Abstract
What controls the fate of the T-cell response remains incompletely defined. Gain of effector function facilitated by costimulation has been thought to be a crucial factor in determining the outcome of the T-cell response, i.e. long-term memory in the presence of costimulation versus tolerance induction in the absence of costimulation. In this study, we show that while costimulation or cognate CD4 helps to promote the acquisition of effector function during the initial phase of the CD8 T-cell response, the fate of effector CD8 T cells is controlled by the duration of subsequent antigenic stimulation. Effector CD8 T cells differentiate into memory cells only after clearance of antigen, whereas in the presence of persistent antigen, effector CD8 T cells are tolerized. Furthermore, protective immunity against tumour cannot develop in the persisting antigen environment. These results suggest that removal of persisting antigen by other means might be a prerequisite for effective immunotherapy in cancer.
Keywords: antigen duration, immunotherapy, T-cell memory, T-cell tolerance
Introduction
The factors that influence the outcome of T-cell responses, i.e. long-term memory versus tolerance, have not been completely defined. Over the years, the ‘two-signal’ hypothesis proposes that when naive T cells receive antigen stimulation (‘signal 1’) via T-cell receptor (TCR), the deciding factor for determining their fate is whether there is costimulation (‘signal 2’) provided by the bone marrow-derived antigen presenting cells such as dendritic cells (DCs).1–4 This model has been used to explain that peripheral CD8 tolerance to self-antigens is the result of antigen presentation by immature DCs which express low levels of costimulatory molecules, leading to abortive T-cell activation and the establishment of tolerance.5,6 Accordingly, successful immunity to pathogens is the result of pathogen-derived signals that promote maturation of DCs, which consequently express high levels of costimulatory molecules, leading to full activation of T cells and the development of effector and memory functions.6,7
Emerging evidence, however, indicated that abortive activation of naive CD8 T cells alone did not lead to tolerance, whereas persistent antigen was required for the deletion of abortively activated CD8 T cells.8 The question remains whether effector CD8 T cells can be tolerized in the presence of persistent antigen as they are considered more functionally ‘fit’.9,10 Previous studies have demonstrated that in several murine models of systemic persistent viral infection, virus-specific CD8 T cells were either deleted or anergized despite a vigorous effector CD8 T-cell response initially.11–13 Similar findings were observed in hosts who were chronically infected with simian or human immunodeficiency viruses or hepatitis C virus.14–16 However, it is often difficult to discern whether this is because of persisting antigen or other mechanisms such as the ability of certain pathogens to down-regulate the function of the antigen-presenting cells and thus inhibiting the activation of naive CD8 T cells into effector T cells.17 In addition, some pathogens can also promote regulatory mechanisms that may influence the outcome of the immune response, such as the induction of regulatory T cells seen in the models of persistent Friend virus infection13 and chronic parasitic infection with Leishmania major.18
To address this question, we have developed models of in vivo CD8 T-cell responses to a model antigen, the influenza virus haemagglutinin (HA), presented as a self-tissue antigen in HA-transgenic mice (C3-HA) versus a viral antigen in vaccinia virus encoding HA (rVV-HA). We previously demonstrated that adoptive transfer of naive HA-specific CD8 T cells into C3-HAhi transgenic mice that express high levels of self-HA in a variety of parenchymal tissues including lung19,20 led to full activation of HA-specific T cells and development of effector function in the absence of costimulation.21 Here we compared the fate of effector CD8 T cells in C3-HAhi mice with those generated in syngeneic B10.D2 mice infected with a recombinant vaccinia virus encoding HA (rVV-HA). We found that despite comparable levels of effector CD8 T-cell development during the initial phase of the CD8 T-cell response, the fate of effector CD8 T cells was drastically different in these two settings: long-term memory formation in rVV-HA-treated B10.D2 mice compared with tolerance induction in C3-HAhi mice. We further found that provision of costimulation or cognate CD4 help did not promote the transition of effector CD8 T cells into memory cells in C3-HAhi mice and the critical factor in controlling the fate of effector CD8 T cells was the duration of subsequent antigen stimulation: effector CD8 T cells developed into memory cells only in the absence of antigen, whereas the fate of effector CD8 T cells was tolerance induction in the presence of persistent antigen. Furthermore, the generation of memory CD8 T cells correlated with the protective immunity against tumour challenge. Our findings indicate that the transition of effector CD8 T cells into long-lived, functional memory CD8 T cells depends upon the duration of subsequent antigenic stimulation and so they provide important insights for the design of vaccine strategies for treating diseases with prolonged antigen exposure such as cancer.
Materials and methods
Mice
B10.D2 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The C3-HA transgenic mice have been described previosuly.19–21 These mice were generously provided by Dr Drew Pardoll (Johns Hopkins University, Baltimore, MD) and were bred and maintained under specific pathogen-free conditions in the animal facility of Duke University Medical Center. Briefly, the influenza virus (A/PR/8/34, Mount Sinai strain) HA gene was placed under the control of the rat C3 promoter. Upon microinjection into B10.D2 embryos, two founder lines, C3-HAhi and C3-HAlo, were established, containing 30–50 and three copies of transgene, respectively. They express HA mRNA in the same set of organs including the lung, heart, skeletal muscle, kidney and male genital organs. The levels of HA mRNA and protein in C3-HAhi and C3-HAlo were measured directly by quantitative reverse transcription—polymerase chain reaction and indirectly by bioassay of tissue extract induced hybridoma cytokine release, respectively. The difference in the lung is roughly 100-fold at RNA level and 1000-fold at protein level.19,20 The Clone 4 HA-TCR transgenic mice that express a TCR recognizing a Kd-restricted HA epitope (518IYSTVASSL526) were kindly provided by Dr Linda Sherman (Scripps Research Institute, La Jolla, CA).22 The 6.5 TCR-HA transgenic mice that express a TCR recognizing an I-Ed-restricted HA epitope (110SFERFEIFPKE120) were kindly provided by Dr Harald von Boehmer (Harvard University, Boston, MA).23 These mice were back-crossed for more than nine generations into the Thy-1.1, B10.D2 genetic background.
