Abstract
Toll-like receptors (TLRs) recognize specific pathogen-associated molecular patterns (PAMPs), which subsequently trigger innate immunity. Recent data also suggest a role for TLRs in the direct activation of adaptive immune cells. In the present study, the expression and function of TLR1–TLR10 were characterized in purified human tonsillar B cells, focusing on differences among CD19+ CD38– CD27– (naïve B cells), CD19+ IgD– CD27–[germinal centre (GC) B cells] and CD19+ CD38– CD27+ (memory B cells) cells. The study was also designed to compare the TLR expression in B cells obtained from infected and hyperplastic tonsils that served as controls. The results demonstrated a distinct repertoire of TLRs, in which TLR1, TLR2, TLR7, TLR9 and TLR10 predominated. No differences were found among naïve, GC and memory B cells. Tonsillar infection did not substantially alter the TLR expression profile in ex vivo-isolated B-cell subsets. Purified CD19+ B cells stimulated with Pam3CSK4, R-837 and CpG oligodeoxynucleotide (ODN) 2006, via TLR1/TLR2, TLR7 and TLR9, respectively, showed an induction of interleukin-6 secretion and an up-regulated expression of human leucocyte antigen (HLA)-DR. Collectively, the present study demonstrates that B cells exhibit constitutively high levels of specific TLRs, which are not developmentally regulated during the B-cell differentiation process. Ongoing microbial infections, such as chronic tonsillitis, do not appear to affect the TLR profile in B cells. Furthermore, the distinct expression of TLRs allows B cells to respond directly to the cognate PAMPs. This further emphasizes the role of TLRs in directly activating adaptive immune cells.
Keywords: infection, PAMPs, Toll-like receptors, tonsillar B cells
Introduction
The Toll-like receptor (TLR) family consists of 10 members (TLR1–TLR10),1 which are transmembrane proteins with an extracellular leucine-rich domain and a conserved cytoplasmic domain. The cytoplasmic domain is homologous to the interleukin (IL)-1 receptor and is referred to as the Toll/IL-1 receptor (TIR) domain.2–4 Each TLR recognizes specific microbial components, so-called pathogen-associated molecular patterns (PAMPs),5 including bacterial lipoproteins and lipoteichoic acids (TLR2), double-stranded viral RNA (dsRNA; TLR3), lipopolysaccharides (LPS; TLR4), flagellin (TLR5), imidazoquinolines and single-stranded viral RNA (ssRNA; TLR7 and TLR8), and unmethylated CpG oligodeoxynucleotides (ODNs; TLR9).1,4,6–8 TLR1 and TLR6 only signal as a dimer when combined with TLR2,9 and the ligand for TLR10 is as yet unknown.2 TLRs are differentially expressed in various immune cells. In general, mononuclear phagocytes and dendritic cells (DCs) express the widest TLR repertoires.10 Recognition of PAMPs by innate immune cells results in a signalling pathway that leads to antigen presentation, up-regulation of costimulatory molecules and release of cytokines, which in turn stimulates the adaptive immune system consisting of B and T lymphocytes.9–11 In addition to this indirect activation of B cells, it is also speculated whether infectious organisms can directly activate the adaptive immune response.10,12
At present, our knowledge about TLR regulation and function in B cells is limited, except for TLR9. Herein, we examined whether the expression of TLRs is developmentally regulated during the differentiation from naïve to germinal centre (GC) and memory B cells in tonsillar tissue, and whether the TLR expression in the various cell subsets is regulated in response to infection. The direct effects of TLR ligands other than CpG have been poorly investigated on purified human B cells. Therefore, we also analysed the functional response of purified human B cells to a range of different TLR ligands.
Materials and methods
Patients
Tonsils were obtained from 33 patients undergoing tonsillectomy at Malmö University Hospital (Malmö, Sweden). The study was approved by the Ethics Committee of Lund University and written informed consent was obtained. After the tonsillectomy, swabs were taken for tonsillar core cultures (representing the microbial flora of the tonsillar crypts) in order to determine the presence of pathogenic β-haemolytic streptococci and anaerobes. The patients providing the tonsils were divided into two groups (referred to as infected or control), based on their clinical diagnoses and the outcome of the core culture. The infected group consisted of 14 patients (age range: 3–21 years, median 7 years) referred for tonsillectomy because of multiple episodes (at least four times during the year preceding the surgery) of group A β-haemolytic streptococci (GAS) tonsillitis (small to substantially enlarged tonsils, positive culture test). The control group consisted of 19 patients (age range: 3–18 years, median 6·5 years) who underwent tonsillectomy because of tonsillar hyperplasia indicated by either obstructive sleep apnoea or occasional failure to thrive (no history of recurrent tonsillitis, negative culture test). Even though the control tonsils were culture-negative and functioned as appropriate controls in regard to infection, they were not normal healthy tissues. Therefore, it cannot be entirely excluded that there was some immunologically relevant cause of the hyperplasia that affected the outcome of the present study. None of the patients displayed symptoms of acute infection at the time of surgery, and none had received any antibiotic treatment for at least 1 month before surgery. Apart from the tonsillar symptoms, all patients were healthy and did not receive any medication.
