Abstract
The present study demonstrates that endogenous production of IL-12 is crucial for survival in Staphylococcus aureus-induced arthritis in mice. Staphylococcal load is enhanced in several organs, because of lack of IL-12. This might be due to decreased production of IFN-γ in IL-12-deficient mice. Although IL-12-deficient mice were exposed to higher staphylococcal load, they demonstrated no increased severity of arthritis as compared with control animals.
Keywords: IL-12, septic arthritis, Staphylococcus aureus
Synopsis
Introduction:
Septic arthritis is a severe disease, which is associated with high mortality and rapid destruction of affected joints. The most common bacterial agent in this condition is S aureus, which is the responsible pathogen in 37–56% of all cases of septic arthritis. Despite eradication of bacteria from the joint cavity, destruction of the joint often continues, resulting in severe sequelae.
In our mouse model of sepsis and septic arthritis we use an intravenous inoculum of a S aureus strain (LS-1) that produces toxic shock syndrome toxin (TSST)-1. This bacterial strain was originally isolated in a mouse with spontaneous staphylococcal arthritis. IL-12 is a heterodimeric cytokine that is composed of the constitutively expressed p35 gene product and the inducible p40 subunit. It is primarily produced by monocytes/macrophages and dendritic cells.
IL-12 has a variety of effects on natural killer cells and T cells, including the ability to facilitate T-helper (Th1)-cell responses, and thereby IFN-γ production. IFN-γ has been shown to be arthritogenic in septic arthritis, but protective with regard to bacterial clearance and survival. IL-12 is protective in several experimental models of bacterial infections, as demonstrated by the fact that neutralization of this cytokine increases susceptibility and addition of recombinant IL-12 ameliorates the severity of the infection. However, IL-12 given at high doses induces a septic shock-like condition, and neutralization of IL-12 protects mice from lipopolysaccharide-induced shock. Thus, the role of IL-12 in cases of severe bacterial infection that culminate in septic shock is not established.
It has recently been shown that IL-12 deficiency exists in humans and that absence of IL-12 gives rise to recurrent infections with S aureus. The role of IL-12 in S aureus infection has not previously been assessed. Inoculation of IL-12 p40-deficient mice and their wild-type counterparts with a TSST-1-producing S aureus strain shows the critical importance of IL-12 for survival during S aureus sepsis, in that it mediates downregulation of staphylococcal growth.
The aim of the present study was to investigate the importance of IL-12 in S aureus arthritis, specifically its impact on survival, bacterial clearance and development of arthritis.
Materials and methods:
Inbred male C57BL/6 mice that were intact or defective with respect to IL-12 production were used throughout the study. At the time of the experiment, IL-12-deficient mice had undergone five backcrosses to C57BL/6. A TSST-1-producing S aureus strain (LS-1), which was originally isolated from a spontaneously arthritic NZB/W mouse, was prepared according to a previously described method and was injected into one of the tail veins.
Results:
Ten days after inoculation with staphylococci all of the IL-12-/- mice were dead, as compared with 22% of the wild-type control animals (Fig. 1; P = 0.002). These results clearly indicate the critical importance of IL-12 production for survival during infection with S aureus.
Figure 1.
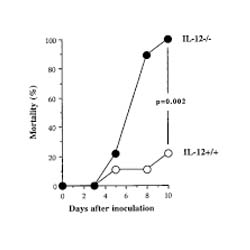
IL-12 protects against S aureus-induced death (n = 9 for both IL-12+/+ and IL-12-/-). Cumulative mortality in response to inoculation of 4 × 108S aureus per mouse. The experiment was finished at day 21, when seven out of the nine IL-12+/+were still alive.
Bacterial growth was determined in liver, kidneys and joints 20 days after intravenous inoculation with 1 × 107 staphylococci/mouse. The clearance of bacteria was clearly diminished in IL-12-deficient mice in all three organs.
Serum levels of IFN-γ were measured in IL-12-deficient mice and wild-type control animals after intravenous inoculation of 1 × 107 staphylococci/mouse. IL-12-deficient mice displayed decreased IFN-γ levels as measured at days 7 and 20 after the inoculation of bacteria as compared with levels in wild-type controls (day 7, 208 ± 83 versus 764 ± 288 U/ml; and day 20, 269 ± 114 versus 444 ± 170 U/ml; these results were not statistically significant).
The disruption of the p40 gene did not result in any significant differences with regard to either the frequency or severity of arthritis as compared with wild-type controls. The clinically observed frequency of septic arthritis was 76% in IL-12+/+ mice, as compared to 54% in IL-12-/- animals 20 days after inoculation of 1 × 107 staphylococci/mouse. Histopathological examination confirmed the clinical data (Table 1).