For tumour challenge experiments, Clone 4-BALB/c F1, B10.D2-BALB/c F1, and C3-HAhi-BALB/c F1 were used because the HA-expressing A20 lymphoma cell line (A20-HA) is derived from BALB/c mice. All mice used in these studies were between 8 and 12 weeks of age. Experimental procedures were performed in accordance with protocols approved by the Animal Care and Use Committee of the Duke University Medical Center.
Adoptive transfer of naive HA-specific transgenic T cells
Naive clonotypic HA-specific CD8 T cells were prepared from Clone 4 TCR transgenic mice. Briefly, single cell suspensions were prepared from spleen and lymph nodes of Clone 4 TCR mice and clonotypic percentage was then determined by flow cytometry analysis of CD8+ Vβ8.2+ cells as described elsewhere.22 The activation marker CD44 was also checked to ensure that these clonotypic cells were naive. CD4 T cells were removed from the pooled lymphocytes using anti-CD4 microbeads according to the manufacturer's instructions (Miltenyi-Biotech, Bergisch-Gladbach, Germany). Recipient mice were injected intravenously with 2 × 106 clonotypic cells in 0·2 ml of Hanks' balanced salt solution (HBSS) on day 0. In some experiments, clonotypic cells were labelled with carboxy-fluorescein diacetate, succinimidyl ester (CFSE) as described previosuly21 before injection.
In vivo anti-CD40 antibody or CpG treatment
The agonistic anti-mouse CD40 monoclonal antibody (3/23)24–26 was purchased from BD PharMingen (San Diego, CA) and the control rat IgG was obtained from Jackson ImmunoResearch (West Grove, PA). Immediately after adoptive transfer of Clone 4 T cells, 10 μg antibody in 200 μl phosphate-buffered saline was injected intravenously. Phosphorothioate-stabilized CpG-oligodeoxynucleotide (ODN; 5′-TCC ATG ACG TTC CTG ATG CT) was synthesized by Integrated DNA Technologies (Coralville, IA) and injected intraperitoneally at 10 nmol.
Recombinant viruses encoding HA
Recombinant vaccinia virus encoding HA (rVV-HA), was kindly provided by Dr Drew Pardoll (Johns Hopkins University, Baltimore, MD); it was grown in TK-143B cells and purified from the cell lysate by sucrose banding.27 The titre of virus was determined by plaque-forming assay on TK-143B cells. Mice were infected with 5 × 106 plaque-forming units (PFU) rVV-HA intraperitoneally.
Recombinant E1-deleted adenoviruses encoding HA (Ad-HA) was generated by homologous recombination in Escherichia coli.28 Ad-HA was grown in 293 cells (American Type Culture Collection, Manassas, VA), purified by two rounds of CsCl density centrifugation and desalted by gel filtration through Sephadex G-25 column (PD-10 column, Amersham Bioscience.25 The titre of virus was determined by plaque-forming assay on 293 cells.29 Mice were infected with 2 × 109 PFU Ad-HA intravenously.
Flow cytometry
All monoclonal antibodies were purchased from BD PharMingen (San Diego, CA). Antibodies used for staining were: anti-CD8 (53-6.7), anti-Thy-1.1 (OX-7), anti-Thy-1.2 (53-2.1), anti-CD44 (1M7), anti-CD62L (MEL-14), anti-CD69 (H1.2F3), anti-Vβ8.2 (F23.1), anti-CD11c (HL3) and anti-interferon-γ (IFN-γ; XMG1.2). KdHA518-526 tetramer was purchased from Beckman Coulter Immunomics (San Diego, CA). FACSCaliber (Becton Dickinson, San Jose, CA) was used for flow cytometry analyses. For sorting of activated clonotypic CD8 T cells, naive Clone 4 T cells (Thy-1.1+) were injected into B10.D2 mice (Thy-1.2+) infected with rVV-HA, C3-HAlo or C3-HAhi mice (Thy-1.2+) and lymphocytes were harvested 4 days later. CD8+Thy-1.1+ T cells were enriched through depleting of non-CD8 cells and Thy-1.2+ cells by a cocktail of biotinylated monoclonal antibodies followed by antibiotin MicroMeads (Miltenyi-Biotech). Enriched T cells were then stained with CD8 and Thy-1.1, and subjected to cell sorting gated on CD8, Thy-1.1 and with at least three divisions by CFSE profile with a high-speed cell sorter FACSVantage (Becton Dickinson, San Jose, CA).
Intracellular IFN-γ staining
To assess the production of IFN-γ intracellularly, cells were incubated in RPMI-1640 supplemented with 10% fetal bovine serum in the presence of 2 μg/ml of the Kd HA518-526 peptide and 5 μg/ml Brefeldin A containing Golgi-Plug (BD PharMingen) for 6 hr at 37°. After washing, cells were stained to detect surface CD8 and Thy-1.1. Cells were then permeabilized and stained to detect intracellular IFN-γ with anti-IFN-γ fluorescein isothiocyanate using the Cytofix/Cytoperm kit (BD PharMingen) according to the manufacturer's instruction.
