Abstract
B-cell chronic lymphocytic leukaemia (B-CLL) is the most prevalent leukaemia in Western countries and is characterized by the gradual accumulation in patients of small mature B cells. Since the vast majority of tumoral cells are quiescent, the accumulation mostly results from deficient apoptosis rather than from acute proliferation. Although the phenomenon is relevant in vivo, B-CLL cells die rapidly in vitro as a consequence of apoptosis, suggesting a lack of essential growth factors in the culture medium. Indeed, the rate of B-CLL cell death in vitro is modulated by different cytokines, some favouring the apoptotic process, others counteracting it. Two related members of the tumour necrosis factor family, BAFF (B-cell activating factor of the TNF family) and APRIL (a proliferation-inducing ligand), already known for their crucial role in normal B-cell survival, differentiation and apoptosis, were recently shown to be expressed by B-CLL cells. These molecules are able to protect the leukaemic cells against spontaneous and drug-induced apoptosis via autocrine and/or paracrine pathways. This review will focus on the role of BAFF and APRIL in the survival of tumoral cells. It will discuss the expression of these molecules by B-CLL cells, their regulation, transduction pathways and their effects on leukaemic cells. The design of reagents able to counteract the effects of these molecules seems to be a new promising therapeutic approach for B-CLL and is already currently developed in the treatment of autoimmune diseases.
Keywords: B-CLL, BAFF, APRIL, apoptosis, mRNA, NF-κB
Introduction
B-cell chronic lymphocytic leukaemia (B-CLL) constitutes the most prevalent leukaemia in Western countries and is still an incurable disease. Only a proportion of patients achieve a complete response to chemotherapy and treatments remain palliative. B-CLL is characterized by a slow accumulation in patients of small, mature B cells typically displaying the CD5, CD19, CD23 and CD20 markers.1–3 A small pool of highly proliferating cells has been detected in the lymph nodes and bone marrow that feed the pool of leukaemic cells in the blood.4 The latter are almost exclusively quiescent cells, 95–98% of them being arrested at the G0 stage of the cell cycle. Therefore, their accumulation mostly results from a deficient apoptosis rather than from an acute proliferation. This deficiency might also explain why cell-cycle-specific agents and other drugs active against proliferating cells are ineffective. Whether defects in the apoptotic pathway are frequently encountered in a variety of cancers, B-CLL represents a paradigm of the tumours that arise as a consequence of alterations in the processes leading to programmed cell death. Indeed, B-CLL cells display multiple intrinsic defects in their apoptotic machinery and dysregulated production of survival signals from their microenvironment (reviewed in refs 5, 6).
The elucidation of the mechanisms that confer on B-CLL cells their remarkable resistance to programmed cell death is thus of great importance, notably for the conception of new therapeutic approaches. Within the last 15 years, many events necessary for the successful achievement of the apoptotic process in normal B lymphocytes have been shown to be altered in CLL leukaemic B cells. These alterations include both defects in the death programme itself and an exacerbated response to survival signals. The resulting benefit is a selective advantage conferred on the leukaemic cells over their normal counterparts.
Our group has contributed to the unravelling of some of the mechanisms underlying this defective apoptosis. A few years ago we showed that the leukaemic cells spontaneously expressed an inducible nitric oxide synthase, iNOS or NOS-2.7 The NO endogenously released impaired the apoptotic pathway, probably in part because of its capacity to inhibit the activity of caspase(s) through its binding and inactivation of the cystein present in the active site of the enzyme.8 Recently, a straight correlation between the level of endogenous NO and the mitochondrial mass in B-CLL has been demonstrated and NO was shown to be a major factor involved in mitochondrial biogenesis. Notably, there was an inverse correlation between the level of NO (and the mitochondrial mass) and the susceptibility of the leukaemic cells to fludarabine-induced apoptosis.9 These data emphasized the crucial role of NO in the resistance of B-CLL towards apoptosis and the interest of blocking its production for therapeutic purpose.
On the other hand, studies from both other laboratories and ours have demonstrated that tumour necrosis factor (TNF) superfamily members contribute to the enhanced survival potential of B-CLL cells. Our interest for the study of these molecules originated in the observation, by DNA microarray analysis, of a difference between the expression of TACI (transmembrane activator and calcium modulator and cyclophilin ligand-interactor) by B lymphocytes from B-CLL patients and those from healthy donors. TACI is a member of the TNF-R family and is a common receptor for two related members of the TNF family, BAFF (B-cell activation factor belonging to the TNF family) and APRIL (a proliferation-inducing ligand). The role of BAFF and APRIL was thus investigated in B-CLL and both molecules were shown to provide strong survival signals to the leukaemic cells through autocrine and/or paracrine pathways, allowing them to overcome spontaneous and drug-induced apoptosis.
The present review will therefore be focused on the role of BAFF and APRIL in the natural history of B-CLL cells, notably their implication in the control of apoptosis.
BAFF and APRIL
Characterization of BAFF and APRIL
BAFF is a molecule discovered independently by several groups. Schneider et al. identified it by sequence homology with the TNF superfamily members.10 Moore et al. named it BLyS (B-lymphocyte stimulator) because it induces B lymphocyte proliferation and immunoglobulin secretion.11 It is also known as THANK (TNF homologue that activates apoptosis, nuclear factor-κB and c-jun N-terminal kinase),12 TALL-1 (TNF- and ApoL-related leucocyte-expressed ligand-1),13 zTNF414 and TNFSF13B (TNF superfamily member 13B).
APRIL was identified as a close homologue of BAFF15 and is also known as TRDL-1α (TNF-related death ligand-1α),16 TALL-2, zTNF2, or TNFSF13.
These molecules belong to a cluster of the TNF superfamily and share several biological characteristics and functions, although their effects are not redundant.17 Both are homotrimeric type II transmembrane proteins but exist also as soluble molecules10,11,15 derived from the cleavage of transmembrane forms by furin-like convertases.18,19 Lopez-Fraga et al. showed that in contrast to BAFF, which is cleaved at the cell surface, APRIL would be cleaved intracellularly, so removing the possibility of detecting its integral form at the plasma membrane.19 However, other studies have reported that APRIL can also be expressed at the cell surface. The soluble forms of BAFF and APRIL, as for the majority of the members of the TNF superfamily, are detected as homotrimeric molecules. They also form active heterotrimers that can be found in the sera of patients presenting with rheumatoid diseases of autoimmune origin.20 Determination of the crystal structure of APRIL has revealed a trimeric ligand very similar to that of BAFF. Yet, whereas BAFF can form 20-trimer assemblies under certain conditions, this was not observed in the APRIL structure.21
Expression of BAFF and APRIL
BAFF was initially found to be expressed by antigen-presenting cells (APC) and to stimulate the proliferation of B lymphocytes and their secretion of immunoglobulins.10,11 Macrophage-derived and dendritic cell-derived BAFF is a key molecule by which these APC regulate human B-cell proliferative responses to T-cell-independent stimuli.22 Expression of both membrane and soluble forms of BAFF is increased after treatment of monocytes in vitro with cytokines, notably interferon-γ and interleukin-10 (IL-10). The membrane expression of BAFF persists during differentiation in macrophages but decreases during maturation in dendritic cells. BAFF binds receptors with high affinity (Kd < 10 nm) at the B-lymphocyte surface, resulting in nuclear factor-κB (NF-κB) and ELF-1 (E74-like factor 1) activation and in Polo-like kinase mRNA induction.23 BAFF is also present and produced by cells outside the lymphoid system, such as astrocytes.
