Abstract
Experimental autoimmune encephalomyelitis (EAE) is mediated by myelin-specific CD4+ T helper 1 (Th1) cells, while recovery from the disease is associated with the presence of Th2 cells. Here we used animals with targeted deletion of the T-bet gene to determine its role in the progression of EAE. T-bet regulates the production of interferon-γ (IFN-γ) in CD4+ and natural killer cells, and CD4+ T cells from T-bet-deficient mice were unable to differentiate into a Th1 phenotype. Moreover BALB/c mice deficient in T-bet were resistant to the induction of EAE disease, with minimal inflammatory infiltrates in the central nervous system. These mice were resistant to EAE induction even when PLP(180−199) peptide specific effector T cells from BALB/c wild type were transferred to BALB/c T-bet-deficient mice. This resistance to EAE is may be caused by the production of the anti-inflammatory cytokine interleukin-10 (IL-10) from the spleen cells upon ex vivo stimulation with PLP(180−199) peptide and in vivo presence in the central nervous system. There was no difference in the recall responses in spleen cells from T-bet-deficient and wild type mice; however, less secretion of IFN-γ was observed from primed splenocytes. The expression of IFN-γ was less in the central nervous system of T-bet-deficient mice whereas IL-10 was significantly higher in T-bet-deficient as compared to wild type mice. These data indicate that T-bet genes play a critical role in maintaining the encephalitogenic nature of CD4+ T cells in autoimmune responses during EAE disease progression.
Keywords: EAE, T-bet, T-cells, IL-10, autoimmunity
Introduction
Experimental autoimmune encephalomyelitis (EAE) shares many of the clinical and histopathological features of multiple sclerosis (MS) and thus serves as a useful animal model for MS.1,2 In EAE, T helper 1 (Th1)/Th2 imbalance contributes to the pathogenesis of autoimmune diseases as Th1 cells that produce pro-inflammatory cytokines (interleukin (IL)-2, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α)) potentiate the disease; however, Th2 cells produce anti-inflammatory cytokines (IL-4, IL-5, IL-10 and IL-13) and have been associated with remissions from the disease.3–5 However, the targeted deletion of individual cytokines led to unexpected results in EAE disease. IFN-γ−/− and IFN-γ receptor−/− mice developed severe disease6,7 and treatment of mice with anti-IFN-γ antibody worsened the disease.8 In case of IL-4 the results were mixed with few reports showing that the development of disease in IL-4−/− mice was very much similar to that in wild type mice,9,10 while others reported more severe disease in IL-4−/− mice.11 These conflicting reports may be caused by the redundancy of cytokine functions as cytokines may have dual roles, either proinflammatory or regulatory, during the course of an immune response.12 Here, we investigated the role of T-bet in EAE disease process by using mice deficient in T-bet gene; T-bet is a key regulator of Th1 cytokine (IFN-γ), that is known to play a pivotal role in development of Th1 immune responses.13 This study may thereby identify the possible genetic target for future therapeutic interventions in T-cell mediated autoimmune diseases.
T-bet is a recently identified transcription factor, which regulates the production of IFN-γ in CD4+ and natural killer (NK) cells.13 The differentiation of Th cells into distinct functional subtypes is critical for mediating effective adaptive immune responses. The molecular mechanism by which a Th precursor cell (Thp) adopts a Th1 or Th2 phenotype involves a complex interplay between the induction of lineage-specific transcription factors, such as T-bet, GATA3, c-Maf and the stimulatory effects of the cytokine milieu which are largely provided by antigen-presenting cells.14 In case of Th1 differentiation, antigen stimulation of a naive Thp causes simultaneous low-level expression of both the Th1- and Th2-specific transcription factor T-bet and GATA3, and coproduction of small amounts of IFN-γ and IL-4.14 The differentiation of Thp into Th1 cell is thought to occur via two different events. First is up-regulation of IL-12Rβ2 expression by T-bet, which amplifies the effects of IL-12 and strengthens signalling through signal transducer and activator of transcription-4 (STAT4).15,16 The second is activation of STAT1 via IFN-γ receptor engagement, which further up-regulates T-bet expression in a positive feedback loop.17 The net effects of T-bet, STAT4, and STAT1 activation then drive high-level production of IFN-γ by Th1 cells, which are necessary Th1-mediated immune functions.18
Previously, we have reported that lovastatin inhibits EAE disease progression by inhibiting the expression of T-bet19 and recently, it has been shown that T-bet is required for EAE induction;20 further it was shown that silencing of T-bet defines the differentiation of autoreactive lymphocytes.21 In this report, we used T-bet-deficient mice to investigate its role in the EAE disease progression induced both by myelin PLP(180–199) peptide and by adoptive transfer of peptide specific effector T-cells.