Table 1.
Histopathological examination of joints
| Synovitis | Bone/cartilage destruction | |||
| IL-12 status | Frequency (%) | Index | Frequency (%) | Index |
| IL-12-/- | 75 | 3.7 ± 0.9 | 63 | 3.4 ± 1.0 |
| IL-12+/+ | 89 | 5.2 ± 1.2 | 78 | 6.2 ± 1.7 |
Histopathological examination of joints from IL-12-deficient mice (n = 16) and their wild-type counterparts (n = 9) 20 days after intravenous inoculation of 1 × 107 staphylococci/mouse. All four limbs were sectioned and stained with haematoxylin and eosin. Synovial hypertrophy or bone/cartilage destruction was given a score of 1–3 for each joint location (elbow, wrist, carpal joints, fingers, knee, ankle, tarsal joints and toes). Values are expressed as percentage or mean ± standard error or the mean.
Discussion:
The importance of functional IL-12 during S aureus arthritis is demonstrated by the present study. The major impact of IL-12 is on survival. This protection seems to be via more efficient bacterial clearance in vivo. All arthritic parameters, clinically and histopathologically, indicated decreased severity in IL-12-deficient mice as compared with wild-type controls; however, statistical significance was not reached. It is possible that differences in joint inflammation and erosion in response to staphylococci were more pronounced in IL-12+/+ versus IL-12-/- mice, if the same amount of bacteria were harboured in the joints. The levels of IFN-γ are decreased in the absence of IL-12 production, although this was not statistically significant. This was expected, because IL-12 induces IFN-γ production by natural killer cells and promotes type 1 T-cell differentiation, cells known to produce IFN-γ. Because IFN-γ is important for phagocytic functions as well as promoting development of septic arthritis, downregulation of IFN-γ production in IL-12-/- mice may explain the outcome of S aureus-induced arthritis.
Introduction
Sepsis caused by S aureus is a life-threatening condition that may cause septic shock, resulting in multiple organ failure and ultimately death [1]. Septic arthritis is a severe disease, involving high mortality and rapid destruction of affected joints [2,3,4,5]. The most common bacterial agent in this condition is S aureus, which is the responsible pathogen in 37-56% of all cases of septic arthritis [6,7,8]. Despite eradication of bacteria from the joint cavity destruction of the joint often continues, resulting in severe sequelae [9]. Several efforts have been made to down-modulate the inflammatory responses that lead to septic death and arthritis [10]. One problem in these attempts is the risk of increasing staphylococcal growth in vivo. In our mouse model of sepsis and septic arthritis, we use an intravenous inoculum of a TSST-1-producing S aureus strain (LS-1) [11]. This bacterial strain was originally isolated from a mouse with spontaneous staphylococcal arthritis [12].
IL-12 is a heterodimeric cytokine that is composed of the constitutively expressed p35 gene product and the inducible p40 subunit [13]. It is primarily produced by monocytes/macrophages and dendritic cells [14,15]. It has a variety of effects on natural killer cells and T cells, including the ability to faclitate Th1-cell responses and thereby IFN-γ production [16].
IFN-γ is a classical Th1 cytokine. Modulation of IFN-γ levels using neutralizing monoclonal antibodies or addition of recombinant cytokines show the beneficial effect of IFN-γ in terms of defence against S aureus infection, decreasing bacterial load in tissues and increasing survival. Results from these experiments also indicate an arthritogenic role of IFN-γ in S aureus infections [17].
The role of IL-12 in several experimental models of bacterial infection is protective, as demonstrated by the fact that neutralization of this cytokine increases susceptibility and addition of recombinant IL-12 ameliorates the severity of the infection [18,19,20]. However, IL-12 given at high doses induces a septic shock-like condition, and neutralization of IL-12 protects mice from lipopolysaccharide-induced shock [21,22]. Thus, the role of IL-12 in cases of severe bacterial infection that culminate ending in septic shock is not established. It has recently been shown that IL-12 deficiency exists in humans [23] and that absence of IL-12 may give rise to recurrent infections with S aureus [24]. The role of IL-12 in S aureus infection has not previously been assessed. Inoculation of IL-12 p40-deficient mice and their wild-type counterparts with a TSST-1-producing S aureus strain demonstrates the critical importance of IL-12 for survival during S aureus sepsis due to IL-12-dependent downregulation of staphylococcal growth, and the arthritogenic role of IL-12 in septic arthritis.