APRIL is practically undetectable in normal tissues but is strongly up-regulated in many tumour cells in vivo and in vitro and stimulates tumour cell growth.15
BAFF and APRIL receptors
BAFF and APRIL bind with high affinity two members of the TNF-receptor (TNF-R) superfamily, B-cell maturation antigen (BCMA) and TACI.14,24–26 BCMA was first discovered in a malignant T-cell lymphoma, where it was fused to the IL-2 gene by a t(4;16)(q26;p13) translocation.27 BCMA is normally expressed by mature B and T lymphocytes.28 Its signalization implicates TNF-R-associated factor 1 (TRAF-1), TRAF-2, and TRAF-3 and results in the activation of NF-κB, Elk-1 (Ets-like transcription factor 1), c-jun N-terminal kinase (JNK) and p38.12,29
TACI is detected in subpopulations of B lymphocytes and activated T cells.30 Transfection of HEK293T cells with TACI confers on them the ability to bind BAFF and APRIL with subnanomolar and nanomolar affinities, respectively; both ligands induce NF-κB activation in these cells.24 Binding of BAFF to TACI also stimulates NF-κB activation in vitro in B-lymphoma cells, whereas a soluble form of TACI inhibits this induction and also the production of immunoglobulin M (IgM) by peripheral B lymphocytes. The TACI intracellular domain interacts with TRAF-2, TRAF-5 and TRAF-6 and activates NF-κB and JNK.25
BAFF, but not APRIL, binds a third receptor named BAFF-R or BR3.31–33 BAFF-R was first identified in A/WySnJ mice that are deficient in B cells and present a mutated gene, Bcmd (B-cell maturation deficiency) in comparison with the parental A/J mice. The Bcmd gene codes for BAFF-R, which binds BAFF specifically (not APRIL); the interaction between BAFF and BAFF-R plays a dominant role in the long-term survival of B lymphocytes.34 Using soluble, monomeric forms of the receptors, it was demonstrated that BAFF-R binds BAFF with a 100-fold selectivity over BCMA, whereas APRIL shows the opposite selectivity.35 The anomaly of the Bcmd/BAFF-R/BR3 gene in A/WySnJ mice results in its inactivation and ultimately in the absence of B2-type peripheral B lymphocytes.32 This deficit in the development of B follicles in A/WySnJ mice can be normalized by survival signals given by Bcl-xL overexpression.36 BAFF-R is expressed by normal B lymphocytes, binds TRAF-3 and the interaction is stimulated by BAFF. TRAF-3 overexpression inhibits the NF-κB activation and IL-10 production induced by BAFF-R, suggesting that TRAF-3 negatively regulates these phenomena.37 Indeed, critical residues in BAFF-R mediate TRAF-3 recognition and ensure its selective binding solely to this member of the TRAF family.38
The existence of a specific receptor for APRIL was postulated several years ago inasmuch as APRIL was found to exert biological effects in cells lacking both TACI and BCMA. Recently, it was shown that a basic amino acid sequence close to the N terminus of mature APRIL was required for binding to the APRIL-specific receptor, identified as sulphated glycosaminoglycan side chains of proteoglycans. Syndecan-1-positive plasma cells and proteoglycan-rich non-haematopoietic cells displayed specific, heparin-sensitive binding to APRIL. A model was proposed whereby APRIL binding to the extracellular matrix or to proteoglycan-positive cells induces APRIL oligomerization, which was the prerequisite for the triggering of TACI- and/or BCMA-mediated activation, migration, or survival signals.39 The specific binding of APRIL to heparan sulphate proteoglycans and its inhibition by heparin was confirmed by Hendriks et al.40. This binding involves critical lysines and is distinct from the site of binding to BCMA. Interestingly, this interaction of APRIL with heparan sulphate proteoglycans is responsible for its tumour growth-promoting activity but not for the proliferation of T lymphocytes.
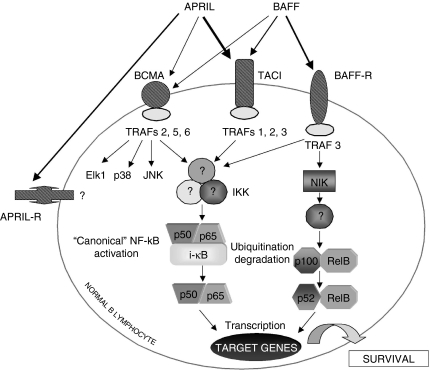
A schematic picture of BAFF and APRIL, their receptors and their main signalling pathways is presented in Fig. 1.
Figure 1.
Schematic representation of BAFF and APRIL signalling pathways. See text for the significance of the various components of these transduction pathways.
Roles of BAFF and APRIL in B-lymphocyte survival and differentiation
BAFF and APRIL and their receptors play a key role in the survival and differentiation of B cells. They therefore provide not only a new insight into the development of autoreactive B cells, but also a paradigm to the interaction between survival, growth and death affecting all cells.41 Even though BAFF and APRIL share many characteristics, their functions and effects are not redundant.
BAFF was first described as a survival and maturation factor for B lymphocytes.10,11,42 This role in the long-term survival of B lymphocytes is based in part on the fact that transgenic mice overexpressing BAFF in lymphoid tissues exhibit hyperplasia of the mature B-cell compartment.14,43,44 In contrast, mice that are deficient in BAFF, like those presenting with a mutation in the BAFF-R/BR3 gene, show a deficit in peripheral B lymphocytes.31,32,34,45 From analysis of these BAFF knockout mice, it was concluded that B-cell development was blocked at the transitional T1 stage corresponding to the earliest B cells migrating from bone marrow to the spleen. However, while the humoral responses to T-dependent antigens were impaired in the BAFF knockout mice, antigen-specific class-switched antibody was still produced. The formation of germinal centres with normal somatic hypermutation after antigenic challenge also took place in these mice.46 These findings suggest that BAFF knockout mice possess more differentiated, mature B cells than was originally believed.
