Abstract
Itching is one of the major clinical symptoms in atopic dermatitis (AD) and complicates the management of this pathological condition. An animal model of AD-like pruritus would contribute to a better understanding of AD and could lead to the development of safe and effective antipruritic agents. DS non-hair (DS-Nh) mice raised under conventional conditions spontaneously develop pruritus, which is associated with a dermatitis similar to human AD. There is a significant positive correlation between disease severity and the period of scratching behaviour in DS-Nh mice. In the present study, we found that levels of histamine and nerve growth factor (NGF) in serum and/or skin tissue were higher in DS-Nh mice with AD-like dermatitis than in age-matched mice without dermatitis. The histopathological data indicated that nerve fibres extend into and mast cells infiltrate the surrounding area of the skin lesion. NGF production by XB-2 cells, which was derived from mouse keratinocytes, was enhanced by histamine via the H1 receptor. We also found that prolonged treatment with an H1-antagonist was effective against pruritus through depression of the production of NGF, which is thought to be generated by keratinocytes. We conclude that DS-Nh mice can serve as a suitable model for gaining a better understanding of pruritus in AD, and that prolonged treatment with an H1-antagonist may be beneficial in patients with AD-associated pruritus.
Keywords: atopic dermatitis, DS-Nh, histamine, nerve growth factor, pruritus
Introduction
Human atopic dermatitis (AD) is a chronic inflammatory skin disease affecting over 10% of children and is also a major cause of occupation-related disability resulting from skin disease,1 which generally produces severe itching of the skin.2 Steroids are the most popular and efficient treatment for AD, but there is a fear that they can cause side effects such as skin atrophy. Because itching behaviour further aggravates skin symptoms,3,4 reduction of itching would be one of the most effective strategies5 for management of AD in patients if undesirable side effects can be avoided. Although histamine H1-antagonists are used widely in the treatment of AD,6,7 they do not have sufficient inhibitory effect against pruritus. Histamine does not therefore play a major role in pruritus treatment of patients with human ad.8–11 What is needed is development of antipruritus agents free from the side effects that occur with the use of steroids.
In 1974, DS non-hair (DS-Nh) mice were developed from a colony of inbred DS strain mice developed in 1954 from outbred dd stock at the Central Institute for Experimental Animals, Tokyo, Japan. DS and DS-Nh mice are currently maintained at Aburahi Laboratories, Shionogi & Co., Ltd, Shiga, Japan. The Nh non-hair phenotype is inherited in an autosomal dominant fashion.12 DS-Nh mice raised under conventional conditions spontaneously develop dermatitis, which is associated with staphylococcal enterotoxin (SE) C-producing bacteria. DS-Nh mice may be similar to patients with human AD with respect to the following features: (1) superantigen-producing Staphylococcus aureus can be isolated from skin lesions; (2) serum levels of immunoglobulin E (IgE) and interleukin (IL)-4 are increased significantly; (3) the numbers of whole mast cells and CD-4 bearing T cells are significantly increased; and (4) itching behaviour becomes very severe.13–15
Nerve growth factor (NGF), discovered more than 50 years ago,16,17 belongs to the neurotrophin family, together with brain-derived neurotrophic factor and neurotrophins. NGF is essential for the survival, development, differentiation and function of peripheral sympathetic and sensory neurons and basal forebrain cholinergic neurons in the central nervous system. NGF also plays an important role as a cytokine through its receptors expressed on inflammatory cells such as mast cells, T cells, B cells, macrophages and basophils.18–24 Recently, plasma levels of NGF in patients with AD and in NC/Nga mice with dermatitis,25,26 which are best known as an AD model, were reported to be significantly increased compared with those in control subjects. Furthermore, some investigators reported that NGF may also be essential for the development of inflammatory skin disease such as psoriasis, AD and allergic contact dermatitis.25,27,28 In these skin diseases, it is considered that keratinocytes produce larger amounts of NGF. Recently, Kanda et al. reported that histamine induces keratinocytes to produce NGF via H1 receptors.29 These results suggest that NGF may play an important role in causing pruritus in patients with AD, and the depressed production of NGF is a new therapeutic target for histamine H1-antagonist treatment.
We considered it necessary to establish a suitable animal model for the development of effective medications and for elucidation of the mechanisms related to pruritus in AD. In this study, we investigated DS-Nh mice from the perspective of pruritus. We also confirmed the feasibility of using loratadine as a histamine H1-antagonist to block the production of NGF in vivo and in vitro.
