Abstract
Lyme borreliosis is a tick-borne disease often manifesting as a circular skin lesion. This cutaneous form of the disease is known as erythema migrans. In a 5-year follow-up study in southern Sweden, 31 of 708 individuals initially diagnosed with erythema migrans and treated with antibiotics were found to be reinfected with Borrelia burgdorferi. Although men and women were tick-bitten to the same extent, 27 of the 31 reinfected individuals were women, all of whom were over 44 years of age. The aim of this study was to determine whether this discrepancy in gender distribution could be a result of differences in immunological response. Twenty single-infected and 21 reinfected women and 18 single-infected and three reinfected men were included in the study. None of the participants showed any sign of an ongoing B. burgdorferi infection, and thus the habitual response was captured. Lymphocytes were separated from blood and stimulated with antigens. The secretion of interleukin (IL)-4, IL-6, IL-10, interferon (IFN)-γ and tumour necrosis factor (TNF)-α was measured by enzyme-linked immunosorbent assay (ELISA), enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) or Immulite. No difference was detected in cytokine secretion between single-infected and reinfected individuals. We also compared the immunological response in men and women, regardless of the number of B. burgdorferi infections. Women displayed a significantly higher spontaneous secretion of all cytokines measured. The ratios of IL-4:IFN-γ and IL-10:TNF-α were significantly higher in women. Gender differences in immune reactivity might in part explain the higher incidence of reinfection in women. The higher IL-4:IFN-γ and IL-10:TNF-α ratios seen in women indicate that postmenopausal women have T helper type 2 (Th2)-directed reactivity with impaired inflammatory responses which might inhibit the elimination of spirochetes.
Keywords: cytokine, erythema migrans, gender, Lyme borreliosis, postmenopausal
Introduction
Lyme borreliosis (LB) is the most common vector-borne disease in Europe, with over 70% of the cases represented by the cutaneous form erythema migrans (EM).1 Several studies confirm that reinfections, i.e. new episodes of LB, can occur in humans.2–7 In a 5-year follow-up study of individuals diagnosed with EM and treated with antibiotics in southern Sweden, 31 out of 708 individuals were found to be reinfected, i.e. had one or several new EM that required a doctor's visit and treatment with antibiotics.3 Although women and men were tick-bitten to the same extent, 27 of these 31 individuals were women, and all of these women were older than 44 years.3 This observation could in part be explained by host immune mechanisms acting differently either in women compared with men, or in reinfected compared with single-infected individuals. Women are known to display different immune responses from men, as strongly indicated by the gender differences in susceptibility to autoimmune diseases.8,9
In terms of the overall distribution of LB there is no gender difference, but, if age is taken into consideration, males in the USA aged 5–19 and ≥60 years have a higher incidence than females in the same age groups.10 By contrast, in the Swedish population aged ≥ 45 years, more women than men develop LB.1
The immune response is produced by interactions between immune cells that, to a large extent, communicate by secretion of cytokines. Tumour necrosis factor (TNF)-α is a pro-inflammatory cytokine which, for example, induces fever, activates endothelial cells and stimulates proliferation of cytotoxic T cells.11 Interleukin (IL)-6 is a pleiotropic cytokine which, for example, initiates production of acute-phase proteins12 and elevates B-cell maturation, thus promoting immunoglobulin production.13 IL-6 also induces IL-4 secretion, thereby polarizing naïve T helper (Th) cells to Th2 effector cells.14 IL-10, in contrast, is a regulatory cytokine with mainly anti-inflammatory properties, such as reduction of the antigen-presenting ability of macrophages.15
Previous studies have shown that the most favourable immune response to an infection with Borrelia burgdorferi, the spirochete causing LB, is of Th1 type with secretion of the cytokine interferon (IFN)-γ.16–20 It seems important that this Th1-like response occurs at an early stage21 and is accompanied by a pro-inflammatory response.22 If, however, the pro-inflammatory response is lacking and the individual responds with a Th2-type pattern, including secretion of IL-4, the infection is not cleared as quickly.21,22
The aim of the study was to determine whether host immune status could explain the increased risk of LB reinfection in women. To this end, we analysed the habitual (i.e. several years after infection) host immune response with the enzyme-linked immunosorbent spot-forming cell assay (ELISPOT), the enzyme-linked immunosorbent assay (ELISA) and Immulite in terms of cytokine secretion patterns of blood cells in vitro and compared responses between individuals who were reinfected with LB and individuals who were single-infected with LB and also between genders. We also investigated possible confounding health factors that might have influenced the uneven distribution of reinfection in women and men.