Materials and methods
Mice
The homozygous breeding pair of T-bet knockout mice on BALB/c background were obtained from the Jackson Laboratories (Bar Harbor, ME) and bred in the animal facility of Medical University of South Carolina and the 4–5-week-old wild type (BALB/c) mice were purchased from Jackson Laboratories when needed. For all the experiments 8–10-week-old female mice were used. All animals were housed under specific pathogen-free conditions and animal protocols were approved by the Animal Care and Use Committee of MUSC, Charleston USA. Paralysed mice were afforded facile access to food and water.
Peptide and reagents
Myelin proteolipid protein (myelin PLP180–199) (WTTCGSIAFPSKTSASIGSL) was purchased from Peptide International Inc. (Louisville, KY). Recombinant murine IL-12 was purchased from BD Pharmingen (San Diego, CA). The purified anti-IFN-γ, IL-10 and CD4 antibodies were purchased from Santa Cruz (Santa Cruz, CA). Tyramide signal amplification kit was from NEN Life Sciences (Boston, MA).
Active EAE induction with myelin PLP(180–199) peptide
EAE in BALB/c mice were induced with myelin PLP(180–199) peptide as described earlier.22 Briefly the wild-type and T-bet-deficient BALB/c mice (six to eight mice per group) were immunized subcutaneously (s.c) in the flanks with 200 µg of PLP(180–199) peptide in 0·1 ml phosphate-buffered saline and 0·1 ml complete Freund's adjuvant containing 0·4 mg Mycobacterium tuberculosis (H37Ra; Difco Laboratories, Detroit, MI) and injected intravenously (i.v) with 400 ng pertussis toxin (Sigma, St Louis, MO) on the day of immunization and 2 days later. EAE was scored as described previously: grade 1, piloerection; 2 limp tail or isolated weakness of gait without limp tail; 2·5, partial hind leg paralysis; grade 3, hind leg paralysis; grade 4, complete hind and fore limb paralysis; grade 5, moribund or death stage.
Adoptive EAE induction
The adoptive EAE was induced as described earlier.22 The female donor mice (6–10 weeks old) were immunized s.c. with 100 µg PLP(180–199) peptide containing 200 µg of M. tuberculosis H37Ra on day 0 and day 7. Ten days after immunization the spleens were taken out and splenocytes were cultured ex vivo for 72 hr in RPMI-complete (containing RPMI-1640; Gibco BRL, 10% fetal bovine serum and 100 µg/ml streptomycin and penicillin (Atlanta Biologicals Norcross, GA), 1 mm glutamine, 1 mm non-essential amino acids and 5 × 10−5 m 2-mercaptoethanol (Sigma-Aldrich), and 10 ng/ml mouse rIL-12 (BD Pharmingen)). After 72 hr incubation, cells were harvested and resuspended (30 × 106 cells/0·3 ml) in buffered salt solution and than adoptively transferred intraperitoneally (i.p.) in T-bet−/− or wild type littermate recipients irradiated at 450 Gy rads. The mice were given two doses (400 ng/mouse), of pertussis toxin i.p. on days 0 and 2 and EAE disease induction was assessed as above.
Recall responses
For evaluation of the T-cell recall responses the spleens of PLP(180−190) immunized T-bet−/− and wild type mice were harvested on day 10, in which red blood cells had been lysed and the cells were plated at a density of 5 × 105 cells/well in a 96-well plate in RPMI-complete in the presence or absence (background) of varying concentrations of PLP(180–199) (1–10 µg/ml) peptide. For proliferation, [3H]thymidine (1 µCi/well) was added at 48 hr and mean incorporation of thymidine in DNA was measured after 72 hr by 1450 Microbeta Wallac Trilux Liquid Scintillation Counter (Perkin Elmer Life Sciences, Foster City, CA).