Antibodies and reagents
The following anti-human immunoglobulins were used for flow cytometry analyses: CD19-ECD (J4.119) from Immunotech (Beckman Coulter, Marseille, France), CD27-phycoerythrin (PE) (M-T271) from DakoCytomation (Copenhagen, Denmark), CD38-fluorescein isothiocyanate (FITC) (HIT2) from BD Pharmingen (Heidelberg, Germany), immunoglobulin D (IgD)-FITC (goat, polyclonal), TLR2-FITC (TL2.1) and TLR9-FITC (26C593) from AMS Biotechnology (Abingdon, UK) and human leucocyte antigen (HLA)-DR-PE (LN3) from eBioscience (San Diego, CA). Unlabelled antibodies against TLR1 (GD2.F4) and TLR7 (rabbit, polyclonal) were purchased from Acris antibodies (Hiddenhausen, Germany), and detected using the Alexa Fluor 488 mouse immunoglobulin G1 (IgG1) labelling kit from Molecular Probes (Eugene, OR) or sheep anti-rabbit IgG-PE from Acris. For immunohistochemical staining, antibodies against CD3 (PSI) from Novocastra (Newcastle upon Tyne, UK), CD20cy (L26) from DakoCytomation, TLR1 (GD2.F4), TLR2 (rabbit, polyclonal), TLR7 (rabbit, polyclonal) and TLR9 (26C593) from Acris were used. For visualization, DakoCytomation Envision+ System-horseradish peroxidase (HRP) kits were utilized. Pam3CSK4, flagellin (from Bacillus subtilis) and imiquimod (R-837) were purchased from Invivogen (San Diego, CA), LPS (from Salmonella minnesota) from Alexis Biochemicals (Lausen, Switzerland) and phosphorothioate-modified CpG ODN 2006 (CpG-2006), 5′-tcgtcgttttgtcgttttgtcgtt-3′13 from DNA Technology A/S (Aarhus, Denmark).
Cell separation and cell culture
Tonsils were minced in complete medium consisting of RPMI-1640 (Sigma-Aldrich, St Louis, MO) supplemented with 0·3 g/l l-glutamine, 10% fetal calf serum (FCS) (AH diagnostics, Aarhus, Denmark), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA), and the T cells were removed by rosetting with neuraminidase-activated sheep red blood cells (SRBCs). Mononuclear T-cell-depleted cells were isolated by density-gradient centrifugation using Ficoll–Paque (Amersham Bioscience, Uppsala, Sweden). The interphase fraction, containing predominantly B cells, was collected and washed in complete medium. Peripheral blood lymphocytes (PBLs) were isolated from blood obtained from six healthy volunteers (age range: 26–56 years, median 34 years), as previously described,14 after the removal of erythrocytes using a mixture of two parts Macrodex® (Meda AB, Solna, Sweden) and one part sodium metrizoate 32·8% (Axis-Shield PoC AS, Oslo, Norway) followed by density-gradient centrifugation using Lymphoprep™ (Axis-Shield PoC AS). The lymphocyte-enriched interphase fraction was collected and washed in complete medium. Different subsets of B cells were isolated using the MACS magnetic labelling system (Miltenyi Biotec, Cologne, Germany), according to the instructions of the manufacturer. Briefly, cells were incubated at 4° with antibody-conjugated microbeads in buffer containing phosphate-buffered saline (PBS) supplemented with 0·5% FCS and 2 mm EDTA, and separated on a large cell selection (LS) or a large cell depletion (LD) column placed on a magnetic separator. Untouched CD19+ B cells from peripheral blood (PB) or tonsils were isolated by an indirect magnetic labelling system (B Cell Isolation KitII; Miltenyi Biotec) using antibodies against CD2, CD14, CD16, CD36, CD43 and CD235a to deplete T cells, natural killer (NK) cells, dendritic cells (DCs), monocytes, granulocytes and erythroid cells (purity >95%). CD19+ tonsillar B cells were occasionally further separated using antibodies against CD38-FITC and IgD-FITC followed by Anti-FITC Microbeads (Miltenyi Biotec). Unlabelled CD38– cells and IgD– cells were collected after separation on LD columns, further incubated with CD27 Microbeads (Miltenyi Biotec) and separated on LS columns. Both the positive and the negative fractions were collected, resulting in CD38– CD27– naïve B cells, CD38– CD27+ memory B cells and IgD– CD27– GC B cells. The purity of the cells was determined by fluorescence-activated cell sorter (FACS) analysis. For the functional studies, seven additional tonsils were obtained. Freshly isolated CD19+ B cells were cultured (1 × 106 cells/ml) in medium alone or with various concentrations of Pam3CSK4, LPS, flagellin, R-837 or CpG-2006, as indicated. After 48 hr, the expression of HLA-DR was analysed by FACS, and IL-6 was measured in the cell-free supernatants by enzyme-linked immunosorbent assay (ELISA).