The capacity of B lymphocytes to bind BAFF is correlated with their maturation state (transitional versus mature B cells) and with a different pattern of expression of its various receptors. Despite a similar effect, the mechanisms favouring the survival induced by BAFF implicate various mediators, according to the differentiation state of the B cells. For instance, BAFF induces overexpression of the A1 and Bcl-xL anti-apoptotic genes in mature B lymphocytes but not in immature lymphocytes.47 In vitro, BAFF also increases the survival of immature B cells, leading to their differentiation into mature B cells, without proliferation. Upon BAFF-influenced differentiation, these cells change their surface-IgM transduction machinery and proliferate rather than undergoing apoptosis.48
As regards mature resting B cells in the periphery, their survival depends on signalling from the B-cell receptor and the BAFF-R, both stimulating the activation of NF-κB through the canonical and alternative pathways, respectively. It should be recalled that BAFF is able to stimulate both the classical and alternative (NEMO/IKKγ-independent) NF-κB pathway.49 It was therefore suggested that B-cell receptor and BAFF-R survival signaling are mutually dependent and that BAFF-R signalling enhances the expression of survival genes through direct chromatin modifications in NF-κB target gene promoters.50
Mice with a TACI gene deletion present with splenomegaly, a spontaneous proliferation of B lymphocytes and a higher level of circulating immunoglobulins. Moreover, antigenic stimulation in vivo induces the production of specific antibodies more than in normal mice.51 Whereas the antibody response against T-dependent antigens was normal in those mice, the response to type II T-independent antigens (NIP-Ficoll, Pneumovax) collapsed.52 This supports the theory that TACI has an inhibitory effect on B-cell activation, contributing to the maintenance of homeostasy in the B-cell lineage.53
Mice transgenic for the soluble form of TACI, with BAFF and APRIL effects neutralized, have B-cell maturation arrested between the transistional T1 and T2 stage cells, with depletion of marginal zones and of B2-type follicular B lymphocytes, but not of peritoneal B1 lymphocytes. In contrast, transgenic mice for a soluble form of murine BCMA, neutralizing APRIL but with little or no effect on murine BAFF, do not have modified B-cell compartments. This suggests a crucial role of BAFF interacting with TACI (or BAFF-R), whereas BCMA and APRIL should not be necessary.54
Whereas, as mentioned above, BAFF knockout mice displayed a close to total disappearance of B lymphocytes in the follicles and marginal zones of the secondary lymphoid organs, mice invalidated for BCMA exhibited apparently normal B-cell compartments.45 Yet both soluble forms of BCMA and TACI prevent APRIL binding to its receptors and block the proliferation of primary B lymphocytes that is induced by APRIL. The soluble form of BCMA also inhibits the antibody response against T-dependent and T-independent antigens.55 This suggests that the roles of BCMA and APRIL, even though they are less important than that of BAFF and its receptors TACI and BAFF-R, must not be neglected.
BAFF facilitates the humoral response to T-dependent and T-independent antigens, in part via an attenuation of the apoptosis, as shown by the prolonged survival in vitro and in vivo of B lymphocytes activated by antigen. BAFF cooperates with CD40 ligand by protecting B cells from apoptosis in the replicative phase and by favouring the survival of quiescent naive B lymphocytes. This attenuation of apoptosis is correlated with a modulation of the protein ratio in the family Bcl-2/Bax in favour of survival and NF-κB activation. This protection of apoptosis underlies the stimulating effects of BAFF during the physiological humoral response and its role in the induction of autoimmunity.56,57
In mice, BAFF injection induces an augmentation of the serum levels of all immunoglobulin classes, of salivary IgA, and also the expansion and the differentiation of splenic B lymphocytes, suggesting that it may be useful in some immune deficits. BAFF, produced by macrophages and dendritic cells, is much more efficient than APRIL as a factor responsible for T cell-independent B-lymphocyte proliferation.22
Soluble forms of receptors TACI-Fc and BAFF-R-Fc block B-cell proliferation induced by BAFF.25,31,32 In vivo, soluble TACI inhibits the antigen-specific response and the formation of germinal centres, underlying the critical role of BAFF in the immune response.58 Moreover, the injection of a soluble BAFF-R form inhibits the development of an antibody response in mice. The hybrid molecule BAFF-R-Fc is more effective than BCMA-Fc in inhibiting BAFF effects.59
BAFF action is not restricted to B lymphocytes because it regulates the activation of T lymphocytes stimulated via their T-cell receptor. BAFF provides these cells with a costimulatory signal inducing IL-2 secretion and entry into S phase but, in contrast to B cells, does not influence their long-term survival.60
As regards APRIL, it stimulates in vitro the proliferation of mouse primary B and T lymphocytes and promotes the survival of T lymphocytes and non-lymphoid cells.17 The proliferation of primary B cells stimulated with APRIL is prevented by soluble forms of BCMA and TACI.55 In vivo, APRIL was shown to induce an augmentation of spleen volume as a result of an accumulation of B cells and of activated T cells, suggesting a role for the molecule in lymphoid homeostasy. Analysis of T cells reveals an augmentation of APRIL mRNA, dependent on the T-cell activation state. In addition, transgenic mice for APRIL showed an increased survival of T cells in vitro and of CD4+ T cells in vivo.61 APRIL also plays a role in the antibody response of B lymphocytes to T-independent type II antigens.17
When stimulated by innate immune signals like interferon-α, -γ or CD40 ligand, dendritic cells express more BAFF and APRIL, which, in the presence of cytokines (IL-10, transforming growth factor-β, IL-4) induce the immunoglobulin gene switch of Cμ to Cγ, Cα and/or Cε in B lymphocytes.62 In mice, both TACI and BAFF-R are able to transduce signals that result in isotype switching.63 BAFF and APRIL expression by dendritic cells, induced by signals resulting from innate immunity, also leads to the plasmacytoid differentiation of B lymphocytes and to plasmablast survival, converting a subliminal B-cell activation into a productive response, but with the danger of favouring the production of autoantibodies.64
BAFF and APRIL are thus able to induce the class-switch and the terminal differentiation of B lymphocytes, thus providing a link between innate and acquired immunity.