Materials and methods
Mice, bacteria and infection
Inbred male C57BL/6 mice that were intact or defective with respect to IL-12 production were used throughout the present study. IL-12 p40-/- mice were kindly provided by Dr J Magram (Nutley, NJ, USA). The procedure of gene disruption and mouse phenotype has previously been described in detail [25]. At the time of experiment, IL-12-deficient mice had undergone five back-crosses to C57BL/6. Mice were maintained in the animal facility of the Department of Rheumatology, University of Göteborg, Sweden, and were kept under standard temperature and light conditions. The mice were housed in a pathogen-free environment. They were fed laboratory chow and water ad libitum.
A TSST-1-producing S aureus strain (LS-1), which was originally isolated from a spontaneously arthritic NZB/W mouse, was prepared according to a previously described method [26] and injected into one of the tail veins. Two different doses of bacteria (1 × 107 and 4 × 108 staphylococci/mouse) were used. Viable counts were used to check the numbers of staphylococci injected.
Clinical evaluation of arthritis and weight
All mice were labelled and monitored individually. Limbs were inspected by two observers at regular intervals (3, 7, 12 and 20 days). Arthritis was defined as visible erythema and/or swelling of at least one joint. In order to evaluate the intensity of arthritis, we used a clinical scoring system in which macroscopic inspection yields a score of 0–3 for each paw (0, normal; 1, mild swelling and/or erythema; 2, moderate swelling and erythema; and 3, marked swelling and occasionally ankylosis), resulting in an arthritic score ranging from 0 to 12 for each mouse. An arthritic index was constructed by dividing the sum of scores from all four limbs in each mouse by the number of animals in each experimental group. Weight was checked at days 0, 3, 7, 12 and 20.
Experimental protocol
In the first experiment 16 IL-12-/- mice and nine IL-12+/+controls were inoculated intravenously with 1 × 107 staphylococci/mouse. Arthritis and weight were checked at regular intervals. The experiment was finished after 20 days, when mice were bled and killed. Kidneys were aseptically removed and bacterial growth was determined by viable counts. All four limbs were histopathologically examined.
In the second experiment, 21 IL-12-/- and 20 IL-12+/+ controls were inoculated with 1 × 107 staphylococci/mouse. Arthritis and weight were checked at regular intervals. Six hours after staphylococcal inoculation, five mice from each group were bled for bacterial examination and cytokine analyses. Two days after inoculation, five mice from each group were killed and bacterial growth in, liver, kidneys and joints were examined. The same procedure was performed at day 7 (IL-12-/-, n = 6; IL-12+/+, n = 5). The surviving mice were bled and killed at day 20.
In a third experiment, a high dose of S aureus (4 × 108 staphylococci/mouse) were given intravenously to nine IL-12-/- mice and nine IL-12 control animals. Survival was checked daily.
Cytokine and immunoglobulin analyses
Microtitre plates were coated with 2 μg/ml rat antimouse IFN-γ monoclonal antibodies (Pharmingen, San Diego, CA, USA) dissolved in sodium bicarbonate (pH 9.6), and blocked with 1% bovine serum albumin dissolved in 0.05 mol/l Tris (pH 7.4) for 1 h before adding samples for a 2-h incubation. Recombinant mouse IFN-γ (Genzyme, Cambridge, MO, USA) was used to create a standard curve. Biotinylated rat antimouse IFN-γ (2 μg/ml; Pharmingen) was employed as the capture antibody. The plates were kept for 2 h at 37°C, and then incubated with streptavidin alkaline phosphatase (Dako A/S, Glostrup, Denmark) and alkaline phosphatase substrate 1 mg/ml (Sigma, St Louis, MO, USA). Absorbance was measured at 405 nm in a Titretec multiscan photometer (Flow Laboratories, McLean, WA, USA). IL-10 was analyzed using an optEIA ELISA (Pharmingen) Assays were performed in accordance with the manufacturer's instructions, except for the addition of 2 mol/l HCl, instead of 2 mol/l H2SO4.
Serum levels of IgG1, IgG2a and IgG3 were measured using the radial immunodiffusion technique [27]. Antisera and immunoglobulin standards were purchased from Sigma.
Determination of bacterial load
Bacterial samples from talocrural and radiocarpal joints were obtained using cotton sticks. Bacterial presence was defined as 15 colony-forming units or more for each joint. The liver and both kidneys were removed aseptically, placed on ice, homogenized and diluted in 10 ml phosphate-buffered saline. Viable counts were performed to determine bacterial concentration. Colonies from every plate were then tested using a Staphaurex kit (Murex Diagnostcs, Dartford, UK).