RNA isolation and real-time reverse transcription–polymerase chain reaction (RT–PCR)
Freshly isolated cells were lysed in RLT buffer (Qiagen, Hilden, Germany), supplemented with 1% 2-mercaptoethanol, and stored at −80° until use. RNA was extracted using an RNeasy Mini Kit (Qiagen). The quantity and quality of the RNA concentration was determined by spectrophotometry based on the absorbance A260/A280 ratio. The Omniscript Reverse Transcriptase kit (Qiagen) and oligo(dT)15 primer (Novagen, Nottingham, UK) were used for first-strand cDNA synthesis with an aliquot of 20 ng RNA as the starting material. The cDNA thus obtained was diluted with water and 18 ng was used for amplification. The real-time PCR was performed on a Smart Cycler (Cepheid, Sunnyvale, CA) using TaqMan Universal PCR Master Mix, No AmpErase UNG and Assay-on-Demand Gene Expression products (Applied Biosystems, Foster City, CA), containing unlabelled primers and MGB probe (FAM™ dye-labelled). To ensure proper function of the probes, neutrophils and T cells were used as controls. The thermal cycler was programmed to perform an initial set-up (95°, 10 min) and 45 cycles of denaturation (95°, 15 seconds) followed by annealing/extension (60°, 1 min). The relative amounts of mRNA for the TLRs were determined by subtracting threshold cycle (Ct) values for these genes from the Ct value for the internal control gene β-actin (ΔCt). Data are depicted as 2ΔCt × 105 and presented as mean values ± standard error of the mean (SEM).
FACS analysis
Flow cytometry analyses were performed on a Coulter Epics XL flow cytometer (Beckman Coulter, Marseille, France). Live lymphocytes were gated based on forward- and side-scatter properties and 10 000–15 000 events were collected and analysed using expo32 adc analysis software (Beckman Coulter). Isotype controls relevant for each antibody were used for background staining. For the detection of TLRs, intracellular staining of the cells was performed. Freshly isolated CD19+ cells were fixed in 4% formaldehyde and permeabilized in PBS containing 0·1% Triton-X-100. PBS supplemented with 2% FCS was used for all labelling and washing steps.
Immunohistochemistry
The morphological localization of TLR proteins in tonsils was investigated using immunohistochemistry. Tissue preparations were embedded in paraffin, cut in 3-µm thick sections, mounted on glass slides and stored at −80° until use. Prior to visualization of proteins, the sections were treated with xylene to remove the paraffin and rehydrated using ethanol. To facilitate binding of the antibodies, the sections were treated with Target retrieval solution (Dako) for 20 min in a microwave oven, followed by 1% Triton-X-100 for increased membrane permeability. To quench endogenous peroxidase activity, sections were incubated in 0·03% hydrogen peroxide for 10–15 min. If needed, sections were incubated for 20 min in PBS containing 2% FCS to block nonspecific binding. Primary mouse antibodies against CD3 (1 : 100 dilution), CD20 (1 : 1500), TLR1 (1 : 50 dilution) and TLR9 (1 : 50 dilution), and rabbit antibodies against TLR2 (1 : 50 dilution) and TLR7 (1 : 50 dilution), were applied to the sections for 1 hr. Subsequently, HRP-labelled goat anti-mouse or goat anti-rabbit polymer (Dako) was incubated with the sections for 30 min, followed by 3,3′-diaminobenzidine (DAB) substrate-chromogen (Dako) for 5–10 min. On some occasions, sections were counterstained with Mayer's haematoxylin. The sections were dehydrated with increasing concentrations of ethanol, rinsed in xylene and mounted in Pertex (Histolab, Gothenburg, Sweden). Tris-buffered saline (TBS) (pH 7·6), supplemented with 0·05% Tween 20, was used for all washing steps.
Cytokine detection by ELISA
Cell-free culture supernatants were assayed for IL-6 (sensitivity 3·12–300 pg/ml) using ELISA plates from R & D Systems (Minneapolis, MN).
Statistical analyses
Statistical analysis was performed using graphpad prism 4 (GraphPad, San Diego, CA). The Student's t-test was used to determine statistical differences for unpaired comparisons, with the Welch test if variances were non-homogenous. P-values of < 0·05 were considered significant.
Results
Generation of naïve, GC and memory B cells
B-cell differentiation is a central process in the humoral immune response. This often involves GC reactions, eventually leading to the generation of memory B cells or plasma cells that produce antibodies against invading pathogens.15,16 A separation protocol for the enrichment of B-cell subsets was developed based on the expression of CD19 and markers that have been suggested to distinguish B cells at different differentiation stages, namely IgD, CD27 and CD38 (Fig. 1a–c).15,17–20 Naïve and memory B cells were isolated as CD38– cells from the CD19+ fraction. The CD38– fraction was further separated into CD27+ memory cells and CD27– naïve cells. The CD19+ fraction was also enriched for IgD– CD27– cells, representing GC B cells that had not gone through somatic hypermutation. The purity of the isolated B cell subsets was consistently ≥90%.
Figure 1.
Separation of naïve, germinal centre (GC) and memory B cells. Tonsils were initially depleted of T cells by sheep red blood cell (SRBC) rosetting and further depleted of any remaining non-CD19+ cells. The CD19+ fraction was divided for isolation of (a) naïve, (b) GC and (c) memory B cells, based on the expression of CD38, immunoglobulin D (IgD) and CD27. The separations were accompanied by flow cytometry analysis of surface phenotype. PE, phycoerythrin; FITC, fluorescein isothiocyanate.
