Abstract
Toll-like receptors (TLRs) are pattern recognition receptors (PRRs) that recognize conserved pathogen-associated molecular patterns (PAMPs) synthesized by micro-organisms. Despite the essential requirement for TLRs in prokaryotic infection, the pattern and regulation of TLR gene expression by Trichomonas vaginalis in the mucocutaneous barrier are still unknown. Our hypothesis is that T. vaginalis-infected epithelial cells are major effector cells in the skin barrier. These cells function as a central regulator of TLR gene expression, thus accelerating the process of barrier dysfunction via increased release of chemokines and proinflammatory cytokines. To test this hypothesis, RT-PCR was performed on TLRs, interleukin (IL)-8 and tumour necrosis factor (TNF)-α. Stimulation of HeLa cells by T. vaginalis was observed to up-regulate TLR2, 4 and 9 mRNA expression as well as that of IL-8 and TNF-α. To further clarify the molecular mechanism of barrier devastation triggered by these up-regulatory stimuli, we examined the profiles of extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB activation in HeLa cells using specific inhibitors. Interestingly, pretreatment of HeLa cells with the p38 MAPK inhibitor SB203580 demonstrated inhibition of T. vaginalis-induced up-regulation of TLR2, 4, and 9 mRNA expression. By contrast, inhibition of ERK or NF-κB activation failed to block T. vaginalis-induced up-regulation of TLR9 mRNA expression or TLR2 and TLR4 mRNA expression, respectively. In addition, pretreatment with SB203580 reduced epithelium-derived IL-8 and TNF-α release evoked by T. vaginalis. Our results show that T. vaginalis infection of the mucocutaneous barrier could up-regulate TLR2, 4 and 9 gene expression via the p38 MAPK signalling pathway in epithelial cells; this process then leads to modulation of p38 MAPK-dependent IL-8 and TNF-α release from the epithelium.
Keywords: HeLa cells, p38 MAPK, TLRs, Trichomonas vaginalis
Introduction
Trichomonas vaginalis is a flagellated protozoan parasite which infects the human genito-urinary tract. It is the causative organism of trichomoniasis, one of the most prevalent sexually transmitted diseases (STDs) in the world. Emerging epidemiological data have implicated T. vaginalis infection in a higher incidence of human immunodeficiency virus (HIV) infection.1 As a plausible mechanism of trichomoniasis with the sexual transmission of HIV-1, it has been proposed that disruption of urogenital epithelial monolayers by T. vaginalis could facilitate passage of HIV-1 to underlying layers. Therefore, interaction between T. vaginalis and epithelial cells is crucial to the pathogenicity of T. vaginalis itself, as well as super-infection with HIV or an equivalent epithelium-breaching pathogen.
Epithelial cells are known to be an efficient protective barrier against infection, and to induce initiation of an innate immune response. For instance, adhesion of T. vaginalis to vaginal epithelial cells (VECs) is an essential primary step in infection with trichomoniasis.2 There are several innate pathogenic molecules, including chemokines and cytokines such as monocyte chemoattractant protein 1 (MCP-1), interleukin (IL)-8, IL-6, and tumour necrosis factor (TNF)-α). Of these, IL-8 is one of major chemoattractants of neutrophils in the mucocutaneous tissues found during an immune response. Like other prototypic epithelium-derived cytokines, TNF-α regulates the survival and death of epithelial cells. Accumulating evidence suggests that these two cytokines might be of prime importance in the local innate defence against T. vaginalis.3–5
In this context of local epithelial defence, Toll-like receptors (TLRs) are the main surface linker between host innate and adaptive immune defences.6 Although TLR regulation of many pathogens is widely accepted, the exact mechanism by which protozoan infection regulates TLR gene expression in epithelial cells has not been determined. Accumulating data indicate that vaginal and cervical epithelial cell lines express TLR1, 2, 3, 5, and 6,7 while uterine epithelial cells express TLR1–9, but fail to express TLR10.8 Such stimulation of epithelial TLRs contributes to the local innate immune defence via a release of diverse immunoactive molecules. For example, endocervical epithelial cells express IL-6 and IL-8 via TLR2 when exposed to the surface antigen of Neisseria gonorrheoa.9 Mitogen-activated protein kinases (MAPKs) may play a key role in parasite proliferation and differentiation, and probably in invasion, in the case of protozoan infection (e.g. by Toxoplasma gondii).10–12 In particular, TLR signalling in the mucocutaneous barrier is closely linked to the MAPK signalling pathway [particularly extracellular signal-regulated kinase (ERK) and p38 MAPK] and nuclear factor (NF)-κB signalling.13,14 However, the overall role of MAPKs and the NF-κB signalling cascade in relation to the regulation of TLRs and control of immunocytokines in T. vaginalis-infected HeLa cells is unknown. Uncovering the underlying mechanisms will allow the development of novel innovative therapeutics such as specific signal inhibitors.