Materials and methods
Mice
Male DS-Nh mice used in this study were obtained as F1 (Nh/+) from male DS-Nh (Nh/Nh) × female DS (+/+) mice kept under specific pathogen-free (SPF) conditions (free from S. aureus). DS-Nh and DS mice were maintained in microisolator cages under a 12 hr light/12 hr dark cycle and were provided with standard feed and water ad libitum. They were housed in a conventional animal room or in a room under SPF conditions. This study was conducted according to the guidelines for animal experimentation at Kobe University School of Medicine and at Shionogi.
Reagents
Histamine dihydrochloride was obtained from Sigma (St Louis, MO). Desloratadine was obtained from Sequoia Research Products Ltd (Oxford, UK).
Evaluation of clinical skin conditions
Symptoms of conventional and SPF DS-Nh mice were assessed in mice from 5 to 25 weeks old. Clinical skin conditions were defined as erythema, oedema, dry skin, erosion and excoriation. These evaluation items, except for dry skin, were assessed by determining the total area of lesions on the face, because the dermatitis is initially limited to the face. Dry skin was evaluated according to its severity, as previously described.13 Itching was evaluated by measuring the cumulative rubbing/scratching time during a 5-min observation period.
Measurement of IgE and NGF in serum from mice
At several time-points, serum from DS-Nh mice at different ages was collected and stored at −80° until measurement of IgE and NGF. Serum levels of IgE were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Yamasa Shuzo Co., Ltd, Chiba, Japan). NGF levels were measured using another ELISA kit (Promega, Madison, WI). The procedures used for ELISA followed the manufacturers' instructions.
Measurement of histamine and NGF in skin tissue from mice
At several time-points, skin tissue from DS-Nh mice at different ages was collected, homogenized and stored at −80° until measurement of histamine and NGF. After these homogenates were dissolved and centrifuged, their supernatants were used to measure NGF levels by ELISA kit as described above. After these homogenates were boiled and centrifuged, their supernatants were used to measure histamine levels with an enzyme-linked colour developing kit (Kikkoman, Chiba, Japan). The procedures were conducted according to the manufacturer's instructions.
Immunohistochemical and histochemical staining of skin tissue sections
Frozen and paraffin sections were prepared from skin lesions for immunohistochemical and histochemical analyses. The frozen sections were pretreated with 2·5 mg/ml purified mouse IgG/1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS). Next, they were immunostained with rat anti-mouse CD4 (clone H129.19; PharMingen, San Diego, CA). After washing in PBS, these slide-embedded sections were treated with biotin-conjugated goat anti-rat IgG (PharMingen). After washing with PBS, antibody-positive cells on these sections were visualized by enzyme-based colour staining and antibody-positive cells for histopathological analysis were counted.
Paraffin sections were stained with acidic toluidine blue for histopathological analysis to enable counting of the number of mast cells, and were immunostained with rabbit anti-mouse S100 (Laboratory Vision Corporation, Fremont, CA). After anti-S100 antibody-treated slides had been washed with PBS, these slide-embedded sections were treated with biotin-conjugated goat anti-rabbit IgG (PharMingen). After washing with PBS, antibody-positive cells on these sections were visualized by peroxide-diaminobenzidine tetrahydrochloride (DAB) staining and antibody-positive cells were counted for histopathological analysis.
Measurement of NGF production after histamine stimulation in the XB-2 keratinocyte cell line
XB-2 cells (2 × 104 cells/cm2) were seeded on 3T3 feeder cells in 96-well collagen-coated microplates (Iwaki, Chiba, Japan) with Dulbecco's modified Eagle's minimal essential medium (DMEM), 20% fetal calf serum (FCS), 100 U/ml penicillin, and 100 mg/ml streptomycin for 2 days and then the cells were cultured with 100 mm histamine and an appropriate concentration of loratadine at 37° for 24 hr. Cell-free supernatants were collected, and NGF concentrations were measured using an ELISA kit (Promega).
Pharmacological analysis in DS-Nh mice
DS-Nh mice raised under conventional conditions for 5 or 20 weeks were treated with loratadine and rinderon V ointment. Pumps (ALZET Osmotic Pumps, Cupertino, CA) filled with loratadine were implanted subcutaneously; the dosage of loratadine was 3 mg/kg/day for 7 days in each DS-Nh mouse. Rinderon V ointment was applied to the skin lesion at 100 mg/2 days five times for each DS-Nh mouse. These mice were assessed pathologically.
Statistics
Statistical significance was determined by Student's t-test for two unpaired comparisons, unless otherwise stated.