Cytotoxicity assays
For in vivo cytotoxic T-lymphocyte assays, naive B10.D2 splenocytes were labelled with either 5 μm CFSE (CFSEhi cells) or 0·5 μm CFSE (CFSElo cells) in HBSS for 7 min at room temperature. CFSEhi cells were pulsed with 2 μm Kd peptide for 1 hr at 37°, whereas CFSElo cells were incubated with a control peptide. After washing, a mixture of 2·5 × 106 CFSEhi and 2·5 × 106 CFSElo cells was injected into mice intravenously. Six hours after injection of CFSE-labelled target cells, spleen and peripheral lymph nodes were harvested and single cell suspensions were analysed by fluorescence-activated cell sorting (FACS). Each population was distinguished by their respective fluorescence intensity. Assuming that the number of Kd HA518-526 peptide-pulsed cells injected is equivalent to the number of control peptide-pulsed cells injected, the percentage of specific killing was determined as: [(%CFSElo − %CFSEhi)/%CFSElo] × 100.
For ex vivo CTL assay, lymphocytes were harvested and tested on A20 lymphoma (H-2d) targets pulsed with Kd-HA peptide or a control peptide in a 6-hr JAM assay as described previously.30
Tumour challenge
A20-HA (kindly provided by Dr Hyam Levitsky, Johns Hopkins Oncology Center) was grown in RPMI-1640 supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 50 μmβ-mercaptoethanol, 100 IU/ml penicillin, 100 IU/ml streptomycin and 400 μg/ml of G418 as previously reported.27 For tumour challenge experiments, 1 × 106 A20-HA cells in 200 μl HBSS were injected intravenously. Tumour-free survival was determined by weekly inspection, and mice were killed after development of a tumour, as evidenced by increasing abdominal girth and palpable abdominal mass. All the mice killed had the presence of tumours (hepatic and splenic nodules and mesenteric nodal enlargement) confirmed at autopsy.
Results
Differential fates of effector CD8 T cells
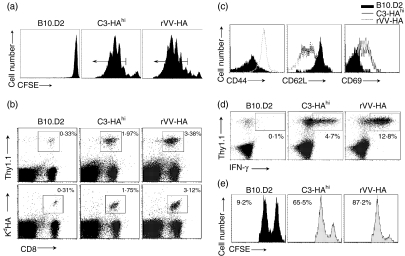
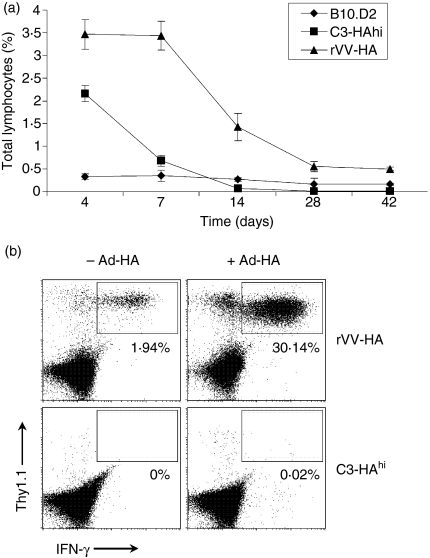
We first examined the behaviour of naive HA-specific CD8 T cells when encountering HA as a self-antigen in C3-HAhi mice or as a viral antigen in the syngeneic B10.D2 mice infected with rVV-HA. Four days after transfer of CFSE-labelled naive HA-specific CD8 T cells (Thy-1.1+) into different recipients (Thy-1.2+), vigorous proliferation was detected in spleen and peripheral lymph nodes with at least six division cycles in both C3-HAhi and rVV-HA-treated B10.D2 mice compared to naive B10.D2 controls (Fig. 1a). This led to roughly sixfold and tenfold expansion of clonotypic CD8 T cells in lymphoid organs of C3-HAhi and rVV-HA recipients, respectively (Fig. 1b). Extensive proliferation resulted in the activation of clonotypic CD8 T cells indicated by the up-regulation of CD44 and CD69, and the down-regulation of CD62L (Fig. 1c), and their differentiation into effector T cells evidenced by their ability to produce IFN-γ (Fig. 1d) and to lyse cognate peptide-pulsed syngeneic target cells in vivo (Fig. 1e) in both C3-HAhi and rVV-HA-treated B10.D2 mice. These results show that high-dose self-antigen could fully activate antigen-specific CD8 T cells in the absence of costimulation, leading to the development of effector function comparable to that achieved with a viral antigen during the initial phase of a CD8 T-cell response. However, despite the gain of effector function, clonotypic CD8 T cells in C3-HAhi mice were rapidly tolerized; this was characterized mainly by deletion as no functionally active HA-specific T cells were detected upon rechallenge with a recombinant adenovirus encoding HA (Ad-HA, Fig. 2). By contrast, after a contraction phase (Fig. 2a), effector CD8 T cells in rVV-HA-treated B10.D2 mice were ultimately differentiated into memory CD8 T cells characterized phenotypically by CD44hi, CD62Lhi and Ly6Chi (data not shown) and functionally by production of IFN-γ upon short-term 5-hr in vitro re-stimulation (Fig. 2b). More importantly, these memory cells rapidly expanded upon rechallenge with Ad-HA (Fig. 2b). These results demonstrate that despite a comparable effector phase initially, effector CD8 T cells in rVV-HA-treated mice developed into memory cells, whereas those in C3-HAhi mice were deleted.
Figure 1.