Expression pattern of TLR1-TLR10 in subsets of human tonsil B cells
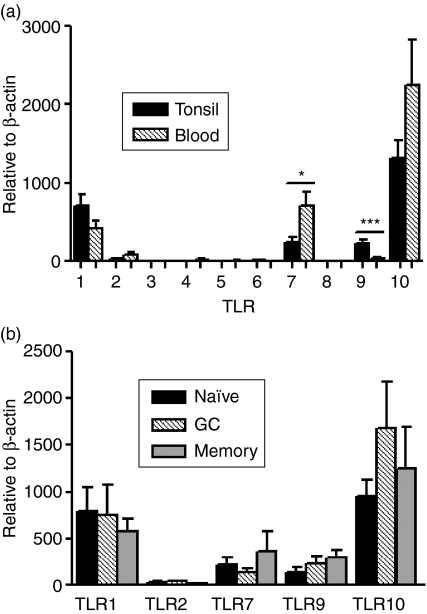
The expression of TLR1–TLR10 transcripts was analysed using quantitative real-time RT–PCR, and all data are presented in relation to the housekeeping gene, β-actin. B cells expressed several TLRs, of which TLR1, TLR7, TLR9 and TLR10 were the most prominent (Fig. 2a). Expression of TLR2 was low, and TLR6 was expressed at the lower detection limit, whereas TLR3, TLR4, TLR5 and TLR8 were undetectable. Unlike previous studies, mainly performed on PB but also on tonsils, showing the presence of TLR1 and TLR6–TLR10 mRNA,21 we failed to detect TLR6 and TLR8. To investigate whether this discrepancy was caused by tissue-specific factors, mRNA was extracted from PB B cells and analysed for TLR1–TLR10 expression. As for tonsillar B cells, no expression of TLR6 or TLR8 was detected (Fig. 2a). However, expression of TLR7 was higher in blood-derived B cells, whereas the expression of TLR9 was considerably lower compared with tonsillar cells.
Figure 2.
Expression pattern of Toll-like receptor (TLR)1–TLR10 transcripts in (a) freshly isolated CD19+ B cells from tonsil (n = 20) and peripheral blood (n = 6) and (b) tonsillar B cells further divided into naïve, germinal centre (GC) and memory B cells (n = 19–23). Expression was determined using quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR) and is depicted in relation to the internal control gene, β-actin, as 2ΔCt × 105. Data from experiments with cells from different donors are summarized and presented as mean ± standard error of the mean (SEM) (*P < 0·05; ***P < 0·001).
Next, we wished to investigate whether the TLR expression was altered during the different stages of B-cell development. The TLR expression in purified subsets of naïve, GC and memory B cells was compared. The three subsets of cells were found to express the detected TLRs constitutively (Fig. 2b), and similar levels of TLR1, TLR2 and TLR10 were expressed by each of the cell subsets. TLR7 and TLR9 were also present in the three subsets, albeit somewhat higher in memory B cells. Neither of these differences did, however, reach statistical significance.
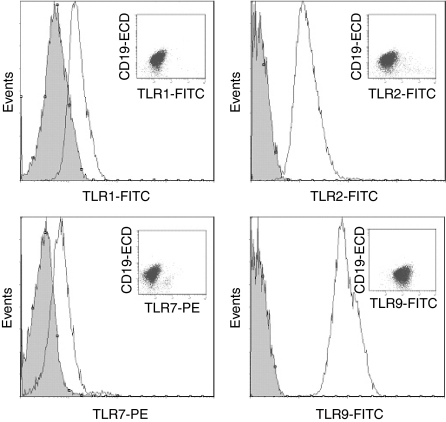
To determine whether the observed mRNA expression pattern actually reflected the functional TLR proteins expressed, flow cytometry and immunohistochemistry were carried out on the TLRs that were transcriptionally expressed. Highly purified CD19+ B cells were stained with antibodies against CD19, TLR1, TLR2, TLR7 and TLR9 (no antibody against TLR10 was commercially available) and analysed by FACS. The cells clearly displayed the receptor proteins, thus verifying the presence of the TLRs (Fig. 3).
Figure 3.
Flow cytometry analysis of Toll-like receptors (TLRs) (open histograms) in freshly isolated human tonsillar CD19+ B cells. Cells were stained intracellularly with mouse monoclonal antibodies (mAbs) against CD19 (J4.119, IgG1), TLR1 (GD2.F4, IgG1), TLR2 (TL2.1, IgG2a) and TLR9 (26C593, IgG1), polyclonal rabbit antibody against TLR7 and appropriate isotype controls (filled histograms). Inserted dot-plots show TLR and CD19. Data from one representative out of four independent experiments are shown. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
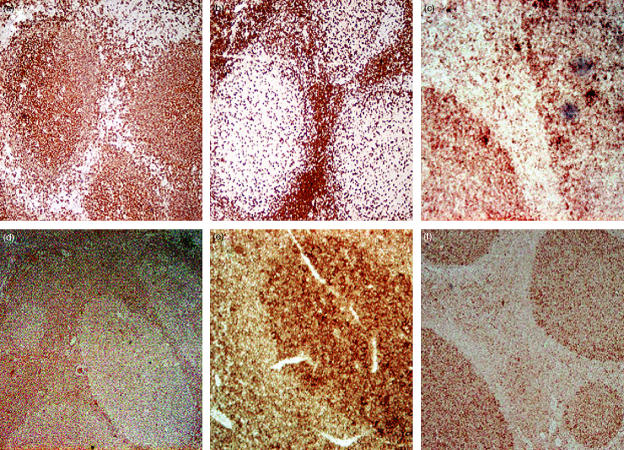
Immunohistochemical staining of tonsils with antibodies against the B- and T-cell markers CD20 and CD3, respectively, as well as against TLR1, TLR2, TLR7 and TLR9, was performed to identify the cellular location of the receptors. CD20+ cells were, as expected, localized mainly within the GCs, whereas CD3+ cells predominantly resided in the T-cell zones (Fig. 4a,b). Cells expressing TLR1, TLR7 and TLR9 were abundant in the GCs, but also present in the adjacent T-cell zones, whereas expression of TLR2 was more intense in the T-cell zones (Fig. 4c–f). The presence of the TLR proteins in the GCs also confirms the presence of the TLR transcripts in B cells.