Results
Kinetic analysis of the clinical condition in DS-Nh mice
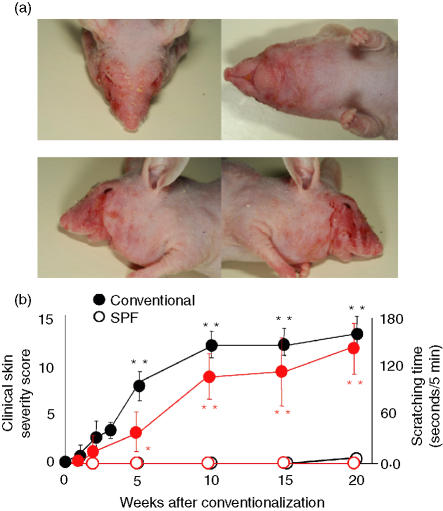
The clinical features in DS-Nh mice with dermatitis at 20 weeks are shown in Fig. 1. DS-Nh mice raised under conventional conditions for 1–2 weeks began to develop AD-like dermatitis, including erythema, oedema, dry skin, erosion and excoriation. These clinical symptoms advanced at a steady pace (Fig. 1b). Erythema, erosion and dry skin appeared in all mice at 7–8 weeks of age, worsened until 15 weeks and remained at this level until 20 weeks of age. Oedema appeared in all mice at 9 weeks of age, worsened up to 14–15 weeks of age and remained at this level until 20 weeks. Excoriation appeared in all mice at 10 weeks, worsened until 15–17 weeks and remained at this level until 20 weeks.
Figure 1.
Clinical features of DS-Nh mice kept under conventional conditions at 20 weeks (a) and fluctuation of clinical skin condition with aging (b). Closed circles show data for conventionally housed DS-Nh mice, and open circles show data for DS-Nh mice raised under specific pathogen-free (SPF) conditions. Total clinical scores for skin conditions were determined from the severity of erythema, oedema, dry skin, erosion and excoriation (open and closed black circle indicate clinical skin score). Rubbing/scratching time was counted per 5 min (open and closed red circle indicate scratching time). Each value represents the mean and standard deviation for 10 conventionally housed DS-Nh mice or five DS-Nh mice raised under SPF conditions.
At 10 weeks, scratching behaviour increased significantly in DS-Nh mice raised under conventional conditions compared with those raised under SPF conditions. This scratching time increased in DS-Nh mice only when they were raised under conventional conditions (Fig. 1b).
Serum levels of IgE, NGF and histamine, and tissue levels of NGF and histamine
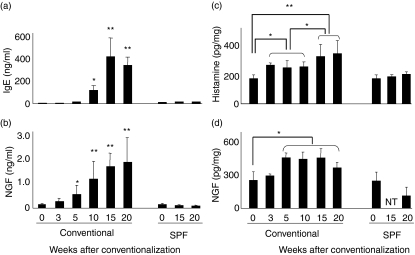
The levels of serum total IgE significantly increased only in conventionally housed DS-Nh mice and not in those raised under SPF conditions. Serum IgE was first detected at 15 weeks of age, and its level subsequently increased with age (Fig. 2a). The data on histamine contents are presented in Fig. 2(c). Histamine was measured in skin homogenates from DS-Nh mice using an enzyme-linked colour developing kit. The levels of tissue histamine significantly increased with age from 8 weeks onwards in DS-Nh mice housed under conventional conditions but not in mice housed under SPF conditions. Next, we measured NGF contents in serum and skin homogenates of DS-Nh mice using ELISA methods. The levels of serum and tissue NGF significantly increased with age only in conventionally housed DS-Nh mice from 10 weeks onwards and not in mice raised under SPF conditions (Figs 2b and d).
Figure 2.
Serological conditions with aging and kinetic levels of nerve growth factor (NGF) and histamine in skin lesions from mice. Levels of immunoglobulin E (IgE) (a) and NGF (b) were measured in sera collected from mice kept under conventional and specific pathogen-free (SPF) conditions. Skin from a series of DS-Nh mice was collected, and histamine and NGF from skin homogenate were eluted to measure histamine (c) and NGF (d) contents using enzyme-linked immunosorbent assay (ELISA) or enzyme-based colour reaction kits. Each value represents the mean and standard deviation of 10 conventionally housed DS-Nh mice or five DS-Nh mice raised under SPF conditions.