Effector CD8 T cells to viral antigen versus self-antigen. Naive HA-specific CD8 T cells were adoptively transferred into non-transgenic B10.D2, C3-HAhi or B10.D2 mice infected with rVV-HA (rVV-HA). After 4 days, lymphocytes from peripheral lymph nodes were harvested for subsequent analyses. (a) In vivo divisions of CFSE-labelled HA-specific T cells. Events were gated on CD8+ Thy-1.1+ T cells. Arrows indicate cells with at least three divisions which were gated for sorting described in Fig. 6. (b) Expansion of HA-specific T cells. Cells were stained with either Thy-1.1 or KdHA tetramer (KdHA518-526) in combination with CD8. Percentage of double-positive cells is indicated. (c) Phenotypic characterization of HA-specific T cells. Cells were stained with activation marker CD44, CD62L or CD69. Events were gated on Thy-1.1+CD8+ T cells. (d) Functional analysis of HA-specific T cells. After in vitro re-stimulation with KdHA peptide (2 µg/ml) for 6 hr, cells were stained with CD8, Thy-1.1 and IFN-γ intracellularly. The percentages of IFN-γ-producing clonotypic CD8 T cells are indicated. Events were gated on CD8+ T cells. (e) In vivo CTL assay. Mice were injected intravenously with an equal number mixture of KdHA peptide-pulsed CFSEhi-labelled (right peak) and control peptide-pulsed CFSElo-labelled (left peak) syngeneic targets. Six hours later, cells from peripheral lymph nodes were examined to detect and quantify CFSE-labelled cells. Percentage of specific lysis is indicated. Representative data of three independent experiments are shown.
Figure 2.
Different fates of effector CD8 T cells. Naive HA-specific CD8 T cells were adoptively transferred into non-transgenic B10.D2, C3-HAhi or B10.D2 mice infected with rVV-HA (rVV-HA). (a) The fate of clonotypic T cells. Cells were harvested 4, 7, 14, 28 or 42 days later for the percentage of HA-specific CD8 T cells (CD8+Thy-1.1+ cells ± SD) in total lymphocytes for all mice in each group is indicated. (b) Recall response: 42 days later, mice were challenged with (+ Ad-HA) or without (– Ad-HA) Ad-HA. Seven days after the challenge, cells were harvested for the percentages of IFN-γ-producing clonotypic CD8 T cells by IFN-γ intracellular staining. Events were gated on CD8+ T cells. (c) Representative data of three independent experiments are shown.
Co-stimulation or cognate CD4 help does not determine the fate of effector CD8 T cells
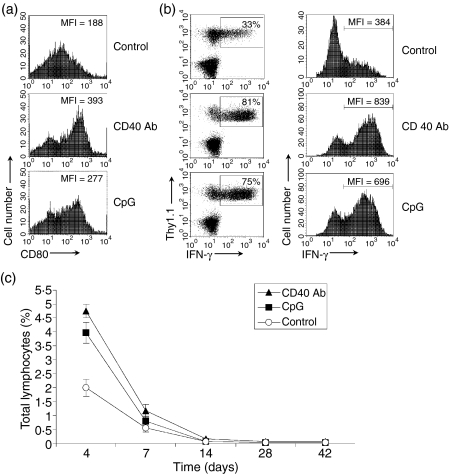
We next set to investigate what may decide the differential fates of effector CD8 T cells in C3-HAhi mice compared with rVV-HA-treated B10.D2 mice. One possibility was that effector CD8 T cells developed in C3-HAhi mice to high-dose self-antigen in the absence of costimulation might not be as functionally ‘fit’ as those derived from rVV-HA-infected mice (i.e. with costimulation), therefore they might die via apoptosis, whereas functionally ‘fit’ effector CD8 T cells in a vaccinia virus-infected host develop into memory cells. To address this question, we examined the fate of HA-specific CD8 T cells in C3-HAhi mice in the presence of costimulation through in vivo ligation of CD40 with an agonistic anti-CD40 antibody or Toll-like receptor 9 (TLR9) with TLR9 ligand, CpG-ODN. These approaches have been used extensively to promote DC activation and maturation in vivo, including up-regulation of costimulatory molecules, CD80 and CD86, the production of interleukin-12 and type I interferon.25,31,32 Naive HA-specific T cells were transferred into C3-HAhi mice that were coadministered with CD40 antibody or CpG. The percentages and functional status of clonotypic CD8 T cells were analysed 4, 7, 14, 28, or 42 days later. In vivo administration of CD40 antibody or CpG promoted DC maturation, as shown by up-regulation of CD80 (Fig. 3a). Both CD40 antibody and CpG-treated C3-HAhi mice accumulated twofold to 2·5-fold more clonotypic T cells in the peripheral lymph nodes on day 4, compared to mice receiving the control antibody (Fig. 3b), leading to an increase not only in the proportion of IFN-γ-secreting clonotypic CD8 T cells but also in the production of IFN-γ per cell, as shown by an elevation of mean fluorescence intensity (Fig. 3b) without a significant enhancement in up-regulation of activation markers such as CD40 and CD69 (data not shown). These results indicate that in vivo ligation of CD40 with CD40 antibody or TLR with CpG promotes the acquisition of effector function by CD8 T cells to high-dose self-antigen. However, effector CD8 T cells developed in the presence of CD40 antibody or CpG were also rapidly deleted after day 4, to nearly undetectable levels by day 28 (Fig. 3c). When these mice were rechallenged at day 42 with Ad-HA, no expansion of clonotypic T cells was detected (data not shown).
Figure 3.
Co-stimulation does not dictate the fate of effector CD8 T cells. Naive HA-specific CD8 T cells were adoptively transferred into C3-HAhi mice treated with 10 µg of anti-CD40 antibody (CD40 Ab) or 10 nmol CpG ODN (CpG), or the control rat IgG (Control). After 24 hr, cells were harvested from the peripheral lymph nodes and stained for CD11c and CD80. Data represent the expression of CD80 in CD11c+ DCs with mean fluorescence intensity (MFI) indicated (a). Four days later, lymphocytes from peripheral lymph nodes were harvested for the percentage and MFI of IFN-γ-secreting cells among clonotypic HA-specific CD8 T cells (b). Four, 7, 14, 28 or 42 days later, the percentages of clonotypic HA-specific CD8 T cells in total lymphocytes were analysed (c). Representative data of two independent experiments are shown.