Phagocytosis and intracellular killing
Intraperitoneal macrophages from noninfected mice were extracted, adjusted to 2 × 106 cells/ml, and incubated in a 42-well plate (Nunc, Roskilde, Denmark) according to procedures detailed previously [28,29]. Adherent macrophages were incubated with 500 ml S aureus at a concentration of 5 × 106 bacteria/ml for 50 min at 37°C, and were subsequently washed three times in Iscove's medium. Macrophage content of bacteria was then measured after two incubation intervals (0 and 4 h) in order to study phagocytosis and intracellular killing capacity, respectively. In order to avoid extracellular bacterial growth in the intracellular study, the incubation medium contained 10 mg/ml gentamycin. The antibiotic was washed away before lysing macrophages with distilled water. Macrophages were extracted from three wild-type mice and three IL-12-deficient mice. Macrophages from IL-12-deficient animals were preincubated for 12 h with 0, 10, or 100 ng/ml of recombinant IL-12 (R & D Systems, Abingdon, Oxon, UK) before adding bacteria. Supernatants were collected from macrophage cultures before exposure to S aureus, and after 4 h of incubation with bacteria.
Histopathologic examination
Limbs were fixed in 4% paraformaldehyde, decalcified and embedded in paraffin. Tissue sections were prepared and stained with haematoxylin and eosin. Sections were examined by two blinded observers, who determined whether synovial hypertrophy (membrane thickness of more than two cell layers), pannus formation (joint cartilage covered with synovial tissue), or destruction of cartilage and bone were present.
Statistical analysis
Mann–Whitney U test and Fisher's exact test were used for statistical analyses. P < 0.05 was considered statistically significant. Values are expressed as mean ± standard error of the mean.
Results
IL-12 production protects the host from fatal S aureus-triggered sepsis
C57BL/6 mice defective (IL-12-/-) or intact (IL-12+/+) with respect to the p40 gene, the inducible part of the heterodimeric IL-12 molecule, were injected intravenously with 4 × 108 staphylococci/mouse. The high dose of bacteria used in this experiment was chosen in order to study the influence of IL-12 on Gram-positive bacteria-triggered septic death. No mice died within the first 4 days. The majority of deaths occurred more than 1 week after inoculation of staphylococci. Ten days after inoculation of staphylococci, all of the IL-12-/- mice had died, as compared with 22% of the wild-type control animals (P = 0.002; Fig. 1). In order to determine whether the mortality rate of the IL-12+/+ mice would increase during the later stage of infection, we terminated the experiment at 3 weeks after the bacterial inoculation. No further control animals died during the course of the experiment. These results clearly indicate the critical importance of IL-12 production for survival during infection with S aureus.
Using a smaller inoculum (1 × 107 staphylococci/mouse) mortality rates were overall low (IL-12-deficient mice 6% [one out of 17] and controls 11% [one out of nine]). Interestingly, with this lower dose of bacteria the weight decrease was more pronounced in the wild-type mice as compared with the IL-12-deficient animals in the early phase (after 3 days) of infection (10.6% in IL-12+/+ mice versus 7.3% in IL-12-/- mice; P = 0.03). The differences between groups regarding weight change then declined, and at the end of the experiment the wild-type mice had regained their initial weight loss, whereas mice that lacked IL-12 production did not (-0.3% versus -4.2%; not significant).
Influence of IL-12 deficiency on S aureus-induced arthritis
The clinically observed frequency of septic arthritis was 76% in IL-12+/+ mice as compared with 54% in IL-12-/- animals 20 days after inoculation of 1 × 107 staphylococci/mouse. At that time, the severity of arthritis was graded as 1.3 in IL-12+/+ mice versus 0.9 in IL-12-/- mice. Histopathological examination confirmed the clinical findings (Table 1). Despite the fact that all parameters regarding development of joint inflammation and destruction of bone and cartilage indicated greater severity in mice with an intact IL-12 gene, none of the parameters tested reached the level of statistical significance.
Staphylococcal load is increased in several tissues due to the absence of IL-12 production
Bacterial content was determined in liver, kidneys and joints 20 days after intravenous inoculation with 1 × 107 staphylococci/mouse. The clearance of bacteria was clearly diminished in IL-12-deficient mice in all three organs. A fivefold increase in bacterial counts was seen in the livers of IL-12-/- mice as compared with wild-type controls (Fig. 2a). In kidneys, the bacterial load was 100-fold higher in IL-12-/-mice as compared with IL-12+/+ controls (Fig. 2b). Finally, S aureus was not detected in joints of IL-12+/+ mice, whereas 75% of IL-12-/- mice had staphylococci in their joints (Fig. 2c). We conclude that the decreased bacterial clearance in IL-12-deficient mice as compared with in control animals is probably the reason for the enhanced incidence of S aureus-induced death in the former.
