Abstract
In the present study, we have analysed the detailed cellular immune mechanisms involved in tumour rejection in carcinoembryonic antigen (CEA) transgenic mice after immunization with dendritic cells (DC) pulsed with an anti-idiotype (Id) antibody, 3H1, which mimics CEA. 3H1-pulsed DC vaccinations resulted in induction of CEA specific cytotoxic T lymphocyte (CTL) responses in vitro and the rejection of CEA-transfected MC-38 murine colon carcinoma cells, C15, in vivo (Saha et al.,Cancer Res 2004; 64: 4995–5003). These CTL mediated major histocompatibility complex (MHC) class I-restricted tumour cell lysis, production of interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), and expression of Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL) in response to C15 cells. CTL used perforin-, FasL-, and TRAIL-mediated death pathways to lyse C15 cells, although perforin-mediated killing was the predominant lytic mechanism in vitro. The cytokines IFN-γ and TNF-α synergistically enhanced surface expression of Fas, TRAIL receptor, MHC class I and class II on C15 cells that increased the sensitivity of tumour cells to CTL lysis. CTL activity generated in 3H1-pulsed DC immunized mice was directed against an epitope defined by the idio-peptide LCD-2, derived from 3H1. In vivo lymphocyte depletion experiments demonstrated that induction of CTL response and antitumour immunity was dependent on both CD4+ and CD8+ T cells. The analysis of splenocytes of immunized mice that had rejected C15 tumour growth revealed up-regulated surface expression of memory phenotype Ly-6C and CD44 on both CD4+ and CD8+ T cells. The adoptive transfer experiments also suggested the role of both CD4+ and CD8+ T cells in this model system. Furthermore, mice that had rejected C15 tumour growth, developed tumour-specific immunological memory.
Keywords: dendritic cells, vaccination, T cells, tumour immunity, transgenic mice
Introduction
Certain anti-idiotype (Id) antibodies that bind to the antigen-combining sites of antibodies can effectively mimic the three-dimensional structures and functions of the external antigens and can be used as surrogate antigens for active specific immunotherapy. Several monoclonal anti-Id antibodies, which mimic distinct human tumour-associated antigens (TAAs) have been developed and characterized by others and us (reviewed in 1). The greatest challenge in immunotherapy by means of anti-Id vaccines is to identify the optimal anti-Id antibody (Ab2β) that will function as a true surrogate antigen for a TAA system and ideally will generate both humoral and cellular immune responses. In most preclinical and clinical studies, immunizations with anti-Id antibody, however, have elicited humoral immunity only and has not induced human leucocyte antigen (HLA) class I antigen restricted TAA-specific cytotoxic T lymphocyte (CTL) response. This is because of the requirement of association of heavy and light chains of anti-Id antibodies for the TAA mimicry in most of the antigenic system analysed. In general, the antigen mimicry by anti-Id antibodies reflects structural homology in majority of the cases. In few cases, the anti-Id antibody that mimics the TAA shows linear amino acid sequence homology with the nominal tumour antigen.2–5 Idio-peptides, which are derived from the heavy and/or light chains of an anti-Id antibody, may be presented by HLA class I antigens to CTL. These particular anti-Id vaccines were capable of inducing both CD4+ T helper activity as well as CD8+ cytolytic activity.2–10 However, little attention has been paid to the detailed mechanism involved in CTL responses after anti-Id vaccination either in animal model or in patients.
We have described a murine monoclonal anti-Id antibody, designated 3H1,11 which mimics a distinct and specific epitope of carcinoembryonic antigen (CEA) over expressed on human colorectal carcinoma (CRC) and other adenocarcinomas. Anti-Id 3H1 can be used as a surrogate for CEA and induced anti-CEA immunity in different species of animals as well as humans and showed promise as a potential vaccine candidate in the phase I/II clinical trials of CRC patients. Anti-Id 3H1 could easily break immune tolerance to CEA and induced high-titre anti-CEA antibody as well as CD4+ T helper 1 (Th1) responses in CRC patients.12–14 In our clinical studies, 3H1 was injected after absorption onto alum, which we believe favours the humoral immune responses. A similar phenomenon was observed when the efficacy of 3H1 as a tumour vaccine was evaluated in a murine tumour model.15 Recently we have reported that immunizations of CEA transgenic (CEA-Tg) mice with 3H1-pulsed DC could reverse CEA unresponsiveness and result in induction of CEA-specific humoral and cellular immune responses and the rejection of CEA cDNA-plasmid transfected MC-38 murine colon carcinoma cells, C1516 in nearly 100% of experimental mice.17
In the present study, we have dissected the cellular mechanisms involved in these events and provided convincing evidence to support the notion that TAA-specific cytotoxic T-cell responses could be generated in CEA-Tg mice after vaccination with DC pulsed with anti-Id antibody, 3H1. The sensitivity of C15 cells to CTL lysis was mediated by, granule, FasL, and tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-dependent pathways. We have also identified a peptide from 3H1 (designated LCD-2) that induces CTLs recognizing CEA positive colon carcinoma cells in a major histocompatibility complex (MHC) class I-restricted fashion. 3H1-pulsed DC immunized mice were immune to tumour rechallenge and adoptive transfer experiments also suggested the induction of long-lived memory response. To the best of our knowledge, this is the first report documenting the details of the mechanisms of T-cell mediated immunity invoked by an anti-Id vaccine in a clinically relevant animal model.
Materials and methods
Mice and cell lines
CEA-Tg mice [C57BL/6J-TgN(CEAGe)18FJP], male and female, were obtained from Dr F. James Primus and have been described previously.16,17 For in vivo studies all mice were used at 6–8 weeks of age. The mice were treated in accordance with Institutional Animal Care and Use Committee guidelines.
The murine chemically induced colon carcinoma cells, MC-38 (C57BL/6, H-2b), and the human CEA-transfected MC-38 clone (C15-4.3) have been described previously.16 EL4 (thymoma, H-2b), B16-F10 (melanoma, H-2b), RMA (lymphoma, H-2b), RMA-S (lymphoma, H-2b– TAP2 deficient), SCC VII (squamous cell carcinoma, H-2k), AG104A (fibrosarcoma, H-2k), TSA (mammary adenocarcinoma, H-2d), and murine natural killer (NK)-sensitive YAC-1 (lymphoma, H-2a) cells were maintained in tissue culture in Dulbecco's modified Eagle's minimal essential medium or RPMI-1640 medium.