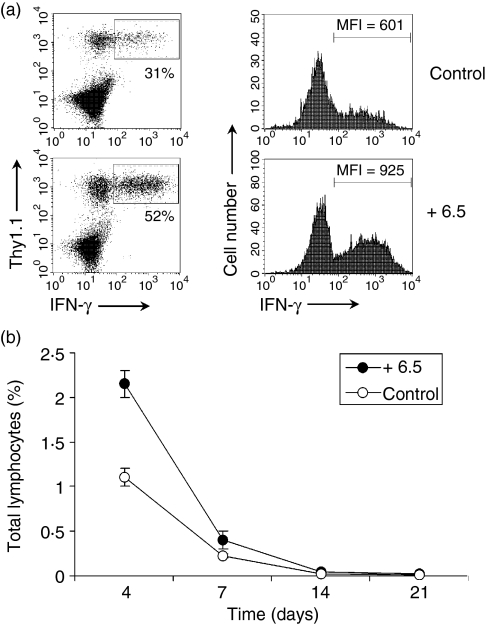
Studies have shown that cognate CD4 help is required for CD8 memory formation.33 These observations prompted us to test whether lack of CD8 memory cells is the result of the absence of cognate CD4 help in C3-HAhi mice. To address this question, we cotransferred HA-specific CD8 T cells with HA-specific CD4 T cells derived from 6.5 TCR-HA transgenic mice into C3-HAhi mice and monitored the behaviour of HA-specific CD8 T cells over time. As shown in Fig. 4(a), cotransfer of cognate HA-specific CD4 T cells promoted the expansion and effector function of HA-specific CD8 T cells on day 4, compared to mice receiving HA-specific CD8 T cells only. However, effector CD8 T cells developed in the presence of cognate CD4 help did not lead to CD8 memory formation as they were rapidly deleted after day 4, to nearly undetectable levels by day 21 (Fig. 4b).
Figure 4.
CD4 help does not result in the formation of memory CD8 T cells. Naive HA-specific CD8 T cells were adoptively transferred into C3-HAhi mice alone (Control) or with HA-specific CD4 T cells derived from 6.5 TCR-HA transgenic mice (+ 6.5). Four days later, lymphocytes from peripheral lymph nodes were harvested for the percentage and MFI of IFN-γ-secreting cells among clonotypic HA-specific CD8 T cells (a). Four, 7, 14, or 21 days later, the percentages of clonotypic HA-specific CD8 T cells in total lymphocytes were analysed (b).
Taken together, these results suggest that while costimulation or cognate CD4 helps to promote the acquisition of effector function, gain of effector function by CD8 T cells in the presence of costimulation or CD4 help does not lead to memory cell formation in C3-HAhi mice.
Potential role for the duration of antigen stimulation in CD8 T-cell response
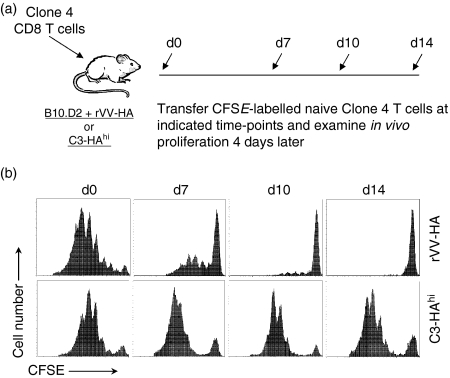
What then might determine the transition of effector CD8 T cells to memory cells? As the immune system is evolved to control infections, the main task of effector CD8 T cells is to clear invading pathogens. However, it might not be conceivable for self-reactive effector CD8 T cells to eliminate self-antigen although such reactivity may precipitate organ-specific autoimmunity as we observed in C3-HAhi mice.21 Therefore, we hypothesized that the duration of HA antigen stimulation in rVV-HA infected B10.D2 mice is transient, whereas that in C3-HAhi mice is persistent. To test this, we adoptively transferred naive HA-specific T cells into C3-HAhi mice or B10.D2 mice infected with rVV-HA on day 0. The presence of HA antigen in vivo was monitored by the extent of in vivo divisions of CFSE-labelled naive HA-specific T cells subsequently transferred at different time-points after the initial encounter (Fig. 5a). Indeed, in rVV-HA-infected B10.D2 mice, the amount of HA antigen decreased over time and was essentially cleared by day 14. By contrast, the HA antigen persisted at the same level in C3-HAhi mice over time (Fig. 5b). These results suggest that the key factor that may influence the fate of effector CD8 T cells is the duration of subsequent antigen stimulation.
Figure 5.
Antigen duration in CD8 T-cell memory versus tolerance. (a) A schematic view of the experiments outlined below. (b) Naive HA-specific CD8 T cells were adoptively transferred into B10.D2 mice infected with rVV-HA (rVV-HA) or C3-HAhi mice on day 0. At days 0 (d0), 7 (d7), 10 (d10) or 14 (d14), CFSE-labelled naive HA-specific T cells were transferred into these mice and in vivo divisions of CFSE-labelled cells harvested from the spleen were analysed 4 days later. Events were gated on CD8+ Thy-1.1+ T cells. Representative data of three independent experiments are shown.