BAFF and APRIL in B-CLL
BAFF and APRIL were rapidly identified as major players in the onset of various autoimmune diseases. Their potent effects on normal B-lymphocyte survival made it also logical to investigate their role in the pathogenesis of haematological B-cell malignancies. Indeed, BAFF/APRIL are produced in excess in patients with various B-lymphoid malignancies, either by the leukaemic cells themselves or by cells in their microenvironment or both.65 A variety of tumoral B cells from non-Hodgkin's lymphoma (NHL) were found to express receptors for BAFF. Comparable levels were detected in B lymphocytes from normal individuals and from patients with diffuse large cell lymphoma (DLCL), mantle cell lymphoma (MCL) and marginal zone lymphoma, whereas those from B-CLL and follicular lymphoma displayed somewhat lower expression.66 According to these authors, the B-cell tumours tested, expressed no or very low levels of membrane-bound BAFF protein Yet, the levels of soluble BAFF in the serum of untreated patients with different NHL subtypes were significantly elevated, compared to those in healthy donors. For instance, patients with follicular lymphomas exhibited a mean value of 13.4 ng/ml in comparison with 4.6 ng/ml for normal donors. A significant increase was also observed for DLCL, whereas no augmentation was seen in MCL patients, but the number of patients tested3 was low. BAFF alone did not induce significant proliferation on tumour cells from follicular lymphoma patients; however, when used in a costimulation assay with anti-IgM, but not CD40 ligand, it stimulated the proliferation of tumour B cells.66
The expression of the three BAFF receptors by B-CLL cells was confirmed by Novak et al.67 who detected in all B-CLL cells the presence of TACI protein by membrane immunofluorescence and that of BAFF-R mRNA by reverse transcription—polymerase chain reaction (RT-PCR); in contrast, BCMA mRNA was only detected in a small percentage of patients. Addition of exogenous BAFF was found to protect leukaemic cells from spontaneous and chlorambucil-induced apoptosis. More importantly, BAFF mRNA was detected by RT-PCR in about half of the B-CLL patients, a result confirmed by semiquantitative real-time PCR; this was correlated with a low expression of the BAFF protein at the membrane of the leukaemic cells. In comparison, APRIL mRNA was detected in only 10% of the patients tested.67
At about the same time, our group similarly observed that BAFF-R, TACI and BCMA were expressed by the B-CLL leukaemic B cells. In addition, we detected the presence of BAFF and APRIL mRNA and that of the corresponding proteins at the surface of the leukaemic cells in nearly all B-CLL patients tested. The addition of exogenous BAFF and APRIL to the leukaemic cells stimulated NF-κB activation (already high in these cells) and protected them from spontaneous and flavopiridol-induced apoptosis. Conversely, the addition of soluble BCMA-Fc or of anti-BAFF or anti-APRIL neutralizing antibodies resulted in apoptosis induction. These results, together with the observed coexpression of BAFF, APRIL and their receptors by B-CLL cells, suggested that the leukaemic B cells could be rescued from entering apoptosis through an autocrine survival pathway mediated by BAFF and APRIL. Finally, a soluble form of BAFF was detected by surface enhanced laser desorption/ionization–time of flight–mass spectrometry (SELDI-TOF-MS) in sera from B-CLL patients, at a higher level than in normal sera.68
APC are the major cells expressing BAFF and, to a lesser extent, APRIL. Inasmuch as B-CLL leukaemic cells display the corresponding receptors, the possibility of a paracrine pathway of survival, in addition to the autocrine loop described above, might therefore be envisaged. Indeed, it was recently reported that nurselike cells, which differentiate from CD14 cells when cultured with CLL B cells, expressed significantly higher levels of APRIL than monocytes and significantly higher levels of BAFF and APRIL than B-CLL cells. The viability of the leukaemic cells cocultured with nurse-like cells was reduced in the presence of decoy receptors binding both BAFF and APRIL, but not with BAFF-R-Fc, which binds only to BAFF.69 In contrast to our observations and those of Novak et al., these authors found that BCMA-Fc, although impairing the sustained viability of the leukaemic cells cocultured with nurse-like cells, had no effect on the leukaemic cells tested alone, but the reasons for this discrepancy are not clear. On the other hand, they observed that BAFF-R-Fc, used as decoy receptor, was unable to impair the viability of B-CLL cells that were cultured either with or without nurse-like cells. Together, these results would suggest that APRIL might play an important role in the protective effect afforded by nurse-like cells to B-CLL cells.69 Indeed, APRIL transgenic mice develop lymphoid tumours with age that originate from expansion of the peritoneal B1 B-cell population.70 Although the population of B1 cells in man has not exactly the same properties as murine B1 cells, this raises the possibility of a crucial role for APRIL in B-CLL leukaemogenesis. In this respect, the leukaemic cells from some of our B-CLL patients were better protected from apoptosis by APRIL than by BAFF (unpublished observations).
Mechanisms of BAFF and APRIL-mediated survival of B-CLL cells
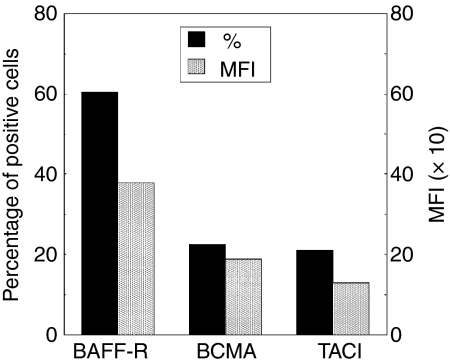
We first investigated the expression of the three known BAFF receptors by membrane immunofluorescence. BAFF-R was the main receptor detected on the leukaemic cells whereas BCMA and TACI, although present, were expressed at a much lower level (Fig. 2). These results are in agreement with recent works reporting that BAFF-R is expressed on most mature B cells and in B-cell lymphoproliferative disorders.71,72
Figure 2.
Membrane expression of the three BAFF receptors on B-CLL cells. The expression of the three BAFF receptors was analysed on purified leukaemic B cells from 18 B-CLL patients by membrane immunofluorescence and flow cytometry with specific fluorescence-labelled antibodies against BAFF-R, TACI and BCMA and their corresponding fluorescence-labelled isotypes. Results are expressed as the percentages of positive cells (%) and their mean fluorescence intensity (MFI), as estimated according to Kolmogorov—Smirnov.
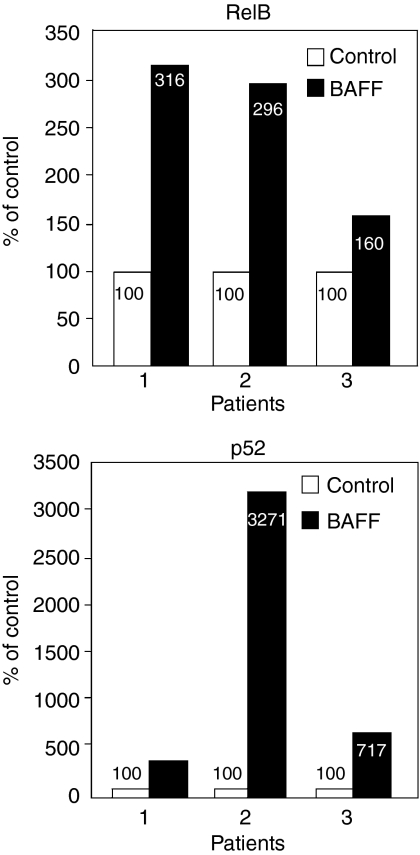
Activation of the transcription factor NF-κB is a common feature downstream of these three receptors in normal B lymphocytes, as mentioned earlier. As seen in Fig. 3, the addition of BAFF to B-CLL cells was found to result in a marked activation of the non-canonical or alternative pathway (Haiat et al. submitted for publication). This activation was more important than that of the classical or canonical pathway previously reported.68 The interaction of BAFF with BAFF-R is likely responsible for this pattern of NF-κB activation, inasmuch as it was previously shown that BAFF-R preferentially induces the noncanonical NF-κB signalling pathway.73 This function is mediated by a sequence motif, PVPAT, the mutation of which abolishes its interaction with TRAF-3 as well as its ability to induce non-canonical NF-κB.73
Figure 3.