Anti-Id vaccine and immunization
Generation, purification, and characterization of anti-Id monoclonal antibody (mAb) 3H1, designated as CeaVac have been described previously.11 Isotype matched control anti-Id mAb 1A718 was also used in this study. Mature DC were generated from bone marrow and were pulsed with 3H1 or 1A7 as described previously.17 After loading, DC were injected subcutaneously (s.c.) in the lower right flank of syngeneic mice and boosted twice on every other week as described.17
Cytotoxicity assays
Assays were performed according to the standard protocols.19 Mice were killed 2 weeks after the third immunization and lymphocytes were isolated from harvested spleen of three mice per group. 3H1-specific CTL were generated as described previously,17 and the CTL activity was determined by a 6–8 hr 51Cr release assay using a variety of target cell lines. Cytotoxicity assays of 6–8 hr were used in preference to standard 4 hr assays which, although adequate for granule-mediated killing, tend to underestimate death receptor-mediated lysis. For inhibition experiments, various monoclonal antibodies (mAbs) were added to the culture at a final concentration of 10 µg/ml. Isotype-matched mAbs were used as control. For the inhibition of perforin-dependent cytotoxicity, CTL were pretreated with concanamycin A (CMA, Sigma-Aldrich, St. Louis, MO) for 2 hr at 500 nm, and then cytotoxicity assays were performed in the continuous presence of CMA. All antibodies used in inhibition experiments were purchased from BD PharMingen (San Diego, CA). In select experiments, purified CD4+ and CD8+ T cells were obtained from antigen-specific CTL culture by using magnetic beads (Miltenyi Biotec, Auburn, CA), and were used in cytotoxicity assays. Spontaneous release was <25% of maximum release.
Flow cytometric analysis
Cell surface antigens on C15 cells were analysed after overnight treatment with rm interferon-γ (rmIFN-γ; 103 U/ml) and/or rmTNF-α (250 U/ml; BD PharMingen) or media alone by flow cytometry using fluoroscein isothiocyanate (FITC)- or phycoerythrin (PE)-labelled mAbs reactive to H-2Kb MHC class I alloantigen, I-Ad and I-Ed MHC class II alloantigens, Fas, TRAIL-R2, or isotype-matched controls. Cells were also stained for surface expression of CEA using an anti-CEA mAb (8019), followed by incubation with a secondary antibody, FITC-labelled goat anti-mouse immunoglobulin (IgG; Southern Biotechnology, Birmingham, AL). After staining cells were washed twice and analysed in a flow cytometer as described previously.17
For the detection of FasL or TRAIL on CTL, CTL (2 × 106/ml) generated from immunized mice splenocytes were first incubated for 6 hr at 37° with media alone, untreated or IFN-γ and TNF-α treated C15 cells (2 × 105/ml), or phorbol 12-myristate 13-acetate (PMA; 20 ng/ml) plus ionomycin (1 µg/ml). Cells were then stained simultaneously with FITC-labelled anti-CD4 or anti-CD8 mAb, and PE-labelled anti-FasL or anti-TRAIL mAb, or isotype-matched controls. After washing, the cells were subjected to flow cytometric analysis. CTL were stained for perforin expression using an intracellular staining technique as described previously.17 Briefly, cells were stained with PE-labelled anti-CD4 or anti-CD8 mAb, washed, then fixed and permeabilized, followed by staining with anti-perforin antibodies (clone KM585; Kamiya Biomedical, Seattle, WA), washed, and then additionally incubated with a secondary antibodies, FITC-labelled goat anti-rat IgG (Southern Biotechnology). The cells were then washed twice and analysed. Cells from CTL culture were also stained and analysed for the surface expression of Ly-6C, CD44, CD122, and CD127 on CD4 and CD8 T cells. Anti-TRAIL-PE and anti-TRAIL-R2-PE mAbs were purchased from eBioscience (San Diego, CA) and all other mAbs were purchased from BD PharMingen.
N-a-benzyloxycarbonyl-l-lysine-thiobenzyl ester hydrochloride (BLT)-esterase release assay
CTL (2 × 105/well) generated from immunized mice splenocytes were incubated with untreated or IFN-γ and TNF-α treated C15 cells (2 × 104/well) at 37° for 6 hr. Cell-free supernatant was harvested and the BLT esterase activity of the supernatant was measured as described previously.20
Detection of Fas-induced cell death
Cell death was measured by 51Cr release assays. Briefly, C15 cells were treated overnight with IFN-γ and/or TNF-α or left untreated. Cells were then labelled with 51Cr and were analysed for Fas-mediated killing by incubation for 8–18 hr with anti-mouse Fas mAb (10 µg/ml, Jo2; BD PharMingen) and protein G (10 µg/ml; Amersham Pharmacia, Piscataway, NJ) to maximize cross-linking of anti-Fas mAb.
Proliferation assays
In vitro T-cell proliferation was measured by [3H]thymidine incorporation as described previously.17 Based on the amino acid sequence homology of 3H1 and CEA, several peptides were designed and synthesized.3 Peptide LCD-2 (TLIYRANRLIDGVP) and HFW-1 (GPELVKPGASL) were obtained from 3H1. Peptide CEA-B (PPAQYSWLIDGN) and CAP-1 (YLSGANLNL) were obtained from CEA. CAP-1 is a class I, HLA-A2 restricted CEA epitope that can induce human T cells for cytolytic activity against CEA-positive tumour cells. In select experiments, these peptides were used as stimulants at a concentration of 5 µg/well. Assays were performed in triplicate wells.
Peptide pulsing of RMA-S cells for CTL-mediated lysis
RMA-S cells were cultured with different peptides (100 µg/ml) for 18–20 hr at 37° and 5% CO2 in RPMI 1640 medium. Ovalbumin (OVA) peptide257−264 (SIINFEKL), which encodes an H-2Kb-restricted epitope,21 was used as control. Following incubation, cells were harvested, washed, and labelled with 51Cr for use as targets in CTL assay. Peptide pulsed RMA-S cells were also stained with FITC-labelled anti-mouse H-2Kb mAb and analysed for MHC class I antigen expression by flow cytometry.
In vivo depletion of immune cell subsets
Depletion studies were performed by intraperitoneal administration of anti-CD4 (GK1·5), anti-CD8 (2·43), anti-NK (PK136; ATCC, Manassas, VA), or isotype control antibody (rat IgG, Sigma). Mice were injected with 200 µg of antibody on days −3 and +3 of each immunization and 2 days before tumour cell challenge. Specific depletion was >85% as determined by flow cytometry (data not shown).
Prophylactic treatment in s.c. tumour model and rechallenge experiments
Groups of mice (n = 8) were immunized as described.17 Two weeks after the third immunization, mice were challenged with 1 × 106 of CEA-transfected murine colon carcinoma cells, C1516 or 5 × 105 of non-transfected parental MC-38 cells s.c. in the lower left flank. Mice were monitored on a regular basis for tumour growth and survival. 3H1-pulsed DC immunized mice that had rejected C15 tumour growth and survived longer than 90 days were also rechallenged with either C15, MC-38, or EL4 (1 × 105) tumour cells s.c. In all rechallenge experiments naïve CEA-Tg mice were included as controls and were challenged with the same tumour dose.
Adoptive transfer experiments
3H1-pulsed DC immunized mice that had rejected C15 tumour growth and survived longer than 90 days served subsequently as donors of lymphocytes for adoptive transfer experiments. Splenocytes were cultured for 5 days in presence of 3H1 and rhIL-2 as described and at the end of culture CD4+ and CD8+ T cells were isolated at >90% purity. Purified T cells or total lymphocytes were then transferred (4 × 106 cells/mouse) intravenously (i.v.) into naïve CEA-Tg mice. Adoptive transfer of in vitro stimulated naïve mice splenocytes served as control. After 1 day, each group of mice were challenged with 1 × 106 of C15 tumour cells s.c.
Statistical analysis
Statistical analyses were performed by Student's two-tailed t-test or non-parametric Mann–Whitney rank-sum test using SigmaStat software (Jandel, San Rafael, CA). The data are presented as mean ± SE. P < 0·05 was considered significant.