Figure 4.
Tonsil sections stained with antibodies against (a) CD20 (diluted 1 : 1500), (b) CD3 (diluted 1 : 100), (c) Toll-like receptor (TLR)1 (diluted 1 : 50), (d) TLR2 (diluted 1 : 50), (e) TLR7 (diluted 1 : 50) and (f) TLR9 (diluted 1 : 50), were localized using 3,3′-diaminobenzidine (DAB), which stains tissues brown, and analysed by microscopy (magnification ×40–100).
Influence of tonsillar infection on the TLR expression pattern
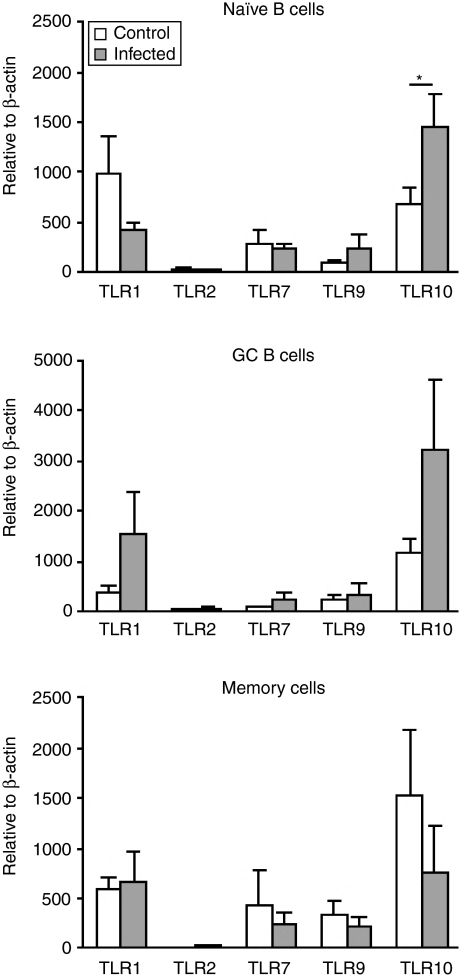
It has previously been described that TLR ligands, such as bacterial and viral components, found at sites of infection or inflammation, affect the expression of their cognate TLRs.22 Tonsils, indeed, are a site of high antigenic burden, being continuously exposed to bacteria, viruses, fungi and other inhaled or ingested substances.23 This led us to compare the expression of TLR mRNA in cells from recurrently infected tonsils with that from non-infected, hyperplastic tonsils that served as controls. In the infected tonsils, several strains of bacteria were found, including β-haemolytic streptococci (group A, C and G) and anaerobes. In general, the expression of TLRs in naïve and GC B cells was higher in cells from infected tonsils, whereas it was the other way round in memory B cells(Fig. 5). The only significant difference was that TLR10 was up-regulated in naïve B cells from infected tonsils. In GC B cells there was also a trend towards a higher expression of TLR10 in cells from infected tonsils compared to control, although not significant. In memory B cells the relationship was reversed.
Figure 5.
Expression pattern of Toll-like receptor (TLR) transcripts in subsets of human tonsillar B cells from infected tonsils and controls. Freshly isolated naïve, germinal centre (GC) and memory B cells were analysed for TLR expression using quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR). Data from experiments with cells from different donors are summarized (infected, n = 7–13; controls n = 12–13), depicted in relation to the internal control gene, β-actin, as 2ΔCt × 105 and presented as mean ± standard error of the mean (SEM) (*P < 0·05).
Stimulation via TLR1/TLR2, TLR7 and TLR9 induce secretion of IL-6 and up-regulate HLA-DR by CD19+ tonsillar B cells
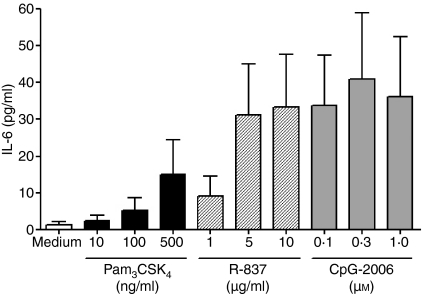
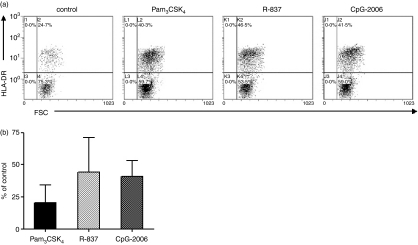
After finding a repertoire of TLRs in B cells comprising TLR1, TLR2, TLR7, TLR9 and TLR10, we next wanted to investigate whether the receptors exhibit functional activity. TLR9 ligation with CpG-2006 is known to activate purified human B cells directly, but little is known about the functional activity of other TLRs.24 Therefore, the responsiveness of purified tonsillar CD19+ B cells to different concentrations of Pam3CSK4 (TLR1/TLR2), R-837 (TLR7) and CpG-2006 (TLR9), was evaluated. In these experiments, R-837 and CpG-2006 induced a profound secretion of IL-6 (Fig. 6), whereas the effect exerted by Pam3CSK4 was less prominent. In addition to cytokine secretion, expression of the costimulatory molecule, HLA-DR [major histocompatibility complex (MHC) class II] was evaluated by FACS analysis. The data showed that Pam3CSK4, R-837 and CpG-2006 induced an up-regulation of HLA-DR (20·6 ± 13·9%, 44·1 ± 27·0% and 40·9 ± 12·1%, respectively) compared to control without stimulus (Fig. 7a,b). In accordance with the lack of TLR4 and TLR5 expression, LPS (0·1–10 µg/ml) and flagellin (0·1–5·0 µg/ml) did not have an effect on HLA-DR expression or IL-6 production (n = 3; data not shown). Together, these results indicate that R-837, CpG-2006 and, to a lesser extent, Pam3CSK4, are capable of directly activating purified human B cells.