Materials and methods
Study design and population
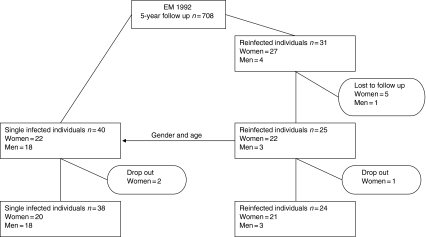
This was a case–control study carried out in southern Sweden based on the 5-year follow up of 708 individuals diagnosed with EM and treated with antibiotics in 1992. None of the patients developed late disseminated forms of LB but 27 women and four men were reinfected, i.e. had a new B. burgdorferi infection resulting in an EM that required a doctor's visit, during the observation period.3 Inclusion criteria were a description of the clinical appearance of the EM, an EM lesion size of at least 5 cm in diameter, a prescription of antibiotics, and information that the EM was preceded by a probable tick bite.3 Serological analyses were not performed because of their limited diagnostic value.6,23
Of the reinfected individuals, twenty-two women and three men participated in the present study, i.e. 81% (25 of 31 patients). They were matched with single-infected individuals according to gender and age. To each reinfected woman we matched one single-infected woman, but to the reinfected men we matched 18 single-infected men because of the lower number of reinfected men (Fig. 1). The reinfected individuals had had one or several reinfections with EM since the initial EM infection in 1992, whereas the single-infected individuals had not had a new EM since 1992. A blood sample was drawn from all participants. The sampling was performed in the period from the autumn of 2002 until the summer of 2003 at different hospitals and blood was transported to the University Hospital in Linköping for analysis within 5 hr.
Figure 1.
Schematic illustration of the participant flow.
The participants answered a health questionnaire including questions about medication and diseases (i.e. occurrence of chronic disease, immediate hypersensitivity reactions, cell-mediated immunity reactions, heredity for autoimmune disease, and heredity for hypersensitivity- or cell-mediated reactions). If necessary, patients were contacted by phone for complementary information.
Two single-infected women and one reinfected woman declined to participate in the study; the final group thus consisted of 21 reinfected women, three reinfected men, 20 single-infected women and 18 single-infected men (Fig. 1).
Preparation of mononuclear cells
Peripheral blood mononuclear cells were separated from heparinized blood using gradient centrifugation on Lymphoprep (Medinor AB, Lidingö, Sweden) as previously described.16 The cells were counted by phase-contrast microscope and the cell density was adjusted to 1 × 106 lymphocytes/ml.
Antigens
In the ELISPOT assay, cells were stimulated with the following antigens: an outer surface protein-enriched fraction of Borrelia garinii strain Ip90 (OF Ip90),21 which previously had been proved to discriminate between healthy and B. burgdorferi-infected individuals,18 in a final concentration of 10 µg/ml, a purified protein derivate of tuberculin (PPD) in a final concentration of 10 µg/ml (Statens Serum Institut, Copenhagen, Denmark) and phytohaemagglutinin (PHA) in a final concentration of 20 µg/ml (Sigma Aldrich, Stockholm, Sweden).
In the ELISA and Immulite assays, the following antigens were used in the primary cell cultures, with final concentrations indicated in parentheses: OF Ip90 (10 µg/ml), peptidoglycan from Staphylococcus aureus (0·01 mg/ml; Sigma-Aldrich) and PHA (20 µg/ml; Sigma Aldrich). OFI p90 was included to trigger memory responses of T cells (IL-4 and IFN-γ ELISPOT) or innate immune responses of mainly monocytes/macrophages (ELISA and Immulite). PPD was included as an unrelated recall antigen and peptidoglycan as another stimulant of innate immune responses with different Toll-like receptor signalling pathways.
ELISPOT
The ELISPOT assay used was performed as described in detail elsewhere.16,18 The plates were coated with the monoclonal antibodies anti-IFN-γ, anti-IL-4 and anti-IL-10 (Mabtech AB, Nacka, Sweden) diluted in sterile phosphate-buffered saline (PBS) to a final concentration of 15 µg/ml. The plates were incubated at 4° overnight and then frozen at −20°.
The coated plates were thawed at 37° and 100 000 lymphocytes/well were added. The spontaneous secretion and stimulation procedures were performed in triplicate. As a negative control, wells were filled with cell culture medium only (i.e. no cells). The plates were incubated at 37° with 5% CO2 and 95% humidity for 48 hr.
The plates were developed with paired monoclonal antibodies anti-IFN-γ, anti-IL-4 and anti-IL-10, conjugated with biotin (Mabtech AB) diluted in PBS-Tween to a final concentration of 1 µg/ml, streptavidin conjugated with alkaline phosphatase (Mabtech AB) diluted 1 : 1000 in PBS-Tween and finally AP Color Development Reagent NBT and BCIP diluted in AP buffer (AP Conjugate Substrate Kit; Bio-Rad Laboratories AB, Sundbyberg, Sweden), with washings between all steps.
The spots were counted by the same person (EY) using AID EliSpot Reader System version 2·6 (AID, Strassberg, Germany).
Cell culture for ELISA and Immulite
Lymphocytes were cultured at a cell density of 1 × 106 cells/ml in tissue culture medium (TCM) consisting of Iscove's modification of Dulbecco's medium (Invitrogen, Taby, Sweden) supplemented with 3024 g/l NaHCO3, 292 mg/l l-glutamine (Sigma Aldrich), 50 IU/ml penicillin (In Vitro Sweden AB, Stockholm, Sweden), 50 µg/ml streptomycine (In Vitro), 10 ml/l 100× non-essential amino acids (Invitrogen) and 5% heat-inactivated fetal bovine serum (Sigma Aldrich) and stimulated with antigens. The cells were cultured at 37° with 5% CO2 and 95% humidity for 48 hr. The supernatants were collected after centrifugation and stored at −70°.