The fate of effector CD8 T cells is determined by the duration of subsequent antigenic stimulation
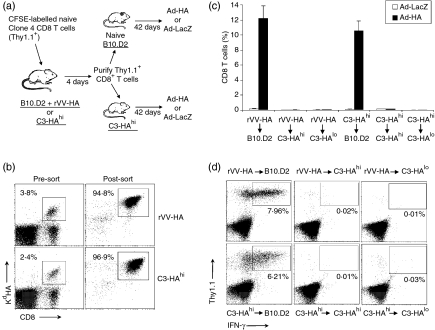
To determine what controls the differentiation of effector CD8 T cells into memory cells, we examined the fate of effector CD8 T cells purified from rVV-HA-infected B10.D2 mice or from C3-HAhi mice in an HA antigen-free (naive B10.D2 mice) versus an HA antigen-persistent (C3-HAhi mice) environment. We transferred CFSE-labelled naive HA-specific CD8 T cells into either C3-HAhi or rVV-HA-treated B10.D2 mice. Four days after transfer, effector CD8 T cells were isolated from C3-HAhi or rVV-HA-treated B10.D2 mice by FACS based on CD8 and Thy-1.1 staining (Fig. 6a). In addition, we only selected those cells that had undergone at least three divisions based on CFSE profile as shown in Fig. 1(a) to ensure isolation of the activated CD8 T cells. The purity of sorted HA-specific effector T cells was confirmed by staining cells with CD8 and Kd-HA tetramer. Nearly all (95–97%) of the sorted T cells were antigen-specific (Kd-HA tetramer positive, Fig. 6b). Purified effector CD8 T cells (5 × 105) were then transferred into either naive B10.D2 mice or C3-HAhi mice. The fate of these T cells in vivo was examined 42 days after transfer upon rechallenge with a recombinant adenovirus encoding HA (Ad-HA) or a control adenovirus, Ad-LacZ (Fig. 6a).
Figure 6.
Effector CD8 T cells fail to differentiate into memory cells in persisting antigen environment. (a) Purification and transfer of effector CD8 T cells into antigen-free versus antigen-persistent hosts. A schematic view of the adoptive transfer experiments. (b) CFSE-labelled naive HA-specific CD8 T cells were adoptively transferred into B10.D2 mice infected with rVV-HA (rVV-HA) or C3-HAhi mice. Four days later, cells were harvested from peripheral lymph nodes and spleen, and CD8+ Thy-1.1+ T cells were enriched by negative selection using magnetic beads before sorting gated on CD8, Thy-1.1 and at least three divisions by CFSE as shown in Fig. 1(a). Cells were stained with CD8 and KdHA tetramer. The percentage of clonotypic T cells before enrichment and sorting (Pre-sort) and the purity of clonotypic T cells after cell sorting (Post-sort) are indicated. (c) HA-specific effector CD8 T cells purified from rVV-HA infected B10.D2 mice (rVV-HA) were transferred into naive B10.D2 (rVV-HA (r) B10.D2), C3-HAhi (rVV-HA (r) C3-HAhi), or C3-HAlo (rVV-HA (r) C3-HAlo), and those effector cells from C3-HAhi mice were transferred into naive B10.D2 (C3-HAhi (r) B10.D2), C3-HAhi (C3-HAhi (r) C3-HAhi), or C3-HAlo (C3-HAhi (r) C3-HAlo). After 42 days, mice were challenged with Ad-HA or Ad-LacZ; 7 days after the challenge, cells were analysed for the expansion of clonotypic T cells. The percentages of clonotypic T cells (CD8+ Thy-1.1+ cells ± SD) in total CD8 T cells for all mice in each group are indicated. (d) Function of clonotypic CD8 T cells after challenge with Ad-HA were analysed by IFN-γ intracellular staining and the percentages of IFN-γ-producing clonotypic CD8 T cells in total CD8 T cells are indicated. Representative data of three independent experiments are shown.
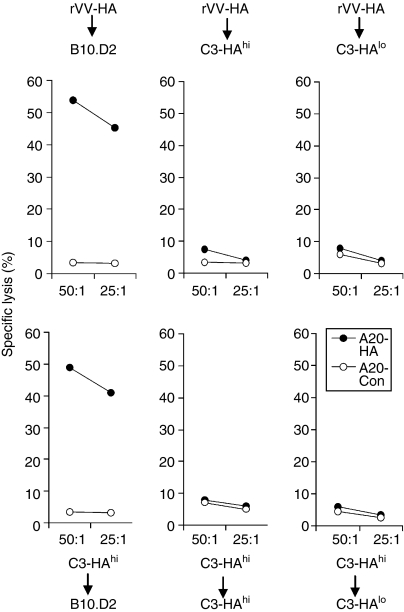
Transfer of HA-specific effector CD8 T cells from rVV-HA-infected mice into antigen-free B10.D2 mice led to the development of memory CD8 T cells as they rapidly expanded (> 64-fold) in vivo upon rechallenge with Ad-HA in comparison to Ad-LacZ (Fig. 6c), and demonstrated the ability to secrete IFN-γ (Fig. 6d) and to lyse HA-specific targets (Fig. 7) ex vivo. Similarly, transfer of effector CD8 T cells isolated from C3-HAhi mice into antigen-free B10.D2 mice also led to the formation of memory CD8 T cells evidenced by rapid expansion of functionally active HA-specific T cells upon Ad-HA rechallenge (Figs 6 and 7), suggesting that effector CD8 T cells derived from C3-HAhi mice can also develop into memory cells in the absence of antigen. However, when the effector CD8 T cells isolated from either rVV-HA-treated B10.D2 mice or C3-HAhi mice were transferred into antigen-persistent C3-HAhi mice, no memory cells were generated because no expansion of functionally active HA-specific T cells was observed upon rechallenge with Ad-HA, indicating that these T cells were deleted (Figs 6 and 7).
Figure 7.
