Abstract
Inflammation in the central nervous system (CNS) can be studied in experimental autoimmune encephalomyelitis (EAE). The proinflammatory cytokines interferon-gamma (IFN-γ) and tumour necrosis factor (TNF) are implicated in EAE pathogenesis. Signals through the type 1 TNF receptor (TNFR1) are required for severe EAE to develop, whereas deficiency in IFN-γ or its receptor result in more severe EAE. We investigated IFN-γ expression in TNFR1-deficient (TNFR1–/–) mice. We describe here that there were more IFN-γ-secreting T cells present in the CNS of TNFR1–/– mice during EAE compared to wild-type (WT) mice, despite that clinical symptoms were mild, with delayed onset. There was greater expression of IL-12/23p40 by antigen-presenting cells in these mice, and in vitro, TNFR1–/– antigen-presenting cells induced greater secretion of IFN-γ but not interleukin (IL)-17 when cultured with primed T cells than did WT antigen presenting cells. TNFR1–/– mice with EAE had significantly higher expression of CXCL10 mRNA (but not CCL5 mRNA) in the CNS compared to WT mice with EAE. These data demonstrate that IFN-γ expression is enhanced in the CNS of TNFR1–/– mice with EAE and suggest that IFN-γ levels do not necessarily correlate with EAE severity.
Keywords: EAE, IFN-γ, TNF
Introduction
Cytokines are critical mediators of inflammation that function in networks, exhibiting pleiotropy and redundancy. Tumour necrosis factor (TNF) and interferon gamma (IFN-γ) are two major proinflammatory cytokines. In the central nervous system (CNS) their roles are complex, and IFN-γ and TNF have been attributed both detrimental and beneficial functions in multiple sclerosis (MS) and the animal model experimental autoimmune encephalomyelitis (EAE).1 In MS and EAE, peripheral immune cells infiltrate the CNS, targeting myelin antigens, and the resulting inflammatory processes lead to damage to myelin, oligodendrocytes and axons. EAE has proved a useful model in which to elucidate molecular interactions involved in the inflammation contributing to MS.
IFN-γ is not present in the normal CNS, but is secreted by activated infiltrating T cells during EAE. IFN-γ-secreting Th1 cells are present in the CNS before onset of clinical symptoms, and disappear during the remission phase of EAE.2,3 IFN-γ+ T cells are present in MS lesions4,5 and treatment with IFN-γ exacerbates MS.6 However, there is increasing evidence from EAE studies where IFN-γ is neutralized or genetically deficient that IFN-γ can also mediate protective actions as EAE severity is increased.7–10 Potential mechanisms by which IFN-γ may exert a protective effect include direct inhibition of inflammatory cytokine expression and induction of cytokine inhibitors (for reviews, see1,11). Infiltrating macrophages and resident microglia can regulate T cell IFN-γ production by secreting IFN-γ-inducing cytokines. These include interleukin (IL)-18 and IL-12 (a heterodimeric protein (p70) consisting of p35 and p40 subunits). Another cytokine, IL-23, also contains the p40 subunit (combined with a p19 subunit). Prior to the discovery of IL-23, results based on p40 were assumed to indicate IL-12 function. Recent studies comparing IL-23p19, IL-12p35 and IL-12/23p40 are clarifying the actions of IL-12 and IL-23.12–15 Evidence from mice deficient in the p40, p35 or p19 subunits has demonstrated that IL-23 rather than IL-12 plays a critical role in EAE induction, as IL-23p19–/– and IL-12/23p40–/– mice but not IL-12p35–/– mice are resistant to EAE.16 However, IL-23 is not a strong inducer of IFN-γ expression, and instead stimulates IL-17 secretion from T cells.15 Consistent with this, IL-23-polarized IL-17 secreting T cells are highly pathogenic, and neutralization of IL-17 ameliorates EAE.15,17
TNF has been detected in MS lesions18,19 and TNF levels in cerebrospinal fluid correlated with disease progression in MS patients.20 In EAE, TNF is up-regulated early by microglia before the onset of clinical symptoms, and is expressed later by T cells and macrophages.2,3 The actions of TNF are mediated through two receptors, tumour necrosis factor receptor (TNFR)1 and TNFR2, which differ in their intracellular domains and therefore their relative roles in mediating TNF actions, through mechanisms that are not yet clearly understood (for reviews see21,22). Mice deficient in TNF or TNFR1 are resistant to EAE, either completely or showing a late-onset phenotype.23–28 This is due, at least in part, to a key role of TNF in early expression of chemokines and adhesion molecules at the blood–brain barrier, promoting infiltration of the CNS by peripheral immune cells.28,29 In contrast, TNFR2–/– mice develop similar or more severe disease than wild-type (WT) mice.24,25,27 Blockade of TNF with neutralizing antibodies or soluble receptors ameliorates EAE in rats and mice.30–35 However, in contrast to other inflammatory diseases, most notably rheumatoid arthritis (for review, see36), therapies aimed at targeting TNF have not proved effective against MS and in some cases have led to an increased relapse rate.37
The aim of this study was to investigate IFN-γ expression and regulation in TNFR1–/– mice with EAE, as this has not been characterized previously. We describe the surprising observation that despite developing milder EAE, TNFR1–/– mice had higher numbers of IFN-γ-secreting T cells in the CNS. We suggest that these observations may be due to enhanced expression of IL-12/23p40 and certain chemokines.
Materials and methods
Mice
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). C57BL/6-back-crossed mice with a targeted disruption of the TNFR1 gene38 were obtained originally from Professor Tak Mak (Amgen, Toronto, Ontario, Canada) and are maintained as a homozygous colony in the Montreal Neurological Institute. All mice were housed in microisolator cages in a pathogen-free facility, with 12 hr:12 hr light:dark cycle and free access to food and water. All procedures were performed in accordance with guidelines of the Canadian Council on Animal Care, as approved by the McGill University Animal Care Committee.
Induction of EAE
EAE was induced by subcutaneous injection on day 0 (tailbase) and day 7 (flanks) with 100 µg myelin oligodendrocyte glycoprotein peptide p35–55 (MOGp35–55; Sheldon Biotechnology Centre, Montreal, Quebec, Canada) in complete Freund's adjuvant (Fisher, Montreal, Quebec, Canada) containing 500 µg heat-killed Mycobacterium tuberculosis (H37RA; Difco, Detroit, MI). An intraperitoneal injection of 200 ng pertussis toxin (Cedarlane, Hornby, Ontario, Canada) was administered on day 0 and day 2. Mice were monitored daily and assessed by weight and clinical score (grade 1: floppy tail; grade 2: 1 + hind limb paresis (assessed as splayed hind limbs when walking and a slowness of the animal to right itself when placed on its back); grade 3: 2 + unilateral hind limb paralysis; grade 4: bilateral hind limb paralysis; grade 5: moribund).