ELISA
The ELISA assay for IL-10 detection was performed as previously described by Jenmalm et al.24 The amount of substrate converted to product was detected as absorbance at 450 nm in an Anthos htIII ELISA reader (Anthos Labtec Instruments, Salzburg, Austria). TCM was used as a negative control and for dilution of samples and standard.
Values were calculated from the absorbance of the standard curve after subtracting the negative controls. The sensitivity limit for quantitative determinations was 3·12 pg/ml.
Immulite
IL-6 and TNF-α were detected using Immulite IL-6 and Immulite TNF-α (Diagnostic Products Corporation, Mölndal, Sweden), according to the manufacturer's instructions. Briefly, granules coated with antibodies directed towards IL-6 or TNF-α were mixed with the samples. After washing, alkaline phosphatase-labelled antibodies were added. Free antibodies were washed away and chemiluminescent reagent was supplied. The reaction between alkaline phosphatase and the chemiluminescent reagent resulted in light production, which was measured in the Immulite Analyzer (Diagnostic Products Corporation).
Values were calculated from a standard curve. The sensitivity limit for quantitative determinations was 5·0 pg/ml for IL-6 and 1·7 pg/ml for TNF-α.
Statistical methods
When comparing the occurrence of health-related variables in different groups, we used Fisher's exact test. From the triplicate wells in ELISPOT, the median value for the number of spots was calculated, and for ELISA the mean value of the duplicate was used. The spontaneous secretion was subtracted to obtain the antigen-specific induced secretion. As data were not normally distributed, the non-parametric Mann–Whitney U-test was used to compare cytokine secretion between groups. To compare the age distribution in the groups, an independent sample t-test was used.
A P-value of <0·01 was considered significant for the laboratory and questionnaire data to compensate for multiple comparisons. However, for the comparison of age distribution, a P-value of <0·05 was considered significant.
Ethical considerations
The study was approved by the Ethical Committee at the University of Lund.
Results
The participants
None of the participants showed any sign of an ongoing B. burgdorferi infection. The mean age of the reinfected women was 68 years (standard deviation (SD) 9·9 years; range 51–81 years) and that of the single-infected women was 66 years (SD 9·6 years; range 53–85 years). The mean age of the reinfected men was 58 years (SD 22 years; range 43–84 years) and that of the single-infected men was 62 years (SD 11 years; range 43–78 years). There was no significant difference in age between women and men, between reinfected women and single-infected women, or between reinfected men and single-infected men.
All except two of the individuals included in this study had a C-reactive protein (CRP) level below 10 mg/l at the time of blood sampling. CRP was slightly elevated (12 mg/l) in one of the reinfected women and in one of the single-infected women (27 mg/l). All individuals were considered free of any inflammatory process that could have influenced the host immune response results.
The questionnaire
In the analysis of the questionnaire, we compared reinfected with single-infected individuals and women with men according to their current health status and medication. None of the participants had immunosuppressive therapy.
We could not find any differences in the history of the occurrence of chronic disease, immediate hypersensitivity reactions (i.e. mediated by IgE and mastcells, such as anaphylaxis, angioedema or wheal-flare reactions), cell-mediated immunity reactions (i.e. mediated by lymphocytes and lymphokines such as allergic contact dermatitis and delayed hypersensitivity reactions), heredity for autoimmune disease, heredity for hypersensitivity- or cell-mediated reactions, or the use of naturopathic preparations or drugs generally in comparing reinfected with single-infected individuals and women with men. We also compared reinfected women with single-infected women for the use of drugs containing hormones and age at menopause, but no differences were found between the two groups. All women except one (in the reinfected group) reported that they were in the menopause. Three women in the single-infected group and three women in the reinfected group were on oestrogen treatment.
