Abstract
Immune defence against microbes depends in part on the production of antimicrobial peptides, a process that occurs in a variety of cell types but is incompletely understood. In this study, the mechanisms responsible for the induction of cathelicidin and β-defensin antimicrobial peptides were found to be independent and specific to the cell type and stimulus. Vitamin D3 induced cathelicidin expression in keratinocytes and monocytes but not in colonic epithelial cells. Conversely, butyrate induced cathelicidin in colonic epithelia but not in keratinocytes or monocytes. Distinct factors induced β-defensin expression. In all cell types, vitamin D3 activated the cathelicidin promoter and was dependent on a functional vitamin D responsive element. However, in colonic epithelia butyrate induced cathelicidin expression without increasing promoter activity and vitamin D3 activated the cathelicidin promoter without a subsequent increase in transcript accumulation. Induction of cathelicidin transcript correlated with increased processed mature peptide and enhanced antimicrobial activity against Staphylococcus aureus. However, induction of β-defensin-2 expression did not alter the innate antimicrobial capacity of cells in culture. These data suggest that antimicrobial peptide expression is regulated in a tissue-specific manner at transcriptional, post-transcriptional and post-translational levels. Furthermore, these data show for the first time that innate antimicrobial activity can be triggered independently of the release of other pro-inflammatory molecules, and suggest strategies for augmenting innate immune defence without increasing inflammation.
Keywords: Cathelicidin, colon mucosa, defensin, gene regulation, human, skin
Introduction
Defence of the skin and gastrointestinal epithelia against microbial pathogens involves both innate and adaptive immune responses. Several elements contribute to the innate immune defence system in these tissues, including physical and chemical barriers (e.g. the stratum corneum or the production of mucin) and recruitment of leukocytes such as neutrophils, macrophages, etc. In addition to these diverse antimicrobial strategies, the synthesis and secretion of small cationic peptides by epithelia has become recognized as an important mechanism for host defence.1 As effectors of innate immunity, antimicrobial peptides (AMPs) directly kill a broad spectrum of microbes including Gram-positive and Gram-negative bacteria as well as fungi and certain viruses. In addition, these peptides interact with the host itself, triggering events that complement their role as antibiotics.2 When combined, the functions of the AMPs suggest that they are an important, and previously underappreciated, component of the human immune defence system.
Most AMPs are cationic molecules binding to and interacting with the negatively charged membranes of microbes. In recent years an array of AMPs expressed by epithelial cells has been identified including the gene families known as defensins and cathelicidins.3,4 Cathelicidins are AMPs of which the pro-peptide precursors contain a conserved N-terminal ‘cathelin’ domain. In man, one cathelicidin gene, named CAMP, has been identified as coding for the pre-pro-protein hCAP18 which includes a C-terminal antimicrobial peptide called LL-37.5 LL-37 can be further processed to smaller peptides with enhanced antimicrobial activity or signalling properties.6,7 The AMP domain from cathelicidins of different species varies widely in sequence, composition and structure. In contrast, defensins are more uniform in their appearance. They are small cysteine-rich AMPs that mainly form β-sheet structures. Several human defensins have been identified and studied with respect to their expression, regulation and function.4
Cathelicidins and defensins are expressed at various epithelial surfaces, including the skin, colon and lower small intestine.8–12 Their relevance in human disease has been illustrated by several clinical correlations. Patients with atopic dermatitis lack appropriate AMP expression and are more susceptible to bacterial superinfections while psoriasis patients, who rarely develop bacterial skin infections, show high expression of epidermal AMPs.13 In the gastrointestinal tract, cathelicidins are involved in the gastric mucosal defence against Helicobacter pylori.14 Crohn's disease patients with NOD2 mutations show decreased levels of β-defensin expression. This deficiency has been suggested to lead to altered tolerance towards the intestinal flora and subsequently to trigger inflammation.15 In the colon, Shigella bacteria are able to penetrate the mucosa after down-regulating epithelial cathelicidin expression.16 Taken together, these clinical observations have supported laboratory models and have established a role for AMPs in the epithelial defence against microbial pathogens.
Despite the importance of AMP expression to immune defence, the molecular mechanisms of AMP regulation are poorly understood. Several studies on the regulation of expression of AMPs have been published, some with conflicting results. Recent work has found that stimuli not previously considered to be involved in innate immune activation, such as vitamin D3, can increase the expression of antimicrobial peptides. A vitamin D responsive element (VDRE) has been identified in the human cathelicidin gene (CAMP) promoter and activation of this VDRE by 1,25-dihydroxy-vitamin D3[1,25(OH)2VD3] will induce cathelicidin expression in keratinocytes and myeloid cells.17,18 Topical treatment of human skin with 1,25(OH)2VD3 has also been shown to enhance cathelicidin peptide expression.19 However, the capacity of 1,25(OH)2VD3 to alter the antimicrobial function of cells or tissues is unknown. Furthermore, the significance of this finding in light of observations of cathelicidin induction in vivo, a process that is initiated upon injury12 and that is not associated with any known alteration in vitamin signalling, is unclear. On the other hand, stimuli that are more consistently present in the wound environment, such as insulin-like growth factor-1 (IGF-1), or molecules present at tissues that express cathelicidin, such as the short-chain fatty acid butyrate, have been reported to increase cathelicidin abundance.11,20 The molecular mechanisms, however, are largely unknown. Short-chain acids such as butyrate inhibit histone deacetylation, which leads to chromatin remodelling and subsequent changes in the expression of various genes and alterations of a variety of signalling pathways.33 Other cationic peptides with antimicrobial activity but less clear function in vivo, such as the human β-defensins (HBD-1 to HBD-4), have been reported to have other stimuli for expression.9,21 It is unclear whether these stimuli overlap or are suitable for therapeutic use.