Previously, we showed that T. vaginalis induced apoptotic cell death via activation of Bcl-xL (Bcl-2-like survival factor) and NF-κB signalling in macrophages.15,16 Based on these findings, we report here that T. vaginalis infection could up-regulate epithelium-derived TLR2, 4, and 9 gene expression via the p38 MAPK signalling pathway, subsequently modulating the expression of the p38 MAPK-dependent prototypic chemokine IL-8 and the proinflammatory cytokine TNF-α in the epithelium.
Materials and methods
Antibodies
Anti-ERK1/2, anti-p38, anti-p65, anti-phospho-specific ERK1/2, and anti-phospho-p38 MAPK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Inhibitors
SB 203580, a specific inhibitor of p38 MAPK, PD 98059, a specific inhibitor of ERK kinase, and pyrrolidinecarbodithioic (PDTC), an inhibitor of NF-κB, were purchased from Calbiochem (San Diego, CA).
Parasites
Trichomonas vaginalis strain KT-4 was kindly provided by Dr J. S. Ryu (Department of Parasitology, University of Han-Yang, Seoul, South Korea). Trichomonads were cultured in Diamond's Trypticasc-yeast extract-maltose (TYM) medium (NAPCO, Winchester, VA) supplemented with 10% fetal bovine serum (FBS) in 5% CO2 at 37° for 24 hr. Only late-logarithmic-phase organisms were used for assays.
Cell culture
HeLa cells were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM) (Gibco BRL, Hercules, CA) supplemented with 10% FBS (Sigma, St Louis, MO), penicillin 100 U/ml, and streptomycin (100 U/ml) in an atmosphere of 5% CO2 at 37°. Parasites were added to HeLa cells at a multiplicity of infection of 10.
Western blot analysis
HeLa cells were seeded in 35-mm plastic dishes (3 × 105 cells per dish) and incubated with T. vaginalis for different times as indicated. Cells were lysed in a lysis buffer (50 mm Tris-HCl, pH 7·4, 150 mm NaCl, 1% Triton X-100, 0·5% sodium deoxycholate, 1 mm sodium orthovanadate, 1 µg/ml aprotinin, 10 µg/ml leupeptin, and 1 µg/ml pepstatin A). After centrifugation at 13 000 g at 4° for 30 min, the supernatant was collected; 20 µg of lysates from each sample was run on 15% sodium dodecyl sulphate (SDS) polyacrylamide gels and then electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes. PVDF membranes were blocked in blocking buffer [Tris buffered saline with Tween (TBST) containing 5% bovine serum albumin (BSA)] overnight at 4°. They were then incubated with primary antibodies overnight at 4°, washed, and incubated with goat anti-rabbit horse raddish peroxidase (HRP) or anti-mouse HRP for 1 hr at room temperature. The membrane was developed with ECL substrate (Amersham Life Sciences, Arlington Heights, IL), and exposed to Biomax MS autoradiography X-ray film (Kodak, Rochester, NY).