Figure 6.
Induction of interleukin-6 (IL-6) secretion by CD19+ tonsillar B cells. Purified cells (1 × 106 cells/ml) were incubated in medium alone or with various concentrations of Pam3CSK4, R-837 and CpG-2006. After 48 hr, the supernatants were recovered and IL-6 was measured by enzyme-linked immunosorbent assay (ELISA) [mean ± standard error of the mean (SEM), n = 4].
Figure 7.
Up-regulation of human leucocyte antigen (HLA)-DR on CD19+ tonsillar B cells in response to Pam3CSK4, R-837 and CpG-2006. Purified cells (1 × 106 cells/ml) incubated for 48 hr with medium alone (control), Pam3CSK4 (500 ng/ml), R-837 (10 µg/ml) or CpG-2006 (0·3 µm) were analysed for HLA-DR using flow cytometry. Results are presented as (a) dot plots with HLA-DR-positive cells from one representative out of four independent experiments, or as (b) the percentage of control [mean ± standard error of the mean (SEM), n = 4].
Discussion
In the present study, the expression pattern of TLR1–TLR10 in purified subsets of B lymphocytes of human origin is presented for the first time at both mRNA and protein levels. Freshly isolated B cells exhibited a distinct TLR repertoire, comprising TLR1, TLR2, TLR7, TLR9 and TLR10. The expression was not found to be developmentally regulated during the differentiation pathway from naïve to GC and memory B cells. Moreover, tonsillar infection only had a limited effect on TLR expression, with an increase in TLR10 mRNA in naïve B cells. It was also demonstrated that purified CD19+ B cells are directly activated by Pam3CSK4, R-837 and CpG-2006, mediated via TLR1/TLR2, TLR7 and TLR9, respectively, to produce IL-6 and induce expression of HLA-DR.
Previous studies have investigated the TLR repertoire in B cells, showing mRNA encoding primarily TLR1 and TLR6–TLR10.21 The present study largely confirmed the existing transcriptional data, but also extended and complemented these results at the protein level, demonstrating the presence of TLR1, TLR2, TLR7 and TLR9 using FACS analysis. The protein level of TLR2 was quite high in contrast to the mRNA expression level. Similar discrepancies when TLR mRNA and protein disconnect have previously been reported for human leucocytes.1,25 Although the PCR techniques currently used are very sensitive, they do not provide definite proof of the presence of TLRs.26 Measuring transcribed mRNA alone is clearly not sufficient to characterize the TLR repertoire, which underscores the importance of analysing protein expression and ultimately functional activity. In contrast to most studies performed on PB,21,22,27 we failed to detect TLR6 and TLR8 in tonsils. As this suggested that TLR expression might be regulated by tissue-specific factors, we also investigated the expression of TLRs in B cells from PB. Expression of both TLR6 and TLR8 was, however, still lacking, indicating that the differences in TLR expression profiles are caused by variations from one study to another.
Herein, we isolated naïve, GC and memory B cells based on the expression of CD19, IgD, CD38 and CD27. CD27 has been defined as a marker for somatically mutated B cells28 in tonsil comprising memory B cells and a portion of the GC B cells. Thus, the IgD– CD27– fraction referred to as GC B cells only represents unmutated cells, not the whole GC B-cell population. Nevertheless, they have still been referred to as GC B cells. No differences were found among naïve, GC and memory B cells, indicating that the differentiation process does not affect the TLR repertoire. A previous study on B cells derived from PB has demonstrated a poor TLR expression in naïve cells (TLR6, TLR7, TLR9 and TLR10) followed by up-regulation during the differentiation into memory cells.27 It is important to recognize here the functional differences between B cells from PB and secondary lymphoid tissues such as tonsils. It is well known that blood lacks GC B cells and contains a larger percentage of resting B cells compared with tonsils.29 In addition, our data clearly show that TLR9 is expressed to a greater extent in B cells from tonsils than in PB, whereas expression of TLR7 is higher in PB. Naïve and memory cells moving from a resting state are thought to induce a general increase in TLR expression when they are activated and re-enter the cell cycle.21 Bourke et al.10 demonstrated a higher expression of TLR7, TLR9 and TLR10 in activated GC B cells compared with resting B cells isolated from tonsils.10 However, activation of these resting B cells with mitogen up-regulated the expression of the receptors. Altogether, this suggests that the differential expression observed by others perhaps is not a result of the differentiation stage of the cell. Instead, the increase in TLR expression might be a consequence of cells progressing from a resting to an activated phase.