Based on current observations we hypothesized that antimicrobial peptides of the cathelicidin and β-defensin family would have distinct regulatory systems. In this study we examined the regulation of AMP expression in different tissues to gain insight into the mechanisms responsible for their expression and function. Distinct factors that reflected the respective microenvironments of the cell were found to modify cathelicidin and β-defensin expression; unique regulatory events were uncovered that depended on cell type. Furthermore, a direct association between the induction of antimicrobial peptide expression and antimicrobial activity was shown for the first time. Factors involved in the increase of antimicrobial activity are distinct from factors that induce classical chemokine responses, suggesting that a mechanism for the regulation of innate antimicrobial defence exists distinct from triggers of inflammation.
Materials and methods
Cell culture and stimuli
Normal human epidermal keratinocytes (NHEK) were grown in serum-free EpiLife® cell culture media (Cascade Biologics; Portland, OR) containing 0·06 mm Ca2+ and 1 × EpiLife® defined growth supplement (EDGS) at 37° under standard tissue culture conditions. Stock cultures were maintained for up to six passages in this medium with the addition of 50 U/ml penicillin and 50 μg/ml streptomycin. HaCaT keratinocytes were cultured in Dulbecco's modified Eagle's medium (DMEM) with 4·5 g/l glucose (BioWhittaker; Walkersville, MD) supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. HT-29 colon cells and U937 monocytes were grown in RPMI-1640 media (Sigma, St Louis, MO) and FET colon cells were grown in DMEM/F12 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. Cells at 50–70% confluence were stimulated with tumour necrosis factor-α (20 ng/ml; Chemicon, Temecula, CA), interleukin-1β (IL-1β; 20 ng/ml), IL-4 (50 ng/ml), IL-6 (50 ng/ml), IL-8 (20 ng/ml), IL-12 (50 ng/ml), IL-13 (50 ng/ml; all interleukins from R & D Systems, Minneapolis, MN), interferon-γ (200 U/ml; Sigma), interferon-β (200 U/ml; Roche, Indianapolis, IN), IGF-1 (100 ng/ml, Sigma), epidermal growth factor (EGF; 20 ng/ml; R & D Systems), flagellin (50 ng/ml; Alexis Biochemicals, Carlsbad, CA), lipopolysaccharide (1 μg/ml; Sigma), peptidoglycan (1 μg/ml; Fluka, Buchs, Switzerland), lipoteichoic acid (10 μg/ml; Sigma), phorbol 12-myristate 13-acetate (PMA; 20 μm; Sigma), Malp-2 (2 μm; Alexis), CpG (10 μg/ml; Sigma), poly(I:C) (25 μg/ml; Amersham, Piscataway, NJ), butyrate (2 mm; Sigma), lithocholic acid (50 μm, Sigma), calcium (1·7 mm), 1,25(OH)2VD3 (1–200 nm; Sigma), 25-OH vitamin D3 (100 nm; Fluka), all-trans retinoic acid (1 μm; Sigma), 9-cis retinoic acid (1 μm; Sigma) or ciglitazone (10 μm; Cayman Chemical, Ann Arbor, MN) for up to 24 hr. Furthermore, cells were irradiated with UVB light (100 mJ/cm2) or stimulated with Staphylococcus aureus or group A Streptococcus extracts. For analysis of the involvement of signalling pathways in the induction of cathelicidin cells were preincubated with the MEK-ERK inhibitor U0126 (20 μm; Calbiochem, San Diego, CA) for 30 min before stimulation. For inhibition of transcriptional activity cells were treated with actinomycin D (5 μg/ml; Sigma).
Real-time reverse transcription–polymerase chain reaction (RT-PCR)
After cell stimulation, total RNA was extracted using Trizol® (Invitrogen) and 1 µg RNA was reverse transcribed using iScript® (Bio-Rad, Hercules, CA). The expression of cathelicidin was evaluated using a FAM-CAGAGGATTGTGACTTCA-MGB probe with primers 5′-CTTCACCAGCCCGTCCTTC-3′ and 5′-CCAGGACGACACAGCAGTCA-3′. For GAPDH expression a VIC-CATCCATGACAACTTTGGTA-MGB probe with primers 5′-CTTAGCACCCCTGGCCAAG-3′ and 5′-TGGTCATGAGTCCTTCCACG-3′ was used. Predeveloped Taqman® assay probes (ABI, Foster City, CA) were used for the analyses of the expression of IL-8, CYP24A1 and involucrin. HBD-1 (forward primer: 5′-GTCGCCATGAGAACTTCCTACC-3′, reverse: 5′-CATTGCCCTCCACTGCTGAC-3′) and HBD-2 (forward: 5′-GGTGTTTTTGGTGGTATAGGCG-3′, reverse: 5′-AGGGCAAAGACTGGATGACA-3′) expression was evaluated using a SYBR Green® protocol according to the manufacturer (ABI). All analyses were performed in triplicate from two to five independent cell stimulation experiments in an ABI Prism® 7000 Sequence detection system. Fold induction relative to the vehicle-treated control was calculated using the 2(–ΔΔCt) method, where ΔCt is ΔCt(stimulant) − ΔCt(vehicle), ΔCt is Ct(cathelicidin) − Ct(GAPDH) and Ct is the cycle at which an arbitrary detection threshold is crossed. Results were considered significant when at least a three-fold difference in expression levels was detected and statistical analysis revealed P-values < 0·05.