Activation by BAFF of the non-canonical NF-κB pathway in B-CLL cells. Leukaemic cells from B-CLL patients were incubated for 18 hr in the presence or in the absence of BAFF 0·25 μm. Total lysates were analysed for the presence of activated NF-κB using a Transam ELISA kit (Active Motif). The active form of NF-κB is detected by its binding to a κB oligonucleotidic consensus sequence immobilized in the wells of an ELISA microplate. The non-canonical pathway is evidenced using either an anti-p52 or anti-RelB antibody that recognizes conformation epitopes of the corresponding proteins bound to κB. A second anti-immunoglobulin antibody conjugated with peroxidase, followed by addition of the peroxidase substrate allows a colorimetric detection at 450 nm of the complexes, using a Victor® 2 microplate spectrophotometer.
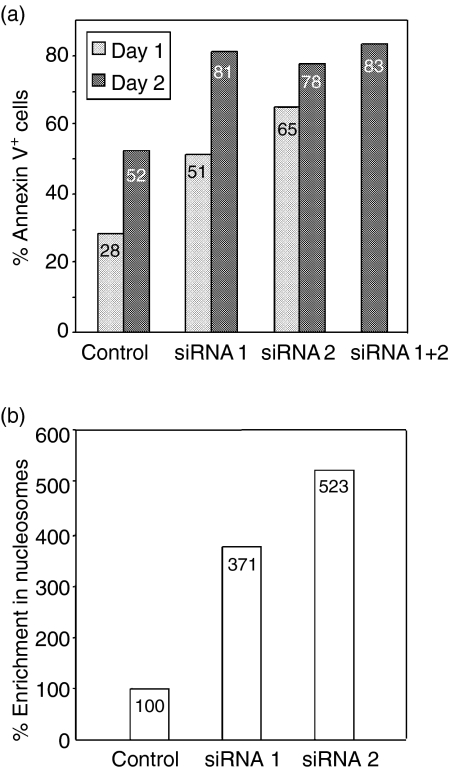
To confirm the essential role of BAFF in the control of B-CLL spontaneous apoptosis, we transfected leukaemic cells from B-CLL patients with BAFF-specific siRNAs. Two 21-mer specific BAFF siRNA were designed according to a modification of the rules defined by Elbashir et al.74 These siRNA were transfected by nucleofection (Amaxa, Köln, Germany) in B-CLL leukaemic cells, either alone or in combination. Leukaemic B-CLL cells maintained in culture in suspension are essentially non-dividing cells with a low metabolism, express small amount of Dicer and die rather rapidly as a result of high spontaneous apoptosis and are thus difficult to transfect. Nevertheless, using the nucleofection system developped by Amaxa, we were able to achieve the transfection of specific BAFF siRNA in these cells with a reasonable yield (80%) and acceptable viability. The efficacy of the transfection was monitored by measuring, by fluorescence-activated cell sorting analysis, the membrane expression of BAFF in the transfected B-CLL cells. Successful inhibition of BAFF expression was accompanied by a stimulation of apoptosis (Haiat et al. submitted for publication). An increase in the percentages of annexin V-labelled cells and in the enrichment of cytoplasmic nucleosomes was observed in the B-CLL cells transfected with BAFF siRNA as compared with untransfected cells or cells transfected with an unrelated siRNA (Fig. 4). This induction of apoptosis was also correlated with a reduction in the level of spontaneous NF-κB activation.
Figure 4.
Transfection of B-CLL cells with BAFF siRNA stimulates spontaneous apoptosis. Leukaemic cells from a B-CLL patient were transfected by nucleofection (Amaxa) with a scramble siRNA (Control) or with one or two BAFF-specific siRNA (si RNA1 and si RNA2). After overnight incubation, apoptosis induction was estimated by measuring the percentage of annexinV-FITC-labelled cells (a) and the enrichment in cytoplasmic nucleosomes, in comparison with control cells taken as 100% (b).
These data confirm the existence of an autocrine loop implicating BAFF in the resistance of B-CLL cells to spontaneous apoptosis and emphasize the crucial role of NF-κB stimulation in this process. Comparable results were obtained by Nishio et al. who showed that BAFF and APRIL triggered the canonical pathway of NF-κB activation and that BAFF also stimulated the noncanonical pathway. These authors reported in addition that both factors stimulated the expression of Mcl-169 an antiapoptotic protein acting at the level of the mitochondria and known to be involved in the resistance of B-CLL cells to drug induced apoptosis.
BAFF was reported to stimulate IL-10 expression in normal B lymphocytes.75 Because IL-10 triggers BAFF production by APC, an amplification loop is therefore conceivable that could provide a link between the autocrine and paracrine mechanism of B-CLL survival.
Of note, BAFF was reported to up-regulate CD23 expression in mouse B lymphocytes.76 If also true for human B cells, and taking into account the role of membrane and soluble CD23 in the control of B-CLL apoptosis, this would suggest an alternative mechanism by which BAFF could favour the survival of these cells.
An excessive lymphocyte survival would allow the accumulation of autoreactive cells not eliminated by the normal cell death pathways. It would be thus interesting to test the BAFF and APRIL dependency of B-CLL cells according to their VH subgroup and their putative response to autoantigens.
Serum levels of BAFF and APRIL as potential predictive factors in leukemia
Patients with certain B-cell lymphomas or multiple myelomas were reported to exhibit serum values of soluble BAFF (sBAFF) and sAPRIL significantly higher than those detected in normal donors. For instance, multiple myeloma patients present levels of sBAFF and sAPRIL about fivefold those found in healthy donors.77 This could be logically expected, taking into account the survival effect of these factors on normal and tumoral B lymphocytes. It should be noted, however, that the values of sBAFF and sAPRIL reported in the literature for normal donors vary in a ratio of 1 to more than 20 and 1 to 50, respectively. This fact raises the question of the standardization of the enzyme-linked immunosorbent assays (ELISAs) used for the detection of these molecules.
By using a commercial ELISA (R & D Systems Europe, Tille, France), we compared the level of sBAFF in the serum of 88 B-CLL patients and 18 normal donors paired for age and sex. Unexpectedly, we found significantly lower levels of sBAFF in the sera of B-CLL patients (920 ± 54 ng/ml) compared to normal donors (1183 ± 76 ng/ml) (P = 0·0334). Moreover, for the 55 patients of known immunoglobulin VH mutational status, we observed significantly lower sBAFF values among the 16 unmutated patients who had an unfavourable outcome (691 ± 84 ng/ml) in comparison with the 39 mutated patients with more favourable outcomes (974 ± 81 ng/ml) (P = 0·0226).
By SELDI-TOF, we observed, in addition to the monomeric form of sBAFF,68 that two molecules of higher molecular weight, able to bind sBAFF, were present in the serum of B-CLL patients (our unpublished results). The presence of sBAFF-containing complexes in the serum of B-CLL patients might explain the differences observed for the detection of sBAFF between the ELISA and SELDI-TOF techniques and also the unexpected correlation between the level of sBAFF and the mutated status, normally associated with poor prognosis.