Cytokine secretion
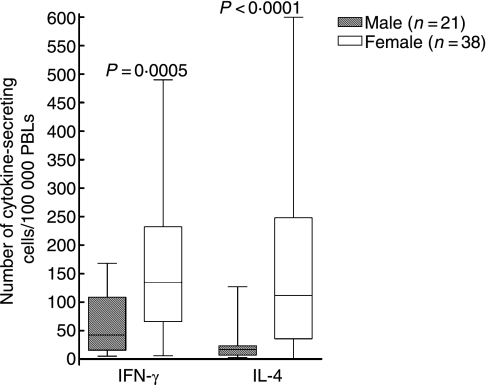
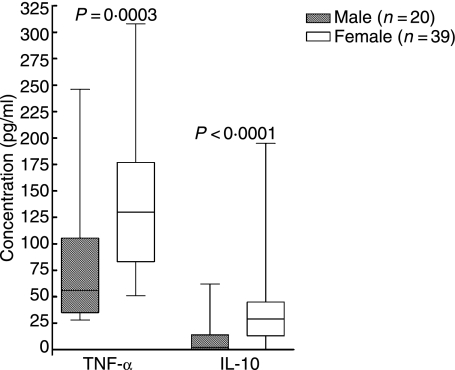
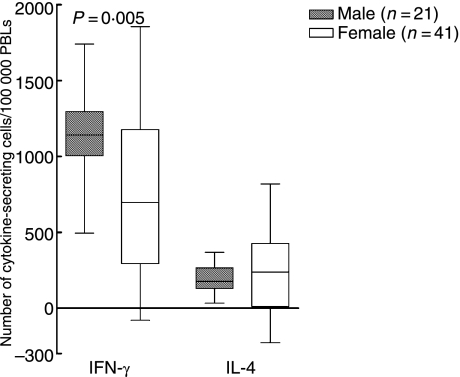
When reinfected individuals were compared with single-infected individuals, no difference was found regarding cytokine secretion (Tables 1 and 2). However, when men were compared with women, regardless of numbers of times infected with B. burgdorferi, we found that women had a higher number of cells spontaneously secreting IFN-γ (P = 0·0005; Fig. 2), IL-4 (P < 0·0001; Fig. 2) and IL-10 (P = 0·0004; Table 2), in analyses with ELISPOT. Women also showed a higher amount of spontaneous secretion of IL-6 (P = 0·0001; Table 1), IL-10 (P < 0·0001; Fig. 3) and TNF-α (P = 0·0003; Fig. 3) when measured with ELISA or Immulite. The men displayed a higher number of cells responding with IFN-γ secretion (P = 0·005; Fig. 4) when stimulated with PHA and with IL-10 secretion when stimulated with OFI p90 (P = 0·009; Table 2).
Table 1.
Comparison of the amount (pg/ml) of cytokine secreted by blood mononuclear cells between individuals reinfected or single-infected with Lyme borreliosis, and between men and women (regardless of number of Borrelia burgdorferi infections), as determined by enzyme-linked immunosorbent assay (ELISA) or Immulite
| Reinfected | Single-infected | Men | Women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Stimulation | Median | (Q1, Q3) | n* | Median | (Q1, Q3) | n* | P | Median | (Q1, Q3) | n* | Median | (Q1, Q3) | n* | p |
| IL-6 | Spontaneous | 1540 | (641, 2590) | 23 | 1069 | (77, 1743) | 36 | ns | 88 | (7, 1483) | 20 | 1540 | (682, 2560) | 39 | 0·0001 |
| Peptidoglycan | 17410 | (7269, 22020) | 13 | 14550 | (6893, 22710) | 29 | ns | 15399 | (9398, 24829) | 17 | 12750 | (4867, 22020) | 25 | ns | |
| OF Ip90 | 15703 | (6606, 22519) | 22 | 8300 | (6092, 13380) | 33 | ns | 7395 | (5034, 12770) | 17 | 12135 | (7086, 21327) | 38 | ns | |
| IL-10 | Spontaneous | 25 | (7, 41) | 23 | 16 | (1, 34) | 36 | ns | 2 | (< 3, 15) | 20 | 29 | (13, 45) | 39 | <0·0001* |
| Peptidoglycan | 697 | (146, 936) | 15 | 1011 | (763, 2476) | 25 | ns | 1016 | (808, 2546) | 16 | 768 | (345, 1413) | 24 | ns | |
| OF Ip90 | 218 | (131, 373) | 22 | 197 | (101, 363) | 33 | ns | 121 | (67, 336) | 17 | 227 | (148, 365) | 38 | ns | |
| TNF-α | Spontaneous | 115 | (80, 160) | 23 | 97 | (52, 166) | 36 | ns | 56 | (35, 107) | 20 | 130 | (83, 177) | 39 | 0·0003* |
| Peptidoglycan | 2227 | (1382, 3584) | 13 | 1349 | (833, 2390) | 29 | ns | 1813 | (980, 2976) | 17 | 1474 | (890, 3206) | 25 | ns | |
| OF Ip90 | 844 | (621, 1345) | 22 | 537 | (349, 704) | 33 | ns | 574 | (280, 765) | 17 | 642 | (439, 1325) | 38 | ns | |
Results are also displayed in Fig. 3.
Values are given as medians and the spread as the first and third quartiles (Q1, Q3). The Mann–Whitney U-test was used to compare reinfected with single-infected individuals and also to compare men with women.
Differences in numbers of individuals are a result of limited sample sizes in some cases.
IL, interleukin; ns, not significant; OF Ip90, outer surface protein-enriched fraction of Borrelia garinii strain Ip90; TNF, tumour necrosis factor.
Table 2.