Fluorescence immunohistochemistry
NHEK, HaCat, FET and HT-29 cells were grown on chamberslides and stimulated with 1,25(OH)2VD3 and butyrate for 24 hr. After methanol fixation and subsequent washings in phosphate-buffered saline (PBS), slides were blocked in 3% bovine serum albumin in PBS for 30 min at room temperature and stained with a polyclonal chicken anti-hCAP-18/LL-37 primary antibody or preimmune serum as described elsewhere.22 After washing in PBS 3 × 15 min, slides were reprobed with a fluorescein isothiocyanate-labelled goat anti-chicken antibody. After subsequent washings with PBS, slides were mounted in ProLong Anti-Fade reagent (Molecular probes, Eugene, OR) and evaluated with an Olympus BX41 microscope (Olympus, Melville, NY) at 400× magnification.
Promoter analysis and site-directed mutagenesis
To analyse cathelicidin promoter activity, different-sized fragments of the 5′ untranslated region of the human cathelicidin gene CAMP were cloned into a luciferase reporter plasmid and transfected into HaCat keratinocytes and HT-29 colon cells. Fragments of the 5′untranslated region of CAMP were amplified with sense 5′-CACACAGCTAGCGGCTCTCTTCCTCTCTGG-3′ (pGL3-300), 5′-CACACAGCTAGCCTCATGCCTCAGCTTGTA-3′ (pGL3-600), 5′-CACACAGCTAGCCTTCAGTGGCCTTCAGCA-3′ (pGL3-1000), 5′-CACACAGCTAGCCTTG GGTGGTGTCTGCCTA-3′ (pGL3-1200), 5′-CACACAGCTAGCGGAACCCCTGGACAACGG-3′ (pGL3-1500) and antisense 5′-GAGAGACTCGAGGTCTGCCTCCCTCTAGCC-3′ primers using human genomic DNA as a template. Primers were designed to introduce an NheI restriction site at the 5′ end and an XhoI restriction site at the 3′ end of the amplicon. The amplification products were cloned into the TOPO vector (Invitrogen) and transformed into Escherichia coli TOP10 OneShot competent cells (Invitrogen), following the manufacturer's guidelines. After DNA purification using the WizardPLus, SV Miniprep purification system (Promega, Madison, WI) constructs were digested with NheI and XhoI and then subcloned into the promoterless pGL3-basic firefly luciferase vector (Promega) to generate reporter plasmid pGL3-300, pGL3-600, pGL3-1000, pGL3-1200 and pGL3-1500. The functional role of a previously described VDRE17 for the transcription of CAMP was studied by site-directed mutagenesis of the VDRE. The VDRE at position −619 base pairs (bp) to −633 bp relative to the translation start site was deleted with sense 5′-AACTTCTGCTTCAGTGATTCTCAT-3′ and antisense 5′-ATGAGAATCACTGAAGCAGAAGTT-3′ primers using a protocol published by Prinzen et al.23 The resulting plasmid lacking the VDRE binding site cloned in the pGL3-basic vector was termed pGL-3–1500-VDRE. All resulting constructs were confirmed by sequencing. All promoter fragments started at position −9 bp relative to the translation initiation site. The actual sizes of the promoter elements were 299 bp for pGL3-300, 602 bp for pGL3-600, 1003 bp for pGL3-1000, 1287 bp for pGL3-1200 and 1569 bp for pGL3-1500.
Transfection and determination of promoter activity
For cathelicidin promoter studies HT-29, U937 and HaCat cells were seeded in 24-well plates (BD Biosciences, San Jose, CA) and used for transfection at 50–70% confluence. Cells were transfected with the indicated CAMP reporter plasmids and 0·1 μg of an internal-control Renilla luciferase expression plasmid (pRL-TK; Promega) by using 1·5 μl transfection reagent Fugene 6 (Roche) according to the manufacturer's instructions. Cells were stimulated with butyrate or 1,25(OH)2VD3 30 min before transfection and incubated for 24 hr before harvesting with 50 μl passive lysis buffer (Promega). Firefly luciferase activity from the CAMP pGL3 reporter vectors and Renilla luciferase activity were measured by the Dual Luciferase Assay system (Promega) in a luminometer (Optocomp I, MGM Instruments, Hamden, CT). Promoter activity was reported as the ratio between firefly and Renilla luciferase activities in each sample.
Western blot
HT-29 and HaCat cells were stimulated with butyrate, 1,25(OH)2VD3 or the vehicle for 24 hr and subsequently lysed in ice-cold lysis buffer (1% Triton-X in PBS containing proteinase inhibitors). After centrifugation, 10 min, 10 000 g, equal amounts of protein were mixed with loading buffer (0·25 m Tris–HCl, 10% sodium dodecyl sulphate (SDS), 10% glycerol, 5%β-mercaptoethanol) and loaded onto a 16% Tris–Tricine gel (GeneMate, Kaysville, UT). After separation, proteins were blotted onto a PVDF membrane (Millipore, Billerica, MA) and blocked in 5% milk (Bio-Rad) in Tris-buffered saline (TBS) with 0·1% Tween-20 for 1 hr at room temperature. After washings in TBS/0·1% Tween-20, membranes were stained with a rabbit polyclonal anti-vitamin D receptor (VDR) antibody (Abcam, Cambridge, MA), washed again in TBS/0·1% Tween-20 and reprobed with a horseradish peroxidase-coupled goat anti-rabbit antibody (DakoCytomation, Glostrup, Denmark). Stained protein was visualized using the Western Lightning® system (Perkin Elmer, Boston, MA). In another experiment HT-29 and HaCat cells were serum starved for 24 hr and subsequently stimulated with butyrate, 1,25(OH)2VD3 or EGF for 15 min with or without prior incubation with the MEK-ERK inhibitor U0126 (20 μm, 30 min). Cells were harvested, subjected to SDS–polyacrylamide gel electrophoresis using a 16% Tris–Tricine gel and blotted. Membranes were stained with a rabbit anti-phospho p44/42 MAPK antibody (Cell Signaling, Beverly, MA) and protein was visualized with the Western Lightning® system.
Surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI-TOF-MS)
Stimulated and control NHEK, HaCat and HT-29 cells were harvested in 1 ml of 1-m HCl and 1% trifluoroacetic acid, sonicated for 3 min on ice, rotated at 4° overnight and subsequently centrifuged for 10 min at 18 000 g. Supernatants were transferred to new tubes, lyophilized, and dissolved in 100 μl of RIPA buffer containing protease inhibitors (Roche). Protein chips (RS100 ProteinChip array®, Ciphergen Biosystems, Fremont, CA) were coated with 4 µl of anti-LL-37 rabbit antibody for 2 hr at room temperature, followed by blocking with 0·5 m ethanolamine in PBS (pH 8·0). After washing three times with PBS/0·5% Triton X-100, proteinchips were assembled in the Bioprocessor™ (Ciphergen) reservoir, samples (50 μl) were applied in duplicates and incubated 2 hr at room temperature. Protein chips were washed twice with RIPA buffer, once with PBS/0·5% Triton X-100, and three times with PBS. Proteinchips were then soaked in 10 mm HEPES buffer and spots were air-dried. Then 0·5 μl of energy absorbance matrix (50% saturated α-cyano-4-hydroxy cinnamic acid in 50% acetonitrile, 0·5% trifluoracetic acid) was applied twice, and all spots were air-dried again. Samples were analysed on a SELDI mass analyser PBS IIC with a linear time-of-flight mass spectrometer (Ciphergen) using time-lag focusing. Mass was calibrated with peptide standards (All-in-1 peptide standard, Ciphergen).
Antimicrobial assay
Antimicrobial activity of stimulated and unstimulated epithelial cells was determined using a modified protocol described elsewhere.24 Cells were grown and stimulated without antibiotics, harvested in 100 μl sterile H2O and sonicated on ice for 20 min. For solution killing assays, S. aureusΔmprF25 was grown in tryptic soy broth (TSB; Sigma) over night and then subcultured in 20% TSB, 25 mm NaHCO3, 1 mm NaHPO4 until log phase was reached. Twenty thousand bacteria (optical density at 600 nm of 1·0 corresponds to 3·75 × 109 colony-forming units/ml) were incubated with various cell lysate concentrations at 37° in 20% TSB, 25 mm NaHCO3, 1 mm NaHPO4. Bacterial growth over time was determined by optical density at 600 nm.
Results
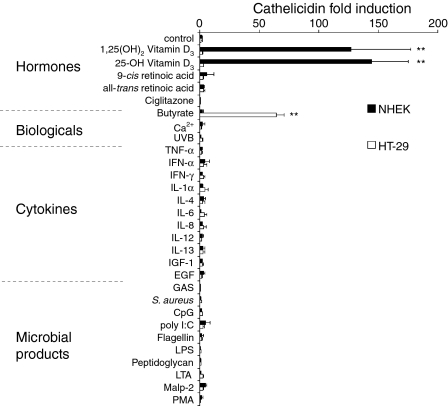
We hypothesized that the regulation of AMP expression would be dependent on the peptide, the tissue environment in which it is expressed, and the stimulus. To investigate if stimuli of AMP gene expression are similar between the skin and the colon, we performed a systematic analysis of the expression of human cathelicidin, HBD-1 and HBD-2. Cathelicidin expression was studied in normal keratinocytes, the keratinocyte cell line HaCat and the colon epithelial cell lines HT-29 and FET. An array of potential stimuli was examined including hormones, biological stimuli, cytokines and microbial products. Of all the factors tested, 1,25(OH)2VD3 and its precursor, 25-OH VD3, induced the largest increase in cathelicidin expression in normal keratinocytes (Fig. 1). Cathelicidin expression was induced after 8 hr with strongest effects after 24 hr, and similar effects were observed in the keratinocyte cell line HaCat (not shown). In contrast, butyrate was the major inducer of cathelicidin expression in colon epithelial cells (Fig. 1). Importantly, the induction of cathelicidin by vitamin D3 in keratinocytes, or butyrate in colon epithelia, was cell-type specific; each was inactive in the other epithelial cell.
Figure 1.
Cathelicidin mRNA expression in keratinocytes and colon epithelial cells. Normal human keratinocytes (NHEK) and colonocytes (HT-29) were stimulated with hormones, biological stimuli, cytokines or microbial products for 24 hr. Concentrations selected for this study exceeded those previously described to be effective in these cells (see Materials and methods). Abundance of mRNA was determined by real-time RT-PCR for cathelicidin and the housekeeping gene GAPDH and normalized to vehicle treated controls. Data shown are means (± SD) of the results from a single stimulation experiment performed in triplicates and are representative of at least three independent experiments. (**: P < 0·01; Student's t-test).