Figure 2.
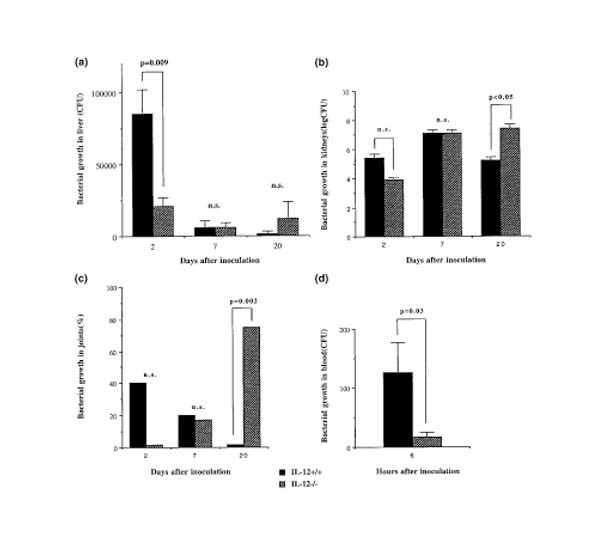
Effect of IL-12 on staphylococcal growth in vivo. (a) Bacterial growth in the liver, at days 2, 7 and 20 after staphylococcal inoculation. At day 2, n = 5 in each group; at day 7, n = 6 IL-12-deficient mice and 5 control animals; and at day 20, n = 8 IL-12-/- mice and 9 wild-type controls. (b) Bacterial growth in the kidneys, at days 2, 7 and 20 after staphylococcal inoculation. At day 2, n = 5 in each group; at day 7, n = 6 IL-12-deficient mice and 5 control animals; and at day 20, n = 8 IL-12-/- mice and 9 wild-type controls. (c) Frequency of mice with bacterial growth in the joints, at days 2, 7 and 20 after staphylococcal inoculation. At day 2, n = 5 in each group; at day 7, n = 6 IL-12-deficient mice and 5 control animals; and at day 20, n = 8 IL-12-/- mice and 9 wild-type controls. Fifteen CFU was regarded as a positive staphylococcal growth. (d) Bacterial growth in blood, 6 h after staphylococcal inoculation; n = 5 for both IL-12+/+ and IL-12-/-.
We also determined staphylococcal load in blood, liver and kidneys earlier during the infection. Surprisingly, bacterial content in blood was decreased in IL-12-deficient mice as compared with that in the wild-type control animals at 6 h, as well as in joints, liver and kidneys at 2 days after inoculation of bacteria (Figs 2a,b,c,d). This initial beneficial effect of IL-12 deficiency on the clearance of staphylococci may explain the significantly lower weight loss measured 3 days after bacterial inoculation in IL-12-/-as compared with IL-12+/+ mice.
In order to study the importance of IL-12 on phagocytosis and intracellular killing capacity, we extracted intraperitoneal macrophages from IL-12-deficient mice and their wild-type counterparts. To simplify our method, we conducted this part of the study in noninfected mice, because in vivo activated macrophages would have encountered different bacterial loads as a result of differences in the in vivo clearance of S aureus, and therefore would have been differentially activated. No differences in phagocytosis or intracellular killing were seen (data not shown). Furthermore, recombinant mouse IL-12 (0, 10 and 100 ng/ml) was added to the IL-12-deficient macrophage cultures 12 h before exposure to staphylococci. Addition of recombinant IL-12 did not affect either phagocytic activity or intracellular killing (data not shown).
Decreased levels but continuous production of IFN-γ during S aureus-induced arthritis in IL-12-deficient mice
Serum levels of IFN-γ were measured in IL-12-deficient mice and wild-type control animals after intravenous inoculation of 1 × 107 staphylococci/mouse; Table 2). IL-12-deficient mice displayed decreased IFN-γ levels, measured at days 7 and 20 after the inoculation of bacteria, as compared with wild-type controls (day 7, 208 ± 83 versus 764 ± 288 U/ml; day 20, 269 ± 114 versus 444 ± 170 U/ml; these findings were not statistically significant). The difference in serum levels of IFN-γ did not reach statistical significance, but results at 7 and 20 days after intravenous inoculation of S aureus indicate, as expected, higher IFN-γ levels in IL-12 p40-intact mice, suggesting a stronger Th1 response in wild-type mice as compared with IL-12 p40-defective mice.
Table 2.