Intracellular cytokine analysis
All reagents and antibodies were from BD Pharmingen unless stated otherwise. Tissues from each animal were processed separately. Single cell suspensions were prepared from the spleen and CNS (pooled brain and spinal cord) of phosphate-buffered saline (PBS)-perfused mice. For spleen, red blood cells were lysed, then cells were washed twice with Hanks's buffered saline solution (HBSS) and resuspended in 10% complete medium (RPMI-1640; Invitrogen, Burlington, Ontario, Canada) supplemented with 10% fetal calf serum (FCS) (Sigma, Oakville, Ontario, Canada), 50 mmβ-mercaptoethanol (Sigma), 2 mm l-glutamine (Life Technologies, Burlington, Ontario, Canada), 100 U/ml penicillin (Life Technologies), and 100 µg/ml streptomycin (Life Technologies). CNS cells were resuspended in 5 ml Percoll (70% in 0·01 N HCl/PBS; Amersham. Baie d'Urfe, Quebec, Canada), and centrifuged at 2000 g, 20° for 20 min. Myelin was removed, and the cell pellet was resuspended in 10% complete RPMI. Spleen or CNS cells were cultured at 4 × 105 cells/ml in uncoated or anti-CD3 (supernatant purified from hybridoma 1452C11, as described previously;39 2 µg/ml; ATCC, Manassas, VA) coated 96-well plates for 5 hr with Brefeldin A (1 µl/1 ml cells; BD GolgiPlug, Mississauga, Ontario, Canada). Cells were harvested and incubated with blocking buffer {supernatant from the anti-FcR 24G2 hybridoma [American Type Culture Collection (ATCC), Manassas, VA] containing 2% FCS (Sigma) and 50 µg/ml hamster IgG (BIO/CAN Scientific, Mississauga, Ontario, Canada)} on ice for 30 min. Cells were washed with fluorescence activated cell sorter (FACS) buffer (2% FCS/0·1% sodium azide in HBSS) and then incubated with antibody for 30 min on ice [CyChrome (CyC)-T cell receptor (TCR)β, peridinin chlorophyll (PerCP)-CD45, allophycocyarin-CD11b]. Cells were washed with FACS buffer, and then permeabilized and intracellular cytokines stained [fluorescein isothiocyanate (FITC)-IFN-γ, phycoerythrin (PE)-p40; PE-IL-17, Groovy Blue Genes Biotech Ltd, Vineland, Ontario, Canada] using a BD Cytofix/Cytoperm kit, according to the manufacturer's instructions. Staining was analysed using cell quest pro on a FACScan (BD). All analyses were performed on 10 000 gated events. Specificity of cytokine staining was confirmed using appropriate isotype controls. Negligible staining with isotype was seen in cells prepared from either WT and TNFR1–/– mice. Absolute cell numbers were calculated using cell counts of spleen or CNS single cell suspensions using trypan blue exclusion and a haemocytometer. These numbers are likely to be underestimations due to cell loss during experimental manipulations.
Histology
Mice were euthanized by terminal anaesthesia (Somnotol; MTC Pharmaceuticals, Hamilton, Ontario, Canada), and perfused with ice-cold PBS. The spinal cord was removed, frozen in Tissue Tek® (Canemco-Marivac, St Laurent, Quebec, Canada) and stored at −80°. Sections (10 µm) were cut and allowed to air-dry before performing haematoxylin and eosin staining (H & E), as described in Tran et al.40 or immunohistochemistry for TCRβ. For immunohistochemistry, sections were fixed in 1% paraformaldehyde (15 min), then incubated in 0·5% Triton X-100/Tris-buffered saline (TBS) for 15 min. Non-specific binding was blocked by incubation in supernatant from the anti-FcR 24G2 hybridoma containing 10% FCS (Sigma) and 10% appropriate normal serum. Sections were then incubated with hamster anti-mouse TCRβ (1/50 in 10% block/TBS; BD Pharmingen) for 2 hr. After washing in TBS, sections were incubated with biotinylated secondary antibody (mouse anti-hamster IgG, 1/100 in 10% block/TBS; BD Pharmingen) for 1 hr. Staining was visualized using the ABC Vectastain ELITE kit, according to the manufacturer's instructions (Vector Laboratories, Burlington, Ontario, Canada), followed by diaminobenzidine reagent (Vector). Sections were washed, dehydrated in ethanol, incubated in xylene and mounted in Permount (Fisher, Montreal, Quebec, Canada). As a negative control, immunohistochemistry was performed with buffer instead of primary antibody, and resulted in no staining.
Co-culture of T cells and macrophages
Macrophages and primed T cells were prepared from spleen or lymph node, respectively, by negative selection using magnetic cell sorting with Dynabeads® (Dynal Biotech ASA, Oslo, Norway) according to the manufacturer's instructions, i.e. unwanted cells were labelled and then magnetically removed. Briefly, lymph nodes were harvested and pooled from four to eight mice 7–10 days after immunization as for EAE (see above) without injection of pertussis toxin. Spleens from two to four unmanipulated mice were harvested and pooled. Single cell suspensions were prepared by homogenizing the tissue, passing through a 70 µm nylon mesh and resuspending in HBSS. Spleen cells were incubated in 0·16 m ammonium chloride (2·5 ml/spleen) for 10 min at room temperature to lyse red blood cells. After washing, lymph node or spleen cells were incubated in blocking buffer (described above) for 20 min on ice. Biotinylated antibodies (BD Pharmingen) were added and incubated for 30 min on ice [for T cells, biotinylated anti-B220 (anti-CD45R; 0·25 µg/106 cells) and biotinylated anti-I-Ab (0·35 µg/106 cells); for macrophages, biotinylated anti-B220 (0·25 µg/106 cells), biotinylated anti-CD3e (0·5 µg/106 cells), biotinylated anti-CD4 (0·5 µg/106 cells) and biotinylated anti-CD8 (0·5 µg/106 cells)]. After washing, cells were incubated with streptavidin-conjugated beads for 20 min at room temperature. Cells conjugated to beads were removed with a magnet and the remaining unlabelled cells were collected. Purity of cell populations was determined by flow cytometry. T cells (B220–, I-Ab–) were at least 95% TCRβ+, macrophages (B220–, CD3e–, CD4–, CD8–) had less than 3% T cell contamination. T cells 105) were cultured with macrophages 105) and MOGp35–55 (50 µg/ml) in a total volume of 200 µl at 37° in 10% complete medium (described above). Cells and supernatant were harvested at various time-points. Cells were stored in TRIzol reagent (Invitrogen, Burlington, Ontario, Canada) at −80° until RNA extraction. Supernatants were centrifuged to remove any cells, then stored at −20°. Experiments were performed in triplicate, and were repeated up to four times with cells prepared from different groups of animals. Note that cultures containing only one cell type in the presence of antigen did not secrete detectable amounts of IFN-γ.