Histochemical and immunohistochemical features of facial skin
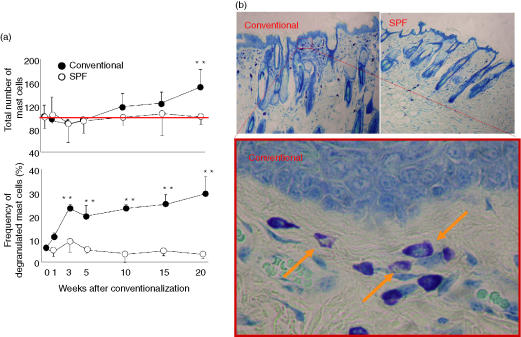
Histological changes such as hyperkeratosis and infiltration of mast cells were observed in skin lesions from all DS-Nh mice raised under conventional conditions (Fig. 3b). The fluctuation data for mast cells in facial skin tissue are shown in Fig. 3(a). The total number of mast cells was increased at 20 weeks onwards, while that of degranulated mast cells increased immediately under conventional conditions and then remained stable after 8 weeks (Fig. 3b).
Figure 3.
Kinetic change in number of mast cells and frequency of degranulated mast cells (a) and histological features of facial skin tissue from DS-Nh conventionally housed mice at 20 weeks (b) The number of mast cells per mm2 of skin area was counted using paraffin sections from conventionally and specific pathogen-free (SPF) housed DS-Nh mice.
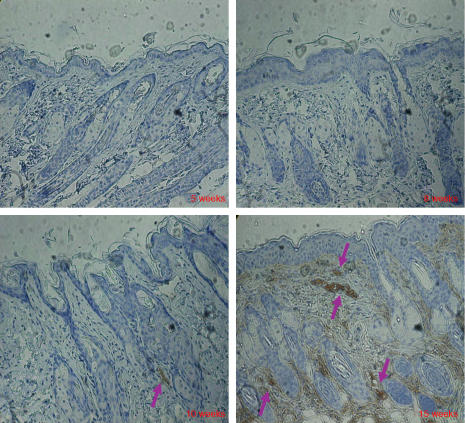
Because the levels of tissue NGF significantly increased and NGF has potent neuroprotective activity, we stained nerve fibres in skin lesions from DS-Nh mice at 5, 8, 10 and 15 weeks using anti-S100 antibody. Many S100-positive cells were found in older DS-Nh mice raised under conventional conditions with AD-like dermatitis (Fig. 4). However, it is difficult to detect S100-positive cells in DS-Nh mice without AD-like dermatitis (data not shown).
Figure 4.
Immunohistological features of facial skin tissue. Frozen sections from conventionally housed DS-Nh mice at 5, 8, 10 and 15 weeks of age were stained with anti-S100 antibodies.
NGF secretion in keratinocytes
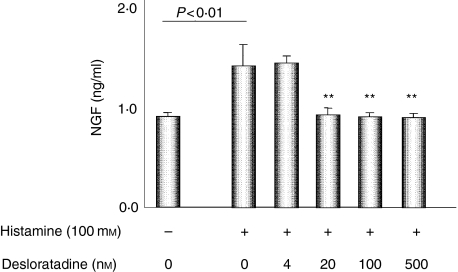
Because the major source of NGF in skin lesions of AD is thought to be keratinocytes, we examined NGF production from keratinocytes stimulated with histamine using the XB-2 cell line. XB-2 cells, which is derived from mouse keratinocytes, secretes a low amount of NGF. The enhanced level of NGF production in XB-2 cells stimulated with histamine was examined to investigate the possibility of new pathogenic roles for histamine in patients with AD. The levels of NGF production in XB-2 cells with stimulation by histamine were significantly higher than in those without stimulation. The stimulatory effects of histamine were suppressed by desloradine, which is an active metabolite of one of the best H1-antagonists, loratadine (Fig. 5).
Figure 5.
Effect of histamine on nerve growth factor (NGF) production and inhibition by desloratadine as a H1 receptor antagonist. The XB-2 cell line was precultured with 0, 4, 20, 100 and 500 nm of loratadine, and then cultured with 100 mm of histamine for 24 hr. The cell-free culture supernatants were assayed for NGF measurement. Each value represents the mean and standard deviation.