Serum levels of IFN-γ
| Days after staphylococcal inoculation | |||
| IL-12 status | 2 | 7 | 20 |
| IL-12+/+ | 12 ± 5 | 764 ± 288 | 444 ± 170 |
| IL-12-/- | 46 ± 17 | 208 ± 83 | 269 ± 114 |
Serum levels of IFN-γ in IL-12-deficient mice (day 2, n = 5; day 7, n = 5; day 20, n = 9) and wild-type controls (day 2, n = 5; day 7, n = 6; day 20, n = 16) after intravenous inoculation of 1 × 107 staphylococci/mouse. Values are expressed as mean ± standard error of the mean (units/ml).
Because IL-10 production is induced by IL-12 [30,31,32,33], and IL-12-induced IL-10 production could contribute to an explanation for the better condition of IL-12-/- mice observed during the early phase of S aureus infection, we analyzed serum levels of IL-10. We did not find detectable levels of IL-10, either in IL-12+/+ or in IL-12-/- mice.
Twenty days after intravenous inoculation of staphylococci, immunoglobulin responses were higher in IL-12-/-mice than in IL-12+/+ mice (Table 3). This was indicated by higher serum levels of IgG1, IgG2a and IgG3 in the knock-out mice. Notably, levels of IgG2a were not reduced, as seen in previous studies on IL-12-deficient mice, which is putatively due to lower levels of IFN-γ [34]. Similar to the present results, IgG2a :IgG1 ratio was not substantially reduced in IFN-γ receptor-deficient mice exposed to superantigen-producing staphylococci [35] or in IL-12-deficient mice subjected to viral infections [36,37]. The increased total IgG production might be a result of a more pronounced Th2 response, and thereby B-cell activation in absence of IL-12 production. Alternatively, an increased staphylococcal load in the IL-12-/- mice might have triggered a more pronounced immunoglobulin production.
Table 3.
Serum IgG isotypes in response to TSST-1 secreting Staphylococcus aureus
| IgG isotype | |||
| IL-12 status | IgG1 | IgG2a | IgG3 |
| IL-12-/- | 5.9 ± 1.1 | 1.8 ± 0.4* | 1.2 ± 0.2 |
| IL-12+/+ | 4.3 ± 0.4 | 0.86 ± 0.0 | 0.97 ± 0.15 |
Levels measured in sera 20 days after inoculation of bacteria. Values are expressed as mean ± standard error of the mean (mg/ml). IL-12-/-, n = 16; IL-12+/+, n = 8. *P = 0.03.
Discussion
The present study demonstrates that IL-12 deficiency leads to striking increase in mortality during S aureus sepsis. These results suggest that the presence of IL-12 is required for efficient control of staphylococcal growth in vivo. Furthermore, although exposed to higher bacterial load, IL-12-/- mice did not display increased joint pathology.
The increased mortality of IL-12-/- mice as compared with their wild-type counterparts was seen in parallel with an increased staphylococcal load in the tissues examined. It is plausible that the increased bacterial growth was the main reason for the sharply increased death rates in the absence of IL-12 production.
In order to determine whether IL-12 itself had any direct effect on the phagocytosis and/or intracellular killing capacity, we employed two different approaches using intraperitoneal macrophages from IL-12-/- and IL-12+/+ mice. No differences in intracellular killing of bacteria were seen. Indeed, supplementing IL-12-/- macrophages with recombinant IL-12 neither affected the uptake nor killing of staphylococci. This is in contrast to the beneficial antifungal effects that IL-12 p40-/- macrophages exert during infection with Candida albicans [38].
IL-12 is known to induce development of Th1 cells and their subsequent differentation to produce IFN-γ [39]. A recent study [17] showed the importance of IFN-γ in recruitment of neutrophils and the increased phagocytosis during staphylococcal infection. Macrophages, despite not being a major producer of IFN-γ, have been shown to synthesize this cytokine after stimulation with IL-12 and IL-18, but not with IL-12 alone [40]. This might have been the main reason for the lack of direct effect of IL-12 on staphylococcal growth in vitro. In vivo data showing decreased serum levels of IFN-γ in IL-12-/-mice as compared with IL-12+/+ control animals support deficient IL-12-dependent IFN-γ production as an explanation for the decreased bacterial clearance in IL-12-/- mice. It is thus plausible that IL-12 is protective with regard to S aureus-triggered death via enhanced phagocytic activity mediated by IFN-γ.