Cytokine analysis
For cytokine assays, myelin PLP(180–199)-immunized spleen cells (5 × 106/ml) were cultured in RPMI-complete in 96-well plates with varying concentration (1–10 µg/ml) of PLP(180–199) peptide. The culture supernatants for IFN-γ (Th1 cytokine) were collected after 48 hr and for IL-4 and IL-10 at 96 hr of culture. The cytokines were measured by enzyme-linked immunosorbent assay (ELISA) OPTEIA system (BD Pharmingen) per the manufacturer's instructions.
Immunohistology
To assess the infiltration of immune cells in CNS of T-bet−/− and wild type mice were killed after 5 days of disease onset by CO2 asphyxiation. The lumbar region of spinal cords were taken out on day 15 and stored in 10% buffered formalin. Paraffin-embedded 4 µm thick transverse sections of the mouse spinal cord (six sections per mouse) were stained with haematoxylin and eosin (H&E) for infiltration of cells. Further, the sections were stained with anti-IFN-γ, IL-10 and CD4 antibodies to determine the in vivo cytokine analysis in the central nervous system (CNS). The sections were incubated with anti-IFN-γ (dilution 1 : 50), IL-10 (dilution 1 : 10) and CD4 (dilution 1 : 10) antibodies overnight at 4°. Respective secondary horseradish peroxidase-labelled antibodies were added for 30 min at room temperature. The signals were amplified using tyramide signal amplification kit per the manufacturer's protocol.
Proliferation and apoptosis of CD4+ T-cells in coculture of spleen cells from wild type and T-bet deficient mice
The T-bet wild type and deficient mice were immunized with PLP(180–199) peptide (100 µg/mouse; s.c.) on days 0 and 7. On day 10, the spleen cells were taken out from wild type and T-bet−/− mice and cells were cultured alone (wild type or T-bet−/−) or in mixed culture at 1 : 1 ratio at 5 × 106 cells/ml in RPMI-complete media for 72 hr for the recall responses in the presence of PLP(180–199) 1 or 5 µg/ml. For recall, [3H]thymidine (1 µCi/well) was added at 48 hr and thymidine incorporation was measured after 72 hr by 1450 Microbeta Wallac Trilux Liquid Scintillation Counter (Perkin Elmer Life Sciences). To measure the apoptosis of CD4+ T cells from wild type immunized mice, the spleen cells were labelled with carboxy-fluorescein diacetate, succinimidyl ester (CFSE; 5 µm, Sigma), for 15 min at 37° and then washed extensively three times with RPMI-1640 to remove all the excess CFSE. The CFSE-labelled cells were used to set up the coculture with naïve spleen cells of T-bet−/− mice. The cells were mixed in a 1 : 1 ratio and cultured in 48-well plates for 72 and 96 hr. After 72 and 96 hr the cells were harvested and stained for apoptosis by Annexin-V phycoerythrin (BD Bioscience). To differentiate the CD4+ T-cells in mixed population, the cells were labelled with anti-CD4 Alexa Fluor 647 (BD Bioscience). For detection of CD4+ T-cells apoptosis the cells were analysed for total gated population of CFSE (wild type) and Annexin-V and Alexa Fluor 647 positive cells.
Statistical analysis
The unpaired Student's t-test was used to evaluate the significance between the T-cell proliferation and cytokine analysis by Graph-Pad Prism 3.0 software. Clinical disease scores are the average maximal scores over the disease course (mean ± SD) and analysed using a non-parametric Kruskal—Wallis test. Statistical significance is set at 0·05 and conducted using SAS statistical software, V8 (Cary, NC). A value of P < 0·05 and above was considered significant.