Effect of topical steroid and H1-antagonist in DS-Nh mice
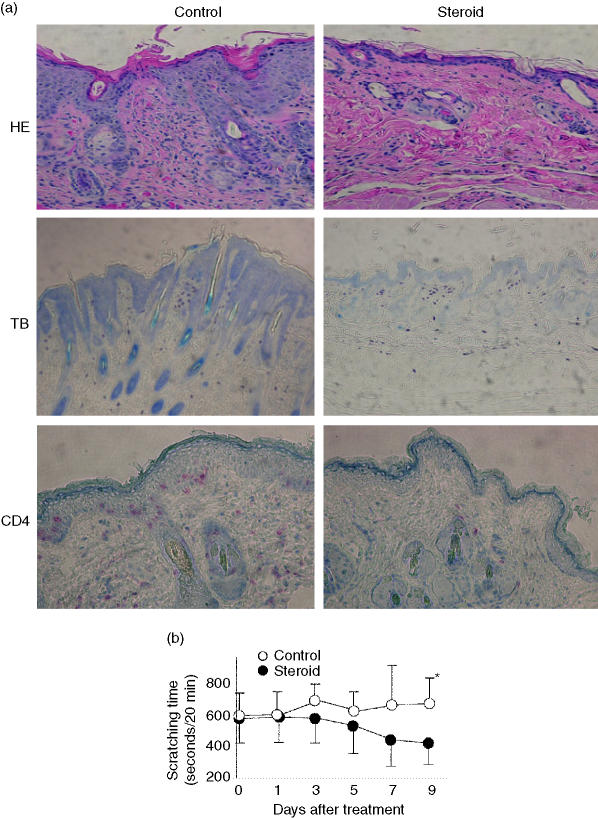
Steroids are commonly used to treat patients with AD. If a model animal is to be used to evaluate new drugs for AD, it is preferable that the model be suitable for treatment with concomitant administration of steroid. We therefore used rinderon V ointment to evaluate pruritus in DS-Nh mice with AD-like dermatitis. After the administration of steroid treatment to DS-Nh mice with skin inflammation, the histological features of facial skin tissue almost returned to the normal predisease state (Fig. 6a), except for the number of mast cells. For example, hyperkeratosis and the number of CD4-bearing T cells were tightly managed by steroid ointment (Fig. 6a). However, steroids do not have a sufficient inhibitory effect on pruritus in DS-Nh mice with AD-like dermatitis at an earlier stage of treatment, as in humans (Fig. 6b).
Figure 6.
Effects of rinderon V in DS-Nh mice with atopic dermatitis (AD)-like dermatitis. Changes in the pathology of skin lesions (a) and scratching times (b) in DS-Nh mice with severe dermatitis after steroid treatment are shown. Paraffin sections were stained with haematoxylin and eosin (HE) acidic toluidine for treatment blue (TB) (left) and non-treatment (right) with steroid, and frozen sections were immunostained for mouse CD4 for treatment (left) and non-treatment (right) with steroid (a). We counted scratching time in DS-Nh mice with severe dermatitis for 20-min periods after steroid or base treatment (b). Each value represents the mean and standard deviation.
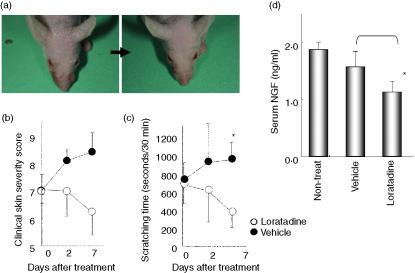
Because loratadine has a short half-life in mice, unlike the case in humans, we used an osmotic pump to administer 3 mg of this H1-antagonist to each DS-Nh mouse per day for 1 week. Clinical severity in DS-Nh mice was alleviated by loratadine treatment and worsened by base material treatment. Significant improvement, based on the effect of H1-antagonist on dry skin and erythema, was noted for all DS-Nh mice at the end-point of loratadine administration (Figs 7a and b). As shown Fig. 7(c), the number of scratches during the 30-min period for DS-Nh mice with loratadine treatment was lower than the number for those given base material treatment. At the end-point of administration, sera were obtained from all DS-Nh mice to measure NGF concentration. The serum levels of NGF from DS-Nh mice given loratadine treatment were significantly lower than those from DS-Nh mice given base material treatment (Fig. 7d).
Figure 7.
Effects of loratadine in DS-Nh mice with atopic dermatitis (AD)-like dermatitis. Changes in the clinical features of facial skin (a), clinical severity (b), and scratching time (c) in DS-Nh mice with weak to moderate dermatitis after loratadine treatment are shown. Levels of nerve growth factor (NGF) in serum from DS-Nh mice were significantly reduced after loratadine treatment compared with those after base material treatment (d) Each value represents the mean and standard deviation or standard error (levels of NGF).