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of TLR2, TLR4, TLR9, IL-8 and TNF-α
Total RNA was extracted from naïve cells or T. vaginalis-treated HeLa cells using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. cDNA was synthesized from 1 µg of the total RNA using SuperScript II (Invitrogen) in a total reaction volume of 25 μl. PCR amplification of the cDNA was performed with magnesium dicholoride, Taq polymerase (TaKaRa, Dalian, China), and 1 µmol/l specific primers in separate PCR reactions. For TLR2, the protocol was 30 cycles of denaturation (95° for 45 seconds), annealing (56° for 45 seconds) and extension (72° for 1 min). For TLR4, the protocol was 28 cycles of denaturation (95° for 45 seconds), annealing (54° for 45 seconds), and extension (72° for 1 min). For TLR9, the protocol was 30 cycles of denaturation (95° for 45 seconds), annealing (55° for 45 seconds) and extension (72° for 1 min). For IL-8, the protocol was 30 cycles of denaturation (95° for 45seconds), annealing (55° for 45 seconds), and extension (72° for 1 min). For TNF-α, the protocol was 30 cycles of denaturation (94° for 30 seconds), annealing (60° for 30 seconds) and extension (72° for 1 min). Primer sequences and PCR product sizes were as follows: for hTLR2, 5′-GCC AAA GTC TTG ATT GAT TGG-3′ (sense) and 5′-TTG AAG TTC TCC AGC TCC TG-3′ (antisense), 346 bp; for hTLR4, 5′-TGG ATA CGT TTC CTT ATA AG-3′ (sense) and 5′-GAA ATG GAG GCA CCC CTT C-3′ (antisense), 506 bp; for hTLR9, 5′-GTG CCC CAC TTC ATG-3′ (sense) and 5′-GGC ACA GTC ATG ATG TTG TTG-3′ (antisense), 260 bp; for IL-8, 5′-ATG ACT TCC AAG CTG GCC GTG GCT-3′ (sense) and 5′-TCT CAG CCC TCT TCA AAA ACT TCT C-3′ (antisense), 292 bp; for TNF-α, 5′-GAG TGA CAA GCC TGT AGC CCA TGT TGT AGC A-3′ (sense) and 5′-GCA ATG ATC CCA AAG TAG ACC TGC CCA GAC-3′ (antisense); for GAPDH, 5′-ACC ACA GTC CAT GCA TCA C-3′ (sense) and 5′-TCC ACC ACC CTG TTG CTG TA-3′ (antisense). Amplification was carried out with a thermal cycler (model 480; Perkin-Elmer, Norwalk, CT). All PCR products were resolved by 2% agarose gel electrophoresis and visualized by staining the gel with ethidium bromide. The RT-PCR products were purified and sequenced to confirm the identities of the DNA bands.
Assay of cytokine production
HeLa cells (107 cells/ml) were cultured in standard 12-well plates and then treated with medium or T. vaginalis for 24 hr in the presence or absence of 50 µm PD98059, 10 µm SB203580 or 15 µm PDTC. Culture supernatants were collected and stored at −70° and IL-8 and TNF-α production in the supernatants was analysed using an enzyme-linked immunosorbent assay (ELISA) test kit (R & D Systems, Minneapolis, MN) according to the manufacturer's protocol. The effect of 60-min pretreatment with 50 µm PD98059, 10 µm SB203580 or 15 µm PDTC on IL-8 and TNF-α production by HeLa cells treated with T. vaginalis was assessed.
Statistical evaluation
All experiments were performed at least three times. Results are presented as means ± standard deviation or standard error. The Mann–Whitney U-test was used for statistical analysis, and a P-value below 0·05 was considered statistically significant.
Results
Up-regulation of TLR2, TLR4 and TLR9 mRNA expression in T. vaginalis-treated HeLa cells
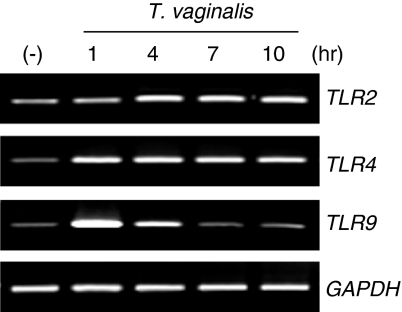
To investigate whether TLR2, 4 and 9 gene expression in HeLa cells is regulated by T. vaginalis, HeLa cells were treated with T. vaginalis for different times. RT-PCR was performed to determine TLR mRNA expression using hTLR2-, hTLR4- and hTLR9-specific primers. As shown in Fig. 1, TLR2 and TLR4 mRNA expression was up-regulated at 1 hr, and sustained in HeLa cells throughout the course of culture with T. vaginalis. By contrast, TLR9 mRNA expression was up-regulated within 1 hr, but returned to baseline levels at 10 hr relative to untreated HeLa cells.