Results
Clinical disease progression in T-bet−/− and wild type mice
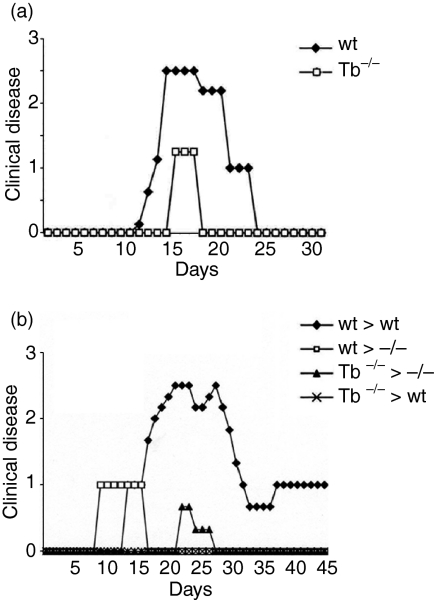
To examine the role of T-bet in EAE disease progression we induced EAE disease in targeted deletion of T-bet−/− mice. As shown in Fig. 1(a) T-bet−/− mice were resistant to the development of clinical EAE disease, while wild type littermates experienced a more severe form of disease. Mice were followed up to 40 days. T-bet−/− mice had a lower incidence of disease (4/12) compared with wild type mice (12/12). The T-bet−/− mice that developed delayed EAE, had a very mild clinical course of the disease as compared to wild type mice (mean maximal disease grade of 1·25 ± 0·1). In contrast, wild type mice had an earlier onset of disease as compared to T-bet−/− mice and attained a higher disease grade (2·5 ± 0·3, P < 0·0001). These data suggest that T-bet plays a very significant role in the development of EAE disease process.
Figure 1.
EAE disease course in T-bet−/− (Tb−/−) and wild type (wt) littermates. (a) Active EAE was induced in T-bet−/− and wild-type littermates by immunization with myelin PLP(180−199) peptide in CFA. (b) Passive EAE was induced in T-bet−/− and wild type mice by adoptive transfer of myelin-PLP(180−199)-sensitized T cells. Clinical disease was monitored as mentioned in methods. Data shown are from 12 mice in each group (both in active and adoptive EAE).
T-bet-deficient mice display decreased EAE severity upon adoptive transfer of effector PLP(180–199)-specific T-cells from wild type to Tbet−/− mice
We adoptively transferred PLP-reactive lymphocytes generated from wild type mice into either T-bet−/− or wild-type littermates. The T-bet−/− mice showed significantly less degree of EAE disease severity in comparison to the wild type littermates (Fig. 1b). The T-bet−/− mice developed mild EAE with significantly decreased severity (P < 0·0001), indicating that host-expressed T-bet is involved in the maintenance of encephalitogenicity during the effector phase of EAE (Fig. 1b). Further these findings suggest that the inflammation in T-bet−/− mice with adoptive transfer of wild type effector T cells were predominantly caused by the presence of IFN-γ. Next, we adoptively transferred the effector T cells from T-bet−/− mice to T-bet-deficient and wild type littermates and determined the disease progression. We observed that the effector T cells from T-bet−/− mice were unable to drive the disease pathology in either T-bet−/− or in wild type mice (Fig. 1b).
Production of cytokines and recall responses in primed T cells from wild type and T-bet−/− mice
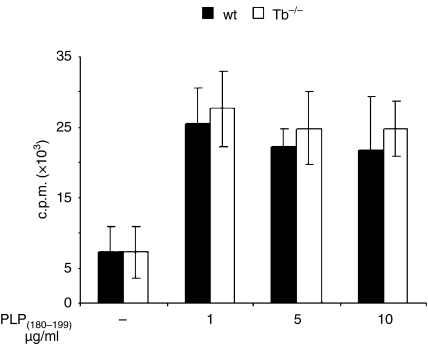
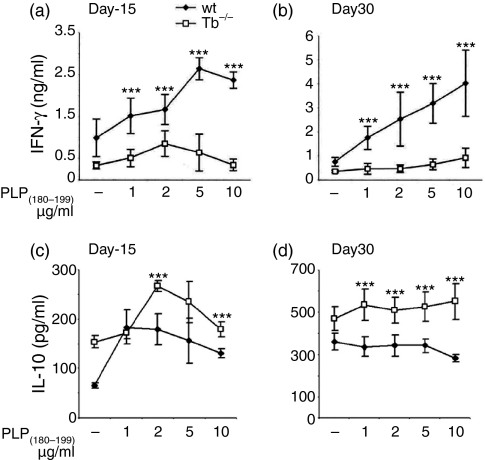
We examined the recall responses to PLP(180–199) peptide in in vivo primed splenocytes isolated from wild type and T-bet−/− littermates on day 10 after immunization. T cells from wild type and T-bet−/− mice proliferated significantly upon ex vivo stimulation with myelin PLP(180–199) peptide (Fig. 2); these results suggest that the cells from T-bet-deficient mice had no problem in the priming antigen-specific T cells. Our results are consistent with the recent publication suggesting that the T-cells from T-bet−/− mice have capacity of recall responses to MOG(35−55) peptide.20 As expected the production of IFN-γ in culture supernatants was significantly higher in splenocytes of wild type mice than in T-bet−/− mice after stimulation with PLP(180–199) peptide (Fig. 3a). However, the production of regulatory cytokine IL-10 was significantly higher (P < 0·0001) in the T-bet−/− mice (Fig. 3c). The levels of IFN-γ was higher at the peak of the disease and it remained higher until day 30 in the wild type mice whereas the level of IL-10 was increased significantly in T-bet−/− mice during the course of disease (Fig. 3b,d). We did not observe any significant difference in the production of Th2 cytokine IL-4 in T-bet−/− and wild type mice (data not shown).