An alternative or parallel explanation for the observed effect of IL-12 deficiency on decreased survival during S aureus-induced sepsis is the previously described effects of this cytokine on IL-10 production. IL-12 induces IL-10 production, and thereby downregulates production of tumour necrosis factor (TNF), IFN-γ and nitric oxide [41], with TNF and IFN-γ being detrimental in septic shock [42,43,44]. Indeed, supplementation with recombinant IL-10 protects against death both from lipopolysaccharide-triggered enotoxemic shock [45,46] and staphylococcal enterotoxin B-induced shock [47]. This might be relevant because the higher bacterial cell counts in IL-12-deficient mice potentially lead to increased production of TSST-1 as compared with controls. However, we were not able to detect IL-10 production in IL-12+/+ or IL-12-/- mice, despite analyzing serum at several time points after bacterial inoculation. Downregulated IL-10 production in the absence of IL-12 could be a reason for the enhanced bacterial clearance seen in IL-12-deficient mice as compared with controls during the early phase of S aureus infection.
It is somewhat surprising that, although IL-12-deficient mice have a defect in bacterial clearance and therefore are exposed to a higher staphylococcal load than their wild-type counterparts, they did not develop a more severe septic arthritis, but rather milder joint involvement. A similar outcome has previously been shown using TNF/lymphotoxin-α double mutants [48]. The mechanisms that underlie these results may again be explained by the decreased production of IFN-γ in IL-12-/- mice versus IL-12+/+ animals. Previous studies implicate IFN-γ as a cytokine that promotes induction of septic arthritis, in addition to its importance in phagocytic activity.
The present results clearly demonstrate the critical role played by endogenous IL-12 in preventing death during S aureus-induced arthritis. The protection results from decreasing the bacterial load, an effect that is possibly due to IL-12-dependent IFN-γ mediation of phagocytic activity.
Acknowledgments
Acknowledgements
This work was supported by grants from the Göteborg Medical Society, the Swedish Association against Rheumatism, King Gustaf V 80 years Foundation, the Nanna Svartz Foundation, the Swedish Medical Research Council, the University of Göteborg, the A-G Crafoord Foundation and Gustaf Dahlen Foundation. We thank Dr I-M Jonsson, M Verdrengh and L Svensson for excellent technical assistance, and Dr J Magram for providing the IL-12 p40-/- mice.
References
- Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Goldenberg DL. Infectious arthritis complicating rheumatoid arthritis and other chronic rheumatic disorders. Arthritis Rheum. 1989;32:496–502. doi: 10.1002/anr.1780320422. [DOI] [PubMed] [Google Scholar]
- Goldenberg DL. Septic arthritis in chronic joint disease. Rheumatol Rev. 1992;4:147–149. [Google Scholar]
- Meijers KAE, Dijkmans BAC, Hermans J, van den Broek PJ, Cats A. Non-gonococcal infectious arthritis: a retrospective study. J Infect. 1987;14:13–20. doi: 10.1016/s0163-4453(87)90704-3. [DOI] [PubMed] [Google Scholar]
- Esterhai JL, Gelb I. Adult Septic Arthritis. Orthop Clin North Am. 1991;22:503–514. [PubMed] [Google Scholar]
- Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117:423–428. doi: 10.1017/s0950268800059070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370–373. doi: 10.1093/rheumatology/36.3.370. [DOI] [PubMed] [Google Scholar]
- Le Dantec L, Maury F, Flipo RM, Laskri S, Cortet B, Duquesnoy B, Delcambre B. Peripheral pyogenic arthritis. A study of one hundred seventy-nine cases. Rev Rheum. 1996;63:103–110. [PubMed] [Google Scholar]
- Goldenberg DL, Reed JI. Bacterial arthritis. N Engl J Med. 1985;312:764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- Fisher CJ, Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- Bremell T, Lange S, Yacoub A, Ryden C, Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremell T, Lange S, Svensson L, Jennische E, Grondahl K, Carlsten H, Tarkowski A. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990;33:1739–1744. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- Podlaski FJ, Nanduri VB, Hulmes JD, Pan YC, Levin W, Danho W, Chizzonite R, Gately MK, Stern AS. Molecular characterization of interleukin-12. Arch Biochem Biophys. 1992;294:230–237. doi: 10.1016/0003-9861(92)90162-p. [DOI] [PubMed] [Google Scholar]