Results
Role of perforin, FasL, and TRAIL pathways in CTL-mediated cytotoxicity of C15 targets in vitro
Previously we have shown that 3H1-pulsed DC immunizations in CEA-Tg mice resulted in induction of CEA-specific effector CD8+ T cells capable of displaying cytotoxic activity against C15 tumour cells in an MHC class I-restricted manner.17 To better understand the potential contribution of antigen-specific CTL in tumour rejection in vivo, we first examined the CTL-mediated cytotoxicity and cytokine production in vitro in response to various cell lines (Table 1). CTL generated from 3H1-pulsed DC immunized mouse splenocytes showed specific lysis of C15 cells but not other syngeneic or allogeneic cell lines. In addition, increased amounts of IFN-γ and TNF-α were produced in response to C15 cells. These specific responses against C15 cells were mostly inhibited (P < 0·001) by anti-CD8 (53-6.7) or anti-H-2Kb/H-2Db (28-8-6) mAb, but not by either anti-CD4 (GK1.5) or anti-I-Ab (KH74) mAb (P > 0·06). The data clearly demonstrate the specificity for C15 cells and H-2Kb restriction of these CTL. Immunization of mice with DC alone generated some non-specific lytic activity (data not shown), which is typically seen with bone-marrow-derived DC as evidenced by previous studies.22
Table 1.
Cytotoxicity and cytokine production by CTL on coincubation with various cell lines1
| Tumour cells | Histology | MHC class I isotype control | mAb | Lysis (%) | IFN-γ (pg/ml) | TNF-α (pg/ml) | IL-4 (pg/ml) |
|---|---|---|---|---|---|---|---|
| C15 | Colon carcinoma | + | 48.7 ± 4.1 | 2000 ± 40* | 450 ± 30* | <20 | |
| Anti-CD4 | 40.0 ± 3.9 | 1260 ± 30 | 285 ± 15 | <20 | |||
| Anti-CD8 | 9.8 ± 1.8 | 360 ± 10 | 180 ± 15 | <20 | |||
| Anti-I-Ab | 38.9 ± 4.2 | 1190 ± 25 | 270 ± 15 | <0 | |||
| Anti-H-2Kb/H-2Db | 10.9 ± 2.1 | 425 ± 15 | 165 ± 15 | <0 | |||
| MC-38 | Colon carcinoma | + | 10.8 ± 2.4 | 1105 ± 25 | 180 ± 15 | <0 | |
| EL4 | Thymoma | + | 4.3 ± 2.0 | 1075 ± 20 | 135 ± 15 | <0 | |
| RMA | Lymphoma | + | 10.9 ± 2.6 | 1030 ± 25 | 150 ± 15 | <0 | |
| B16-F10 | Melanoma | – | 7.5 ± 2.1 | 990 ± 20 | 135 ± 15 | <0 | |
| SCC VII | Squamous cell carcinoma | + | 10.6 ± 3.0 | 885 ± 20 | 135 ± 15 | <0 | |
| AG104A | Fibrosarcoma | + | 2.3 ± 1.6 | 955 ± 20 | 150 ± 15 | <0 | |
| TSA | Mammary carcinoma | + | 5.2 ± 2.0 | 825 ± 15 | 165 ± 15 | <0 | |
| YAC-1 | Lymphoma | ND | 8.8 ± 2.8 | 1010 ± 20 | 150 ± 15 | <0 | |
| – | 1090 ± 25 | 165 ± 15 | <0 |
Cytotoxicity against various cell lines was measured in a 6 hr 51Cr release assay at an E : T ratio of 25 : 1. CTL were generated from 3H1-pulsed DC immunized mice as described. CTL (2 × 105/well) were incubated with tumour cells (2 × 104/well) or media alone in 96-well tissue culture plates at 37° for 6 hr. Cell-free supernatants were harvested and analysed for the production of IFN-γ, TNF-α, and IL-4 by standard ELISA kits.17 For inhibition experiments, blocking mAb was added to the culture at a concentration of 10 µg/ml. Surface MHC class I antigen expression was analysed by flow cytometry. The representative data of three independent experiments is shown.
P < 0.002 with respect to other culture conditions for IFN-γ and TNF-α production.
ND, not determined.
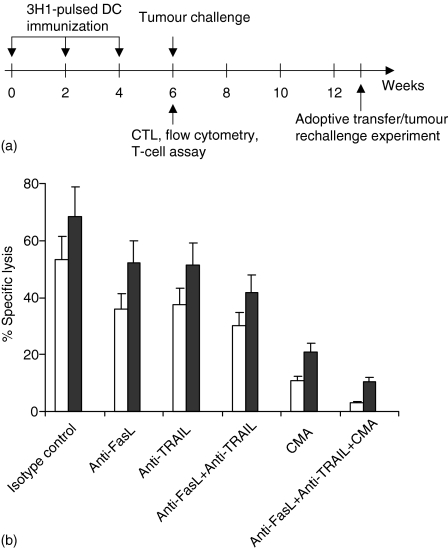
CTL showed cytotoxic activity against C15 cells in relatively short-term assay (6–8 hr) and the overall lytic activity did not increase significantly in long-term (18 hr) assay (e.g. at 25 : 1 ratio, 48·0 versus 52·9). However, if C15 cells were pretreated for overnight with IFN-γ and TNF-α before the cytotoxicity assay, these cells became more susceptible to CTL lysis in short-term assay (Fig. 1b, closed bars). Most of the CTL lysis was blocked (80% inhibition) in the presence of CMA, a potent inhibitor of perforin-mediated lysis,23 and the inhibition was significant (P < 0·005), suggesting a major effector role for granule-mediated lysis in vitro (Fig. 1b). To delineate the nature of the perforin-independent pathways, CTL activity was examined in the presence of neutralizing anti-FasL (MFL3) and anti-TRAIL (N2B2) mAbs. The inclusion of anti-FasL and/or anti-TRAIL mAb had a partial inhibitory effect (29·8–43·6%), suggesting that the perforin pathway was still the dominant effector mechanism under these in vitro assay conditions (Fig. 1b). However, the combination of CMA, anti-FasL mAb, and anti-TRAIL mAb exerted maximal inhibition of lysis compared with isotype-matched control mAb (P < 0·0004). Thus under conditions in which the perforin pathway was blocked, the contribution of the CTL-directed FasL and TRAIL pathways were more easily revealed. Taken together, these data suggest that maximal CTL-mediated lysis of C15 targets predominantly involved perforin-, FasL-, and TRAIL-based effector mechanisms.
Figure 1.
(a) Schematic diagram depicting immunization protocol and experiments. (b) Role of perforin, FasL, and TRAIL on CTL-mediated lysis of C15 tumour cells. Mice were immunized with 3H1-pulsed DC and CTL were generated from splenocytes as described in Materials and methods. Lytic activity of CTL was tested against untreated (open bars) or IFN-γ- and TNF-α-treated (closed bars) C15 cells by 51Cr release assay. The 8 hr cytotoxicity assays (E : T, 50 : 1) were performed in the presence of CMA (500 nm), neutralizing mAb (10 µg/ml), isotype-matched control, or combinations of these agents as indicated. A representative experiment of three is shown.