The existence of an alternative splice isoform of BAFF, called ΔBAFF and lacking 57 nucleotides encoding the A-A1 loop, has been reported to be coexpressed with BAFF in many mouse and human myeloid cells.78ΔBAFF, unlike BAFF, is inefficiently released by proteolysis but can associate with BAFF in heteromultimers and diminish BAFF bioactivity and release.78 Mice transgenic for ΔBAFF have reduced B-cell numbers and T-cell-dependent antibody responses in contrast to BAFF transgenic mice that display elevated immunoglobulin levels and increases in subsets of B cells, yet both BAFF and ΔBAFF modulate the numbers of B1 phenotype B cells.79 Therefore, the presence of the ΔBAFF isoform in B-CLL could perhaps also account for the unexpected correlation between sBAFF and immunoglobulin VH mutational status and experiments are in progress in our laboratory to identify the molecules complexed with sBAFF in the serum of B-CLL patients and to elucidate their biological role.
In a similar way, we measured the levels of sAPRIL by ELISA (Abcys). Even if some sera of B-CLL patients presented high values of sAPRIL, no statistical differences were detected between normal donors (n = 18; 19·3 ±4·0 ng/ml) and B-CLL patients (n = 52; 23·9 ± 5·6 ng/ml; P = 0·64), nor between mutated (n = 39; 26·2 ±7·3 ng/ml) and unmutated (n = 13; 17·2 ± 3·1 ng/ml; P = 0·6961) patients. However, and in contrast with our data, significantly elevated sAPRIL levels, as compared to normal blood donors, were reported in the serum of B-CLL patients by other groups (M. Hahne, oral communication, Meeting of the French cooperative group on B-CLL, Paris, 17 November 2005). The reason for this discrepancy is not clear, but, as for sBAFF, there is considerable variation in the values reported in the literature for normal serum sAPRIL. A technological workshop aimed at comparing the various commercial and laboratory-made ELISAs for the determination of sBAFF and sAPRIL would certainly be of interest for all the researchers working in the field. Nevertheless, the aforementioned data emphasize the interest of sBAFF and sAPRIL as predictive markers of B-CLL.
Role of BAFF and APRIL in other lymphoid malignancies
In addition to B-CLL, and as mentioned previously, other NHL B cells were found to express BAFF, APRIL and their receptors (BAFF-R > TACI > BCMA). Addition of BAFF to these cells reduced the level of apoptosis and enhanced their survival. BAFF was more expressed in aggressive diseases (DLCL and MCL), whereas it was not detected in follicular lymphoma. The expression of BAFF increased as tumours transformed to a more aggressive phenotype. In addition, sBAFF levels were found to be elevated in a subgroup of patients with NHL. In patients with de novo large B-cell lymphoma, a high BAFF level correlated with a poorer median overall survival, the presence of constitutional symptoms, and elevated values of lactic dehydrogenase. BAFF levels were correlated with response to therapy in all patients, but responding patients had a significantly lower BAFF level than those with progressive disease. BAFF and its receptors thus represent potentially important therapeutic targets in B-cell lymphoma.80
As for B-CLL cells, neutralization of endogenous BAFF and APRIL by soluble decoy receptors attenuated the survival of various NHL cells, correlated with a decreased activation of NF-κB, a down-regulation of Bcl-2 and Bcl-xL and an up-regulation of Bax.81 Conversely, exposure of NHL cells to exogenous BAFF and APRIL attenuated apoptosis, increased NF-κB activation, up-regulated Bcl-2 and Bcl-xL and down-regulated Bax. For some of these NHL, BAFF and APRIL were also found to up-regulate c-Myc (an inducer of cell proliferation), to down-regulate p53 and to increase Bcl-6, an inhibitor of B-cell differentiation.81 NHL B cells therefore deregulate an otherwise physiological autocrine survival pathway to evade apoptosis. Neutralization of BAFF and APRIL by soluble TACI and BCMA decoy receptors could thus be useful to dampen the accumulation of malignant B cells in NHL patients.
Using gene expression profiling, the receptors for APRIL and BAFF were detected in primary leukaemic cells of multiple myeloma patients. APRIL and BAFF activated NF-κB, phosphoinositol-3 kinase/AKT (protein kinase B) and mitogen-activated protein kinase pathways and up-regulated Mcl-1 and Bcl-2 anti-apoptotic proteins in myeloma cells. BAFF or APRIL protected these cells from drug-induced apoptosis and played a role in their survival within their bone marrow environment.77 BAFF and APRIL promote multiple myeloma cell growth. The main source of production for BAFF and APRIL is provided by the monocytes and neutrophils present in the bone marrow, APRIL being also produced at high levels by osteoclasts. Patients with multiple myeloma expressing a low amount of TACI on their leukaemic cells have worse prognosis than those expressing high amounts of TACI. By DNA microarray analysis, it was found that TACIhi multiple myeloma patients displayed a mature plasma cell gene signature indicating dependence on the bone marrow environment whereas TACIlo multiple myeloma patients displayed a gene signature of plasmablasts suggesting an attenuated dependence on the bone marrow environment.82
BAFF, APRIL and their receptors as potential targets for B-CLL therapy
BAFF and APRIL have now been recognized to be involved in the pathogenesis and maintenance of various mature B lineage haematological malignancies (reviewed in ref. 83). This dependence of certain lymphoid tumours on BAFF or APRIL might offer a new therapeutic approach and suggest that APRIL/BAFF inhibitors may be of clinical value in these pathologies.
By the technique of human phage display, a human monoclonal antibody against human BLyS, LymphoStat-B, was obtained after affinity optimization mutagenesis. This antibody inhibited the binding of BLyS to its three receptors and neutralized human BLyS bioactivity in vitro and in vivo.84 This antibody is currently being tested in several clinical trials for the treatment of autoimmune diseases, notably systemic lupus erythematosus and rheumatoid arthritis. It would therefore be worth testing Lymphostat B on B-CLL cells, as well as other neutralizing anti-BAFF antibodies.
By phage display, a human single-chain anti-BAFF antibody fragment scFv able to neutralize BAFF bioactivity was recently isolated. This antibody may also be screened for its potential interest in the therapy of autoimmune and tumoral diseases involving BAFF.85
As an alternative approach, the phage display technique was used to identify peptides binding BAFF with high selectivity and affinity. This resulted in the identification of a core decapeptide motif (WYDPLTKLWL). Selected peptides containing this core motif exhibited Kd values as low as 26 nm and were able to disrupt the interaction between BAFF and TACI.86 These peptides, provided they are adequately stabilized and targeted to the leukaemic cells, could also be valuable candidates for the therapy of B-CLL and other B-cell malignancies where a dysregulated expression of BAFF is implicated.