Comparison of number of cytokine-secreting blood mononuclear cells between individuals reinfected or single-infected with Lyme borreliosis, and between men and women (regardless of number of Borrelia burgdorferi infections), as determined by enzyme-linked immunosorbent spot-forming cell assay (ELISPOT)
| Reinfected | Single-infected | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 24) | (n = 38) | (n = 21) | (n = 41) | ||||||||
| Cytokine | Stimulation | Median | (Q1, Q3) | Median | (Q1, Q3) | P | Median | (Q1, Q3) | Median | (Q1, Q3) | P |
| IFN-γ | Spontaneous* | 110 | (58, 222) | 102 | (21, 169) | ns | 42 | (16, 109) | 135 | (68, 231) | 0.0005† |
| PHA | 824 | (348, 1286) | 1052 | (492, 1204) | ns | 1143 | (1005, 1297) | 697 | (294, 1178) | 0.005‡ | |
| PPD | 10 | (−18, 39) | 14 | (−6, 35) | ns | 20 | (1, 50) | 8 | (−23, 36) | ns | |
| OF Ip90 | 20 | (−8, 58) | 12 | (−2, 31) | ns | 12 | (5, 26) | 19 | (−13, 46) | ns | |
| IL-4 | Spontaneous* | 68 | (27, 163) | 34 | (9, 158) | ns | 17 | (7, 24) | 112 | (36, 243) | <0.0001† |
| PHA | 287 | (28, 422) | 183 | (75, 275) | ns | 177 | (130, 266) | 238 | (11, 426) | ns‡ | |
| PPD | −5 | (−23, 6) | −5 | (−15, 4) | ns | 0 | (−8, 6) | −9 | (−57, 1) | ns | |
| OF Ip90 | −1 | (−13, 11) | 2 | (−4, 7) | ns | 1 | (−3, 4) | 0 | (−13, 17) | ns | |
| IL-10 | Spontaneous* | 750 | (436-1024) | 550 | (113, 813) | ns | 156 | (78, 697) | 738 | (431, 1006) | 0.0004 |
| PHA | 244 | (62, 425) | 263 | (44, 729) | ns | 341 | (80, 809) | 172 | (34, 423) | ns | |
| PPD | 32 | (−18, 105) | 65 | (4, 266) | ns | 133 | (8, 564) | 36 | (−19, 104) | ns | |
| OF Ip90 | 47 | (−17, 122) | 60 | (−5, 291) | ns | 145 | (32, 656) | 45 | (−17, 119) | 0.009 | |
The spontaneous secretion was subtracted to obtain the specific secretion. Values are given as medians and the spread as the first and third quartiles (Q1, Q3). The Mann–Whitney U-test was used to compare reinfected with single-infected individuals and also to compare men with women.
Reinfected, n = 22; single-infected, n = 37; women, n = 38.
Results are also displayed in Fig. 2.
Results are also displayed in Fig. 4.
IFN, interferon; ns, not significant; IL, interleukin; PHA, phytohaemagglutinin; PPD, purified protein derivate of tuberculin; OF Ip90, outer surface protein-enriched fraction of Borrelia garinii strain Ip90.
Figure 2.
Number of spontaneously cytokine-secreting cells/100 000 peripheral blood lymphocytes (PBLs) detected by enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). P-values show statistically significant differences using the Mann–Whitney U-test. The median (line), interquartile range (box) and maximum–minimum (whiskers) are marked. IFN, interferon; IL, interleukin.
Figure 3.
Amount (pg/ml) of cytokine spontaneously secreted from peripheral blood lymphocytes (PBLs) detected by enzyme-linked immunosorbent assay (ELISA) [interleukin (IL)-10] or Immulite [tumour necrosis factor (TNF)-α]. P-values show statistically significant differences using the Mann–Whitney U-test. The median (line), interquartile range (box) and maximum–minimum (whiskers) are marked.
Figure 4.
Number of phytohaemagglutinin (PHA)-induced cytokine-secreting cells/100 000 peripheral blood lymphocytes (PBLs) detected by enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). Values are net values, where the number of spontaneously cytokine-secreting cells has been subtracted. P-values show statistically significant differences using the Mann–Whitney U-test. The median (line), interquartile range (box) and maximum–minimum (whiskers) are marked. IFN, interferon; IL, interleukin.
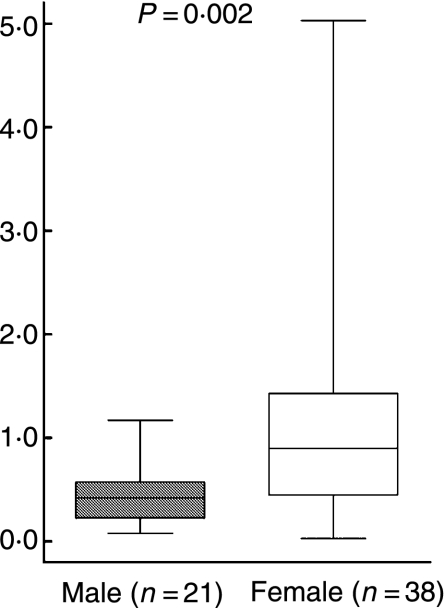
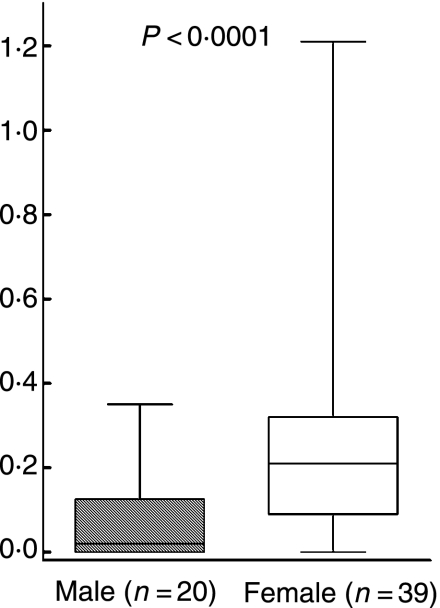
To examine the Th1/Th2 balance, the ratio of cells spontaneously secreting IL-4 to those spontaneously secreting IFN-γ was calculated, and to assess the pro-inflammatory/anti-inflammatory balance, the ratio of cells spontaneously secreting IL-10 to those spontaneously secreting TNF-α was calculated. The women had significantly higher ratios than the men regarding spontaneous secretion for both IL-4/IFN-γ (P = 0·002; Fig. 5) and IL-10/TNF-α (P < 0·0001; Fig. 6).