Fas and TRAIL-R2 expression by C15 tumour cells
The cytokines IFN-γ and TNF-α enhanced susceptibility of C15 cells to CTL lysis (Fig. 1b). Therefore, the effects of these cytokines on target cells were examined in more detail. C15 cells express low levels of Fas; however, treatment with IFN-γ or TNF-α up-regulated Fas expression, and the combination of these cytokines synergistically enhanced surface Fas expression on C15 cells (Table 2). Several studies have suggested that cell surface Fas expression on both mouse and human tumour cell lines can be enhanced by treatment with proinflammatory cytokines, namely, IFN-γ and/or TNF-α.24,25 The expression of TRAIL receptor 2 (TRAIL-R226); on C15 cells was also increased by treatment with TNF-α and the combination of IFN-γ with TNF-α. C15 cells express high endogenous levels of MHC class I. IFN-γ and the combination of IFN-γ with TNF-α further enhanced expression of this molecule on C15 cells. The surface expression of MHC class II was also enhanced to some extent with IFN-γ and/or TNF-α (Table 2). Functionally, treatment of C15 cells with either IFN-γ or TNF-α led to a moderate increase in sensitivity to Fas-mediated death. However, the combination of IFN-γ-? with TNF-α-sensitized C15 cells to Fas-mediated death more efficiently (data not shown). Similar results were observed with soluble TRAIL as a TRAIL-R2 stimulus (data not shown). Soluble TRAIL-induced apoptosis of tumour cell lines has also been reported in other studies.27
Table 2.
IFN-γ and TNF-α treatment of targets resulted increased expression of surface antigens1
| Cytokine treatment of C15 cells | ||||
|---|---|---|---|---|
| Surface antigen expression | None | IFN-γ | TNF-α | IFN-γ + TNF-α |
| CEA | 97.4 | 97.7 | 97.8 | 98.6 |
| MHC class I | 87.0 | 99.2 | 87.3 | 99.5 |
| MHC class II | 6.2 | 11.6 | 16.4 | 20.3 |
| Fas | 19.1 | 33.5 | 31.4 | 56.0 |
| TRAIL-R2 | 36.1 | 36.3 | 49.1 | 54.5 |
C15 cells were cultured overnight in the presence or absence of IFN-γ and/or TNF-α. These cells were then stained with FITC- or PE-labelled mAb or isotype-matched control, washed, and analysed in a flow cytometer. Results are presented as percentage of positive cells after subtraction of isotype control values. The representative data of three independent experiments is shown.
Interactions between CTL and cytokine-treated tumour cells resulted in enhanced granule exocytosis and up-regulated expression of FasL and TRAIL
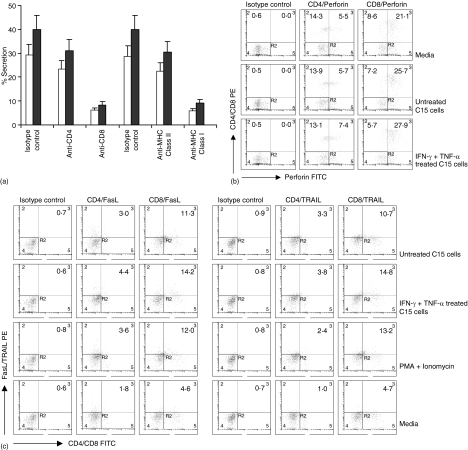
In vitro tumour cell lysis by CTL was mostly granule-mediated and cytokine treatment of tumour targets increased the extent of lysis (Fig. 1b). Therefore, we wanted to examine whether there was any direct correlation between granule exocytosis and tumour killing. Degranulation of CTL was estimated by the release of BLT esterase activity (Fig. 2a). BLT esterase is stored in the granules together with perforin/granzymes, and its secretion correlates with the exocytosis of lytic granules. Supernatants from the coculture of CTL and cytokines treated C15 cells showed higher levels of BLT esterase activity (closed bars) than those from CTL cocultured with untreated C15 cells (open bars). The BLT esterase activity was largely inhibited in presence of anti-CD8 or anti-MHC class I mAb and the inhibition was significant (P < 0·005) compared with isotype-matched control antibody. The perforin expression by CD8+ T cells from CTL culture indicated that IFN-γ- and TNF-α-treated C15 cells induced higher accumulation of intracellular perforin compared with untreated C15 cells (Fig. 2b).
Figure 2.
BLT esterase release activity and expression of perforin, FasL, and TRAIL by CTL in presence of various stimuli. Mice were immunized with 3H1-pulsed DC and CTL were generated from splenocytes as described. (a) CTL were incubated with untreated (open bars) or IFN-γ- and TNF-α-treated (closed bars) C15 cells for 6 hr in 96-well tissue culture plates in presence of neutralizing mAb or isotype-matched control (10 µg/ml). Next, the BLT activity in the supernatant was measured. (b and c) CTL were cocultured with untreated or IFN-γ- and TNF-α-treated C15 cells, media alone, or combination of PMA and ionomycin for 6 hr. These CTL were then stained and analyzed in a flow cytometer for the detection of intracellular accumulation of perforin (b) and surface expression of FasL or TRAIL (c) by CD4+ and CD8+ T cells. Results are presented as percentage of positive cells. (a–c) A representative experiment of three is shown.
Next, we investigated the expression of FasL and TRAIL on CTL at different culture conditions. FasL expression was up-regulated on CTL after 6 hr specific interaction with C15 cells (Fig. 2c). The expression of FasL was increased further when CTL were cocultured with IFN-γ- and TNF-α-treated C15 cells. FasL expression by CTL was also increased after stimulation with PMA and ionomycin. A similar phenomenon was observed when TRAIL expression on CTL was analysed (Fig. 2c).
Peptide-specific proliferative responses in immunized mice
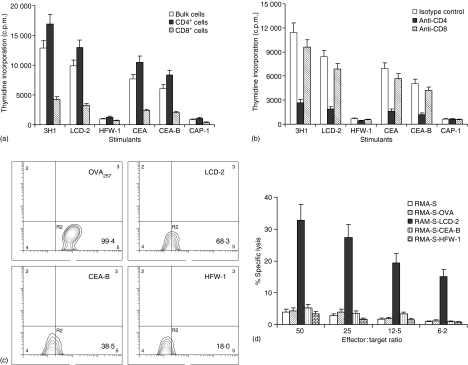
Immunization of mice with 3H1 can induce CEA-specific T-cell responses because of linear homology of amino acid sequence between 3H1 and CEA.3,5 We have found several regions of homology in 3H1 heavy and light chain variable domains, as well as in the framework regions. To search for potential cross-reactive T-cell epitopes, a number of peptides were synthesized based on 3H1 and CEA homology and two partially homologous peptides, designated as LCD-2 (from 3H1) and CEA-B (from CEA) containing the common sequence LIDG were identified.3 Therefore, we tested whether mice immunized with 3H1 had T cells that can recognize peptides from anti-Id mAb as well as nominal antigen CEA. As shown in Fig. 3(a), when incubated with LCD-2 or CEA-B peptide, bulk splenocyte populations and purified CD4+ cells proliferated significantly (P < 0·005), whereas CD8+ T cells were relatively unresponsive. Control peptides HFW-1 (from 3H1) and CAP-1 (from CEA) did not stimulate the proliferation. Furthermore, antigen-induced T-cell proliferation was inhibited significantly in the presence of anti-CD4 (P < 0·002) but not against anti-CD8 mAb (P > 0·06) (Fig. 3b).