Real-time PCR
Spleens and spinal cords were removed from PBS-perfused mice, snap-frozen on dry ice and stored at −80° until processing. RNA was extracted from frozen tissues and cells using TRIzol, according to the manufacturer's protocol (Invitrogen). Glycogen (5 µg/sample; Invitrogen) was used as a carrier for cell samples. cDNA was synthesized by incubating 3 µg total RNA with random hexamer primers (10 µm; Roche Diagnostics, Mannheim, Germany), RNA guard RNase inhibitor (16 U; Amersham Biosciences, Piscataway, NJ), deoxynucleotide triphosphates (dNTPs) (0·5 mm), dithiothreitol (DTT) (3 mm) in first-strand buffer (1×), with Moloney murine leukaemia virus-RT (400 U; Invitrogen) in a total volume of 30 µl at 42° for 1 hr. The reaction was terminated by incubating at 75° for 10 min. Real time PCR analysis was performed on cDNA equivalent to 0·1 µg total RNA by incubating with optimized concentrations of probe, forward and reverse primers in TaqMan® Universal Master Mix (1×; PE Applied Biosystems, Foster City, CA) in a total volume of 25 µl. Primer Express software (PE Applied Biosystems) was used to design specific primers and probes for IFN-γ, IL-12p35, IL-12/23p40, IL-23p19, IL-17, IL-18, CCL5 and CXCL10. IFN-γ 5′ primer: CATTGAAAGCCTAGAAAGTCTGAATAAC; IFN-γ-3′ primer: TGGCTCTGCAGGATTTTCATG; IFN-γ probe: FAM-TCACCATCCTTTTGCCAGTTCCTCCAG-TAMRA; IL-12/23p40 5′ primer: GGAAGCACGGCAGCAGAATA; IL-12/23p40 3′ primer: AACTTGAGGGAGAAGTAGGAATGG; IL-12/23p40 probe: FAM-CATCATCAAACCAGACCCGCCCAA-TAMRA; IL-12p35 5′ primer: AAGACATCACACGGGACCAAA; IL-12p35 3′ primer: CAGGCAACTCTCGTTCTTGTGTA; IL-12p35 probe: FAM-CAGCACATTGAAGACCTGTTTACCACTGGA-TAMRA; IL-23p19 5′ primer: TCC CTA CTA GGA CTC AGC CAA CTC; IL-23p19 3′ primer: ACT CAG GCT GGG CAT CTG TT; IL-23p19 probe: FAM-CCA GCC AGA GGA TCA CCC CCG-MGB; IL-17 5′ primer: CTCCAGAAGGCCCTCAGACTAC; IL-17 3′ primer: TGTGGTGGTCCAGCTTTCC; IL-17 probe: FAM-ACTCTCCACCGCAATGA-MGB; IL-18 5′ primer: GACTCTTGCGTCAACTTCAAGGA; IL-18 3′ primer: TTGTCTGATTCCAGGTCTCCATT; IL-18 probe: FAM-TGATGTTTATTGACAACACGCTTTACTTTATACCTGAAGA-TAMRA; CCL5 5′ primer: GGAGTATTTCTACACCAGCAGCAA; CCL5 3′ primer: CACACACTTGGCGGTTCCTT; CCL5 probe: FAM-TGCAGTCGTGTTTGTC-MGB; CXCL10 5′ primer: GCCGTCATTTTCTGCCTCAT; CXCL10 3′ primer: GGCCCGTCATCGATATGG; CXCL10 probe: FAM-GGACTCAAGGGATCC-MGB. Primers were purchased from Sigma and probes from PE Applied Biosystems. A TaqMan® ribosomal RNA control reagent kit was used to quantify 18S ribosomal RNA expression (PE Applied Biosystems). Real-time PCR was performed in 96-well optical reaction plates using the ABI-Prism 7000 Sequence Detection System (PE Applied Biosystems) and the following cycle conditions: (1) 2 min at 50°, 10 min at 95°; 1 cycle and (2) 15 seconds at 95°, 1 min at 60°; 40 cycles. Controls included reverse transcription–polymerase chain reaction (RT-PCR) with water replacing RNA, and PCR with water replacing cDNA. These controls gave a cycle threshold (Ct) value of 40, indicating no detectable PCR product under these cycle conditions. Results were analysed using the ABI Prism 7000 Sequence Detection System software version 1·1 (PE Applied Biosystems), normalized to 18S, and expressed as arbitrary units.
Enzyme-linked immunosorbent assay (ELISA)
ELISAs for IFN-γ, IL-12p70, IL-17, IL-18 and IL-12/23p40 were used according to the manufacturer's instructions (BD Pharmingen, except IL-17; R & D Biosystems, Minneapolis, MN). Detection limits are stated for each assay.
Statistics
Data were analysed using a Student's t-test, or one-way analysis of variance (anova) with Tukey's post-hoc analysis, as appropriate. P < 0·05 was considered statistically significant.
Results
TNFR1–/– mice have milder EAE symptoms than WT mice but higher IFN-γ mRNA expression
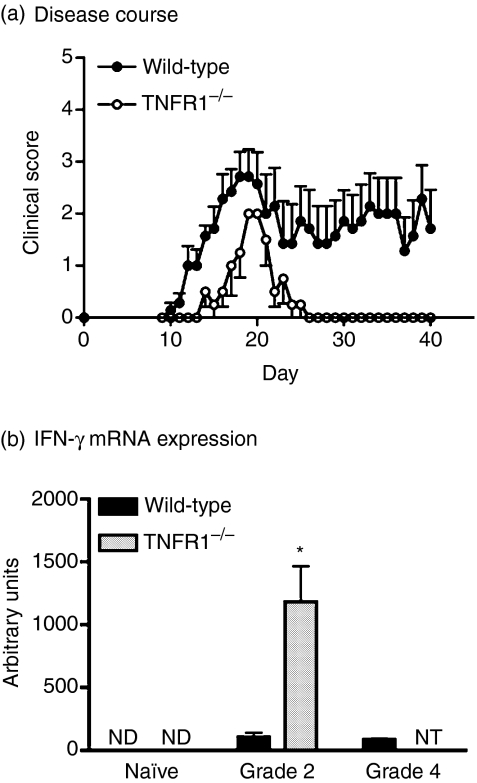
It has been described previously that TNFR1–/– mice develop milder EAE than WT mice.24,25,27,29,41 To confirm our ability to repeat this finding, EAE was induced in WT and TNFR1–/– mice by immunization with MOGp35–55/CFA and pertussis toxin (Fig. 1a). There was a significant delay in the onset of clinical symptoms in TNFR1–/– mice compared to WT mice (day of onset 16·3 ± 1·3 versus 12·3 ± 0·6, P = 0·01). Furthermore, TNFR1–/– mice exhibited significantly milder symptoms, never progressing beyond grade 2 disease, compared to severities of up to grade 4 in WT mice (maximum disease score 2·0 ± 0·0 versus 3·1 ± 0·4). TNFR1–/– mice with EAE either remitted completely or retained mild (sub-grade 1) chronic abnormality, e.g. in tail tone.
Figure 1.
Disease course and interferon-gamma (IFN-γ) expression in wild-type (WT) and tumour necrosis factor receptor (TNFR1)–/– mice. (a) WT (black circles, n = 7) and TNFR1–/– (white circles, n = 4) mice were immunized with myelin olidodendrocyte glycoprotein peptide 35–55 (MOGp35–55)/complete Freund's adjuvant (CFA) on day 0 and day 7, and pertussis toxin was administered intraperitoneally (i.p.) on day 0 and day 2. Mice were scored daily as described in Methods. Results are shown as mean ± SEM. Representative of four replicate experiments (n = 4–8 WT or TNFR1–/– mice per group in each experiment). (b) IFN-γ mRNA expression was assessed by real-time polymerase chain reaction (PCR) in spinal cords of wild-type (black bars) and TNFR1–/– (grey bars) unmanipulated mice (naive) or mice with grade 2 or grade 4 disease. Values are expressed as arbitrary units normalized to 18S expression, mean ± SEM, n = 4 mice. *P < 0·05. ND, not detectable; NT, not tested, as TNFR1–/– mice did not develop disease more severe than grade 2.
We next investigated IFN-γ expression. IFN-γ mRNA was expressed in the spinal cords of mice with EAE, but not in unimmunized animals (Fig. 1b). Surprisingly, TNFR1–/– mice with grade 2 disease had significantly higher levels of IFN-γ mRNA than WT mice with either grade 2 or grade 4 disease. There was no difference in IFN-γ mRNA expression in the spleen between WT and TNFR1KO mice (data not shown).