Discussion
We have reported that DS-Nh mice spontaneously develop dermatitis only when raised under conventional conditions, and this spontaneous itchy dermatitis in DS-Nh mice is comparable to a certain type of human AD.13,14 In this study, we re-examined the issue of spontaneous dermatitis and habitual scratching or rubbing in DS-Nh mice kept in a new breeding room which is not as tightly controlled as an SPF room, in the light of studies indicating that sideration of human and mouse AD is influenced by the growth environment.30–33 For example, the NC/Nga mouse, which is the most extensively studied animal model of AD and is reported to develop AD-like dermatitis when kept under conventional conditions, does not develop dermatitis spontaneously in many laboratories.34 In contrast, DS-Nh mice developed almost the same dermatitis as previously reported, except for earlier onset of dermatitis when mice were raised under our new breeding conditions. This earlier onset of dermatitis may have caused a change in the quantity and characteristics of the S. aureus that exist in breeding rooms, as S. aureus was detected in DS-Nh mice at 3 weeks post exposure in this study and detected in mice at 5 weeks post exposure in a previous study (data not shown). Furthermore, the frequency of SEC-positive S. aureus in this study was higher than that found in a previous study (data not shown). Recently, SEC-positive bacteria were most frequently detected in skin lesions from patients with AD and may play an important role in the development of AD in humans, as in DS-Nh mice.14 These results indicated that DS-Nh mice developed AD-like dermatitis much more easily than NC/Nga mice.
In this study, we confirmed the pharmacological effectiveness of using rinderon V ointment as a steroid-type substance in DS-Nh mice. After such treatment, infiltration of CD4-bearing T cells into the epidermic layer and hyperkeratosis were restored to normal levels. However, the number of mast cells, which have a high ability to secrete pruritogen, remained the same as before steroid treatment. This may partially contribute to the difficulty of managing pruritus with only steroids in patients with AD.
As a greater number of mast cells were observed in DS-Nh mice without dermatitis at 5 weeks of age compared with DS mice13,14 and mast cells have the largest potential for histamine production, we measured the histamine content in DS-Nh mice. Histamine levels in serum and skin tissue from DS-Nh mice at 5 weeks of age (without dermatitis) were significantly higher than in those from DS mice (data not shown). Furthermore, the histamine concentration was increased in the skin tissue in parallel with the quantity and quality of mast cells. Briefly, the increase of histamine in conventionally housed DS-Nh mice at the ages of 8, 10 and 15 weeks might be caused by degranulation of mast cells, and this increase in histamine at the ages of 20 and 25 weeks might be caused by the increased number of mast cells. In patients with AD, the levels of histamine have also been found to be increased.35,36 These results suggest that histamine may play some role in maintaining the medical condition of AD, and the higher levels of histamine in DS-Nh mice compared with DS mice may genetically characterize this dermatitis-prone mouse.
Toyoda et al. reported that (i) plasma levels of NGF were significantly higher in patients with AD and (ii) plasma NGF levels in patients with AD correlated significantly with disease severity.25 Also, NGF has an essential role in neuronal development and survival.16,17 Overexpression of sensory nerve fibres in AD patients, which causes skin hypersensitivity, may be evoked by such elevation of NGF content. In this study, we found that levels of NGF in serum and skin lesions from DS-Nh mice with AD-like dermatitis were significantly increased compared with those from age-matched DS-Nh mice without dermatitis. The levels of NGF significantly increased with age, indicating that serum and tissue NGF levels in DS-Nh mice with AD correlated significantly with disease severity. We also demonstrated that anti-S100 antibody-positive nerve fibres were significantly increased in skin lesions from DS-Nh mice with AD, and that there was a correlation between the skin levels of the positive reaction of this antibody and disease severity. In humans and DS-Nh mice, NGF may play an important role in the development of spontaneous pruritus via the mechanism of neuronal development and survival. However, NGF should be discussed from the standpoint of inflammatory reaction, because NGF receptors were expressed in most inflammatory cells.19–24 In skin from DS-Nh mice with AD-like dermatitis, we observed larger amounts of total and degranulated mast cells (Fig. 5), T cells (Fig. 6) and eosiophils.13 These findings are very similar to those obtained for skin from patients with AD. In vivo and in vitro studies by some investigators have revealed that NGF stimulates T-lymphocyte differentiation and proliferation, mast cell accumulation, proliferation, differentiation, activation and degradation, and eosinophil proliferation. In the light of these findings, we hypothesized that NGF in patients and DS-Nh mice with AD (or AD-like dermatitis) may play an important role in the development of both pruritus and inflammation, which are thought to be exacerbating factors for AD.