The TLR expression in various cell types has been described to be affected locally by an ongoing infection30–32 and we have, in a recent study, demonstrated that the TLR expression profile in tonsillar T cells is altered in response to infection. CD8+ T cells from patients with chronic tonsillitis were found to express higher levels of TLR2, TLR3 and TLR5, whereas CD4+ T cells expressed a lower level of TLR9.33 However, infection only induced minor changes in TLR expression in B-cell subsets ex vivo isolated from human tonsils, with an infection-dependent increase in TLR10 in naïve B cells. As the ligand for TLR10 is unknown, it is impossible to speculate the significance and meaning of this finding. Hornung et al.22 have shown in vitro a decrease in TLR9 in response to CpG-DNA stimulation, and others have demonstrated an increase in TLR7, TLR9 and TLR10 following stimulation with microbial products such as Staphylococcus aureus Cowan I bacteria and CpG-DNA, or activation through the B-cell receptor and CD40.10,27 Although previous studies on how TLR expression is regulated in B cells in response to microbial stimuli are contradictory, it is generally agreed that some regulation occurs, at least in vitro. The infected tonsils were found to harbour Gram-positive streptococci, such as GAS. Structures on Gram-positive bacteria, including streptococci, signal through TLR2,2 which is why a regulation in TLR2 expression could be expected as a result of tonsillar infection. No such regulation was seen, however. Although a clear expression of TLR2 proteins was observed, the mRNA levels were very low. An infection-dependent alteration would consequently not be detected at the mRNA level. One straightforward explanation as to why no alterations in expression were seen for the remaining TLRs might be related to the possible lack of natural ligands for TLR1, TLR7, TLR9 and TLR10. Thus, what happens to the TLR expression in B cells during an ongoing infection currently remains unknown.
All TLRs expressed in tonsillar B cells, for which specific ligands are available, showed functional activity upon stimulation with the corresponding ligand. R-837, CpG-2006 and, to a lesser extent, Pam3CSK4, up-regulated the expression of HLA-DR and induced the secretion of IL-6 by purified CD19+ B cells. TLR9 ligation directly through CpG-2006 is known to activate human B cells to proliferate, secrete IL-6 and induce expression of costimulatory molecules.13,34 Information about the functional activity of R-837 (also known as imiquimod) and Pam3CSK4 is, however, limited on B cells, and to our knowledge, we are the first to demonstrate that they are capable of directly activating purified human B cells. The B-cell-activating capability of Pam3CSK4 was, however, somewhat weaker than that of R-837 and CpG-2006, probably because the plateau of the concentration–response curve was not reached. A recent study, using the imidazoquinoline R-848 (agonist for both TLR7 and TLR8, structurally related to R-837) and loxoribine (selective TLR7 ligand), showed that purified human B cells were unable to respond to both R-848 and loxoribine unless they were cocultured with plasmacytoid dendritic cells (PDCs) or PDC-derived type I interferons (IFNs).24 The same group showed that Pam3CSK4 was incapable of activating human B cells, both in the absence or presence of PDCs. Another group reported that both imiquimod and R-848 induced proliferation, immunoglobulin secretion and up-regulation of MHC class II and B7.2 in murine B cells.35 They further demonstrated that human B cells isolated from peripheral blood mononuclear cells (PBMC) were induced to proliferate in response to R-848 (no data available for R-837). In another study, human CD19+ B cells from PB did not respond markedly when exposed to imiquimod or R-848.36 Furthermore, LPS and flagellin showed no B-cell activating ability, which is an expected finding given the absence of TLR4 and TLR5 expression. Contrasting data have, however, recently been presented in a study by Pasare & Medzhitov.37 They showed that TLR4 and TLR5 signalling in B cells is required for the induction of optimal antibody responses to T-dependent antigens in mice. However, species-related differences clearly exist.21,24 It is well established that murine B cells respond to LPS via TLR4, but there are no existing data showing that human B cells respond to stimulation with LPS or flagellin. Collectively, the direct effects mediated by Pam3CSK4, R-837 and CpG-2006, shown in the present study, highlight the potential of TLRs as regulators of B-cell activation.
Taken together, tonsillar B cells exhibit a constitutively high level of several TLRs, in particular TLR1 and TLR10, which are not developmentally regulated during the differentiation process. In contrast to T cells, the TLR expression levels do not appear to be affected by an ongoing microbial infection such as chronic tonsillitis. In addition to the role as antibody-producing cells during the adaptive immune response, our study further emphasizes the unique ability of B cells to respond directly, via TLRs, to a certain range of microbial structures. This further stresses the importance of TLRs in our defence against invading pathogens.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council, the Swedish Heart Lung Foundation, the Swedish Association for Allergology, the Swedish Foundation for Health Care Science and Allergic Research, and by Lund University.