TNFR1–/– mice with EAE have more IFN-γ+ T cells in the CNS than WT mice
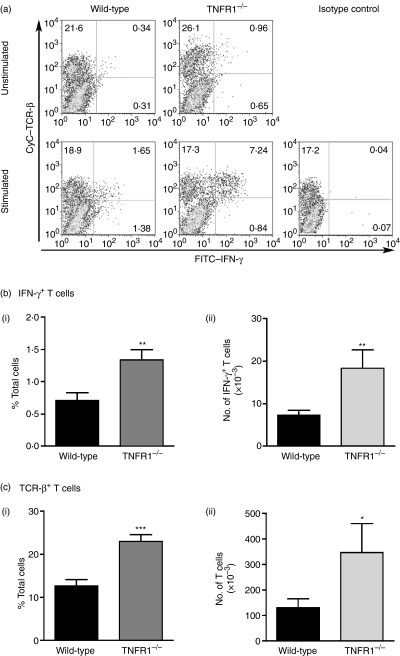
IFN-γ is expressed by CD4+ T cells in the inflamed CNS in EAE.3 IFN-γ+ cells in the CNS of mice with EAE were assessed by flow cytometry, and were predominantly TCRβ+(Fig. 2). Consistent with the unexpectedly high IFN-γ mRNA expression, TNFR1–/– mice with EAE had more IFN-γ+ T cells (both in terms of percentage of total cells and absolute number of cells) in the CNS than WT mice with either the same severity of EAE (grade 2) or more severe clinical disease (grade 4, data not shown). Indeed, there were also more T cells in the CNS of TNFR1–/– mice with EAE, compared to WT mice, the majority (approximately 75%) being CD4+. The proportion of T cells that were IFN-γ+ was not different between WT and TNFR1–/– mice (5·8 ± 3·5% and 5·8 ± 1·7%, respectively; mean ± SD).
Figure 2.
Interferon-gamma (IFN-γ)-positive T cells in the central nervous system (CNS) of mice with experimental autoimmune encephalomyelitis (EAE) CNS cells pooled from brain and spinal cord were prepared from individual wild-type (WT) or tumour necrosis factor receptor (TNFR1)–/– mice with grade 2 EAE and cultured in the presence of Brefeldin A for 5 h with (stimulated) or without (unstimulated) anti-CD3 stimulation. Cells were then labelled for cell surface markers and intracellular IFN-γ and analysed by flow cytometry. (a) Representative flow cytometry profiles for WT and TNFR1–/– mice and isotype control show T cell receptor (TCR)β and IFN-γ expression in live cells. IFN-γ+ TCRβ+ T cells (b) and total TCRβ+ T cells (c) were quantified from WT (black bars, n = 8 mice) and TNFR1–/– (grey bars, n = 5 mice) unstimulated CNS cells (see also Table 1). Results are shown as mean ± SEM both in terms of percentage of total live cells (i) and absolute cell numbers (ii). *P < 0·05, **P < 0·01, ***P < 0·001.
These results were obtained by analysis of CNS cells that did not receive any additional ex vivo stimulation. A more pronounced difference was seen on analysis of anti-CD3-stimulated cells (Table 1). Taken together, these data demonstrate that TNFR1–/– mice have more IFN-γ in their CNS during EAE, due to an overall increase in the number of IFN-γ-producing T cells. By contrast, there was no difference in T cell numbers or the number of IFN-γ+ T cells in the spleens of WT or TNFR1–/– mice (data not shown).
Table 1.
Interferon-gamma (IFN-γ)-positive T cells in the central nervous system (CNS) of mice with EAE
| Unstimulated | Anti-CD3 stimulated | |||
|---|---|---|---|---|
| IFN-c+ T cells | Total T cells | IFN-c+ T cells | Total T cells | |
| % Total cells | ||||
| Wild-type | 0·7 ± 0·4% | 12·6 ± 4·3% | 2·1 ± 1·0% | 11·9 ± 5·3% |
| TNFR1–/– | 1·3 ± 0·4% | 22·9 ± 3·5% | 6·5 ± 3·9% | 20·8 ± 6·2% |
| Significance | P < 0·01 | P < 0·001 | P < 0·01 | P < 0·01 |
| Fold increase | 1·9 | 1·8 | 3·1 | 1·7 |
| No. of cells (× 103) | ||||
| Wild-type | 7·3 ± 3·4 | 130·1 ± 100·4 | 20·0 ± 4·6 | 119·2 ± 116·0 |
| TNFR1–/– | 18·3 ± 9·6 | 346·9 ± 253·6 | 53·0 ± 19·1 | 301·0 ± 222·1 |
| Significance | P < 0·01 | P < 0·005 | P < 0·01 | P < 0·07 |
| Fold increase | 2·5 | 2·7 | 2·7 | 2·5 |
Results shown are mean ± s.d., n = 6–10 mice. TNFR1 –/–: tumour necrosis factor receptor.
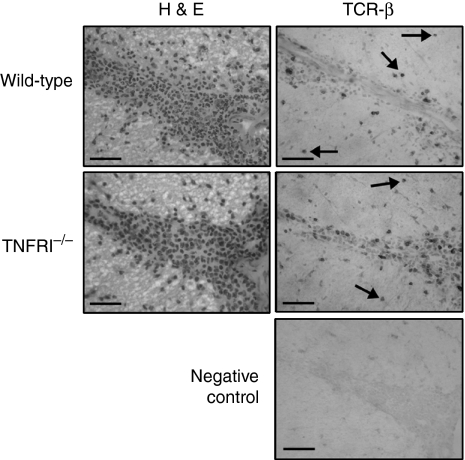
It has been reported that T cell migration is altered in TNFR1–/– mice,29 and that TNF plays an important role in regulation of immune cell infiltration in EAE.28,32 It was therefore important to establish the location of T cells within the CNS of TNFR1–/– mice. Histological analysis did not reveal any significant difference between infiltrates in WT and TNFR1–/– mice with EAE (Fig. 3). TCRβ+ T cells were present in perivascular infiltrates as well as in the adjacent parenchyma, indicating that the migration of these cells was not impaired in TNFR1–/– mice. This is consistent with previous observations in TNF-deficient mice with EAE.28
Figure 3.
Localization of T cells in the spinal cords of mice with experimental autoimmune encephalomyelitis (EAE). Frozen sections (10 µm) were prepared from spinal cords of wild-type (WT) or tumour necrosis factor receptor (TNFR1)–/– mice with grade 2 EAE. Adjacent sections were stained by haematoxylin and eosin (H & E) or immunohistochemistry for T cell receptor (TCR)β. T cells were present in inflammatory cell infiltrates and in the adjacent parenchyma (example cells indicated by arrows) in the spinal cord of WT and TNFR1–/– mice. Negative control, immunohistochemistry performed without primary antibody. Scale bar = 100 µm.