The specific inhibition of BAFF by antisense oligonucleotides or siRNA silencing can also be envisaged. Of course, these considerations also apply for APRIL neutralization and it could be expected that a simultaneous inhibition of the interaction of BAFF and APRIL with their receptors on B-CLL cells would be more efficient to re-induce the apoptotic process in the leukaemic cells.
In addition to preventing the binding of BAFF and APRIL to their receptors, it could be envisaged to inhibit their downstream signalling pathways leading to enhanced survival, notably the activation of the NF-κB canonical and non-canonical pathways. Drugs such as Bortezomib (Velcade, PS-341) that target the degradation of I-κB by the proteasome are already in use for the therapy of various B-cell malignancies, such as MCL. More specific inhibitors of both pathways are currently under study and are likely to constitute a new class of reagents for the treatment of B-CLL.
Acknowledgments
This work was supported by Cancéropôle Ile-de-France. S. Haiat was supported by a grant from ARC.
References
- 1.Keating MJ. Chronic lymphocytic leukemia. Semin Oncol. 1999;26:107–14. [PubMed] [Google Scholar]
- 2.Kay NE, Hamblin TJ, Jelinek DF, Dewald GW, Byrd JC, Farag S, Lucas M, Lin T. Chronic lymphocytic leukemia. Hematology (Am Soc Hematol Educ Program) 2002:193–213. doi: 10.1182/asheducation-2002.1.193. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Stilgenbauer S, Flinn IW. Chronic lymphocytic leukemia. Hematology (Am Soc Hematol Educ Program) 2004:163–83. doi: 10.1182/asheducation-2004.1.163. [DOI] [PubMed] [Google Scholar]
- 4.Decker T, Hipp S, Ringshausen I, Bogner C, Oelsner M, Schneller F, Peschel C. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood. 2003;101:278–85. doi: 10.1182/blood-2002-01-0189. [DOI] [PubMed] [Google Scholar]
- 5.Caligaris-Cappio F. Biology of chronic lymphocytic leukemia. Rev Clin Exp Hematol. 2000;4:5–21. doi: 10.1046/j.1468-0734.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 6.Kolb JP, Kern C, Quiney C, Roman V, Billard C. Re-establishment of a normal apoptotic process as a therapeutic approach in B-CLL. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:261–86. doi: 10.2174/1568006033481384. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Dugas N, Mathiot C, Delmer A, Dugas B, Sigaux F, Kolb JP. B-cell chronic lymphocytic leukemia cells express a functional inducible nitric oxide synthase displaying anti-apoptotic activity. Blood. 1998;92:1031–43. [PubMed] [Google Scholar]
- 8.Kolb JP. Mechanisms involved in the pro- and anti-apoptotic role of NO in human leukaemia. Leukemia. 2000;14:1685–94. doi: 10.1038/sj.leu.2401896. [DOI] [PubMed] [Google Scholar]
- 9.Carew JS, Nawrocki ST, Xu RH, Dunner K, McConkey DJ, Wierda WG, Keating MJ, Huang P. Increased mitochondrial biogenesis in primary leukemia cells: the role of endogenous nitric oxide and impact on sensitivity to fludarabine. Leukemia. 2004;18:1934–40. doi: 10.1038/sj.leu.2403545. [DOI] [PubMed] [Google Scholar]
- 10.Schneider P, Mackay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay A, Ni J, Zhai Y, Yu GL, Aggarwal BB. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J Biol Chem. 1999;274:15978–81. doi: 10.1074/jbc.274.23.15978. [DOI] [PubMed] [Google Scholar]
- 13.Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999;65:680–3. [PubMed] [Google Scholar]
- 14.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–9. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 15.Hahne M, Kataoka T, Schroter M, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–90. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly K, Manos E, Jensen G, Nadauld L, Jones DA. APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res. 2000;60:1021–7. [PubMed] [Google Scholar]
- 17.Mackay F, Ambrose C. The TNF family members BAFF and APRIL. The growing complexity. Cytokine Growth Factor Rev. 2003;14:311–24. doi: 10.1016/s1359-6101(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 18.Nardelli B, Belvedere O, Roschke V, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Fraga M, Fernandez R, Albar JP, Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2:945–51. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roschke V, Sosnovtseva S, Ward CD, et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol. 2002;169:4314–21. doi: 10.4049/jimmunol.169.8.4314. [DOI] [PubMed] [Google Scholar]
- 21.Wallweber HJ, Compaan DM, Starovasnik MA, Hymowitz SG. The crystal structure of a proliferation-inducing ligand, APRIL. J Mol Biol. 2004;343:283–90. doi: 10.1016/j.jmb.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell-dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–71. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 23.Kanakaraj P, Migone TS, Nardelli B, et al. BLyS binds to B cells with high affinity and induces activation of the transcription factors NF-κB and ELF-1. Cytokine. 2001;13:25–31. doi: 10.1006/cyto.2000.0793. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Bressette D, Carrell J, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000;275:35478–85. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 25.Xia XZ, Treanor J, Senaldi G, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–43. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu HB, Johnson H. B cell maturation protein is a receptor for the tumor necrosis factor family member TALL-1. Proc Natl Acad Sci USA. 2000;97:9156–61. doi: 10.1073/pnas.160213497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laabi Y, Gras MP, Carbonnel F, et al. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO J. 1992;11:3897–904. doi: 10.1002/j.1460-2075.1992.tb05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laabi Y, Gras MP, Brouet JC, Berger R, Larsen CJ, Tsapis A. The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucl Acids Res. 1994;22:1147–54. doi: 10.1093/nar/22.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatzoglou A, Roussel J, Bourgeade MF, Rogier E, Madry C, Inoue J, Devergne O, Tsapis A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J Immunol. 2000;165:1322–30. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- 30.Von Bulow GU, Bram RJ. NF-AT activation induced by CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–41. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–11. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 32.Yan M, Brady JR, Chan B, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–52. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 33.Ambrose CM. BAFF-R. J Biol Regul Homeost Agents. 2002;16:211–13. [PubMed] [Google Scholar]
- 34.Harless SM, Lentz VM, Sah AP, Hsu BL, Clise-Dwyer K, Hilbert DM, Hayes CE, Cancro MP. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol. 2001;11:1986–9. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 35.Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, Hsu YM, Whitty A. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005;44:1919–31. doi: 10.1021/bi048227k. [DOI] [PubMed] [Google Scholar]
- 36.Amanna IJ, Dingwall JP, Hayes CE. Enforced bcl-xL gene expression restored splenic B lymphocyte development in BAFF-R mutant mice. J Immunol. 2003;170:4593–600. doi: 10.4049/jimmunol.170.9.4593. [DOI] [PubMed] [Google Scholar]
- 37.Xu LG, Shu HB. TNFR-associated factor-3 is associated with BAFF-R and negatively regulates BAFF-R-mediated NF-kappa B activation and IL-10 production. J Immunol. 2002;169:6883–9. doi: 10.4049/jimmunol.169.12.6883. [DOI] [PubMed] [Google Scholar]
- 38.Ni CZ, Oganesyan G, Welsh K, Zhu X, Reed JC, Satterthwait AC, Cheng G, Ely KR. Key molecular contacts promote recognition of the BAFF receptor by TNF receptor-associated factor 3: implications for intracellular signaling regulation. J Immunol. 2004;173:7394–400. doi: 10.4049/jimmunol.173.12.7394. [DOI] [PubMed] [Google Scholar]