Figure 1.
Up-regulation of Toll-like receptor 2 (TLR2), TLR4 and TLR9 mRNA expression by Trichomonas vaginalis in HeLa cells. HeLa cells were treated with T. vaginalis for 1, 4, 7, 10 and 13 hr. (−) stands for T. vaginalis non-treated cells. After T. vaginalis treatment, total RNA was prepared and TLR gene expression was examined by RT-PCR. GAPDH expression was used as a control. Similar results were obtained in three independent experiments.
Involvement of ERK, p38 MAPK, and NF-κB in T. vaginalis-treated HeLa cells
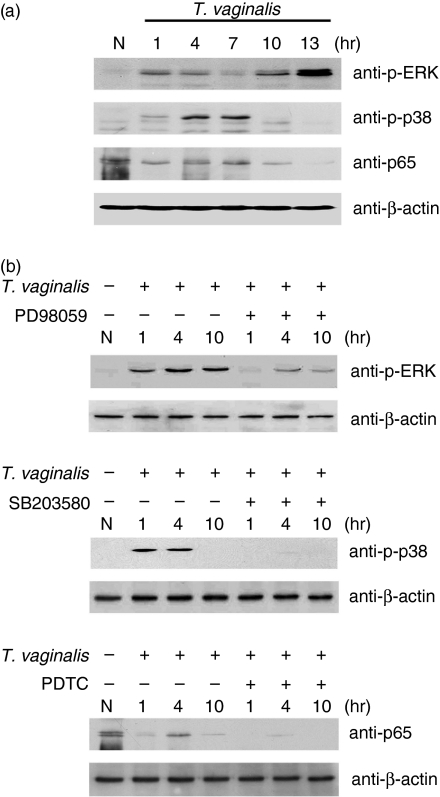
ERK, p38 MAPK and NF-κB play important roles in the inflammatory and immune response by regulating proinflammatory cytokines such as IL-6 and IL-8 and proinflammatory mediators such as intercellular adhesion molecule type 1 (ICAM-1), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Therefore, to evaluate the ability of T. vaginalis to trigger activity of ERK and p38 MAPK in HeLa cells, we examined the kinetics of ERK and p38 MAPK by western blotting at 1, 4, 7, 10 and 13 hr post-treatment. As shown in Fig. 2(a), treatment of HeLa cells with T. vaginalis induced phosphorylation of p38 MAPK. The phosphorylation started to increase at 1 hr and peaked at 7 hr after T. vaginalis treatment. Activation of ERK was detected at an early time-point, and the phosphorylation was sustained and gradually increased in HeLa cells throughout the course of culture with T. vaginalis, and returned to baseline levels after 10 hr. When nuclear translocation of the NF-κB p65 subunit was detected by western blot, T. vaginalis was noted to induce nuclear translocation of the NF-κB p65 subunit within 1 hr.
Figure 2.
The time–course of extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB activation in response to Trichomonas vaginalis infection. (a) Cells were treated with T. vaginalis for 1–13 hr. Lysates were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti-phospho-p38 (anti-p-p38), anti-phospho-ERK1/2, and anti-p65 antibodies. (b) T. vaginalis-induced activation of ERK, p38 MAPK and NF-κB was inhibited by 50 µm PD98059, 10 µm SB203580 or 15 µm pyrrolidinecarbodithioic (PDTC). HeLa cells were pretreated with or without PD98059, SB203580 or PDTC for 1 hr, and then treated with T. vaginalis for the indicated times. Lysates were subjected to SDS-PAGE and immunoblotted with anti-phospho-p38, anti-phospho-ERK1/2 and anti-p65 antibodies. Equal amounts of protein were loaded in each lane, confirmed by immunoblotting with anti-β-actin antibody. N, T. vaginalis and inhibitors non-treated cells.
Specific inhibitors of the ERK, p38 MAPK and NF-κB pathways were then used to analyse their roles in TLR2, 4, and 9 mRNA expression of T. vaginalis-treated HeLa cells. As shown in Fig. 2(b), pretreatment of cells with 50 µm PD98059 and 10 µm SB203580 inhibited T. vaginalis-induced activation of ERK and p38 MAPK, respectively. In addition, 15 µm PDTC inhibited T. vaginalis-induced nuclear translocation of the NF-κB p65 subunit at a dose of 15 µm. These data suggest that PD98059, SB203580 and PDTC inhibited the activation of these signalling pathways in HeLa cells induced by T. vaginalis.