Figure 3.
Peptide-specific proliferation and CD8+ T cell-mediated lysis of peptide pulsed RMA-S cells. (a and b) 3H1-pulsed DC immunized mice splenocytes were cultured in vitro in presence of peptides (LCD-2, HFW-1, CEA-B, and CAP-1), antigen (CEA), or anti-Id mAb (3H1) for 5 days and T-cell proliferation was measured by determining [3H]thymidine incorporation. Proliferation in the presence of media had been subtracted from values obtained in the presence of different stimulants. (a) CD4+ and CD8+ T cells were separated by magnetic-activated cell sorting micro beads as described. Macrophages isolated from splenocytes were added as antigen presenting cells in this assay. (b) Antibodies (10 µg/ml) were added at the beginning of culture. (c) RMA-S cells were cultured overnight in the presence or absence of different peptides. These cells were then stained with FITC-labelled anti-MHC class I mAb and analysed by flow cytometry. Results are presented as percentage of positive cells. (d) CTL were generated from 3H1-pulsed DC immunized mice splenocytes and lytic activity of purified CD8+ T cells was determined by 6 hr 51Cr release assay using peptide-pulsed RMA-S cells as targets. (a–d) A representative experiment of three is shown.
RMA-S cells pulsed with 3H1-derived peptide is target for antigen-specific MHC class I-restricted lysis
HLA motif analysis of cross-reacting peptides (LCD-2 and CEA-B) shows low but positive scores with H-2Kb molecules.28 To assess whether any of these peptide contain CTL epitope, peptide-pulsed RMA-S cells29 were used as targets for lysis by antigen-specific CTL. As shown in Fig. 3(c) 99·4% of RMA-S cells were positive for MHC class I expression when pulsed with OVA peptide (amino acids 257–264), whereas 68·3% and 38·5% cells expressed MHC class I molecules when pulsed with LCD-2 and CEA-B peptides, respectively. CD8+ T cells obtained from 3H1-pulsed DC immunized mice splenocytes could lyse target cells only when RMA-S cells were pulsed with LCD-2 peptide (Fig. 3d). The presence of CEA-B peptide on RMA-S cells was probably suboptimal for lysis by CTL. HFW-1 peptide, derived from 3H1 heavy chain framework region was used as a control in this assay system. These results confirm that CTL activity generated in 3H1-pulsed DC immunized mice was directed against the epitope defined by the LCD-2 peptide.
CD8+ as well as CD4+ T cells are required for DC-mediated antitumour immune response in vivo
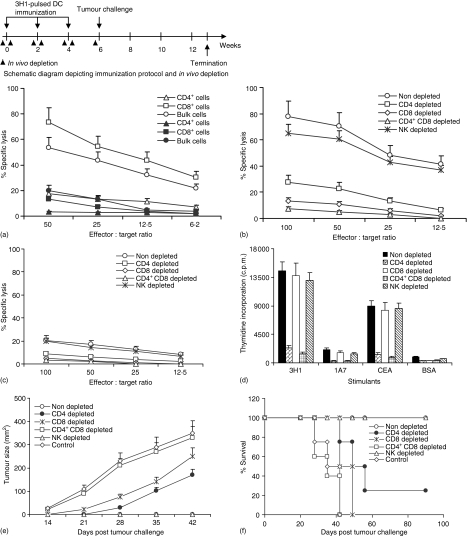
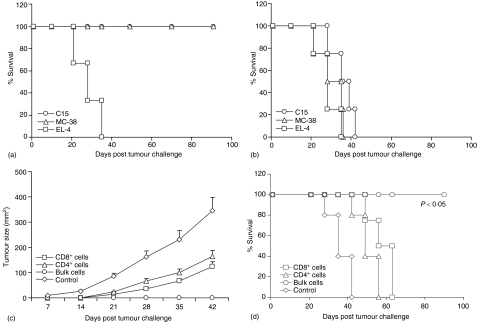
We next implemented in vivo depletion studies with anti-NK, anti-CD4, and/or anti-CD8 antibodies in order to assess the relative contribution of immune cell subsets in antitumour immunity. As shown in Fig. 4(a), in non-depleted immunized mice, in vitro lysis against C15 target cells was mostly mediated by purified CD8+ T cells (P < 0·007), whereas, CD4+ T cells did not display any significant cytolytic activity. However, in vivo depletion of CD4+ and/or CD8+ T cells led to significant reduction of antigen-specific in vitro cytolytic activity (P < 0·006), indicating that both T-cell subsets were required for antigen specific lytic activity, whereas in vivo depletion of NK cells had no effect in cytolysis (Fig. 4b, c). Interestingly, in vitro proliferation of splenocytes from depleted mice indicated that antigen-specific proliferation was mostly mediated by CD4+ T cells (Fig. 4d). Of note, all of the non-depleted or NK-depleted mice were protected from C15 tumour development, whereas control mice or mice depleted of both CD4+ and CD8+ T cells succumbed to death at the same time (Fig. 4e, f). Mice depleted of CD8+ T cells alone or mice depleted of CD4+ T cells alone had partial survival benefit. Therefore, these data suggest that neither T-cell subset alone was sufficient to confer complete antitumour immunity after immunization with 3H1-pulsed DC. This protection was specific to CEA-expressing tumours, as 3H1-pulsed DC immunized mice did not mount an antitumour response when inoculated with the parental MC-38 cells.17
Figure 4.
Involvement of T-cell subsets in the induction of immune response and rejection of C15 tumour cells in vivo. (a) CTL were generated from 3H1-pulsed DC immunized mice and cytotoxicity was measured by 6 hr 51Cr release assay using C15 (open symbol) and MC-38 (closed symbols) as target cells. (b–f) mice were depleted from CD4+ and/or CD8+ T cells, or NK cells during immunizations with 3H1-pulsed DC and before challenge with C15 tumour cells by repeated injections of specific mAbs as described in Materials and methods. CTL were generated from splenocytes and cytotoxicity was measured against C15 (b) and MC-38 (c) target cells. (d) Splenocytes were cultured in vitro in presence of anti-Id mAbs (3H1, 1A7), antigen (CEA), or BSA for 5 days and T-cell proliferation was measured by determining [3H]thymidine incorporation. Proliferation in the presence of media had been subtracted from values obtained in the presence of different stimulants. (e and f) Two weeks after the third immunization, mice were challenged with 1 × 106 of CEA-transfected C15 tumour cells s.c. Mice immunized with 1A7-pulsed DC and challenged with C15 tumour cells served as control. Tumour growth (e) and survival (f) were recorded over time. Mice were killed when tumours became ulcerated or when they reached a size >250 mm2 and survival was recorded accordingly. (a–f) Similar results were obtained in three independent experiments.