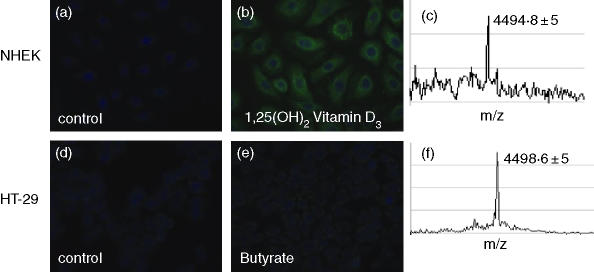
To investigate whether induction of cathelicidin mRNA correlates with cathelicidin protein expression, stimulated cells were stained with an antibody specific to LL-37. Keratinocytes (NHEK) showed enhanced immunoreactivity after stimulation with 1,25(OH)2VD3 while colon cells (HT-29) showed more cathelicidin peptide expression after butyrate treatment (Fig. 2). Butyrate had no effect on cathelicidin immunoreactivity in keratinocytes and 1,25(OH)2VD3 had no effect on colon epithelial cells (not shown). To confirm that immunostaining correlates with the presence of active cathelicidin peptide, stimulated cells were subjected to acid extraction and SELDI-TOF analysis. In both cell types a peptide with a molecular mass of 4494 Da corresponding to LL-37 was detected (Fig. 2).
Figure 2.
Cathelicidin peptide expression in keratinocytes and colon epithelial cells. (a–c) NHEK grown on chamber-slides were stimulated with the vehicle, or 1,25(OH)2 VD3; (d–f) colonocytes (HT-29) were stimulated with the vehicle or butyrate. Cells were stained with a polyclonal anti-LL-37 antibody and nuclei were detected with DAPI in (a,b,d,e). Immunofluorescence is displayed at 400× magnification. Processing to active cathelicidin peptide was evaluated by SELDI-TOF analyses of NHEK and HT-29 cells in (c) and (f) after stimulation with VD3 or butyrate, respectively. A peak at 4 494 Da corresponding to the mature LL-37 peptide was detected in NHEK and HT-29 cells. Data from one representative of three independent experiments are displayed.
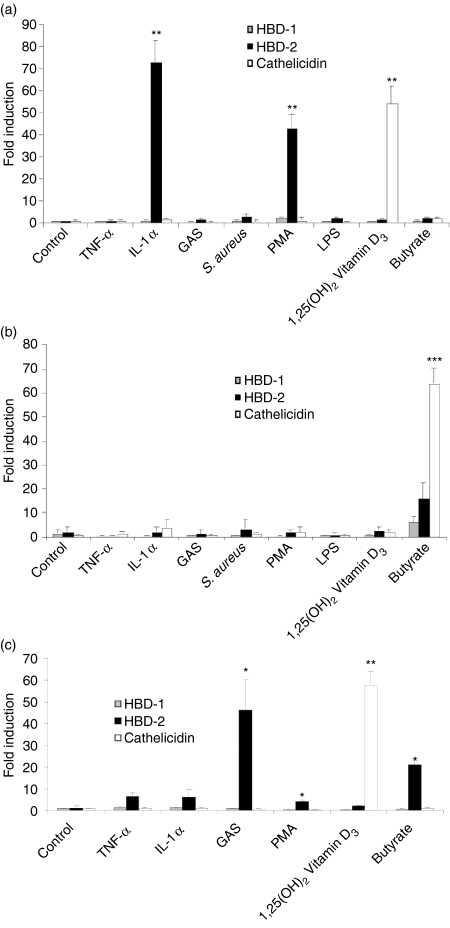
To investigate cell-specific expression of HBD-1 and HBD-2, and directly compare with cathelicidin expression, keratinocytes, colon epithelial cells and monocytes were exposed to a similar panel of potential stimuli. IL-1β and PMA were identified as strong inducers of HBD-2 in keratinocytes (Fig. 3a) and HaCat cells (not shown). None of the factors tested induced HBD-2 expression in HT-29 (Fig. 3b) and FET colon epithelial cells (not shown). Tumour necrosis factor-α, an extract of group A Streptococcus, PMA and butyrate were found to induce HBD-2 in monocytes (Fig. 3c). None of the stimuli changed HBD-1 expression under these conditions.
Figure 3.
Differential expression of HBD-1, HBD-2 and cathelicidin. The induction of human β-defensin 1 (HBD-1), human β-defensin 2 (HBD-2) and cathelicidin were directly compared by real-time RT-PCR. (a) Normal human keratinocytes, (b) HT-29 colon epithelial cells and (c) U937 monocytes were stimulated with a panel of potential stimuli at concentrations described in Materials and methods for 24 hr. Data shown are means (± SD) of a single experiment performed in triplicates and is representative of three independent experiments. (*P < 0·05; **P < 0·01; ***P < 0·001; Student's t-test).
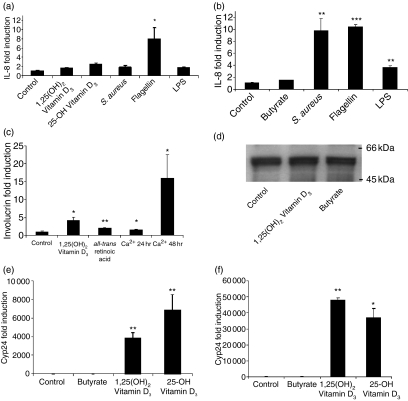
To test whether keratinocytes and colon cells show a correlation between the expression of cathelicidin and the induction of pro-inflammatory chemokines, changes in IL-8 mRNA expression were evaluated. Flagellin was the strongest inducer of IL-8 in keratinocytes (Fig. 4a), flagellin, Staphylococcus aureus extract and lipopolysaccharide were the strongest inducers of IL-8 in colon cells (Fig. 4b). Vitamin D3 and butyrate had no effect on IL-8 expression in these assays.