Role of ERK, p38 MAPK and NF-κB in T. vaginalis-induced TLR2, 4 and 9 mRNA expression
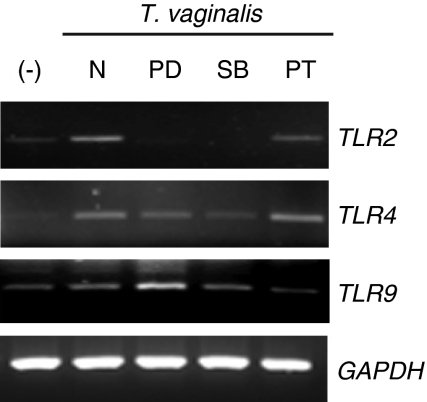
Next, to investigate the effect of ERK, p38 MAPK, and NF-κB activation on TLR gene expression in T. vaginalis-treated HeLa cells, the cells were pretreated with specific inhibitors of the ERK, p38 MAPK or NF-κB pathways prior to T. vaginalis treatment. Pretreatment of HeLa cells with SB203580 significantly inhibited T. vaginalis-induced up-regulation of TLR2, 4 and 9 mRNA expression. In contrast, pretreatment with PD98059 inhibited T. vaginalis-induced up-regulation of TLR2 and TLR4 mRNA expression but did not inhibit TLR9 mRNA expression (Fig. 3). Interestingly, PDTC inhibited TLR9 mRNA expression but did not prevent TLR2 and TLR4 mRNA expression. These data suggest the possibility that the activation of the p38 MAPK signalling pathway plays a role in T. vaginalis-induced up-regulation of TLR2, 4 and 9 gene expression in HeLa cells.
Figure 3.
The involvement of extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB pathways in the regulation of TLR2, 4 and 9 mRNA expression by Trichomonas vaginalis in HeLa cells. HeLa cells were pretreated with or without 50 µm PD98059 (PD), 10 µm SB203580 (SB) or 15 µm pyrrolidinecarbodithioic (PT) for 1 hr, and then treated with T. vaginalis for 10 hr. After T. vaginalis treatment, total RNA was prepared and Toll-like receptor (TLR) gene expression was examined by RT-PCR. GAPDH expression was used as a control. Similar results were obtained in three independent experiments. −, T. vaginalis non-treated cells; N, inhibitors non-treated cells.
Induction of TNF-α and IL-8 mRNA expression by T. vaginalis– involvement of MAPKs
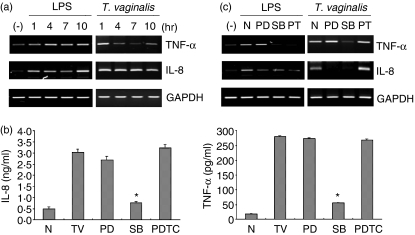
In response to T. vaginalis, IL-8 expression was increased and sustained during the culture, while TNF-α expression peaked at 1 hr, and remained at decreased levels during the culture; it then slightly recovered after 10 hr in the epithelial cells (Fig. 4a). Consistent with the mRNA data, treatment with T. vaginalis showed increased IL-8 production (Fig. 4b). A 1-hr pretreatment of cells with the p38 MAPK inhibitor SB203580 markedly inhibited T. vaginalis-induced IL-8 and TNF-α production and mRNA expression in T. vaginalis-treated HeLa cells. By contrast, PD98059, an ERK inhibitor, inhibited IL-8 production and mRNA expression, but enhanced TNF-α production and mRNA expression. Moreover, lipopolysaccharide (LPS) stimulation of HeLa cells induced mRNA expression of IL-8 and TNF-α in a NF-κB-dependent manner, while pretreatment with the inhibitor of NF-κB also did not affect IL-8 and TNF-α production and mRNA expression in T. vaginalis-treated HeLa cells (Figs 4a, b and c). These data suggest that T. vaginalis-induced regulation of IL-8 and TNF-α gene expression is at least partially dependent on p38 MAPK in HeLa cells.