Figure 5.
Ratio of the number of spontaneously IL-4 to IFN-γ-secreting cells/100 000 peripheral blood lymphocytes (PBLs) detected by enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). P-values show statistically significant differences using the Mann–Whitney U-test. The median (line), interquartile range (box) and maximum-minimum (whiskers) are marked.
Figure 6.
Ratio of the amount (pg/ml) of IL-10 to IFN-α spontaneously secreted from peripheral blood lymphocytes (PBLs) detected by enzyme-linked immunosorbent assay (ELISA) [interleukin (IL)-10] or Immulite [tumour necrosis factor (TNF)-α]. P-values show statistically significant differences using the Mann–Whitney U-test. The median (line), interquartile range (box) and maximum–minimum (whiskers) are marked.
Discussion
We investigated the immune response in individuals who, during a 5-year period (1993–1998), were reinfected with LB and developed EM, and compared these individuals with those who, during the same period, were only infected once. The reinfected and single-infected individuals were matched according to gender and age. None of the participants showed any sign of an ongoing B. burgdorferi infection at the time of this study. Mononuclear cells were stimulated in vitro with Borrelia antigen, the recall antigen PPD, the mitogen PHA and the bacterial cell wall component peptidoglycan. Cytokine secretion was measured with ELISPOT as number of cytokine-secreting cells and with ELISA or Immulite as concentration of cytokine. The cytokines were chosen to represent both innate and adaptive levels of the immune response. In addition, we also studied possible confounding health factors.
We did not find a difference in the immunological response or in the confounding health factors between reinfected and single-infected individuals. Given that the reinfected group consisted primarily of women, we also compared men with women, regardless of number of B. burgdorferi infections, to explore whether there were differences in immune responses between men and women that could explain why mainly women were reinfected. No difference was detected in the use of medication or health status between men and women. The latter is surprising as it is known that, for example, women to a greater extent than men suffer from autoimmune diseases.9 The sample size in our study might have been too small to detect these differences in occurrence of uncommon disease. However, for the immunological response there were major differences between men and women. The women displayed higher spontaneous secretion of all cytokines measured, i.e. IL-4, IL-6, IL-10, IFN-γ and TNF-α. Spontaneous secretion, at an infection-free time-point, reflects the habitual immune status and may suggest what type of immunological defence an individual generally displays. For instance, allergy has been considered a Th2-type related condition and, accordingly, atopic individuals have higher spontaneous IL-4 expression than non-atopic controls.25
Women of reproductive age are believed to handle infections better than men, having a stronger tendency to show Th1-type responses and expression of higher levels of pro-inflammatory cytokines, and they also develop higher antibody titres than men when vaccinated.9,26 However, the female immune response fluctuates with the menstrual cycle. In general, oestrogen has a stimulatory effect on the immune system whereas testosterone acts as a suppressor. When women enter the menopause their levels of oestrogen decrease and thereby the stimulatory effect diminishes, leading to an altered immune status.27 All except one of the women in our study were postmenopausal, and this could be a factor explaining why more women than men became reinfected with B. burgdorferi.
Although the immune system, in terms of cytokine secretion pattern, significantly differed between men and women in this study, the functional consequences must be interpreted carefully because cytokines may have different functions at different concentrations and in different circumstances.28
Because cytokines work in a complex network and stimulate or inhibit one another, it is important to consider not only the concentrations of particular cytokines, but also how they interrelate. IFN-γ and IL-4 are classical examples of antagonistic signature cytokines for Th1 and Th2, respectively, and their ratio mirrors the Th1/Th2 balance.29 Also, the ratio of the anti-inflammatory IL-10 and the pro-inflammatory TNF-α was evaluated to elucidate the balance between inflammatory and anti-inflammatory forces. The findings of increased IL-10/TNF-α ratios, indicating an amplified anti-inflammatory response, and higher IL-4/IFN-γ ratios in women compared with men strongly indicate that postmenopausal women, despite their strong spontaneous secretion of cytokines, might have a Th2-deviated immune response as well as an impaired pro-inflammatory response. This possibility is further supported by the finding of increased PHA-induced IFN-γ responses in men. PHA is a mitogen that activates T cells polyclonally via binding to the T-cell receptor and CD2.30 CD2 ligation induces IFN-γ secretion in T cells31 which in turn induces IL-12 secretion by macrophages. Thus, PHA can be considered as a mainly Th1-inducing agent, and PHA stimulation could be used as a tool for evaluation of the ability to mount strong Th1 responses; for example, children with atopic heredity have been found to display lower PHA responsiveness than children without such heredity.32
Taken together with our previous observations of strong inflammatory responses combined with strong Th1 responses being beneficial for eradication of B. burgdorferi,16,17,21,22 the present findings of Th2 and anti-inflammatory responses in postmenopausal women may partly explain the increased frequency of recurrent LB infection in this group of individuals.