Figure 4.
AMP expression dissociates from cytokine expression and induction of differentiation or vitamin D response. IL-8 expression was evaluated by real-time RT-PCR in (a) Normal keratinocytes and (b) HT-29 colon cells after stimulation with different factors as described in Materials and methods. (c) Involucrin expression, as a surrogate marker for keratinocyte differentiation, was analysed by real-time RT-PCR. (d) The expression of the vitamin D receptor and the effect of 1,25(OH)2 vitamin D3 on vitamin D responsive genes was investigated in colon epithelial cells. HT-29 cells were stimulated with the control (lane 1), 1,25(OH)2 VD3 (lane 2) or butyrate (lane 3), harvested and analysed by Western blot employing a specific anti-VDR antibody. A band at approx. 50 kDa was detected corresponding to VDR protein. In (e) and (f), the expression of the vitamin D responsive gene Cyp24A1 (24-hydroxylase) was analysed by real-time RT-PCR in colon epithelial cells (e) or NHEK (f) stimulated with butyrate, 1,25(OH)2 VD3, 25-OH VD3 or the vehicle control. All data shown are means (± SD) of a single experiment performed in triplicate and representative of one of at least three independent experiments. (*P < 0·05; **P < 0·01; ***P < 0·001; Student's t-test).
As 1,25(OH)2VD3 is known to induce differentiation in keratinocytes,26 we next investigated the relationship between keratinocyte differentiation and cathelicidin expression. As expected, an increase in extracellular Ca2+, 1,25(OH)2VD3 and all-trans retinoic acid each induced keratinocyte differentiation as indicated by an increase in involucrin expression. However, only 1,25(OH)2VD3 induced cathelicidin (Figs 1 and 4c).
To investigate if the inability of vitamin D3 to induce cathelicidin in colon cells was a result of a defect in vitamin D signalling, the expression of the VDR and the effect of 1,25(OH)2VD3 on vitamin D responsive genes was investigated in HT-29 and FET colon cells. Colon cells express the VDR, and this expression is not changed after butyrate or 1,25(OH)2VD3 treatment (Fig. 4d). Furthermore, colon cells (HT-29, FET) responded to 1,25(OH)2VD3 with an increase in the vitamin D responsive gene Cyp24A1 (24-hydroxylase) (Fig. 4e). Lithocholic acid – a recently identified VDR ligand in colon cells – also induced Cyp24A1 in colon cells but failed to induce cathelicidin expression in these cells (not shown). As expected 1,25(OH)2VD3 induced the expression of Cyp24A1 in NHEK and HaCat cells (Fig. 4f).
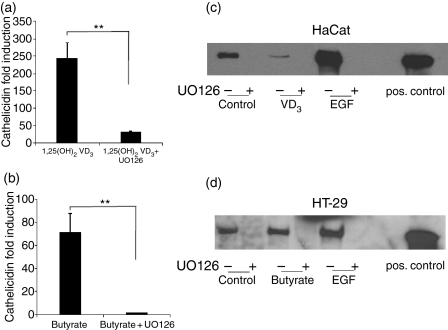
The MEK-ERK signalling pathway has been shown to be involved in butyrate-mediated cathelicidin induction in colon epithelial cells.11 To determine if this pathway is also required for vitamin D3 activity, keratinocytes were first treated with inhibitors of MEK-ERK or other signalling intermediates before attempted induction of cathelicidin expression. The MEK-ERK inhibitor (U0126) blocked the induction of cathelicidin by 1,25(OH)2VD3 in keratinocytes (Fig. 5a) and by butyrate in colon cells (Fig. 5b). Inhibition of EGF signalling (AG1478) or pretreatment with a metalloproteinase inhibitor (Galardin) had no effect on cathelicidin induction in either cell type (not shown). To determine if ERK phosphorylation was critical for cathelicidin induction, both cell types were evaluated for phospho-ERK by Western blot analyses (Fig. 5c,d). These data confirm the activity of the ERK inhibitor U0126 and confirm that EGF induces ERK phosphorylation despite not inducing cathelicidin expression. Therefore ERK phosphorylation was necessary but not sufficient for cathelicidin induction.
Figure 5.
MEK-ERK activation is necessary but not sufficient for induction of cathelicidin expression in both skin and gut epithelial cells. (a) Keratinocytes (HaCat) were stimulated with 1,25(OH)2 VD3 with or without prior incubation with the MEK-ERK inhibitor U0126 (20 μm, 30 min) and cathelicidin expression assessed by real-time RT-PCR. (b) HT-29 colon cells were stimulated with butyrate with or without prior U0126 treatment and cathelicidin expression was analysed. Data shown are means (± SD) of a single experiment performed in triplicates and are representative of three independent experiments. (**P < 0·01; Student's t-test). (c) To determine if MEK-ERK activation was critical for cathelicidin induction keratinocytes were stimulated with the control (lane 1), 1,25(OH)2 VD3 (lane 3) or EGF (lane 5) for 15 min with or without prior incubation with U0126 (lanes 2,4,6) and subjected to SDS–PAGE and Western blot. (d) HT-29 colon cells were stimulated with the control (lane 1), butyrate (lane 3) or EGF (lane 5) for 15 min with or without prior incubation with U0126 (lanes 2,4,6) and analysed by Western blot. As a positive control a phosphorylated ERK protein was used on both blots (lane 8). Blots were stained with a specific phospho-ERK antibody and visualized by chemiluminescence.