Long-lived memory T cells are maintained in mice that have rejected C15 tumour
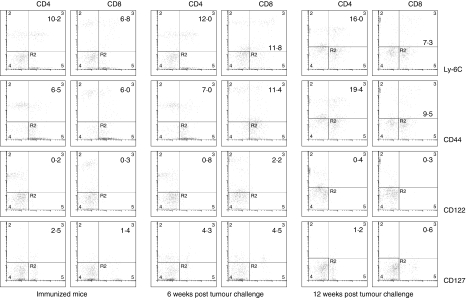
The goal of vaccination strategies is to elicit effective long-term immunity. Our findings show that immunization of CEA-Tg mice with 3H1-pulsed DC was effective at inducing CEA-specific antitumour immunity, which helped those mice to reject a lethal challenge of CEA-transfected tumour cells. We sought to investigate at different time points the expression of Ly-6C, CD44, and CD122, which are associated with memory T-cell populations.30–32 The data presented in Fig. 5 show that expression of Ly-6C and CD44 increased in tumour-rejected mice. The expression on CD4+ T cells was maximal at 12 weeks post tumour challenge, whereas the expression on CD8+ T cells was maximal at 6 weeks post tumour challenge. However, there was no difference of the CD122 (the β-subunit of interleukin (IL)-2 and IL-15 receptor) expression levels following immunization and tumour cells challenge. IL-7 has been implicated in the regulation of homeostasis and survival of CD8+ memory T cells in vivo33 and surface expression of CD127 (the α-subunit of IL-7 receptor) has been described as a marker for long-lived memory T cells.34 The analysis of CD127 expression on CD4+ and CD8+ T cells indicated two- to threefold increases at 6 weeks post tumour challenge (Fig. 5). However, the expression was diminished at later time points. Together, these results suggest that tumour rejected mice developed antigen-specific memory T cells as determined by higher expression of Ly-6C and CD44.
Figure 5.
CD4+ and CD8+ T cells up-regulated surface expression of memory phenotype Ly-6C, CD44, and CD127 following tumour challenge. Splenocytes harvested 2 weeks after the third immunization or 6 weeks and 12 weeks post tumour challenge were stimulated in vitro in the presence of 3H1 for 5 days. For the detection of surface expression of Ly-6C, CD44, or CD122 cells were stained simultaneously with FITC-labelled anti-Ly-6C, anti-CD44, or anti-CD122 mAb, and PE-labelled anti-CD4 or anti-CD8 mAb. For the detection of surface expression of CD127 cells were stained simultaneously with PE-labelled anti-CD127 mAb and FITC-labelled anti-CD4 or anti-CD8 mAb. Cells were also stained with respective isotype-matched controls. After staining cells were washed and analysed by flow cytometry. Results are presented as the percentage of positive cells. A representative experiment of three is shown.
Development of tumour-protective immunological memory
3H1-pulsed DC immunized mice that had rejected CEA-transfected C15 tumour remained tumour free for more than 90 days after challenge (Fig. 4f). To determine whether these mice developed tumour-protective long-term immunity in vivo, they were rechallenged with the same tumour cells used for the first challenge or with other syngeneic tumour cells. Mice cured from C15 tumour rejected subsequent challenges with the same tumour or non-transfected parental MC-38 and remained tumour free for more than 90 days when the experiment was terminated, while mice could not reject syngeneic unrelated tumour, EL4 (Fig. 6a). The results indicate that the tumour protection was mediated by persistent antitumour immunity specific for antigens relevant to C15 tumour. It is of interest that the tumour-free mice also had protective immunity against subsequent challenge with the parental MC-38 cells, suggesting that mice rejecting CEA-transfected carcinoma developed immunity to other antigens expressed on C15 and shared with the non-transfected parental MC-38 cell line, resulting in long-lasting memory against these tumours. Induction of immunity to shared tumour antigens has also been observed in other tumour models during tumour rejection after particular antigen immunization.35,36 All control CEA-Tg mice were previously untreated and died of progressive tumour growth, irrespective of challenge with C15, MC-38, or EL4 cells (Fig. 6b).
Figure 6.
3H1-pulsed DC immunized mice that had rejected C15 tumour cells developed tumour-specific long-lived memory response. (a) Surviving mice that had been immunized and challenged as described (Fig. 4) were rechallenged either with 1 × 106 of CEA-transfected C15, 5 × 105 of non-transfected parental MC-38, or 1 × 105 of syngeneic unrelated EL4 tumour cells s.c. 13 weeks after first tumour inoculation. (b) A set of age-matched naive CEA-Tg mice were also challenged either with C15, MC-38, or EL4 tumour cells and used as controls. Survival was recorded as described. (c and d) Adoptive transfer of immune splenocytes to prevent the growth of subsequent tumour challenge. 3H1-specific lymphocytes were generated from C15 tumour-free mice at 13 weeks of post tumour challenge as described in Materials and methods. Stimulated cells (4 × 106) were infused i.v. into naïve mice and on the next day mice were challenged with 1 × 106 of C15 tumour cells s.c. Tumour growth (c) and survival (d) were recorded over time. (a–d) Similar results were obtained in two independent experiments.
We finally investigated whether this immunological memory could be transferred into naïve (non tumour-bearing and untreated) adult CEA-Tg mice. Recipient mice were challenged with C15 tumour cells 1 day after the adoptive transfer of in vitro stimulated splenocytes. The data depicted in Fig. 6(c, d) indicate that adoptive transfer of total splenocytes from tumour-free mice resulted tumour protection in 100% mice and these mice remained tumour free until day 90, when the experiment was terminated. Whereas C15 tumour developed rapidly in control group of mice adoptively transferred with total splenocytes from naïve CEA-Tg mice, and all of these mice died within 42 days. Tumour growth was relatively slower in groups of mice where adoptive transfer was performed with either CD4+ or CD8+ T cells which resulted in partial protection. Thus, both tumour antigen-specific CD4+ and CD8+ T cells played an important role in the control of tumour growth in this murine model of colon carcinoma.