Ex vivo cytolytic assay. The same clonotypic CD8 T cells harvested from the experiments outlined in Fig. 6 were assayed for their cytolytic activity on A20 lymphoma targets (H-2d) pulsed with Kd-HA peptide (A20-HA) or an irrelevant peptide (A20-Con) by a 6-hr JAM test. Percentage of specific lysis as a function of effector to target ratio is indicated. Representative data of three independent experiments are shown.
To rule out the possibility that the failure of memory cell formation in antigen-persistent hosts was not the result of unusually high levels of HA antigen in C3-HAhi mice, we transferred effector CD8 T cells isolated from either rVV-HA-treated B10.D2 mice or C3-HAhi mice into C3-HAlo mice that express low levels of HA antigen (∼500–1000-fold lower than that of C3-HAhi mice). Again, no memory CD8 T cells were detected 42 days after transfer, suggesting that memory cells cannot be formed in the presence of low-dose persistent antigen either (Figs 6 and 7). Taken together, these results demonstrate that effector CD8 T cells differentiate into long-lived memory cells only in the absence of antigen, whereas effector CD8 T cells are deleted in the presence of persistent antigen.
Protective immunity against tumour cannot be generated in the persisting antigen environment
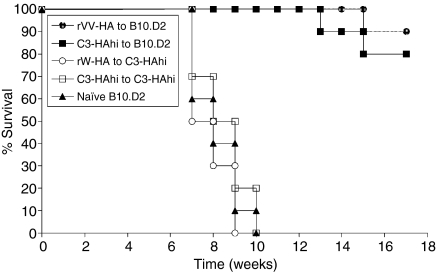
Tolerance induction of effector CD8 T cells in the persisting antigen environment suggests that protective immunity cannot be generated in this setting. To address this question, we utilized a murine model of A20 lymphoma engineered to express HA (A20-HA) as previously described.27 B10.D2-BALB/c F1 or C3-HAhi-BALB/c F1 mice were used for this study as A20 is a BALB/c lymphoma. HA-specific effector CD8 T cells were isolated from rVV-HA-immunized B10.D2-BALB/c F1 mice or C3-HAhi BALB/c F1 mice by sorting as described and subsequently transferred into antigen-free B10.D2-BALB/c F1 mice or antigen-persistent C3-HAhi-BALB/c F1 mice. Forty-two days after transfer, these mice were challenged with 2 × 105 A20-HA lymphoma cells intravenously and monitored for tumour-free survival over time from tumour inoculation. In antigen-free recipients that received HA-effector CD8 T cells from 42 days before, 80–90% of the mice were alive without evidence of tumour at the termination of the experiment (Fig. 8). This is statistically significant relative to B10.D2-BALB/c F1 mice that did not receive effector CD8 T cells (P < 0·001). In contrast, in antigen-persistent recipients that received HA-effector CD8 T cells from 42 days before, all mice succumbed to tumour within 10 weeks after tumour inoculation (Fig. 8). These results indicate that protective immunity against tumour cannot be generated in the persisting antigen environment because of the lack of long-lived CD8 memory cells.
Figure 8.
Protective immunity against tumour cannot be generated in the persisting antigen environment. Purified HA-specific effector CD8 T cells from rVV-HA infected B10.D2-BALB/c F1 mice were transferred into B10.D2-BALB/c F1 (rVV-HA (r) B10.D2, n = 10) or C3-HAhi-BALB/c F1 (rVV-HA (r) C3-HAhi, n = 10), and those effector cells from C3-HAhi-BALB/c F1 mice were transferred into B10.D2-BALB/c F1 (C3-HAhi (r) B10.D2, n = 10) or C3-HAhi-BALB/c F1 (C3-HAhi (r) C3-HAhi, n = 10). After 42 days, these mice along with naive B10.D2-BALB/c F1 mice (Naive B10.D2, n = 10) were challenged with A20-HA lymphoma intravenously. The graph indicates the percentage of tumour-free survival over time from tumour inoculation. Representative results of two independent experiments are shown.
Discussion
In this paper, we show that while costimulation or cognate CD4 help promotes the acquisition of effector function, the critical factor in controlling the fate of effector CD8 T cells in vivo is the duration of subsequent antigen stimulation. Effector CD8 T cells differentiate into long-term memory cells only in the absence of antigen, whereas tolerance is the fate of effector CD8 T cells in the presence of persistent antigen. In addition, protective immunity against tumour challenge cannot be generated in the persisting antigen environment.
Our observation that deletion rather than long-term memory formation is the fate of fully activated effector CD8 T cells in the presence of persistent antigen extends the previous work that persisting antigen is required for the deletion of abortively activated CD8 T cells8 and may provide a basis for the previous observations that pathogen-specific T cells are often deleted or become dysfunctional and lose effector functions in the setting of chronic infections in mice11,12,34,35 and humans.14,16 Furthermore, this study can also explain why tumour-specific T cells can be elicited de novo in patients with malignant melanoma, but are tolerized with tumour progression.36 However, in some of these models of chronic infection, the development of suboptimal memory or anergy was observed rather than total deletion as indicated by our study.34,35 This could be because of the high affinity of TCR-HA transgenic T cells, or the dose or the location of persisting antigen as suggested.37
It has been shown that in some other forms of chronic infections, particularly localized parasitic or bacterial infections, antigen persistence may be necessary to maintain protective immunity. In a model of Leishamania major infection, the protective immunity to L. major in a different site is lost when the primary subcutaneous infection is eliminated.18,38 Also, pathogen-specific T cells are reduced when antibiotics are given to bacillus Calmette—Guérin-infected mice.39 However, under these conditions, it is not clear if the protective immunity is derived from the true memory cells because these pathogen-specific T cells usually do not survive after antigen clearance. It is possible that in these studies the protective immunity may be conferred by circulating effector cells that are yet to be tolerized because these infections are usually localized and may not able to induce systemic T-cell deletion as suggested.40
How persistent antigen stimulation results in tolerance of effector CD8 T cells remains to be defined. One possibility is that under the condition of persistent TCR ligation, effector CD8 T cells may be deleted through ‘activation-induced cell death’ (AICD) or the extrinsic apoptotic pathway. Previous reports have suggested that AICD is mediated by Fas (CD95/Apo-1) or tumour necrosis factor receptor 1 (TNF-R1) of the TNF-R superfamily.41 Although it has been shown that Fas plays a key role in antigen-induced cell death of effector CD4 T cells,42,43 the role of Fas in the peripheral deletion of effector CD8 T cells in vivo remains controversial.44–46 A recent report suggests that Bim (a pro-apoptotic protein in the intrinsic apoptotic pathway47), but not Fas, was involved in the deletion of CD8 T cells.46 Future work should focus on delineating the pathway(s) leading to deletion of effector CD8 T cells in vivo. Understanding of this pathway will help to design strategies to prevent deletion of CD8 T cells in the settings of persistent antigen such as cancer and chronic infections.