Figure 2.
Recall responses in T-bet−/− (Tb−/−) and wild type (wt) littermates. Spleen cells from PLP(180−199)-immunized mice were re-stimulated ex vivo in the presence of Ag PLP(180−199) (1–10 µg/ml) peptide for recall responses. [3H]thymidine (1 µCi/well) was added at 48 hr, and the uptake of radioactivity was measured after 24 hr. The results expressed as mean ± SD of three independent experiments.
Figure 3.
Cytokine responses in T-bet−/− (Tb−/−) and wild type (wt) littermates. Spleen cells from PLP(180−199)-immunized mice were harvested at two different time points of disease course [days 15 (a and c) and 30 (b and d)] and cells re-stimulated ex vivo with antigen PLP(180−199) (1–10 µg/ml) peptide for cytokine (IFN-γ and IL-10) responses. The supernatants were collected at 48 hr (for IFN-γ) and 96 hr (for IL-10) for cytokine measurements. The IFN-γ and IL-10 secretion at day 15 (a and c) and day 30 (b and d) are shown here. The data represents results (mean ± SD) of three independent experiments. ***P < 0·0001 compared to T-bet−/− at different antigen concentrations.
Immune cells infiltration to CNS
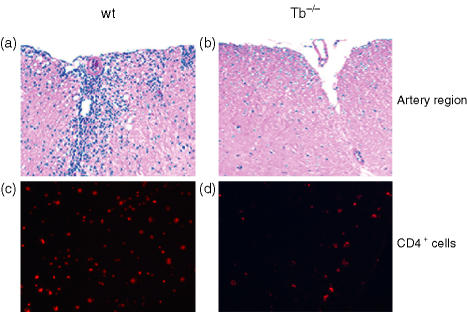
Pathological examination of the CNS from T-bet−/− and wild type littermates harvested 5 days following disease onset (day 15) showed minimal infiltrates in the CNS of T-bet−/− mice. As shown in Fig. 4(a,b), the wild type and T-bet−/− EAE mice had significant differences in the degree of cellular infiltrates in the spinal cord, whereas the wild type mice showed significant increases in mononuclear cell infiltration. However, the T-bet-deficient mice had very little infiltration of cells. To characterize the infiltrated cells in CNS, the sections were stained for CD4+ T-cell marker. As shown in Fig. 4(c,d) more CD4+ T cells were infiltrated in the CNS of wild type mice, whereas in the T-bet-deficient mice we observed very little CD4+ T-cell infiltration (Fig. 4c,d). These results suggest the lower disease incidence and less severe disease in T-bet−/− mice may be because of the absence of pathogenic CD4+ T cells in the CNS.
Figure 4.
Infiltration of leucocytes into the CNS of T-bet−/− (Tb−/−) and wild type (wt) littermates. Animals were killed on day 15 at the peak of disease, and spinal cords were fixed (10% buffered formalin) and infiltration was assessed by H&E staining and visualized at ×10 for wild type (a) and T-bet−/− (b) mice in the arterial region. The tissue sections were stained for the CD4+ T cells in wild-type (c) and T-bet−/− mice (d) Data shown are representative of eight different animals.