TNFR1–/– APCs induce greater IFN-γ production by T cells in vitro
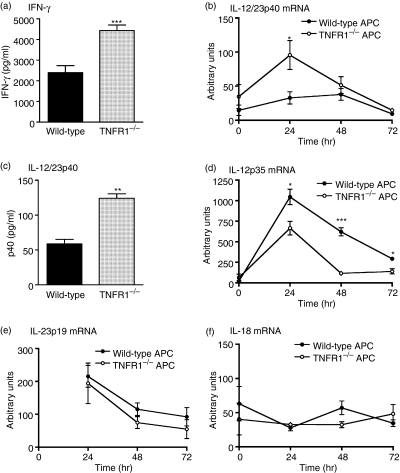
A critical step that controls T cell production of IFN-γ in EAE is the recognition by primed T cells of antigen presented in the context of MHCII on APCs. In the CNS, APCs include macrophages, microglia and dendritic cells. Factors such as IL-12 (p40/p35) and IL-18 that are secreted by APCs regulate T cell IFN-γ production. TNF acting through TNFR1 has been shown to inhibit IL-12 expression.42 We therefore compared the ability of WT and TNFR1–/– APCs to induce T cell IFN-γ production, and investigated whether there was altered expression of IFN-γ-inducing cytokines. Although antigen-specific proliferation was comparable in all co-cultures (data not shown), primed T cells secreted significantly more IFN-γ when cultured with TNFR1–/– APCs than when cultured with WT APCs (Fig. 4a). Similarly, IL-12/23p40 mRNA was expressed at significantly higher levels in co-cultures containing TNFR1–/– APCs (Fig. 4b). This corresponded to greater levels of IL-12/23p40 in supernatants from co-cultures of T cells with TNFR1–/– macrophages (Fig. 4c). In contrast, IL-12p35 mRNA expression was higher in co-cultures of T cells with WT APCs than with TNFR1–/– APCs (Fig. 4d), and IL-12p70 protein was below detectable levels in all co-cultures (detection limit 125 pg/ml). Neither IL-23p19 nor IL-18 mRNA expression differed between cultures containing WT or TNFR1–/– APCs (Fig. 4e–f) and IL-18 protein was below detection limits in all co-cultures (detection limit 31·2 pg/ml). The p40 subunit is a component of IL-12 and IL-23. Whereas IL-12 is a key IFN-γ-inducing cytokine, IL-23 has been described to induce IL-17.13 We therefore investigated IL-17 secretion in these co-cultures. T cells cultured with WT or TNFR1–/– APCs produced significant and comparable amounts of IL-17 (WT 1837 ± 175 pg/ml versus TNFR1–/– 1915 ± 435 pg/ml, P = 0·79; mean ± SD, n = 3, representative of two independent experiments), despite that in the same experiments, co-cultures containing TNFR1–/– APCs produced more IFN-γ protein (WT, 5113 ± 1457 pg/ml; TNFR1–/–, 8539 ± 1133 pg/ml, P < 0·05, mean ± SD, n = 3).
Figure 4.
Co-cultures of T cells with wild-type (WT) or tumour necrosis factor receptor (TNFR1)–/– antigen-presenting cells (APCs). WT T cells were cultured with WT (black bars; black circles) or TNFR1–/– (grey bars; white circles) macrophages (APC) in the presence of myelin olidodendrocyte glycoprotein peptide 35–55 (MOGp35–55) (50 µg/ml) for 72 hr. Interferon-gamma (IFN-γ) (a) and interleukin (IL)-12/23p40 (c) protein levels in the cell supernatants were measured by enzyme-linked immunosorbent assay (ELISA). Results are mean ± SEM, n = 3, representative of four replicate experiments. Detection limits: IFN-γ, 75 pg/ml; IL-12/23p40, 31·2 pg/ml (b, d–f) At various time-points, cells were harvested and expression of IL-12/23p40 (b), IL-12p35 (d), IL-23p19 (e) and (f) IL-18 mRNA was assessed by real-time polymerase chain reaction (PCR). Data are expressed as arbitrary units normalized to 18S. Results are shown as mean ± SEM, n = 4. *P < 0·05, **P < 0·01, **P < 0·001. Note that IL-23p19 mRNA was undetectable in untreated cultures (t = 0 hr).
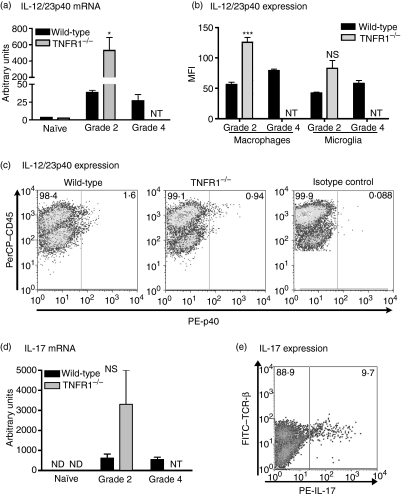
Enhanced expression of IL-12/23p40 by CNS APCs during EAE in TNFR1–/– mice
Our in vitro data suggested that increased IFN-γ production by T cells was associated with higher IL-12/23p40 expression by TNFR1–/– APCs. We therefore investigated the expression of IL-12/23p40 in the CNS during EAE. Levels of IL-12/23p40 mRNA were higher in the spinal cords of TNFR1–/– mice with grade 2 EAE than in WT mice with either grade 2 or grade 4 disease (P < 0·05; Fig. 5a). Both IL-12p35 and IL-23p19 mRNAs were expressed in the spinal cords of untreated mice and mice with EAE, but there were no differences between WT and TNFR1–/– mice (data not shown). Flow cytometry analysis identified both macrophages and microglia as sources of IL-12/23p40 (Fig. 5c). We observed no difference between WT and TNFR1–/– mice in the percentage of IL-12/23p40-positive cells, or the percentage of macrophages or microglia that were IL-12/23p40+. However, TNFR1–/– macrophages in the CNS produced more IL-12/23p40 per cell than WT macrophages (indicated by higher mean fluorescent intensity (MFI), Fig. 5(b). A similar trend was seen for microglia, although this did not reach significance. As mentioned above, IL-12/23p40 is a subunit of IL-23, which can induce IL-17. We investigated IL-17 expression in TNFR1–/– mice with EAE, but found that consistent with our co-culture data, IL-17 was not significantly affected by TNFR1–/– deficiency. There was a trend towards enhanced IL-17 mRNA expression in the spinal cord of TNFR1–/– mice with EAE compared to WT mice, but this did not reach significance (Fig. 5d). Similarly, there was no difference in the percentage or number of IL-17-secreting T cells in the CNS between TNFR1–/– mice and WT mice with EAE (data not shown).
Figure 5.
Expression of IL-12/23p40 in the central nervous system (CNS) of mice with experimental autoimmune encephalomyelitis (EAE). (a) Interleukin (IL)-12/23p40 and (d) IL-17 mRNA expression was assessed by real-time polymerase chain reaction (PCR) in spinal cords from wild-type (WT) (black bars) and tumour necrosis factor receptor (TNFR1)–/– (grey bars) unmanipulated mice (naive) or mice with grade 2 or grade 4 EAE. Values are expressed as arbitrary units normalized to 18S expression, mean ± SEM, n = 4 mice. *P < 0·05. (b, c, e) CNS cells pooled from brain and spinal cord were prepared from individual WT or TNFR1–/– mice with EAE and cultured in the presence of Brefeldin A for 5 hr. Cells were labelled for cell surface markers and intracellular IL-12/23p40 or IL-17 and analysed by flow cytometry. (b) The mean fluorescence intensity of IL-12/23p40+ macrophages (CD11b+, CD45hi) and microglia (CD11b+, CD45lo) was determined in cells prepared from WT (black bars, n = 6) or TNFR1–/– (grey bars, n = 7) mice with grade 2 or grade 4 EAE. Results are shown as mean ± SEM. ***P < 0·001. (c) Representative flow cytometry profile shows CD45 and IL12/23p40 expression in CD11b+ live cells. (e) Representative flow cytometry profile shows T cell receptor (TCR)β and IL-17 expression in CD45hi live cells prepared from WT mice. ND: not detected, NT: not tested, as TNFR1–/– mice did not develop disease more severe than grade 2; NS: no significant difference between WT and TNFR1–/– groups.