Figure 4.
The p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 attenuates Trichomonas vaginalis-induced cytokine production. (a) HeLa cells were treated for increasing times with lipopolysaccharide (LPS) or T. vaginalis. After T. vaginalis treatment, total RNA was prepared and interleukin-8 (IL-8) and tumour necrosis factor-α (TNF-α) mRNA expression was examined by RT-PCR. GAPDH expression was used as a control. Similar results were obtained in three independent experiments. (b) HeLa cells were pretreated with or without 50 µm PD98059 (PD), 10 µm SB203580 (SB) or 15 µm pyrrolidinecarbodithioic (PDTC) for 1 hr, and then treated with T. vaginalis (TV) for 10 hr. Culture supernatants were analysed by enzyme-linked immunosorbent assay (ELISA) for IL-8 or TNF-α protein expression (pg/ml). ND stands for not detected. The average of three separate cell platings (n = 3) was determined (± standard error of the mean). The experiment was repeated three times with similar results. Asterisks indicate statistically significant decreases in IL-8 and TNF-α production induced by treatment with 15 µm SB203580 (P < 0·05). (c) HeLa cells were pretreated with or without 50 µm PD98059 (PD), 10 µm SB203580 (SB) or 15 µm PDTC (PT) for 1 hr, and then treated with T. vaginalis for 10 hr. After LPS or T. vaginalis treatment, total RNA was prepared and IL-8 and TNF-α mRNA expression was examined by RT-PCR. GAPDH expression was used as a control. Similar results were obtained in three independent experiments. N, LPS and T. vaginalis non-treated cells.
Discussion
This study clearly indicates that T. vaginalis infection in HeLa cells could up-regulate TLR2, 4 and 9 gene expression via the p38 MAPK signalling pathway; this leads to modulation of the release of the p38 MAPK-dependent prototypic chemokine IL-8 and the proinflammatory cytokine TNF-α from the epithelium.
Although the regulation of many pathogens (e.g. bacteria, mycoplasma and viruses) by TLRs is widely accepted in T lymphocytes, dendritic cells, macrophages, and epithelial cells,6,17 there is relatively little information regarding the regulation of TLR genes in epithelial cells during a protozoan infection. In part, our data support our main hypothesis that T. vaginalis-infected epithelial cells, the major effector cells in the skin barrier, are a central regulator of TLR gene expression. In addition, TLR gene expression suggests a specific innate immune response through interaction between specific components of T. vaginalis and epithelium-derived TLR2, 4 and 9. However, the exact molecular nature of the T. vaginalis-derived TLR ligand has not been determined. This is a limitation of our study. For instance, Trypanosoma cruzi (the aetiological agent of Chagas' disease) and its components act as exogeneous mediators of inflammation that are recognized through TLR2/CD14.18 In the case of TLR4-deficient mice, parasite survival is increased with a higher activity of arginase, an enzyme known to promote parasite growth.19 In addition to TLR2 and TLR4, it has been reported that the TLR9-mediated innate immune activation by the malaria pigment hemozoin plays an important role in malaria parasite–host interactions.20 In gut epithelia, TLR9 mRNA is expressed and can respond to Escherichia coli DNA by increasing IL-8 production.21 In this study, we observed that T. vaginalis induced up-regulation of TLR2, 4 and 9 gene expression in HeLa cells. After T. vaginalis treatment, TLR2, 4 and 9 mRNA expression in HeLa cells was increased significantly. These data therefore show that, in T. vaginalis-infected cells, PAMP initiates immune responses via the regulation of TLRs which are essential to host defence and survival.