Cytokine analysis in the CNS following EAE disease induction
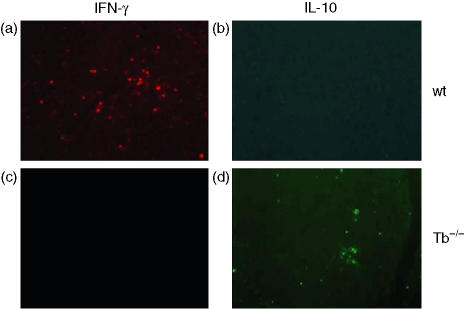
Further, the presence of inflammatory and regulatory (IFN-γ and IL-10) cytokines were determined in the CNS of T-bet−/− and wild type mice. The spinal cord sections from wild type mice showed the presence of IFN-γ; however, T-bet−/− mice had no positive staining for IFN-γ, as shown in Fig. 5(a, b). In contrast to IFN-γ the spinal cord sections from T-bet−/− mice had high expression of IL-10 in comparison to the wild-type littermates (Fig. 5c,d. The increased expression of IL-10 may be a regulatory factor in low disease severity and incidence in T-bet−/− mice.
Figure 5.
The cytokine expression in the CNS of T-bet−/− (Tb−/−) and wild type (wt) littermates. The fixed (10% buffered formalin) spinal cord sections were stained for the presence of IFN-γ and IL-10 expression in the CNS. The tissue sections for IFN-γ (a and b) and IL-10 (c and d) from T-bet−/− and wild type mice are shown here. Data shown are representative of eight different animals.
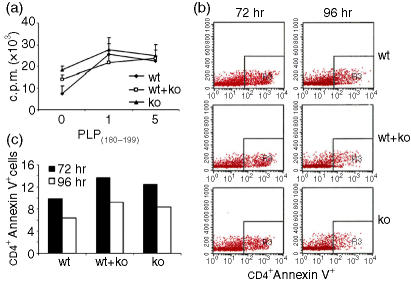
Analysis of proliferation and apoptosis of wild type CD4+ T cells cocultured with spleen cells of T-bet-deficient mice
We analysed the reason why the adoptive transfer of wild-type spleen cells primed with PLP(180–199) into T-bet−/− mice were unable to induce EAE. The possibilities are either the transferred wild type cells were not able to proliferate or undergoing apoptosis in microenvironment of T-bet−/− cells. To examine this possibility, we set up in vitro assays, for proliferation, the wild type spleen cells from immunized mice were cocultured with spleen cells from immunized T-bet−/− mice. As shown in Fig. 6(a), there was no inhibition in T-cell proliferation when cells from wild type and T-bet−/− mice were cultured alone or together at 1 : 1 ratio. To examine the apoptosis, we used the spleen cells of PLP(180–199) immunized wild type mice and labelled them with CFSE. The CFSE-labelled cells were cocultured with T-bet−/− spleen cells from unimmunized mice (we used the T-bet−/− cells from naïve mice to mimic the adoptive EAE model as there the cells were transferred to the naïve mice). As shown in Fig. 6(b,c). there was not much difference in the apoptosis of CD4+ T-cells in wild type or T-bet−/− alone or when they were cocultured (the CD4+ T-cells were analysed as CFSE, AlexaFluor647 and Annexin-V positive cells). These results suggest that the presence of T-bet−/− cells neither in interfere with expansion of wild type T-cells and nor compromised them by inducing apoptosis.
Figure 6.
T-cell proliferation and apoptosis in CD4+ T cells. The wild type (wt) and T-bet−/− (Tb−/−) spleen cells from immunized mice were cultured alone or cocultured at the ratio of 1 : 1. Cell proliferation was examined using thymidine(1 µCi/well) at 72 hr (a) data is from five individual mice spleen cells expressed mean ± SD. Wild type spleen cells from immunized mice were cocultured with naïve spleen cells from T-bet−/− mice for 72 and 96 hr. Apoptosis of CD4+ T cells was measured using Annexin-V staining kit (b and c).
Discussion
Investigations into the role of individual cytokine gene knockout in EAE disease induction have provided contradictory results. These observations point to the potential of therapeutic advantage of targeting genes that regulate Th1 and Th2 immune responses as opposed to individual cytokine genes. The Th1 cytokines, IFN-γ and TNF-α have been linked to disease expression in EAE and play a dual role in disease initiation or remission.6,7,23,24 However, targeted deletion of IFN-γ−/− resulted in higher degree of disease as compared to control.6 Here we report the effect of targeted disruption of T-bet gene in the development of EAE and our data shows that T-bet−/− mice are protected from EAE disease.