We observed that the men displayed a higher number of Borrelia-specific IL-10-secreting cells than the women. However, we did not find a similar difference when the amount of IL-10 was investigated, i.e. measured with ELISA. The relevance of this finding is uncertain and needs to be further explored.
An alternate or complementary explanation for the increased frequency of reinfection in women is reinfection with a different strain of B. burgdorferi, which because of antigenic variation evades the memory cells generated in the primary infection.29 The participants in our study were initially included in a retrospective, long-term follow-up study3 that was not designed to include biopsy. Therefore, phenotyping was not possible. There is, however, no obvious reason why postmenopausal women should be more frequently exposed to different strains of B. burgdorferi than men. Neither could difference in behaviour pattern explain the uneven gender distribution in LB reinfections, as men and women were tick-bitten to the same extent.3
We analysed the immunological response several years after the infection, and a possible objection to our findings is therefore that the results we obtained are of no importance in terms of the acute response. The immune system does change during the lifespan. These changes, however, occur slowly, except during puberty and menopause, and therefore we assume that no substantial variation has occurred in the immune response between the primary infection and the time of cytokine analyse for the individuals in this study.
Serology was not performed on the individuals in this study because, at the time of EM diagnosis, only 30–40% of patients displayed antibodies to Borrelia.6,23 Studies following patients with culture-confirmed EM have shown that, although antibodies can be detected 10–20 years after initial infection,33 titres decline gradually during the first year.23
In conclusion, we could not find any significant difference in the immune response or in confounding health factors between individuals who were reinfected with LB and individuals who were single-infected with LB. We did, however, find a gender difference in the immunological response, with women displaying higher spontaneous secretion of all cytokines measured, i.e. IL-4, IL-6, IL-10, IFN-γ and TNF-α. The increases in the IL-4/IFN-γ and IL-10/TNF-α ratios compared with men suggest that postmenopausal women have a Th2-directed and anti-inflammatory immune reactivity with increased IL-10-mediated suppression of the pro-inflammatory response compared with men. This may in part explain the increased frequency of reinfection in postmenopausal women.
Acknowledgments
We are most grateful to Professor Sven Bergström, Department of Microbiology, Umeå University, Sweden for kindly supplying us with B. burgdorferi. We would also like to thank the staff at the hospitals in Kalmar, Malmö and Linköping for helping with the collection of blood samples and Maria Petersson for excellent laboratory assistance.
This work was supported by the Medical Research Council of South-East Sweden, the County Council of Blekinge, Sweden, the Health Research Council in the South-East of Sweden and the Swedish Research Council (K2005-06X-14631-03A).
References
- 1.Berglund J, Eitrem R, Ornstein K, et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–27. doi: 10.1056/NEJM199511163332004. [DOI] [PubMed] [Google Scholar]
- 2.Asch ES, Bujak DI, Weiss M, Peterson MG, Weinstein A. Lyme disease: an infectious and postinfectious syndrome. J Rheumatol. 1994;21:454–61. [PubMed] [Google Scholar]
- 3.Bennet L, Berglund J. Reinfection with Lyme borreliosis: a retrospective follow-up study in southern Sweden. Scand J Infect Dis. 2002;34:183–6. doi: 10.1080/00365540110080070. [DOI] [PubMed] [Google Scholar]
- 4.Berglund J, Eitrem R, Norrby SR. Long-term study of Lyme borreliosis in a highly endemic area in Sweden. Scand J Infect Dis. 1996;28:473–8. doi: 10.3109/00365549609037943. [DOI] [PubMed] [Google Scholar]
- 5.Salazar JC, Gerber MA, Goff CW. Long-term outcome of Lyme disease in children given early treatment. J Pediatr. 1993;122:591–3. doi: 10.1016/s0022-3476(05)83541-3. [DOI] [PubMed] [Google Scholar]
- 6.Nowakowski J, Nadelman RB, Sell R, et al. Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med. 2003;115:91–6. doi: 10.1016/s0002-9343(03)00308-5. [DOI] [PubMed] [Google Scholar]
- 7.Nowakowski J, Schwartz I, Nadelman RB, Liveris D, Aguero-Rosenfeld M, Wormser GP. Culture-confirmed infection and reinfection with Borrelia burgdorferi. Ann Intern Med. 1997;127:130–2. doi: 10.7326/0003-4819-127-2-199707150-00006. [DOI] [PubMed] [Google Scholar]
- 8.Lockshin MD. Sex ratio and rheumatic disease. Isr Med Assoc J. 2001;3:511–6. [PubMed] [Google Scholar]
- 9.Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–8. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 10.Orloski KA, Hayes EB, Campbell GL, Dennis DT. Surveillance for Lyme disease – United States 1992–98. MMWR CDC Surveill Summ. 2000;49:1–11. [PubMed] [Google Scholar]