MAPKs as regulatory signal mediators in the immunocytes play a pivotal role in host innate immune responses. Both ERK and p38 MAPK are central to the signalling pathway that leads to proinflammatory cytokine production.22–24 For example, ethanol exposure is reported to down-regulate the TLR2-, 4- and 9-mediated macrophage inflammatory response by limiting p38 MAPK and ERK activation.11 Mason et al. reported that the production of IL-12 in response to T. gondii infection is dependent upon TRAF6 (tumor necrosis factor receptor-associated factor 6) and p38 MAPK25. Prior to this study, we reported that T. vaginalis-induced apoptotic cell death is regulated via p38 MAPK signalling pathways in macrophages (data not shown). The present data reveal that TLR signalling in the epithelium barrier is closely associated with MAPK (particularly ERK and p38 MAPK) and NF-κB signalling. However, the overall role of the MAPK and NF-κB signalling cascade in relation to regulation and control of immunocytokines by TLRs in T. vaginalis-infected HeLa cells is unknown. Regarding this question, our data revealed that only p38 MAPK is activated in cultured HeLa cells in a time-dependent manner in response to T. vaginalis treatment. Based on experiments with ERK, p38 MAPK and NF-κB inhibitors, we might speculate that TLR2, 4 and 9 gene expression is regulated by different mechanisms in HeLa cells, and that the p38 MAPK pathway plays a central role in the regulation of TLR expression in HeLa cells. This is supported by the results shown in Fig. 3. However, these phenomena cannot be generalized to other epithelial cells.
We explored the relationships amongst ERK, p38 MAPK, NF-κB and the prototypic immunocytokines IL-8 and TNF-α. IL-8 is a potent chemoattractant that strongly induces neutrophil chemotaxis. It has been reported that the regulation of epithelial IL-8 expression is controlled by the p38 MAPK pathway26. Our data show that, when cells were treated with T. vaginalis, IL-8 mRNA expression was up-regulated in HeLa cells. Furthermore, the ability of T. vaginalis to induce epithelial IL-8 production was reduced by pretreatment with inhibitors specific for the ERK or the p38 MAPK pathway, suggesting that IL-8 expression induced by T. vaginalis is down-regulated by inhibition of ERK and p38 MAPK.
NF-κB is a key regulator of the expression of many proinflammatory genes and of IL-8 synthesis.27 Unexpectedly, we observed that pretreatment with a specific inhibitor of NF-κB activity, PDTC, failed to reduce T. vaginalis-induced IL-8 production in HeLa cells. These data suggest that T. vaginalis-induced epithelial IL-8 synthesis occurs via an NF-κB-independent mechanism.
Production of IL-8, usually in concert with TNF-α expression, induces the recruitment and activation of macrophages and neutrophils to the site of infection and enhances the T-cell response.28 It has been reported that the p38 MAPK pathway is involved in TNF-α expression.29 Consistent with this, our findings indicated that TNF-α gene expression is also increased in response to T. vaginalis treatment in HeLa cells. More interestingly, inhibition of p38 MAPK activity led to reduced TNF-α mRNA expression, and therefore reduced TNF-α production. By contrast, specific inhibitors of ERK and NF-κB failed to reduce T. vaginalis-induced TNF-α gene expression, suggesting that only the p38 MAPK pathway is involved in T. vaginalis-induced TNF-α production in HeLa cells.
Collectively, these results indicate that T. vaginalis infection of the mucocutaneous barrier up-regulates TLR2, 4 and 9 gene expression via the p38 MAPK signalling pathway in epithelial cells; this leads to modulation of the release of the p38 MAPK-dependent prototypic chemokine IL-8 and the proinflammatory cytokine TNF-α from the epithelium. These findings suggest that regulation of the TLR, IL-8 and TNF-α genes via the p38 MAPK pathway may constitute the initial host response against T. vaginalis infection.
Acknowledgments
This study was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (A05-0485-AA0718-05N1-00010A).
