen because of its presence in a larger number of APCs. If antigenic dominance were a function of the larger number of macrophages presenting ESAT-6, rather than to a larger number of M. tuberculosis bacilli making this antigen, it would serve to explain why a 50-fold higher level of transcription of esat6 than fbpB per lung was associated with only a three- to fourfold larger number of ESAT-6-specific T cells.
It should be remembered that the onset of stationary level lung infection results from inhibition of M. tuberculosis growth by host macrophages, and that this is associated with the acquisition of a ‘dormancy’ transcriptome on the part of M. tuberculosis that involves induction of dosR (Rv3133c) and numerous other genes of the dosR regulon,25 including Rv2626c, a gene of unknown function, and Rv2031c, the alpha-crystallin gene (acr), both of which are expressed at high levels. A previous study in this laboratory11 showed that the expression of Th1 immunity against airborne M. tuberculosis infection in B6 mice is associated with a significant increase in the level of expression of Rv2626c and acr per CFU of M. tuberculosis. The acquisition of a dormancy transcriptome by M. tuberculosis occurs in response to a transcriptional change on the part of infected macrophages26 that results in them acquiring the ability to synthesize inducible nitric oxide synthase and to generate nitric oxide that can act as a signal for M. tuberculosis to undergo its protective dormancy transcriptional response.27 As shown here, inhibition of M. tuberculosis growth and its persistence in the lungs in an apparent non-replicating state was associated with an approximately 11-fold decrease in the copy number of esat6 transcripts per CFU, a 15-fold reduction in the number of fbpB transcripts, and a 20-fold reduction in transcript number of ftsZ. The function of ESAT-6 is not known but is believed to be essential for M. tuberculosis pathogenicity, 28–30 whereas antigen Ag85B, being a mycolyl transferase, is involved in cell wall lipid biosynthesis.10 Therefore, synthesis of Ag85B would be needed for M. tuberculosis replication. FtsZ is a protein essential for bacterial septum formation7,31,32 and therefore for M. tuberculosis division. According to this study there were about 5 × 104 transcripts of ftsZ per lung and therefore per 5 × 106 CFU of M. tuberculosis during stationary level infection. Thus only 5 × 104 bacilli (1% of the population) could each have contained one transcript of ftsZ, or only 5 × 103 bacilli could each have contained 10 transcripts. In any case, the very small number of transcripts of this gene per total M. tuberculosis population argues against the notion that the M. tuberculosis population was turning over to a significant extent during stationary level infection. Thus stationary level infection was not the result of equilibrium between bacterial destruction and growth, but was the result of almost all bacilli being in a state of non-replicating persistence.11,33 This would be in keeping with the conclusions recently reached by others,34 who showed that in spite of the resistance of DNA in dead bacilli to degradation, stationary lung infection in mice is associated with the presence of a stationary number of chromosome equivalents of M. tuberculosis DNA.
Acknowledgments
This work was supported by NIH grants AI-37844 and HL-64565 and by NSF grants 0116165 and 0420639.