There are some potential explanations for the lack of disease in T-bet−/− mice. First, it is possible that cells in the T-bet−/− mice fail to be primed. Our proliferation data show that T-bet−/− mice derived spleen cells had no difference in the recall responses as compared to the wild type littermates. These results are consistent with an earlier published study in B6 T-bet−/− mice in the MOG(35−55) primed cells.20 Second, the cells may fail to differentiate and express the appropriate cytokine profile conducive to disease and our data are consistent with this hypothesis, because cells from T-bet−/− mice produce low levels of IFN-γ and high levels of IL-10, an anti-inflammatory cytokine. The third possibility is that T-bet−/− primed cells lack appropriate chemokine signals to enter the CNS. Th1 and Th2 cytokines play an important role in production of key chemokines that control migration of effector T cells.25 Thus, the decrease in IFN-γ in T-bet−/− mice may decrease the production of Th1-dependent chemokines such as inducible protein-10 (IP-10) and monokine induced by interferon-gamma (MIG), leading to milder disease.26,27 Recently it is reported that T-bet- and STAT1-deficient mice have differential expression of chemokines20 and silencing of T-bet with antisense oligonucleotides or small interfering RNAs (siRNA) inhibited the production and phosphorylation of STAT1 in antigen-specific T-cell differentiation.21 Accordingly IFN-γ−/− mice had undetectable levels of monocyte chemotactic protein-1 (MCP-1) and RANTES (regulated on activation, normal, T-cell expressed, and secreted) during the course of EAE,28 and STAT1−/− mice had low IP-10, MCP-1, RANTES and increased macrophage inflammatory protein-1β (MIP-1β), MIP-2 and MIP-1α.20 The STAT1−/− mice had more neutrophils and foamy macrophage infiltration than did T cells because of the high expression of MIP-1β, MIP-2 and MIP-1α rather than IP-10, MCP-1 and RANTES in the diseased brain.20
We have observed that the adoptive transfer of PLP(180–199)-primed T-cells to T-bet−/− mice unable to induce EAE. Our ex vivo data indicate that the microenvironment of T-bet−/− spleen cells either in primed or unprimed state does not affect the T-cell expansion or induce apoptosis in the donor wild type cells. These results suggest that the T-bet−/− host environment is controlling the nature of encephalitogenicity of transferred wild type cells in vivo. It was shown that T-bet regulates the binding of CD4+ T cells to E-selectin, P-selectin and CXCR3 expression which are needed for Th1 cell migration.29,30 It may be possible that wild type cells are unable to traffic to the CNS in the T-bet−/− mice and because of that they are resistant to EAE. In vivo survival and migration of wild-type CD4 Th1 cells in the T-bet−/− host microenvironment need further investigation, and analysis of these cells will further enhance the role of T-bet in autoimmune diseases.
The second observation in our study is that primed T cells from T-bet−/− mice were unable to produce IL-4, a key Th2 cytokine, but still provided protection against EAE disease. Although the role of Th2 cells in EAE remains controversial, the Th2 cells have been shown to protect against Th1 cell-mediated inflammatory diseases and CNS-specific Th2 clones have been shown to be protective in EAE.9–12 Our data documents the lack of encephalitogenicity of T-bet−/− cells in terms of key Th1 (IFN-γ) cytokine production and disease induction. The disease progression was monitored until day 40 and T-bet−/− animals remained resistant and developed very mild clinical disease with delayed onset and minimal cellular infiltrates. These observations underscore the complexity of the Th1/Th2 paradigm in autoimmune diseases in general and particularly in the EAE disease process. The studies described here indicate that targeting of T-bet gene extends beyond the simple effect of a particular cytokine deficiency or dysregulation.