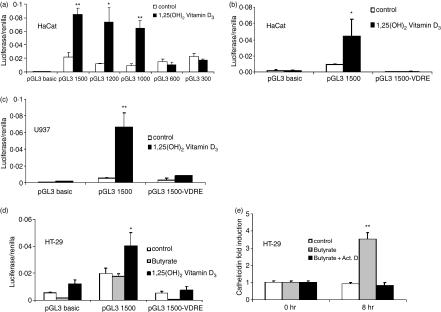
To identify the promoter elements responsible for cathelicidin regulation, different-sized fragments of the 5′ untranslated region of the human cathelicidin gene CAMP were cloned into a luciferase reporter plasmid and transfected into HaCat keratinocytes and HT-29 colon cells. Site directed mutagenesis was used to generate reporter plasmids lacking the VDRE in this promoter. Constructs lacking the VDRE (pGL3-300 and pGL3-600, Fig. 6a) or constructs with a deleted VDRE site (pGL3 1500-VDRE), lost responsiveness to 1,25(OH)2VD3 stimulation when transfected into HaCat cells and monocytes (Fig. 6b,c). Interestingly, 1,25(OH)2VD3 resulted in enhanced promoter activity in HT-29 cells (Fig. 6d) despite an inability of 1,25(OH)2VD3 to increase steady-state levels of cathelicidin transcript or protein in these cells. In contrast, none of the CAMP promoter constructs was induced in colon cells stimulated with butyrate but baseline activity was decreased in constructs with a deleted VDRE site. Despite the inability of butyrate to increase CAMP promoter activity in colon cells, butyrate-induced cathelicidin mRNA expression in colon cells was blocked by inhibition of mRNA transcription with actinomycin D (Fig. 6e).
Figure 6.
CAMP promoter activity in keratinocytes, colon cells and monocytes. Fragments of the 5′UTR of the human cathelicidin gene CAMP were cloned into a luciferase reporter plasmid. (a) HaCat keratinocytes were transfected with different fragments of the CAMP promoter and stimulated with 1,25(OH)2 VD3 (100 nm). After 24 hr cells were harvested and luciferase activity assayed. Only fragments containing the VDRE at −619 bp to −633 bp relative to the translation start site showed increased transcriptional activity after stimulation. HaCat keratinocytes (b) and U937 monocytes (c) were transfected with promoter constructs containing an intact (pGL3 1500) or deleted VDRE (pGL3 1500-VDRE), stimulated with 1,25(OH)2 VD3 and subsequently luciferase activity measured. Deletion of the VDRE completely blocked VD3-induced transcriptional activity in both cell types. (d) HT-29 colon cells were transfected with pGL3 1500 and pGL3 1500-VDRE construct and stimulated with butyrate (2 mm) or 1,25(OH)2 VD3. Vitamin D3 increased transcriptional activity in HT-29 cells but did not increase mRNA abundance (Fig. 1), while butyrate had no effect on transcriptional activity of the pGL3 1500 construct. (e) To investigate if butyrate increases cathelicidin expression through a transcriptional mechanism, HT-29 cells were stimulated in the presence of Actinomycin D and cathelicidin evaluated by real-time PCR after 8 hr. Despite an inability to CAMP promoter activity, butyrate induced cathelicidin mRNA expression in colon cells was blocked by inhibition of mRNA transcription by Actinomycin D. All data shown are means (± SD) of single experiments performed in triplicates and are representative of at least three independent experiments. (*P < 0·05; **P < 0·01; Student's t-test).
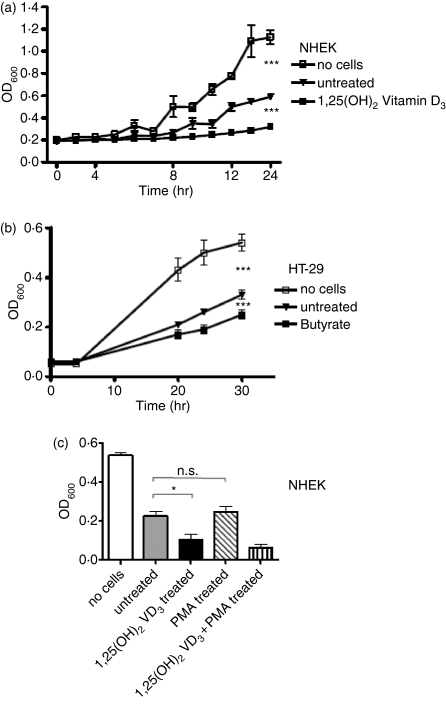
Observations that induction of AMP expression is cell-type specific and differs between cathelicidins, HBD-1 and HBD-2, permitted direct evaluation of a correlation between AMP expression and antimicrobial activity. Lysates of cells induced to increase each AMP were tested for their ability to inhibit the growth of S. aureusΔmprF (Fig. 7). Lysates derived from keratinocytes stimulated to increase cathelicidin expression with 1,25(OH)2VD3 increased their ability to inhibit bacterial growth compared to unstimulated cells (Fig. 7a). Similarly, the cathelicidin increase in colon cells stimulated with butyrate also increased antimicrobial activity (Fig. 7b). Notably, 1,25(OH)2VD3 and butyrate showed no antimicrobial activity themselves (data not shown). Conversely, keratinocyte stimulation with PMA, a strong inducer of HBD-2 expression, did not enhance antimicrobial activity (Fig. 7c). Treatment of keratinocytes with a combination of 1,25(OH)2VD3 and PMA showed similar activity compared to stimulation with 1,25(OH)2VD3 alone.