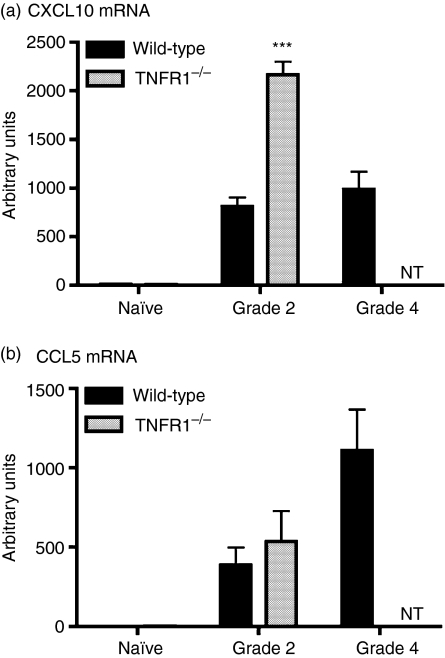
TNFR1–/– mice express higher levels of an IFN-γ-inducible chemokine
Our results describe two striking features of EAE in TNFR1–/– mice: increased expression of IFN-γ and the presence of more T cells in the CNS, compared to WT mice. We therefore investigated expression of two T cell-attracting chemokines that can be regulated by IFN-γ, and are normally strongly up-regulated in EAE: CCL5 [regulated upon activation, normal, T cell expressed and secreted (RANTES)] and CXCL10 (IP-10).28,43,44 As shown in Fig. 6, mRNA for both chemokines was expressed at very low levels in the spinal cords of unmanipulated WT and TNFR1–/– mice, but was strongly up-regulated during EAE. However, there was a much greater increase in CXCL10 mRNA expression in TNFR1–/– mice with EAE (grade 2) than in WT mice with either grade 2 or grade 4 disease. This difference was not seen for CCL5. These data suggest a selective effect of release of IFN-γ from TNFR1-mediated regulation in the CNS.
Figure 6.
CXCL10 mRNA expression in the spinal cords of mice with experimental autoimmune encephalomyelitis (EAE) (a) CXCL10 and (b) CCL5 mRNA expression was assessed by real-time polymerase chain reaction (PCR) in spinal cords of wild-type (WT) (black bars) and tumour necrosis factor receptor (TNFR1)–/– (grey bars) unmanipulated mice (naive) or mice with grade 2 or grade 4 disease. Values are expressed as arbitrary units normalized to 18S expression, mean ± SEM, n = 4 mice. ***P < 0·001. NT: not tested, as TNFR1–/– mice did not develop disease more severe than grade 2. Note that both CXL10 and CCL5 mRNAs were expressed at very low levels in the spinal cords of naive mice.
Discussion
In this study, we describe the unexpected finding that TNFR1–/– mice with EAE have more IFN-γ-producing T cells in the CNS than WT mice, despite displaying a milder EAE phenotype (delayed onset of disease, symptoms never advancing beyond hind limb weakness). This is accompanied by increased T cell infiltration, enhanced IL-12/23p40 expression by CNS APCs and up-regulation of CXCL10. It is important to note that, throughout this study, TNFR1–/– mice were compared with WT mice both of equivalent grade (grade 2, killed before TNFR1–/– mice) and of higher grade (grade 4, killed on the same day as TNFR1–/– mice). Thus, the severity of disease and the duration of symptoms are compared.
The presence of more T cells is in agreement with previous reports that TNFR1–/– mice with EAE have more inflammatory infiltrates in the CNS.25,41 There was no evidence of abnormal or impaired T cell migration, e.g. in restricted localization to perivascular cuffs. Similarly, a previous study on TNF–/– mice has described unimpaired migration of T cells into the CNS during EAE.28 Gimenez and colleagues described delayed but unimpaired T cell trafficking to the CNS, but impaired migration into the parenchyma.29 Their study used an adoptive transfer model of EAE to which TNFR1–/– mice were nearly completely resistant. In contrast, we used an active model of EAE that induced disease in TNFR1–/– mice, albeit with milder disease, making it easier for us to compare symptomatic WT and TNFR1–/– mice. It is likely that the different disease models used could explain the differences in observed T cell migration between our study and the Gimenez study.29
The observation that IFN-γ levels are greater in mice with a milder EAE phenotype is unexpected, because it is widely assumed that IFN-γ levels correlate with EAE severity. IFN-γ is not expressed in the healthy CNS, but is strongly expressed in the CNS during EAE (our data2,3). Changes in IFN-γ expression during the disease indicate disease progression, with levels increasing towards peak disease then decreasing as disease remits.2,3 What our data show is that during ongoing inflammation, the level of IFN-γ expression does not necessarily correlate with disease severity.
Our data provide evidence for at least two potential mechanisms acting in the TNFR1-deficient CNS that contribute to elevated IFN-γ levels. First, TNFR1–/– APCs have an enhanced IFN-γ-inducing effect. Perivascular APCs at the blood–brain barrier are critical for disease initiation45 and regulate IFN-γ secretion by the infiltrating antigen-specific T cells. Infiltrating TNFR1–/– macrophages and resident microglia would also provide continuing enhanced stimulation once immune cells begin to infiltrate the CNS. Secondly, increased CXCL10 expression induced by increased levels of IFN-γ would recruit more T cells, creating a positive feedback loop that results in increased T cell numbers. Our observation of altered expression of CXCL10, but not CCL5, in TNFR1–/– mice with EAE is likely to reflect the relative responsiveness of these genes, and perhaps different cell types, to IFN-γ. CXCL10 [‘IFN-inducible protein-10 (IP-10)’] is up-regulated early in EAE, and is expressed primarily by astrocytes adjacent to lesions.43,44 CCL5 can be up-regulated by proinflammatory cytokines such as IL-1 and TNF, as well as IFN-γ,46 and is expressed mainly by T cells. CCL5 expression parallels infiltration of the CNS by immune cells.47 Another factor that may be relevant is T cell life span. Bachmann and colleagues described reduced T cell apoptosis in the CNS of TNFR1–/– mice with EAE compared to WT controls.41 The absence of this TNF-mediated apoptosis for limiting and resolving inflammatory T cell responses may mean that IFN-γ-expressing TNFR1–/– T cells survive longer than WT T cells. IL-17 expression was not increased significantly in the CNS of TNFR1–/– mice with EAE, despite more T cells being present. This supports our hypothesis that the higher IFN-γ levels are not due simply to the presence of more T cells, but are up-regulated by a specific mechanism. Consistent with this, TNFR1–/– APCs induced greater secretion of IFN-γ but not IL-17 in vitro.
In vivo, it is not clear which TNFR1–/– cell type is responsible for the observed differences in TNFR1–/– mice. The critical step could be due to enhanced effects of TNFR1–/– APCs early in the process, or later due to a lack of regulatory feedback. Alternatively, the lack of TNFR1 on T cells could be key, e.g. through reduced apoptosis, although our in vitro data indicate a clear impact of TNFR1–/– APCs on T cell function. Another possibility is that compensatory mechanisms have developed in TNFR1–/– mice due to the permanent deficiency in this receptor.