Observed resistance to EAE in T-bet−/− animals may be a result of the regulatory mechanism induced by the IL-10 production. The observed increased production of IL-10 throughout the study, may be the main driving factor in disease attenuation. Recently, it was shown that regulatory T-cells (CD4+ CD25+) efficiently inhibit the proliferation of effector cells from T-bet−/− and STAT1−/− mice.20 Earlier it was shown that IL-10 producing regulatory CD4+ CD25+ T-cells suppress the induction of EAE.31,32 The observed higher levels of IL-10 indicates that T-bet−/− mice have more IL-10-producing immunoregulatory cells; however, whether these cells are truly regulatory is yet to be investigated. Whether the regulatory environment in T-bet−/− animals is mediated by regulatory T cells or by a state of Th2 immune deviation requires further investigations to assess the direct effect of regulatory or antigen specific Th2 cell on EAE disease progression.
Collectively these results document that T-bet gene controls pathway central to the induction of EAE disease. Disruption of the T-bet gene attenuates clinical disease and reduces inflammatory infiltrates in the CNS. Further studies are required in relapsing models of T-bet−/−-deficient mice in order to elucidate the mechanisms of function of these genes in EAE.
Acknowledgments
We thank Drs Mohamad Labib Salem and Azizul Haque for critical reading of the manuscript and Ms. Joyce Bryan, Ms Carrie Barnes and Hope Terry for laboratory assistance.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Swanborg RH. Experimental autoimmune encephalomyelitis inrodents as a model for human demyelinating disease. Clin Immunol Immunopathol. 1995;77:4. doi: 10.1016/0090-1229(95)90130-2. [DOI] [PubMed] [Google Scholar]
- 3.Chitnis T, Khoury SJ. Cytokine shifts and tolerance in experimental autoimmune encephalomyelitis. Immunol Res. 2003;28:223. doi: 10.1385/IR:28:3:223. [DOI] [PubMed] [Google Scholar]
- 4.Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 5.Sredni-Kenigsbuch D. TH1/TH2 cytokines in the central nervous system. Int J Neurosci. 2002;112:665. doi: 10.1080/00207450290025725. [DOI] [PubMed] [Google Scholar]
- 6.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5. [PubMed] [Google Scholar]
- 7.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223. [PubMed] [Google Scholar]
- 8.Lublin FD, Knobler RL, Kalman B, et al. Monoclonal anti-gamma interferon antibodies enhance experimental allergic encephalomyelitis. Autoimmunity. 1993;16:267. doi: 10.3109/08916939309014645. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299. [PubMed] [Google Scholar]
- 10.Liblau R, Steinman L, Brocke S. Experimental autoimmune encephalomyelitis in IL-4-deficient mice. Int Immunol. 1997;9:799. doi: 10.1093/intimm/9.5.799. [DOI] [PubMed] [Google Scholar]
- 11.Falcone M, Rajan AJ, Bloom BR, Brosnan CF. A critical role for IL-4 in regulating disease severity in experimental allergic encephalomyelitis as demonstrated in IL-4-deficient C57BL/6 mice and BALB/c mice. J Immunol. 1998;160:4822. [PubMed] [Google Scholar]
- 12.Lin JX, Migone TS, Tsang M, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 13.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 14.O'Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 15.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 16.Mullen AC, High FA, Hutchins AS, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 17.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilliard BA, Mason N, Xu L, Sun J, Lamhamedi-Cherradi SE, Liou HC, Hunter C, Chen YH. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172:1273. doi: 10.4049/jimmunol.172.2.1273. [DOI] [PubMed] [Google Scholar]
- 20.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Lyons JA, Ramsbottom MJ, Trotter JL, Cross AH. Identification of the encephalitogenic epitopes of CNS proteolipid protein in BALB/c mice. J Autoimmun. 2002;19:195. doi: 10.1006/jaut.2002.0619. [DOI] [PubMed] [Google Scholar]
- 23.Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frei K, Eugster HP, Bopst M, Constantinescu CS, Lavi E, Fontana A. Tumor necrosis factor alpha and lymphotoxin alpha are not required for induction of acute experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:2177. doi: 10.1084/jem.185.12.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 26.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246. [PubMed] [Google Scholar]
- 27.Luster AD. Chemokines — chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 28.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 29.Underhill GH, Zisoulis DG, Kolli KP, Ellies LG, Marth JD, Kansas GS. A crucial role for T-bet in selectin ligand expression in T helper 1 (Th1) cells. Blood. 2005;106:3867. doi: 10.1182/blood-2005-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol. 1999;11:1625. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+ CD4+ regulatory T cells. Int Immunol. 2004;16:249. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]