Abbreviations
- Ct
threshold cycle
- DAB
3,3′-diaminobenzidine
- DC
dendritic cell
- dsRNA
double-stranded viral RNA
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- FACS
fluorescence-activated cell sorter
- FITC
fluorescein isothiocyanate
- GAS
group A β-haemolytic streptococci
- GC
germinal centre
- HLA
human leucocyte antigen
- HRP
horseradish peroxidase
- IFN
interferon
- IgD
immunoglobulin D
- IgG
immunoglobulin G
- IL
interleukin
- LPS
lipopolysaccharides
- NK
natural killer
- ODN
oligodeoxynucleotide
- PAMP
pathogen-associated molecular pattern
- PB
peripheral blood
- PBL
peripheral blood lymphocytes
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PDC
plasmacytoid dendritic cells
- PE
phycoerythrin
- RT–PCR
reverse transcription–polymerase chain reaction
- SRBC
sheep red blood cells
- ssRNA
single-stranded viral RNA
- TBS
Tris-buffered saline
- TIR
Toll/IL-1 receptor
- TLR
Toll-like receptor
References
- 1.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 2.Abreu MT, Arditi M. Innate immunity and toll-like receptors: clinical implications of basic science research. J Pediatr. 2004;144:421–9. doi: 10.1016/j.jpeds.2004.01.057. [DOI] [PubMed] [Google Scholar]
- 3.Hallman M, Ramet M, Ezekowitz RA. Toll-like receptors as sensors of pathogens. Pediatr Res. 2001;50:315–21. doi: 10.1203/00006450-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Horner AA, Raz E. Do microbes influence the pathogenesis of allergic diseases? Building the case for Toll-like receptor ligands. Curr Opin Immunol. 2003;15:614–9. doi: 10.1016/j.coi.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 7.Heine H, Lien E. Toll-like receptors and their function in innate and adaptive immunity. Int Arch Allergy Immunol. 2003;130:180–92. doi: 10.1159/000069517. [DOI] [PubMed] [Google Scholar]
- 8.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 9.Wagner H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr Opin Microbiol. 2002;5:62–9. doi: 10.1016/s1369-5274(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 10.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes. inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–63. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 11.Bauer M, Heeg K, Wagner H, Lipford GB. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology. 1999;97:699–705. doi: 10.1046/j.1365-2567.1999.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–73. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–53. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 14.Kinhult J, Uddman R, Laan M, Linden A, Cardell LO. Pituitary adenylate cyclase-activating peptide inhibits neutrophil chemotaxis. Peptides. 2001;22:2151–4. doi: 10.1016/s0196-9781(01)00568-x. [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ, Banchereau J. The paths and molecular controls of peripheral B-cell development. Immunologist. 1996;4:55–66. [Google Scholar]
- 16.Hogerkorp CM, Bilke S, Breslin T, Ingvarsson S, Borrebaeck CA. CD44-stimulated human B cells express transcripts specifically involved in immunomodulation and inflammation as analyzed by DNA microarrays. Blood. 2003;101:2307–13. doi: 10.1182/blood-2002-06-1837. [DOI] [PubMed] [Google Scholar]
- 17.Airoldi I, Raffaghello L, Cocco C, Guglielmino R, Roncella S, Fedeli F, Gambini C, Pistoia V. Heterogeneous expression of interleukin-18 and its receptor in B-cell lymphoproliferative disorders deriving from naive, germinal center, and memory B lymphocytes. Clin Cancer Res. 2004;10:144–54. doi: 10.1158/1078-0432.ccr-1026-3. [DOI] [PubMed] [Google Scholar]
- 18.Gagro A, Toellner KM, Grafton G, et al. Naive and memory B cells respond differentially to T-dependent signaling but display an equal potential for differentiation toward the centroblast-restricted CD77/globotriaosylceramide phenotype. Eur J Immunol. 2003;33:1889–98. doi: 10.1002/eji.200323357. [DOI] [PubMed] [Google Scholar]
- 19.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–26. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen T, Lodahl M, Hancke S, Johnsen HE. In multiple myeloma clonotypic CD38- /CD19+ / CD27+ memory B cells recirculate through bone marrow, peripheral blood and lymph nodes. Leuk Lymphoma. 2004;45:1413–7. doi: 10.1080/10428190410001655157. [DOI] [PubMed] [Google Scholar]
- 21.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–6. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 23.Johansson-Lindbom B, Ingvarsson S, Borrebaeck CA. Germinal centers regulate human Th2 development. J Immunol. 2003;171:1657–66. doi: 10.4049/jimmunol.171.4.1657. [DOI] [PubMed] [Google Scholar]
- 24.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–50. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 25.Nagase H, Okugawa S, Ota Y, et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–82. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 26.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. Expression of toll-like receptors on B lymphocytes. Cell Immunol. 2005;236:140–5. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971–7. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohnhorst JO, Bjorgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjogren's syndrome. J Immunol. 2001;167:3610–8. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 30.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 31.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 32.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 33.Mansson A, Adner M, Cardell LO. Toll-like receptors in cellular subsets of human tonsil T cells: altered expression during recurrent tonsillitis. Respir Res. 2006;7:36. doi: 10.1186/1465-9921-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann G, Battiany J, Poeck H, Wagner M, Kerkmann M, Lubenow N, Rothenfusser S, Endres S. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33:1633–41. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 35.Tomai MA, Imbertson LM, Stanczak TL, Tygrett LT, Waldschmidt TJ. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell Immunol. 2000;203:55–65. doi: 10.1006/cimm.2000.1673. [DOI] [PubMed] [Google Scholar]
- 36.Lore K, Betts MR, Brenchley JM, et al. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171:4320–8. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 37.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]