Our observation that TNFR1–/– APCs produce more IL-12/23p40 than WT APCs is consistent with previous studies describing the ability of TNF to inhibit IL-12/23p40 gene expression in macrophages through TNFR1.42,48,49 This contrasts with another study that showed inhibition of IL-12/23p40 expression with a soluble TNFR2-IgG1 fusion protein, when human microglia were stimulated with lipopolysaccharide (LPS), but not when stimulated with LPS + IFN-γ.50 However, it seems clear that under certain conditions, TNF down-regulates IL-12/23p40 expression. Therefore, the increase in IL-12/23p40 expression is due probably to the absence of a TNFR1-mediated regulatory mechanism. The significance of elevated IL-12/23p40 is not clear. The IL-12/23p40 subunit forms part of IL-12, IL-23 or can form p40 homodimers. We observed reduced IL-12p35 mRNA expression in co-cultures containing TNFR1–/– APCs and no difference in IL-23p19 mRNA expression. This suggests an excess of IL-12/23p40, which could form homodimers. The actions of this molecule are still unclear, as it has been proposed to act as an antagonist to IL-12 or to actively induce macrophage recruitment, through actions at IL-12Rβ1.51–53 Our data show that TNFR1–/– APCs induce T cells to secrete more IFN-γ, but not IL-17, than WT APCs in vitro. These data suggest enhanced IL-12 actions in our co-cultures rather than IL-23, and are not consistent with increased IL-12/23p40 antagonist actions. It may therefore be that IL-12p35 mRNA expression is not limiting in these cultures, or at these time-points, so that increased IL-12/23p40 expression can result in more IL-12 activity.
The remaining enigma is that, despite this apparent amplification of the T cell immune response, TNFR1–/– mice do not develop severe disease. One possibility is that IFN-γ exerts a protective effect. Studies on EAE in rodents have shown that inhibition or a lack of IFN-γ exacerbates EAE, and can make resistant strains susceptible to disease.8–10,54 This suggests that IFN-γ may fulfil some protective function during disease. Several mechanisms for this have been proposed, including induction of cytokine inhibitors, suppression of cytokine release, nitric oxide-mediated suppression of effector cells and promotion of caspase-mediated apoptosis of APCs (for review, see11). However, it is unlikely that IFN-γ is providing the only protective mechanism. TNF mediates numerous processes important in EAE pathogenesis that the absence of TNFR1 is likely to impair, e.g. damage to oligodendrocytes and myelin, immune cell infiltration of the CNS through regulation of chemokines and adhesion molecules.29,55,56 The key role of IL-17 in EAE pathogenesis is probably mediated at least in part via TNF actions on TNFR1.16,17 Vascular cell adhesion molecule (VCAM)-1 expression on CNS vessels is regulated by TNF and TNFR1-dependent VCAM-1 expression on astrocytes is involved in T cell migration in the CNS in EAE.29,32,57 In the absence of TNFR1, there may be enhanced signalling through TNFR2. TNFR2 is thought to mediate protective actions, including oligodendrocyte regeneration and suppression of microglial activation.58,59 Thus, the mild EAE phenotype of TNFR1–/– mice may be due to an altered balance between detrimental and protective mechanisms.
In summary, we have demonstrated that TNFR1–/– mice with EAE have more IFN-γ-secreting T cells in the CNS than WT mice. TNFR1–/– APCs express more IL-12/23p40, and induce T cells to secrete more IFN-γ. Our data indicate that IFN-γ levels are regulated via TNFR1 and do not necessarily correlate with disease severity.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research and the Multiple Sclerosis Society of Canada. The authors would like to thank Maria Caruso for animal breeding and screening, Leah Remington for technical assistance and Nathalie Arbour for advice on flow cytometry. This work was supported by the Canadian Institutes of Health Research and the Multiple Sclerosis Society of Canada.
Abbreviations
- APC
antigen-presenting cell
- CFA
complete Freund's adjuvant
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- FCS
fetal calf serum
- HBSS
Hanks's buffered saline solution
- IFN-
interferon
- IL
interleukin
- MFI
mean fluorescent intensity
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- TCR
T cell receptor
- TNFR1
type 1 tumour necrosis factor receptor
- WT
wild-type
References
- 1.Owens T, Wekerle H, Antel J. Genetic models for CNS inflammation. Nat Med. 2001;7:161–6. doi: 10.1038/84603. [DOI] [PubMed] [Google Scholar]
- 2.Juedes AE, Hjelmstrom P, Bergman CM, Neild AL, Ruddle NH. Kinetics and cellular origin of cytokines in the central nervous system: insight into mechanisms of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2000;164:419–26. doi: 10.4049/jimmunol.164.1.419. [DOI] [PubMed] [Google Scholar]
- 3.Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944–53. [PubMed] [Google Scholar]
- 4.Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–10. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- 5.Hofman FM, Hinton DR, Baemayr J, Weil M, Merrill JE. Lymphokines and immunoregulatory molecules in subacute sclerosing panencephalitis. Clin Immunol Immunopathol. 1991;58:331–42. doi: 10.1016/0090-1229(91)90124-s. [DOI] [PubMed] [Google Scholar]
- 6.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 7.Duong TT, St Louis J, Gilbert JJ, Finkelman FD, Strejan GH. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J Neuroimmunol. 1992;36:105–15. doi: 10.1016/0165-5728(92)90042-j. [DOI] [PubMed] [Google Scholar]
- 8.Duong TT, Finkelman FD, Singh B, Strejan GH. Effect of anti-interferon-gamma monoclonal antibody treatment on the development of experimental allergic encephalomyelitis in resistant mouse strains. J Neuroimmunol. 1994;53:101–7. doi: 10.1016/0165-5728(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 9.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–6. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 10.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–7. [PubMed] [Google Scholar]
- 11.Muhl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol. 2003;3:1247–55. doi: 10.1016/S1567-5769(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 12.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–14. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 15.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 17.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Theraprutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–30. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumour necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–12. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selmaj K, Raine CS, Cannella B, Brosnan CF. Identification of lymphotoxin and tumour necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991;87:949–54. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharief MK, Hentges R. Association between tumour necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–72. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- 21.Bazzoni F, Beutler B. The tumour necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–25. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 22.Hehlgans T, Mannel DN. The TNF–TNF receptor system. Biol Chem. 2002;383:1581–5. doi: 10.1515/BC.2002.178. [DOI] [PubMed] [Google Scholar]
- 23.Riminton D, Korner H, Strickland DH, Lemckert FA, Pollard JD, Sedgwick JD. Challenging cytokine redundancy: inflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumour necrosis factor-deficient, mice. J Exp Med. 1998;187:1517–28. doi: 10.1084/jem.187.9.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willenborg DO, Fordham SA, O'Brien NC, Cowden WB, Ramshaw IA. Tumour necrosis factor-alpha and lymphotoxin-alpha in the pathology of experimental autoimmune encephalomyelitis: is either one responsible or is there another ligand-mediating disease? Res Immunol. 1998;149:804–10. doi: 10.1016/s0923-2494(99)80008-x. discussion 49–50, 55–60. [DOI] [PubMed] [Google Scholar]