Discussion
Previous studies have shown that anti-idiotypes are powerful stimulators of CD4 responses, while the evidence for stimulation of CD8 responses has been less convincing. This was thought to be caused by the inability of exogenous antigens to enter MHC class I pathways. However, Sigal et al.37 have suggested that exogenous cross priming may indeed be the preferred route for CTL priming to viral antigens. This mechanism may also account for the generation of TAA specific CTL following immunizations with anti-Id antibodies. It is of interest that DC pulsed with soluble proteins can present peptide epitopes derived from these exogenous antigens to MHC class I molecules and induce an antigen-specific CTL response.38 This is probably related to Fc receptor-mediated endocytosis or macro-pinocytosis of soluble antigens by DC for presentation on MHC class I molecules.39 Therefore, it is expected that anti-Id 3H1 would be internalized and degraded to peptides by DC and those peptides bound to the MHC molecules would be presented to T cells. T cells with appropriate receptors will be expanded and expected to constitute the anti3H1 cytotoxic, helper, and memory cells. The presence of candidate CTL epitopes in 3H1-peptides has been indicated by us3 and others.5
Our findings clearly demonstrated that after 3H1-pulsed DC immunizations CD8+ T cells could lyse C15 tumour cells in vitro by granule-, FasL-, and TRAIL-mediated pathways, although the antigen-specific CTL response was primarily mediated through the perforin/granzyme pathway. Interestingly, proinflammatory cytokines, such as IFN-γ and TNF-α, sensitized the tumour cells to be lysed by these pathways, suggesting that these cytokines could play an important role not only in the induction but also in the effector phase of the immune response. The interaction between cytokine-treated tumour cells and CTL produced enhanced expression of FasL, TRAIL, and intracellular accumulation of perforin by CD8+ T cells, which implicated the importance of these cytokines on lysis of target cells. Physiologically, IFN-γ and TNF-α may have been secreted by activated CTL following TCR engagement and our in vitro studies demonstrated that CTL produced these cytokines in response to tumour-specific recognition. Several studies in humans suggest that host Fas/FasL system may be important for regulation of tumour growth25,40,41 and TRAIL induced apoptosis in a variety of tumour cells makes it an excellent therapeutic candidate for treating cancer patients.42,43In vivo antitumour activity of TRAIL has also been demonstrated in athymic nude or SCID mice bearing human tumour xenografts derived from several carcinomas including colon carcinoma.44–46
Human CEA consists of three homologous repetitive domains and one 3H1 peptide (LCD-2) in particular showed high homology to this CEA region. The homology of the 3H1 peptide is maximum with repeat II domain showing 4 of 10 identity and results of the amino acid sequence alignment and reverse hydropathy analysis suggested that CDR2 of the 3H1 VL and the region of CEA repeat domain may be the common epitope recognized by the anti-CEA Ab1, 8019.3 Based on 3H1 and CEA sequences, eight different peptides were synthesized and used for in vitro proliferation of peripheral blood mononuclear cells (PBMCs) from colon cancer patients immunized with anti-Id mAb 3H1. Among these, only LCD-2 and CEA-B peptides containing the common sequence LIDG showed consistent stimulation in proliferation assays.3 In our present study, we found that 3H1-pulsed DC immunized mice splenocytes could proliferate significantly in presence of LCD-2 and CEA-B peptides and the peptide LCD-2 also contains the epitope recognized by CD8+ T cells for in vitro tumour cell lysis. Our results are in agreement with the recent findings of Murray et al.10 who have reported that the peptide VH3-11 derived from the anti-Id mAb MF11-30 induced proliferation and cytokine production by T cells, and induced HMW-MAA-specific CTL from PBMCs obtained from melanoma patients immunized with MELIMMUNE, a combination of murine anti-Id mAb MEL-2 and MF11-30. Recently it has also been reported that, PBMCs obtained from normal individuals could generate helper and cytotoxic T-cell responses that lysed CEA expressing human tumour cells after in vitro stimulation with a deimmunized anti-Id antibody that mimics CEA.47 It is obvious that, CD8+ T cells are important effector cells in our model because antigen-specific CTL activity could be detected in vitro and in vivo depletion of CD8+ T cells abrogated the tumour protective immunity.
The development of long-lived immunological memory, as well as the ability to transfer immunity by the infusion of splenocytes suggests the presence of antigen-specific T cells in tumour-protected mice. The adoptive transfer experiment suggests that both CD4+ and CD8+ T cells are required to achieve tumour protection. It is possible that the cytokines produced by the transferred CD4+ T cells are required for the maintenance of transferred CD8+ T cells and/or activation of host CD4+ or CD8+ T cells. Several recent studies have documented that CD4+ T-cell help is critical to the maintenance of CD8+ T-cell population.48–50
Taken together, our findings strongly suggest that 3H1-pulsed DC vaccination induced antigen-specific CD8+ T-cell responses in CEA-Tg mice and the results revealed involvement of perforin-, FasL-, and TRAIL-mediated pathways in tumour cell lysis in vitro. The cytokines IFN-γ and TNF-α had an important role for optimal target cell lysis by antigen-specific CTL. The immunity developed in immunized mice resulted in complete rejection of CEA-expressing murine colon carcinoma cells and the generation of long-lived memory response. These data suggest that 3H1-pulsed DC vaccination could be of future benefit for the treatment of colon cancer patients with minimal residual disease after surgery.
Acknowledgments
We thank Dr F. James Primus (Vanderbilt University Medical Center, Nashville, TN) for kindly providing CEA-Tg mice and CEA-transfected MC-38 cell line, C15. We also acknowledge the help of Mary B. Palascak and Peter Ciraolo for flow cytometry. This work was supported by National Institutes of Health grants RO1 CA86025 and RO1 CA104804.
Abbreviations
- CEA
carcinoembryonic antigen
- DC
dendritic cells
- Id
idiotype
- FasL
Fas ligand
- TRAIL
TNF-related apoptosis-inducing ligand
- TAA
tumour-associated antigen
- CRC
colorectal carcinoma
- CEA-Tg
CEA transgenic
- CMA
concanamycin A
- BLT
N-a-benzyloxycarbonyl-l-lysine-thiobenzyl ester hydrochloride
References
- 1.Bhattacharya-Chatterjee M, Chatterjee SK, Foon KA. Anti-idiotype antibody vaccine therapy for cancer. Expert Opin Biol Ther. 2002;2:869–81. doi: 10.1517/14712598.2.8.869. [DOI] [PubMed] [Google Scholar]
- 2.Pride MW, Shi H, Anchin JM, Linthicum DS, LoVerde PT, Thakur A, Thanavala Y. Molecular mimicry of hepatitis B surface antigen by an anti-idiotype-derived synthetic peptide. Proc Natl Acad Sci USA. 1992;89:11900–4. doi: 10.1073/pnas.89.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee SK, Tripathi PK, Chakraborty M, et al. Molecular mimicry of carcinoembryonic antigen by peptides derived from the structure of an anti-idiotype antibody. Cancer Res. 1998;58:1217–24. [PubMed] [Google Scholar]
- 4.Spendlove L, Li L, Potter V, Christiansen D, Loveland BE, Durrant LG. A therapeutic human anti-idiotype antibody mimics CD55 in three distinct regions. Eur J Immunol. 2000;30:2944–53. doi: 10.1002/1521-4141(200010)30:10<2944::AID-IMMU2944>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Kawano K, Ferrone S, Ioannides CG. Functional idiotopes. tumor antigen-directed expression of CD8+ T-cell epitopes nested in unique NH2-terminal VH sequence of anti-idiotypic antibodies? Cancer Res. 2005;65:6002–5. doi: 10.1158/0008-5472.CAN-04-3400. [DOI] [PubMed] [Google Scholar]