Abbreviations
- IL-8
interleukin-8
- MAPKs
mitogen-activated protein kinases
- PAMP
pathogen-associated molecular pattern
- PDTC
pyrrolidinecarbodithioic
- PRRs
pattern recognition receptors
- STD
sexually transmitted disease
- TLRs
Toll-like receptors
- TNF-α
tumour necrosis factor-α
- VECs
vaginal epithelial cells
References
- 1.Petrin D, Renuka Bhatt KD, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–17. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo R, Engbring J, Alderete JF. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992;6:853–62. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 3.Arici A, MacDonald PC, Casey ML. Regulation of monocyte chemotactic protein-1 gene expression in human endometrial cells in cultures. Mol Cell Endocrinol. 1995;107:189–97. doi: 10.1016/0303-7207(94)03442-v. [DOI] [PubMed] [Google Scholar]
- 4.Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94:195–204. doi: 10.1016/0303-7207(93)90168-j. [DOI] [PubMed] [Google Scholar]
- 5.Laird SM, Li TC, Bolton AE. The production of placental protein 14 and interleukin 6 by human endometrial cells in culture. Hum Reprod. 1993;8:793–8. doi: 10.1093/oxfordjournals.humrep.a138144. [DOI] [PubMed] [Google Scholar]
- 6.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 7.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–32. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–36. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–60. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 10.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–37. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 11.Robert-Gangneux F, Creuzet C, Dupouy-Camet J, Roisin MP. Involvement of the mitogen-activated protein (MAP) kinase signalling pathway in host cell invasion by Toxoplasma gondii. Parasite. 2000;7:95–101. doi: 10.1051/parasite/2000072095. [DOI] [PubMed] [Google Scholar]
- 12.De Souza EM, Araujo-Jorge TC, Bailly C, Lansiaux A, Batista MM, Oliveira GM, Soeiro MN. Host and parasite apoptosis following Trypanosoma cruzi infection in in vitro and in vivo models. Cell Tissue Res. 2003;314:223–35. doi: 10.1007/s00441-003-0782-5. [DOI] [PubMed] [Google Scholar]
- 13.An H, Yu Y, Zhang M, et al. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002;106:38–45. doi: 10.1046/j.1365-2567.2002.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174:456–63. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- 15.Chang JH, Ryang YS, Kim SK, Park JY. Trichomonas vaginalis-induced apoptosis in RAW264.7 cells is regulated through Bcl-xL, but not Bcl-2. Parasite Immunol. 2004;26:141–50. doi: 10.1111/j.0141-9838.2004.00693.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang JH, Ryang YS, Morio T, Lee SK, Chang EJ. Trichomonas vaginalis inhibits proinflammatory cytokine production in macrophages by suppressing NF-kappaB activation. Mol Cells. 2004;18:177–85. [PubMed] [Google Scholar]
- 17.Yang RB, Mark MR, Gray A, et al. Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant cone-rod dystrophy. Hum Mol Genet. 1998;7:1179–84. doi: 10.1093/hmg/7.7.1179. [DOI] [PubMed] [Google Scholar]
- 18.Campos MA, Gazzinelli RT. Trypanosoma cruzi and its components as exogenous mediators of inflammation recognized through Toll-like receptors. Med Inflamm. 2004;13:139–43. doi: 10.1080/09511920410001713565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kropf P, Freudenberg MA, Modolell M, et al. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect Immun. 2004;72:1920–8. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engwerda CR, Good MF. Interactions between malaria parasites and the host immune system. Curr Opin Immunol. 2005;17:381–7. doi: 10.1016/j.coi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. FASEB J. 2003;17:1319–21. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JP, Wong CK, Lam CW. Role of caspases in dexamethasone-induced apoptosis and activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase in human eosinophils. Clin Exp Immunol. 2000;122:20–7. doi: 10.1046/j.1365-2249.2000.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 24.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–6. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 25.Mason NJ, Fiore J, Kobayashi T, Masek KS, Choi Y, Hunter CA. TRAF6-dependent mitogen-activated protein kinase activation differentially regulates the production of interleukin-12 by macrophages in response to Toxoplasma gondii. Infect Immun. 2004;72:5662–7. doi: 10.1128/IAI.72.10.5662-5667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jijon HB, Panenka WJ, Madsen KL, Parsons HG. MAP kinases contribute to IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism. Am J Physiol Cell Physiol. 2002;283:C31–41. doi: 10.1152/ajpcell.00113.2001. [DOI] [PubMed] [Google Scholar]
- 27.Brandt S, Kwok T, Hartig R, Konig W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300–5. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsirpouchtsidis A, Hurwitz R, Brinkmann V, Meyer TF, Haas G. Neisserial immunoglobulin A1 protease induces specific T-cell responses in humans. Infect Immun. 2002;70:335–44. doi: 10.1128/IAI.70.1.335-344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JA, Kim DK, Kang OH, et al. Inhibitory effect of luteolin on TNF-alpha-induced IL-8 production in human colon epithelial cells. Int Immunopharmacol. 2005;5:209–17. doi: 10.1016/j.intimp.2004.09.027. [DOI] [PubMed] [Google Scholar]