Pruritus with scratching is a typical symptom of AD, and the itch–scratching cycle causes further pruritus, which exacerbates the condition of AD. Reduction of pruritus and scratching has come to be recognized as the best approach for preventing aggravation of skin lesions and improving the quality of life of patients with AD. Although histamine is the major secretory product of mast cells and could cause pruritus, it is not thought to be a major pruritogen in AD. This is because the histamine H1 receptor antagonist generally does not have a sufficient antipruritic effect in patients with AD. Recently, Kanda et al. reported that histamine could enhance the expression of NGF in human keratinocytes via H1 receptors,29 which are considered to be a major source of NGF in the epidermis. In this study, we demonstrated that histamine could enhance the expression of NGF in mouse keratinocytes via H1 receptors. Next, we examined the pharmacological effect of an H1-antagonist in DS-Nh mice using loratadine, which shows a higher potency as an antipruritic than other H1-antagonists.37 Our study demonstrated that, in DS-Nh mice, chronic administration of loratadine at a dose of 3 mg/kg/day for 1 week significantly inhibited spontaneous scratching and reduced the serum levels of NGF. Although single administration of loratadine at a dose of 10 mg/kg slightly inhibited spontaneous scratching in DS-Nh mice, this dose had a smaller antipruritic effect than chronic administration. This indicates that histamine leads to the development of pruritus via different pathways, at least two in AD. In the first, histamine causes the development of a pruritus directory, which is reduced by single administration of loratadine. In the other, pruritus develops via NGF production by keratinocytes, which were reduced in number by chronic administration of this medicine.
In conclusion, we have established DS-Nh mice as an AD-like pruritus model by histological and serological analysis. It is often helpful to assess drugs in combination with steroids in vivo, and DS-Nh mice were found to be sensitive to steroid, unlike NC/Nga mice.38 We have also described for the first time, to our knowledge, the antipruritic effect of an H1-antagonist via suppression of the secretion of NGF from keratinocytes in vivo.
Abbreviations
- AD
atopic dermatitis
- NGF
nerve growth factor
References
- 1.Leung DY. Atopic dermatitis: New insight and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000;105:860–76. doi: 10.1067/mai.2000.106484. [DOI] [PubMed] [Google Scholar]
- 2.Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party's Diagnostic Criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406–16. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 3.Kimura T, Miyazawa H. The ‘butterfly’ sign in patients with atopic dermatitis. evidence for the role of scratching in the development of skin manifestations. J Am Acad Dermatol. 1989;21:579–80. doi: 10.1016/s0190-9622(89)80235-x. [DOI] [PubMed] [Google Scholar]
- 4.Wahlgren CF. Itch and atopic dermatitis: an overview. J Dermatol. 1999;26:770–9. doi: 10.1111/j.1346-8138.1999.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 5.Koblenzer CS. Itching and the atopic skin. J Allergy Clin Immunol. 1999;104:S109–13. doi: 10.1016/s0091-6749(99)70052-7. [DOI] [PubMed] [Google Scholar]
- 6.Leung DY, Hanifin JM, Charlesworth EN, et al. Disease management of atopic dermatitis: a practice parameter. Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Work Group on Atopic Dermatitis. Ann Allergy Asthma Immunol. 1997;79:197–211. doi: 10.1016/s1081-1206(10)63003-7. [DOI] [PubMed] [Google Scholar]
- 7.Doherty V, Sylvester DG, Kennedy CT, Harvey SG, Calthrop JG, Gibson JR. Treatment of itching in atopic eczema with antihistamines with a low sedative profile. Br Med J. 1989;298:96. doi: 10.1136/bmj.298.6666.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berth-Jones J, Graham-Brown RA. Failure of terfenadine in relieving the pruritus of atopic dermatitis. Br J Dermatol. 1989;121:635–7. doi: 10.1111/j.1365-2133.1989.tb08196.x. [DOI] [PubMed] [Google Scholar]
- 9.Wahlgren CF, Hagermark O, Bergstrom R. Patients' perception of itch induced by histamine, compound 48/80 and wool fibres in atopic dermatitis. Acta Dermatol Venereol. 1991;71:488–94. [PubMed] [Google Scholar]
- 10.Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522–5. doi: 10.1001/archderm.135.12.1522. [DOI] [PubMed] [Google Scholar]
- 11.Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40–5. doi: 10.1159/000063138. [DOI] [PubMed] [Google Scholar]
- 12.Haraguchi M, Hino M, Tanaka H, Maru M. Naturally occurring dermatitis associated with Staphylococcus aureus in DS-Nh mice. Exp Anim. 1997;46:225–9. doi: 10.1538/expanim.46.225. [DOI] [PubMed] [Google Scholar]