- 6.Somasundaram R, Zaloudik J, Jacob L, et al. Induction of antigen-specific T and B cell immunity in colon carcinoma patients by anti-idiotypic antibody. J Immunol. 1995;155:3253–61. [PubMed] [Google Scholar]
- 7.Pride MW, Shuey S, Grillo-Lopez A, et al. Enhancement of cell-mediated immunity in melanoma patients immunized with murine anti-idiotypic monoclonal antibodies (MELIMMUNE) that mimics the high molecular weight proteoglycan antigen. Clin Cancer Res. 1998;4:2363–70. [PubMed] [Google Scholar]
- 8.Durrant LG, Buckley DJ, Robins RA, Spendlove I. 105AD7 cancer vaccine stimulates anti-tumour helper and cytotoxic T-cell responses in colorectal cancer patients but repeated immunisations are required to maintain these responses. Int J Cancer. 2000;85:87–92. doi: 10.1002/(sici)1097-0215(20000101)85:1<87::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Wagner U, Kohler S, Reinartz S, et al. Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti-idiotype antibody ACA125: immune responses and survival in palliative treatment. Clin Cancer Res. 2001;7:1154–62. [PubMed] [Google Scholar]
- 10.Murray JL, Gillogly M, Kawano K, et al. Fine specificity of high molecular weight- melanoma-associated antigen-specific cytotoxic T lymphocytes elicited by anti-idiotypic monoclonal antibodies in patients with melanoma. Cancer Res. 2004;64:5481–8. doi: 10.1158/0008-5472.CAN-04-0517. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya-Chatterjee M, Mukherjee S, Biddle W, Foon KA, Kohler H. Murine monoclonal anti-idiotype antibody as a potential network antigen for human carcinoembryonic antigen. J Immunol. 1990;145:2758–65. [PubMed] [Google Scholar]
- 12.Foon KA, Chakraborty M, John WJ, Sherratt A, Kohler H, Bhattacharya-Chatterjee M. Immune response to the carcinoembryonic antigen in patients treated with an anti-idiotype antibody vaccine. J Clin Invest. 1995;96:334–42. doi: 10.1172/JCI118039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foon KA, John WJ, Chakraborty M, Sherratt A, Garrison J, Flett M, Bhattacharya-Chatterjee M. Clinical and immune responses in advanced colorectal cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. Clin Cancer Res. 1997;3:1267–76. [PubMed] [Google Scholar]
- 14.Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol. 1999;17:2889–95. doi: 10.1200/JCO.1999.17.9.2889. [DOI] [PubMed] [Google Scholar]
- 15.Pervin S, Chakraborty M, Bhattacharya-Chatterjee M, Zeytin H, Foon KA, Chatterjee SK. Induction of antitumor immunity by an anti-idiotype antibody mimicking carcinoembryonic antigen. Cancer Res. 1997;57:728–34. [PubMed] [Google Scholar]
- 16.Clarke P, Mann J, Simpson JF, Rickard-Dickson K, Primus FJ. Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res. 1998;58:1469–77. [PubMed] [Google Scholar]
- 17.Saha A, Chatterjee SK, Foon KA, Primus FJ, Sreedharan S, Mohanty K, Bhattacharya-Chatterjee M. Dendritic cells pulsed with an anti-idiotype antibody mimicking carcinoembryonic antigen (CEA) can reverse immunological tolerance to CEA and induce antitumor immunity in CEA transgenic mice. Cancer Res. 2004;64:4995–5003. doi: 10.1158/0008-5472.CAN-04-0626. [DOI] [PubMed] [Google Scholar]
- 18.Sen G, Chakraborty M, Foon KA, Reisfeld RA, Bhattacharya-Chatterjee M. Preclinical evaluation in nonhuman primates of murine monoclonal anti-idiotype antibody that mimics the disialoganglioside GD2. Clin Cancer Res. 1997;3:1969–76. [PubMed] [Google Scholar]
- 19.Lu J, Higashimoto Y, Appella E, Celis E. Multiepitope trojan antigen peptide vaccines for the induction of antitumor CTL and Th immune responses. J Immunol. 2004;172:4575–82. doi: 10.4049/jimmunol.172.7.4575. [DOI] [PubMed] [Google Scholar]
- 20.Cho DH, Song HK, Kang HS, et al. Ligation of ICAM-1 molecules inhibits target cell- induced granule exocytosis of IL-12-activated natural killer cells. Cell Immunol. 2000;199:1–7. doi: 10.1006/cimm.1999.1592. [DOI] [PubMed] [Google Scholar]
- 21.Carbone FR, Moore MW, Sheil JM, Bevan MJ. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J Exp Med. 1988;167:1767–79. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porgador A, Snyder D, Gilboa E. Induction of antitumor immunity using bone marrow-generated dendritic cells. J Immunol. 1996;156:2918–26. [PubMed] [Google Scholar]
- 23.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–86. [PubMed] [Google Scholar]
- 24.Lee JK, Sayers TJ, Brooks AD, Back TC, Young HA, Komschlies KL, Wigginton JM, Wiltrout RH. IFN-γ-dependent delay of in vivo tumor progression by Fas overexpression on murine renal cancer cells. J Immunol. 2000;164:231–9. doi: 10.4049/jimmunol.164.1.231. [DOI] [PubMed] [Google Scholar]
- 25.Bergmann-Leitner ES, Abrams SI. Differential role of Fas/Fas ligand interactions in cytolysis of primary and metastatic colon carcinoma cell lines by human antigen-specific CD8+ CTL. J Immunol. 2000;164:4941–54. doi: 10.4049/jimmunol.164.9.4941. [DOI] [PubMed] [Google Scholar]
- 26.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–5. [PubMed] [Google Scholar]
- 27.Uno K, Inukai T, Kayagaki N, et al. TNF-related apoptosis- inducing ligand (TRAIL) frequently induces apoptosis in Philadelphia chromosome-positive leukemia cells. Blood. 2003;101:3658–67. doi: 10.1182/blood-2002-06-1770. [DOI] [PubMed] [Google Scholar]
- 28.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–75. [PubMed] [Google Scholar]
- 29.De Bruijn ML, Schumacher TN, Nieland JD, Ploegh HL, Kast WM, Melief CJ. Peptide loading of empty major histocompatibility complex molecules on RMA-S cells allows the induction of primary cytotoxic T lymphocyte responses. Eur J Immunol. 1991;21:2963–70. doi: 10.1002/eji.1830211210. [DOI] [PubMed] [Google Scholar]
- 30.Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naïve cells (CD44low, Ly-6C–) to TCR/CD8 signaling in response to antigen. J Immunol. 1998;160:3236–43. [PubMed] [Google Scholar]
- 31.Cho BK, Wang C, Sugawa S, Eisen HN, Chen J. Functional differences between memory and naïve CD8 T cells. Proc Natl Acad Sci USA. 1999;96:2976–81. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–8. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 33.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 34.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falo LD, Jr, Kovacsovics-Bankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumor immunity. Nat Med. 1995;1:649–53. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- 36.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific, CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283–7. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non- haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 38.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–22. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 40.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 41.Owen-Schaub L, Chan H, Cusack JC, Roth J, Hill LL. Fas and Fas ligand interactions in malignant disease. Int J Oncol. 2000;17:5–12. [PubMed] [Google Scholar]
- 42.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 43.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 44.Naka T, Sugamura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients' colon tumors grown in SCID mice. Cancer Res. 2002;62:5800–6. [PubMed] [Google Scholar]
- 45.Ray S, Almasan A. Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Cancer Res. 2003;63:4713–23. [PubMed] [Google Scholar]
- 46.Jin H, Yang R, Fong S, et al. Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res. 2004;64:4900–5. doi: 10.1158/0008-5472.CAN-04-0408. [DOI] [PubMed] [Google Scholar]
- 47.Parsons T, Spendlove I, Nirula R, Writer M, Carter G, Carr F, Durrant LG. A novel CEA vaccine stimulates T cell proliferation, γIFN secretion and CEA specific CTL responses. Vaccine. 2004;22:3487–94. doi: 10.1016/j.vaccine.2004.01.070. [DOI] [PubMed] [Google Scholar]
- 48.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 49.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 50.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]