- 11.Borish LC, Steinke JW. 2, Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–75. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 12.Gregory MS, Duffner LA, Faunce DE, Kovacs EJ. Estrogen mediates the sex difference in post-burn immunosuppression. J Endocrinol. 2000;164:129–38. doi: 10.1677/joe.0.1640129. [DOI] [PubMed] [Google Scholar]
- 13.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–75. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 14.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekerfelt C, Ernerudh J, Bunikis J, et al. Compartmentalization of antigen specific cytokine responses to the central nervous system in CNS borreliosis: secretion of IFN-gamma predominates over IL-4 secretion in response to outer surface proteins of Lyme disease Borrelia spirochetes. J Neuroimmunol. 1997;79:155–62. doi: 10.1016/s0165-5728(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 17.Ekerfelt C, Forsberg P, Svenvik M, Roberg M, Bergström S, Ernerudh J. Asymptomatic Borrelia-seropositive individuals display the same incidence of Borrelia-specific interferon-gamma (IFN-gamma)-secreting cells in blood as patients with clinical Borrelia infection. Clin Exp Immunol. 1999;115:498–502. doi: 10.1046/j.1365-2249.1999.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsberg P, Ernerudh J, Ekerfelt C, Roberg M, Vrethem M, Bergström S. The outer surface proteins of Lyme disease borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFN-gamma): diagnostic and pathogenic implications. Clin Exp Immunol. 1995;101:453–60. doi: 10.1111/j.1365-2249.1995.tb03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–11. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oksi J, Savolainen J, Pene J, Bousquet J, Laippala P, Viljanen MK. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with Lyme borreliosis. Infect Immun. 1996;64:3620–3. doi: 10.1128/iai.64.9.3620-3623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widhe M, Jarefors S, Ekerfelt C, Vrethem M, Bergström S, Forsberg P, Ernerudh J. Borrelia-specific interferon-gamma and interleukin-4 secretion in cerebrospinal fluid and blood during Lyme borreliosis in humans: association with clinical outcome. J Infect Dis. 2004;189:1881–91. doi: 10.1086/382893. [DOI] [PubMed] [Google Scholar]
- 22.Widhe M, Grusell M, Ekerfelt C, Vrethem M, Forsberg P, Ernerudh J. Cytokines in Lyme borreliosis: lack of early tumour necrosis factor-alpha and transforming growth factor-beta1 responses are associated with chronic neuroborreliosis. Immunology. 2002;107:46–55. doi: 10.1046/j.1365-2567.2002.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomholt H, Lebech AM, Hansen K, Brandrup F, Halkier-Sorensen L. Long-term serological follow-up of patients treated for chronic cutaneous borreliosis or culture-positive erythema migrans. Acta Dermatol Venereol. 2000;80:362–6. doi: 10.1080/000155500459312. [DOI] [PubMed] [Google Scholar]
- 24.Jenmalm MC, Van Snick J, Cormont F, Salman B. Allergen-induced Th1 and Th2 cytokine secretion in relation to specific allergen sensitization and atopic symptoms in children. Clin Exp Allergy. 2001;31:1528–35. doi: 10.1046/j.1365-2222.2001.01190.x. [DOI] [PubMed] [Google Scholar]
- 25.Esnault S, Benbernou N, Lavaud F, Shin HC, Potron G, Guenounou M. Differential spontaneous expression of mRNA for IL-4, IL-10, IL-13, IL-2 and interferon-gamma (IFN-gamma) in peripheral blood mononuclear cells (PBMC) from atopic patients. Clin Exp Immunol. 1996;103:111–8. doi: 10.1046/j.1365-2249.1996.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Struve J, Aronsson B, Frenning B, Granath F, von Sydow M, Weiland O. Intramuscular versus intradermal administration of a recombinant hepatitis B vaccine: a comparison of response rates and analysis of factors influencing the antibody response. Scand J Infect Dis. 1992;24:423–9. doi: 10.3109/00365549209052627. [DOI] [PubMed] [Google Scholar]
- 27.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–84. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 28.O'Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 29.Barthold SW. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect Immun. 1999;67:36–42. doi: 10.1128/iai.67.1.36-42.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Flynn K, Krensky AM, Beverley PC, Burakoff SJ, Linch DC. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature. 1985;313:686–7. doi: 10.1038/313686a0. [DOI] [PubMed] [Google Scholar]
- 31.Wingren AG, Dahlenborg K, Bjorklund M, et al. Monocyte-regulated IFN-gamma production in human T cells involves CD2 signaling. J Immunol. 1993;151:1328–36. [PubMed] [Google Scholar]
- 32.Holt PG, Somerville C, Baron-Hay MJ, Holt BJ, Sly PD. Functional assessment of CD2, CD3 and CD28 on the surface of peripheral blood T-cells from infants at low versus high genetic risk for atopy. Pediatr Allergy Immunol. 1995;6:80–4. doi: 10.1111/j.1399-3038.1995.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 33.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis. 2001;33:780–5. doi: 10.1086/322669. [DOI] [PubMed] [Google Scholar]