- D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (NKSF/IL-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Zhao Y-X, Nilsson I-M, Tarkowski A. The dual role of IFNγ in experimental Staphylococcus aureus septicemia versus arthritis. Immunology. 1998;93:80–86. doi: 10.1046/j.1365-2567.1998.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-γ. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- Metzger DW, Raeder R, van Cleave VH, Boyle MD. Protection of mice from group A streptococcal skin infection by interleukin-12. J Infect Dis. 1995;171:1643–1645. doi: 10.1093/infdis/171.6.1643. [DOI] [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- Cauwles A, Fiers W, Brouckaert P. Murine IL-12 is involved in Calmette Guerin bacillus-induced sensitization and is by itself sufficient to sensitize mice to the lethal effects of human TNF. J Immunol. 1996;156:4686–4690. [PubMed] [Google Scholar]
- Wysocka M, Kubin M, Vieira LQ, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- Altare F, Lammas D, Revy P, Jouanguy E, Doffinger R, Lamhamedi S, Drysdale P, Scheel-Toellner D, Girdlestone J, Darbyshire P, Wadhwa M, Dockrell H, Salmon M, Fischer A, Durandy A, Casanova JL, Kumararatne DS. Inherited interleukin-12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteriditis disseminated infection. J Clin Invest. 1998;102:2035–2040. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi S, Day NK, Nelson RP, Jr, Emmanuel P, Duplantier JE, Christodoulou CS, Good RA. Interleukin 12 deficiency associated with recurrent infections. Proc Natl Acad Sci USA. 1998;95:13125–13129. doi: 10.1073/pnas.95.22.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Sakiniene E, Bremell T, Tarkowski A. Complement depletion aggravates Staphylococcus aureus septicaemia and septic arthritis. Clin Exp Immunol. 1999;115:92–102. doi: 10.1046/j.1365-2249.1999.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965;2:235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Lissner CR, Swanson RN, O'Brien AD. Genetic control of the innate resistance of mice to Salmonella typhimurium : expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–3013. [PubMed] [Google Scholar]
- Wiedermann U, Tarkowski A, Bremell T, Hanson L, Kahu H, Dahlgren UI. Vitamin A deficiency predisposes to Staphylococcus aureus infection. Infect Immun. 1996;64:1–7. doi: 10.1128/iai.64.1.209-214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J Exp Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- Romani L, Mencacci A, Cenci E, Puccetti P, Bistoni F. Neutrophils and the adaptive immune response to Candida albicans. Res Immunol. 1997;147:512–518. doi: 10.1016/s0923-2494(97)85216-9. [DOI] [PubMed] [Google Scholar]
- Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- McIntyre KW, Shuster DJ, Gillooly KM, Warrier RR, Connaughton SE, Hall LB, Arp LH, Gately MK, Magram J. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur J Immunol. 1996;26:2933–2938. doi: 10.1002/eji.1830261219. [DOI] [PubMed] [Google Scholar]
- Zhao Y-X, Tarkowski A. Impact of interferon-γ receptor deficiency on experimental Staphylococcus aureus septicemia and arthritis. J Immunol. 1995;155:5736–5742. [PubMed] [Google Scholar]
- Schijns VE, Haagmans BL, Wierda CM, Kruithof B, Heijnen IA, Alber G, Horzinek MC. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J Immunol. 1998;160:3958–3964. [PubMed] [Google Scholar]
- Oxenius A, Karrer U, Zinkernagel RM, Hengartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J Immunol. 1999;162:965–973. [PubMed] [Google Scholar]
- Mencacci A, Cenci E, Del Sero G, Fe d'Ostiani C, Mosci P, Trinchieri G, Adorini L, Romani L. IL-10 is required for development of protective Th1 responses in IL-12-deficient mice upon Candida albicans infection. J Immunol. 1998;161:6228–6237. [PubMed] [Google Scholar]
- Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Steege JC, van den Ven MW, Forget PP, Brouckaert P, Buurman WA. The role of endogenous IFN-gamma, TNF-alpha and IL-10 in LPS-induced nitric oxide release in a mouse model. Cytokine. 1998;10:115–123. doi: 10.1006/cyto.1997.0263. [DOI] [PubMed] [Google Scholar]
- Hinshaw LB, Emerson TE, Jr, Taylor FB, Jr, Chang AC, Duerr M, Peer GT, Flournoy DJ, White GL, Kosanke SD, Murray CK, et al. Lethal Staphylococcus aureus-induced shock in primates: prevention of death with anti-TNF antibody. J Trauma. 1992;33:568–573. [PubMed] [Google Scholar]
- Car BD, Eng VM, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Aguet M, Ryffel B. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean AG, Freiburg RA, Andrade S, Menon S, Zlotnik A. Interleukin 10 protects mice against staphylococcal enterotoxin B-induced lethal shock. Infect Immun. 1993;61:4937–4939. doi: 10.1128/iai.61.11.4937-4939.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren O, Eugster H-P, Sedgwick JD, Körner H, Tarkowski A. TNF/LTa double mutant mice resist septic arthritis but display increased mortality in response to Staphylococcus aureus. J Immunol. 1998;161:5937–5942. [PubMed] [Google Scholar]