Another possibility for defective memory CD8 T-cell formation in the settings of persistent antigen is the lack of interleukin-7 (IL-7) and IL-15 responsiveness. A body of literature has shown that both IL-7 and IL-15 play an essential role in the generation and maintenance of memory CD8 T cells during an acute infection.48–52 However, studies have shown that CD8 T cells during chronic infections were unable to undergo homeostatic proliferation, responded poorly to IL-7 and IL-15, and expressed reduced levels of IL-7 and IL-15 receptors35. It remains to be seen whether this altered IL-7 and IL-15 responsiveness is also the resulst of persistent TCR stimulation.
Our findings have important implications for the design of immunotherapeutic strategies for treating cancer. The formation of long-lived memory CD8 T cells only after clearance of antigen suggests that in the setting of preventive vaccinations, the booster vaccination should be timed for the formation of memory cells after the antigen introduced by the first vaccination has been cleared. Frequent and repetitive vaccinations might not be desired. On the other hand, the failure in formation of long-term memory cells in persisting antigen environment suggests that removal of the persisting antigen by other means such as chemotherapy, radiation, and/or debulking surgery in the tumour-bearing hosts, might be a prerequisite for successful vaccination in treating cancer.
Acknowledgments
Supported in part by NIH grants CA093659 and CA047741 to Y.Y.
References
- 1.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–16. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1:126–34. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 8.Redmond WL, Hernandez J, Sherman LA. Deletion of naive CD8 T cells requires persistent antigen and is not programmed by an initial signal from the tolerogenic APC. J Immunol. 2003;171:6349–54. doi: 10.4049/jimmunol.171.12.6349. [DOI] [PubMed] [Google Scholar]
- 9.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–60. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 10.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–5. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 11.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–61. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 12.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmer U, He H, Messer RJ, et al. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 14.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Y, Luscher MA, Altman JD, Hulsey M, Robinson HL, Ostrowski M, Barber BH, MacDonald KS. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8(+) T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J Virol. 2001;75:3028–33. doi: 10.1128/JVI.75.6.3028-3033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–45. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 19.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–64. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–55. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Yang Y. Transient gain of effector function by CD8+ T cells undergoing peripheral tolerance to high-dose self-antigen. Eur J Immunol. 2004;34:1351–60. doi: 10.1002/eji.200324734. [DOI] [PubMed] [Google Scholar]
- 22.Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–83. [PubMed] [Google Scholar]
- 23.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasbold J, Johnson-Leger C, Atkins CJ, Clark EA, Klaus GG. Properties of mouse CD40. cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur J Immunol. 1994;24:1835–42. doi: 10.1002/eji.1830240817. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation. Requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–4. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 26.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–53. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 27.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–83. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 30.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Meth. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 31.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 33.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 34.Bronstein-Sitton N, Cohen-Daniel L, Vaknin I, et al. Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat Immunol. 2003;4:957–64. doi: 10.1038/ni975. [DOI] [PubMed] [Google Scholar]
- 35.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–9. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 37.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uzonna JE, Wei G, Yurkowski D, Bretscher P. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J Immunol. 2001;167:6967–74. doi: 10.4049/jimmunol.167.12.6967. [DOI] [PubMed] [Google Scholar]
- 39.Dudani R, Chapdelaine Y, Faassen Hv H, Smith DK, Shen H, Krishnan L, Sad S. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168:5737–45. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 40.Zinkernagel RM. Localization dose and time of antigens determine immune reactivity. Semin Immunol. 2000;12:163–71. doi: 10.1006/smim.2000.0253. discussion 257–344. [DOI] [PubMed] [Google Scholar]
- 41.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. Embo J. 1995;14:6136–47. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 43.Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–8. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 44.Kurts C, Heath WR, Kosaka H, Miller JF, Carbone FR. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1) J Exp Med. 1998;188:415–20. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertolino P, Trescol-Biemont MC, Thomas J, Fazekas de St Groth B, Pihlgren M, Marvel J, Rabourdin-Combe C. Death by neglect as a deletional mechanism of peripheral tolerance. Int Immunol. 1999;11:1225–38. doi: 10.1093/intimm/11.8.1225. [DOI] [PubMed] [Google Scholar]
- 46.Davey GM, Kurts C, Miller JF, Bouillet P, Strasser A, Brooks AG, Carbone FR, Heath WR. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J Exp Med. 2002;196:947–55. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 48.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 49.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–31. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 50.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL) -15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–22. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]