- 39.Ingold K, Zumsteg A, Tardivel A, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–83. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendriks J, Planelles L, de Jong-Odding J, Hardenberg G, Pals ST, Hahne M, Spaargaren M, Medema JP. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–48. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- 41.Mackay F, Schneider P, Rennert P, Browning J. BAFF and APRIL. A tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 42.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–66. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khare SD, Sarosi I, Xia XZ, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci USA. 2000;97:3370–5. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–14. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 46.Vora KA, Wang LC, Rao SP, et al. Cutting edge. germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003;171:547–51. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- 47.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge. BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168:5993–6. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 48.Rolink AG, Melchers F. BAFFled B cells survive and thrive: roles of BAFF in B-cell development. Curr Opin Immunol. 2002;14:266–75. doi: 10.1016/s0952-7915(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 49.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB-inducing kinase. Immunity. 2004;21:477–89. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Patke A, Mecklenbrauker I, Tarakhovsky A. Survival signaling in resting B cells. Curr Opin Immunol. 2004;16:251–5. doi: 10.1016/j.coi.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Yan M, Wang H, Chan B, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2:638–43. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 52.Von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 53.Siegel RM, Lenardo MJ. To B or not to B. TNF family signaling in lymphocytes. Nat Immunol. 2001;2:577–8. doi: 10.1038/89715. [DOI] [PubMed] [Google Scholar]
- 54.Schneider P, Takatsuka H, Wilson A, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194:1691–7. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.YuG, Boone T, Delaney J, et al. APRIL and TALL-I and receptors BCMA and TACI. System for regulating humoral immunity. Nat Immunol. 2000;1:252–6. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- 56.Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000;192:953–64. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laabi Y, Strasser A Immunology. Lymphocyte survival — ignorance is BLyS. Science. 2000;289:883–4. doi: 10.1126/science.289.5481.883. [DOI] [PubMed] [Google Scholar]
- 58.Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, Dixit VM. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 59.Pelletier M, Thompson JS, Qian F, et al. Comparison of soluble decoy IgG fusion proteins of BAFF-R and BCMA as antagonists for BAFF. J Biol Chem. 2003;278:33127–33. doi: 10.1074/jbc.M305754200. [DOI] [PubMed] [Google Scholar]
- 60.Huard B, Schneider P, Mauri D, Tschopp J, French LE. T cell costimulation by the TNF ligand BAFF. J Immunol. 2001;167:6225–31. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 61.Stein JV, Lopez-Fraga M, Elustondo FA, et al. APRIL modulates B and T cell immunity. J Clin Invest. 2002;109:1587–98. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL. Innate players in adaptative antibody responses. Immunity. 2002;17:235–8. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 65.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin Pharmacol. 2004;4:347–54. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Briones J, Timmerman JM, Hilbert DM, Levy R. BLyS and BLyS receptor expression in non-Hodgkin's lymphoma. Exp Hematol. 2002;30:135–41. doi: 10.1016/s0301-472x(01)00774-3. [DOI] [PubMed] [Google Scholar]
- 67.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100:2973–9. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 68.Kern C, Cornuel JF, Billard C, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–88. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 69.Nishio M, Endo T, Tsukada N, Ohata J, Kitada S, Reed JC, Zvaifler NJ, Kipps TJ. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1. Blood. 2005;106:1012–20. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Planelles L, Carvalho-Pinto CE, Hardenberg G, et al. APRIL promotes B-1 cell-associated neoplasm. Cancer Cell. 2004;6:399–408. doi: 10.1016/j.ccr.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 71.Rodig SJ, Shahsafaei A, Li B, Mackay CR, Dorfman DM. BAFF-R, the major B cell-activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum Pathol. 2005;36:1113–19. doi: 10.1016/j.humpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura N, Hase H, Sakurai D, et al. Expression of BAFF-R (BR 3) in normal and neoplastic lymphoid tissues characterized with a newly developed monoclonal antibody. Virchows Arch. 2005;447:53–60. doi: 10.1007/s00428-005-1275-6. [DOI] [PubMed] [Google Scholar]
- 73.Morrison MD, Reiley W, Zhang M, Sun SC. An atypical tumor necrosis factor (TNF) receptor-associated factor-binding motif of B cell-activating factor belonging to the TNF family (BAFF) receptor mediates induction of the noncanonical NF-kappaB signaling pathway. J Biol Chem. 2005;280:10018–24. doi: 10.1074/jbc.M413634200. [DOI] [PubMed] [Google Scholar]
- 74.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 75.Xu LG, Wu M, Hu J, Zhai Z, Shu HB. Identification of downstream genes up-regulated by the tumor necrosis factor family member TALL-1. J Leukoc Biol. 2002;72:410–16. [PubMed] [Google Scholar]
- 76.Gorelik L, Cutler AH, Thill G, et al. Cutting edge: BAFF regulates CD21/35 and CD23 expression independent of its B cell survival function. J Immunol. 2004;172:762–6. doi: 10.4049/jimmunol.172.2.762. [DOI] [PubMed] [Google Scholar]
- 77.Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–57. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gavin AL, Ait-Azzouzene D, Ware CF, Nemazee D. DeltaBAFF, an alternate splice isoform that regulates receptor binding and biopresentation of the B cell survival cytokine, BAFF. J Biol Chem. 2003;278:38220–8. doi: 10.1074/jbc.M306852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gavin AL, Duong B, Skog P, Ait-Azzouzene D, Greaves DR, Scott ML, Nemazee D. DeltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J Immunol. 2005;175:319–28. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 80.Novak AJ, Grote DM, Stenson M, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104:2247–53. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 81.He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172:3268–79. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 82.Moreaux J, Cremer FW, Reme T, et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood. 2005;106:1021–30. doi: 10.1182/blood-2004-11-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jelinek DF, Darce JR. Human B lymphocyte malignancies. exploitation of BLyS and APRIL and their receptors. Curr Dir Autoimmun. 2005;8:266–88. doi: 10.1159/000082107. [DOI] [PubMed] [Google Scholar]
- 84.Baker KP, Edwards BM, Main SH, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253–65. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- 85.Cao P, Xia Z, Song W, Zhang S. Neutralizing human anti-B-cell-activating factor of the TNF family (BAFF) scFv selected from phage antibody library. Immunol Lett. 2005;101:87–94. doi: 10.1016/j.imlet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 86.Fleming TJ, Sachdeva M, Delic M, et al. Discovery of high-affinity peptide binders to BLyS by phage display. J Mol Recognit. 2005;18:94–102. doi: 10.1002/jmr.722. [DOI] [PubMed] [Google Scholar]