Abbreviations
- Ag85B
antigen 85B
- APC
antigen-presenting cell
- BCG
bacillus Calmette–Guérin
- CFU
colony-forming unit
- DEPC
diethylpyrocarbonate
- ESAT-6
early secretory antigen-6
- FCS
fetal calf serum
- IFN-γ
interferon-γ
- mAb
monoclonal antibody
- PBS
phosphate-buffered saline
- RT-PCR
reverse transcription-polymerase chain reaction
References
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 3.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 4.Huygen K. On the use of DNA vaccines for the prophylaxis of mycobacterial diseases. Infect Immun. 2003;71:1613–21. doi: 10.1128/IAI.71.4.1613-1621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton WJ, Palendira U. Improving vaccines against tuberculosis. Immunol Cell Biol. 2003;81:34–45. doi: 10.1046/j.0818-9641.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- 6.Brandt L, Oettinger T, Holm A, Andersen AB, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–33. [PubMed] [Google Scholar]
- 7.Roy S, Ajitkumar P. Transcriptional analysis of the principal cell division gene, ftsZ, of Mycobacterium tuberculosis. J Bacteriol. 2005;187:2540–50. doi: 10.1128/JB.187.7.2540-2550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2005;201:1915–24. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Souza S, Rosseels V, Romano M, et al. Mapping of murine Th1 helper T-Cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect Immun. 2003;71:483–93. doi: 10.1128/IAI.71.1.483-493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–2. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 11.Shi L, Jung YJ, Tyagi S, Gennaro ML, North RJ. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci USA. 2003;100:241–6. doi: 10.1073/pnas.0136863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winslow GM, Roberts AD, Blackman MA, Woodland DL. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J Immunol. 2003;170:2046–52. doi: 10.4049/jimmunol.170.4.2046. [DOI] [PubMed] [Google Scholar]
- 13.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;62:5407–16. [PubMed] [Google Scholar]
- 14.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scanga CA, Mohan VP, Yu K, Joseph H, Tanaka K, Chan J, Flynn JL. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–58. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4+ T cell response. J Exp Med. 2005;201:1555–65. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guptasarma P. Does replication-induced transcription regulate synthesis of the myriad low copy number proteins of Escherichia coli? Bioessays. 1995;17:987–97. doi: 10.1002/bies.950171112. [DOI] [PubMed] [Google Scholar]
- 18.Fey A, Eichler S, Flavier S, Christen R, Hofle MG, Guzman CA. Establishment of a real-time PCR-based approach for accurate quantification of bacterial RNA targets in water, using Salmonella as a model organism. Appl Environ Microbiol. 2004;70:3618–23. doi: 10.1128/AEM.70.6.3618-3623.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meckenstock R, Steinle P, van der Meer JR, Snozzi M. Quantification of bacterial mRNA involved in degradation of 1,2,4-trichlorobenzene by Pseudomonas sp. strain 51 from liquid culture and from river sediment by reverse transcriptase PCR (RT/PCR) FEMS Microbiol Lett. 1998;167:123–9. doi: 10.1111/j.1574-6968.1998.tb13217.x. [DOI] [PubMed] [Google Scholar]
- 20.Garrido T, Sanchez M, Palacios P, Aldea M, Vicente M. Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J. 1993;12:3957–65. doi: 10.1002/j.1460-2075.1993.tb06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko MSH. Induction mechanism of a single gene molecule: stochastic or deterministic? Bioessays. 1992;14:341–6. doi: 10.1002/bies.950140510. [DOI] [PubMed] [Google Scholar]
- 22.McAdams HH, Arkin A. Stochastic mechanisms in gene expression. Proc Natl Acad Sci USA. 1997;94:814–19. doi: 10.1073/pnas.94.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiering S, Whitelaw E, Martin DIK. To be or not to be active: the stochastic nature of enhancer action. Bioessays. 2000;22:381–7. doi: 10.1002/(SICI)1521-1878(200004)22:4<381::AID-BIES8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–6. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 25.Schnappinger D, Ehrt S, Voskuil MI, et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrt S, Schnappinger D, Bekiranov S, et al. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med. 2001;194:1123–40. doi: 10.1084/jem.194.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med. 2003;198:705–13. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette–Guerin attenuation. J Infect Dis. 2003;187:117–23. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–70. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodin P, de Jonge MI, Majlessi L, Leclerc C, Nilges M, Cole ST, Brosch R. Functional analysis of ESAT-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex-formation, virulence and immunogenicity. J Biol Chem. 2005;280:33953–9. doi: 10.1074/jbc.M503515200. [DOI] [PubMed] [Google Scholar]
- 31.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature (London) 1991;354:161–4. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 32.Rothfield L, Justice S, Garcia-Lara J. Bacterial cell division. Annu Rev Genet. 1999;33:423–8. doi: 10.1146/annurev.genet.33.1.423. [DOI] [PubMed] [Google Scholar]
- 33.Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol. 2001;55:139–63. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 34.Munoz-Elias EJ, Timm J, Botha T, Chan WT, Gomez JE, McKinney JD. Replication dynamics of Mycobacterium tuberculosis in chronically infected mice. Infect Immun. 2005;73:546–51. doi: 10.1128/IAI.73.1.546-551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]