- 13.Hikita I, Yoshioka T, Mizoguchi T, et al. Characterization of dermatitis arising spontaneously in DS-Nh mice maintained under conventional conditions: another possible model for atopic dermatitis. J Dermatol Sci. 2002;30:142–53. doi: 10.1016/s0923-1811(02)00070-1. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka T, Hikita I, Matsutani T, et al. DS-Nh as an experimental model of atopic dermatitis induced by Staphylococcus aureus producing staphylococcal enterotoxin C. Immunology. 2003;108:562–9. doi: 10.1046/j.1365-2567.2003.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asakawa M, Yoshioka T, Hikita I, Matsutani T, Hirasawa T, Arimura A, Sakata T, Horikawa T. WBN/Kob-Ht rats spontaneously develop dermatitis under conventional conditions: another possible model for atopic dermatitis. Exp Anim. 2005;54:461–5. doi: 10.1538/expanim.54.461. [DOI] [PubMed] [Google Scholar]
- 16.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 17.Levi-Montalcini R, Dal Toso R, della Valle F, Skaper SD, Leon A. Update of the NGF saga. J Neurol Sci. 1995;130:119–27. doi: 10.1016/0022-510x(95)00007-o. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallbook F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27:2295–301. doi: 10.1002/eji.1830270925. [DOI] [PubMed] [Google Scholar]
- 19.Tam SY, Tsai M, Yamaguchi M, Yano K, Butterfield JH, Galli SJ. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997;90:1807–20. [PubMed] [Google Scholar]
- 20.Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci USA. 1993;90:10984–8. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambiase A, Bracci-Laudiero L, Bonini S, Bonini S, Starace G, D'Elios MM, De Carli M, Aloe L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100:408–14. doi: 10.1016/s0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- 22.Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–56. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 23.Barouch R, Kazimirsky G, Appel E, Brodie C. Nerve growth factor regulates TNF-alpha production in mouse macrophages via MAP kinase activation. J Leukoc Biol. 2001;69:1019–26. [PubMed] [Google Scholar]
- 24.Burgi B, Otten UH, Ochensberger B, Rihs S, Heese K, Ehrhard PB, Ibanez CF, Dahinden CA. Basophil printing by neurotrophic factors. Activation through the trk receptor. J Immunol. 1996;157:5582–8. [PubMed] [Google Scholar]
- 25.Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71–9. doi: 10.1046/j.1365-2133.2002.04803.x. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka A, Matsuda H. Expression of nerve growth factor in itchy skins of atopic NC/Nga mice. J Vet Med Sci. 2005;67:915–9. doi: 10.1292/jvms.67.915. [DOI] [PubMed] [Google Scholar]
- 27.Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Dermatol Venereol. 1998;78:84–6. doi: 10.1080/000155598433368. [DOI] [PubMed] [Google Scholar]
- 28.Kinkelin I, Motzing S, Koltenzenburg M, Brocker EB. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–7. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]
- 29.Kanda N, Watanabe S. Histamine enhances the production of nerve growth factor in human keratinocytes. J Invest Dermatol. 2003;121:570–7. doi: 10.1046/j.1523-1747.2003.12428.x. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T, Kawakami N, Kondo N, Agata H, Fukutomi O, Shimizu H, Orii T. Prevalence of and risk factors for allergic diseases: comparison of two cities in Japan. Ann Allergy Asthma Immunol. 1995;75:525–9. [PubMed] [Google Scholar]
- 31.Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–38. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 32.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649–55. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 33.Hamada M, Furusyo N, Urabe K, et al. Prevalence of atopic dermatitis and serum IgE values in nursery school children in Ishigaki Island, Okinawa, Japan. J Dermatol. 2005;32:248–55. doi: 10.1111/j.1346-8138.2005.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 34.Shiohara T, Hayakawa J, Mizukawa Y. Animal models for atopic dermatitis: are they relevant to human disease? J Dermatol Sci. 2004;36:1–9. doi: 10.1016/j.jdermsci.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545–52. doi: 10.1111/j.1365-2222.1983.tb02636.x. [DOI] [PubMed] [Google Scholar]
- 36.Ruzicka T, Gluck S. Cutaneous histamine levels and histamine releasability from the skin in atopic dermatitis and hyper-IgE syndrome. Arch Dermatol Res. 1983;275:41–4. doi: 10.1007/BF00516553. [DOI] [PubMed] [Google Scholar]
- 37.Hossen MA, Fujii Y, Ogawa M, Takubo M, Tsumuro T, Kamei C. Effect of loratadine on mouse models of atopic dermatitis associated pruritus. Int Immunopharmacol. 2005;5:1331–6. doi: 10.1016/j.intimp.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Hiroi J, Sengoku T, Morita K, et al. Effect of tacrolimus hydrate (FK506) ointment on spontaneous dermatitis in NC/Nga mice. Jpn J Pharmacol. 1998;76:175–83. doi: 10.1254/jjp.76.175. [DOI] [PubMed] [Google Scholar]