- 25.Eugster HP, Frei K, Bachmann R, Bluethmann H, Lassmann H, Fontana A. Severity of symptoms and demyelination in MOG-induced EAE depends on TNFR1. Eur J Immunol. 1999;29:626–32. doi: 10.1002/(SICI)1521-4141(199902)29:02<626::AID-IMMU626>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Kassiotis G, Pasparakis M, Kollias G, Probert L. TNF accelerates the onset but does not alter the incidence and severity of myelin basic protein-induced experimental autoimmune encephalomyelitis. Eur J Immunol. 1999;29:774–80. doi: 10.1002/(SICI)1521-4141(199903)29:03<774::AID-IMMU774>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Suvannavejh GC, Lee HO, Padilla J, Dal Canto MC, Barrett TA, Miller SD. Divergent roles for p55 and p75 tumour necrosis factor receptors in the pathogenesis of MOG (35–55)-induced experimental autoimmune encephalomyelitis. Cell Immunol. 2000;205:24–33. doi: 10.1006/cimm.2000.1706. [DOI] [PubMed] [Google Scholar]
- 28.Murphy CA, Hoek RM, Wiekowski MT, Lira SA, Sedgwick JD. Interactions between hemopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. J Immunol. 2002;169:7054–62. doi: 10.4049/jimmunol.169.12.7054. [DOI] [PubMed] [Google Scholar]
- 29.Gimenez MA, Sim JE, Russell JH. TNFR1-dependent VCAM-1 expression by astrocytes exposes the CNS to destructive inflammation. J Neuroimmunol. 2004;151:116–25. doi: 10.1016/j.jneuroim.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB. An antibody to lymphotoxin and tumour necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193–200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selmaj K, Raine CS, Cross AH. Anti-tumour necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991;30:694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- 32.Barten DM, Ruddle NH. Vascular cell adhesion molecule-1 modulation by tumour necrosis factor in experimental allergic encephalomyelitis. J Neuroimmunol. 1994;51:123–33. doi: 10.1016/0165-5728(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 33.Korner H, Goodsall AL, Lemckert FA, Scallon BJ, Ghrayeb J, Ford AL, Sedgwick JD. Unimpaired autoreactive T-cell traffic within the central nervous system during tumour necrosis factor receptor-mediated inhibition of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 1995;92:11066–70. doi: 10.1073/pnas.92.24.11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selmaj KW, Raine CS. Experimental autoimmune encephalomyelitis: immunotherapy with anti-tumour necrosis factor antibodies and soluble tumour necrosis factor receptors. Neurology. 1995;45:S44–9. doi: 10.1212/wnl.45.6_suppl_6.s44. [DOI] [PubMed] [Google Scholar]
- 35.Klinkert WE, Kojima K, Lesslauer W, Rinner W, Lassmann H, Wekerle H. TNF-alpha receptor fusion protein prevents experimental auto-immune encephalomyelitis and demyelination in Lewis rats: an overview. J Neuroimmunol. 1997;72:163–8. doi: 10.1016/s0165-5728(96)00183-x. [DOI] [PubMed] [Google Scholar]
- 36.Shanahan JC, St Clair W. Tumour necrosis factor-alpha blockade: a novel therapy for rheumatic disease. Clin Immunol. 2002;103:231–42. doi: 10.1006/clim.2002.5191. [DOI] [PubMed] [Google Scholar]
- 37.Lenercept Multiple Sclerosis Study Group and University of British Columbia MS/MRI Analysis Group. TNF neutralization in MS. results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457–65. [PubMed] [Google Scholar]
- 38.Pfeffer K, Matsuyama T, Kundig TM, et al. Mice deficient for the 55 kD tumour necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–67. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 39.Poudrier J, Owens T. CD54/intercellular adhesion molecule 1 major histocompatibility complex II signalling induces B cells to express interleukin 2 receptors and complements help provided through CD40 ligation. J Exp Med. 1994;179:1417–27. doi: 10.1084/jem.179.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–68. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 41.Bachmann R, Eugster HP, Frei K, Fontana A, Lassmann H. Impairment of TNF-receptor-1 signaling but not fas signaling diminishes T-cell apoptosis in myelin oligodendrocyte glycoprotein peptide-induced chronic demyelinating autoimmune encephalomyelitis in mice. Am J Pathol. 1999;15:1417–22. doi: 10.1016/S0002-9440(10)65395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma X, Sun J, Papasavvas E, et al. Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J Immunol. 2000;164:1722–9. doi: 10.4049/jimmunol.164.4.1722. [DOI] [PubMed] [Google Scholar]
- 43.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol. 1997;150:617–30. [PMC free article] [PubMed] [Google Scholar]
- 44.Ransohoff RM, Hamilton TA, Tani M, et al. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 45.Greter M, Heppner FL, Lemos MP, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–34. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 46.Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–53. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- 47.Miyagashi R, Kikuchi S, Takayama C, Inoue Y, Tashiro K. Identification of cell types producing RANTES, MIP-1alpha and MIP-1beta in rat experimental autoimmune encephalomyelitis by in situ hybridisation. J Neuroimmunol. 1997;77:17–26. doi: 10.1016/s0165-5728(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 48.Hodge-Dufour J, Marino MW, Horton MR, et al. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumour necrosis factor. Proc Natl Acad Sci USA. 1998;95:13806–11. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zakharova M, Ziegler HK. Paradoxical anti-inflammatory actions of TNF-alpha: inhibition of IL-12 and IL-23 via TNF receptor 1 in macrophages and dendritic cells. J Immunol. 2005;175:5024–33. doi: 10.4049/jimmunol.175.8.5024. [DOI] [PubMed] [Google Scholar]
- 50.Becher B, Dodelet V, Fedorowicz V, Antel JP. Soluble tumour necrosis factor receptor inhibits interleukin 12 production by stimulated human adult microglial cells in vitro. J Clin Invest. 1996;98:1539–43. doi: 10.1172/JCI118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Germann T, Rude E, Mattner F, Gately MK. The IL-12 p40 homodimer as a specific antagonist of the IL-12 heterodimer. Immunol Today. 1995;16:500–1. doi: 10.1016/0167-5699(95)80035-2. [DOI] [PubMed] [Google Scholar]
- 52.Russell TD, Yan Q, Fan G, Khalifah AP, Bishop DK, Brody SL, Walter MJ. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J Immunol. 2003;171:6866–74. doi: 10.4049/jimmunol.171.12.6866. [DOI] [PubMed] [Google Scholar]
- 53.Shimozato O, Ugai S, Chiyo M, et al. The secreted form of the p40 subunit of interleukin (IL)-12 inhibits IL-23 functions and abrogates IL-12-mediated antitumour effects. Immunology. 2006;117:22–8. doi: 10.1111/j.1365-2567.2005.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 55.Korner H, Lemckert FA, Chaudhri G, Etteldorf S, Sedgwick JD. Tumour necrosis factor blockade in actively induced experimental autoimmune encephalomyelitis prevents clinical disease despite activated T cell infiltration to the central nervous system. Eur J Immunol. 1997;27:1973–81. doi: 10.1002/eji.1830270822. [DOI] [PubMed] [Google Scholar]
- 56.Selmaj KW, Raine CS. Tumour necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–46. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- 57.Vajkoczy P, Laschinger M, Eugelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–65. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 59.Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumour necrosis factor receptors in rat glia. J Neuroimmunol. 1997;75:104–12. doi: 10.1016/s0165-5728(97)00009-x. [DOI] [PubMed] [Google Scholar]