Figure 7.
Antimicrobial activity of stimulated keratinocytes and colon epithelial cells. (a) NHEK were stimulated with 1,25(OH)2 VD3 for 24 hr, cells were harvested and cell lysates were coincubated with S. aureusΔmprF and bacterial growth was monitored over time to determine antimicrobial activity. Conditions not containing cell lysates or containing cell lysates from unstimulated cells were used as controls. (b) HT-29 colon cells were stimulated with butyrate for 24 hr and cell lysates were incubated with S. aureusΔmprF. (c) NHEK were stimulated with 1,25(OH)2 VD3, or PMA to induce HBD2 expression and cell lysates were incubated with S. aureusΔmprF. Bacterial growth was measured by OD600. All data shown are means (± SD) of triplicates. (*P < 0·05; **P < 0·01; Student's t-test).
Discussion
Epithelial surfaces like the skin and gastrointestinal mucosa depend on the expression of antimicrobial peptides (AMP) for protection against microbes. This conclusion is supported by observations in humans that deficient AMP expression is associated with increased infection13,27 and by similar observations in animal models.28–32 Little was known about the mechanisms that control AMP expression and function. In the current study we show that a unique system to control AMP function exists by demonstrating that AMP expression is differentially regulated between cell types and between AMP gene families, and that expression is regulated at transcriptional and post-transcriptional levels.
We found that different factors induced cathelicidin and human β-defensin expression in the two epithelial cell types investigated here. The hormonally active form of vitamin D3, 1,25(OH)2VD3, and its precursor were the only large inducers of cathelicidin in keratinocytes and butyrate was the sole inducer of cathelicidin in colon epithelial cells. These responses were cell-type specific. Other biological, hormonal and microbial factors had no effect on cathelicidin expression in either cell type. IGF-1 has been reported to induce cathelicidin in keratinocytes grown in organotypic culture.20 However, in our experiments IGF-1 had no significant effect on cathelicidin mRNA abundance in monolayer keratinocytes. This difference probably reflects both the difference in culture conditions from the previous study as well as the much greater relative potency of 1,25(OH)2VD3 to increase expression. In contrast to observations of cathelicidin expression, experiments performed under identical culture conditions showed that cytokines like IL-1β induced HBD-2 in keratinocytes and monocytes but did not affect cathelicidin expression. HBD-1 expression was not changed after stimulation in our experiments in either cell type.
In contrast to observations in keratinocytes, colon cells did not respond to 1,25(OH)2VD3 treatment. However, the short-chain fatty acid butyrate, which was inactive in keratinocytes, did increase cathelicidin in colon cells. Interestingly, butyrate did not increase activity of the cathelicidin promoter constructs that were activated in keratinocytes by 1,25(OH)2VD3. This lack of proximal promoter activity was observed despite use of up to 6 kilobases 5′ upstream to the CAMP open reading frame and evidence that inhibition of transcriptional activity by actinomycin blocked an increase in steady-state cathelicidin mRNA in colon cells. Further illuminating these findings were the observations that 1,25(OH)2VD3 did activate the cathelicidin promoter in colon epithelial cells but had no effect on mRNA abundance and peptide expression in these cells. These data suggest that control of cathelicidin abundance can be influenced by trans-acting enhancer elements as well as changes in transcript stability. Importantly, the current work shows that such factors involved in the control of cathelicidin expression operate in a cell-specific manner and that, dependent on the cell, control may occur transcriptionally or post-transcriptionally.
Enhanced cathelicidin expression following stimulation of keratinocytes and monocytes with 1,25(OH)2VD3 has been reported earlier17,18 but the mechanism, relative potency and functional consequences of this observation were unclear. We confirmed by site-directed mutagenesis that a VDRE is necessary for 1,25(OH)2VD3 to induce cathelicidin transcription in keratinocytes and monocytes. Overall, 1,25(OH)2VD3 induced an increase in keratinocyte cathelicidin mRNA abundance, promoter activity, immunoreactive protein and the biologically active form LL-37. This correlated with increased antimicrobial activity, thus demonstrating that a single molecule can both increase cathelicidin expression and activation.
Active LL-37 peptide was detected by SELDI-TOF in both investigated epithelial cell types. Recently we identified serine proteases of the kallikrein family to be responsible for cathelicidin hCAP18 processing in skin (Yamasaki et al. in press). The proteases involved in cathelicidin processing in colon epithelium are unidentified. Also, the site of hCAP-18 processing in our cell systems is unknown. However, detection of active LL-37 and increased antimicrobial activity in cell lysates suggests that either processed cathelicidin peptide is stored in the cells or is attached to the cell membrane after secretion.
Short-chain fatty acids such as butyrate are generated in the colonic lumen by fermentation of ingested carbohydrates.33 In contrast, skin is the major source of 1,25(OH)2VD3 in humans. Under the influence of UVB irradiation vitamin D3 is produced and hydroxylated to hormonally active 1,25(OH)2VD3. Keratinocytes possess the enzymatic machinery to produce 1,25(OH)2VD3 from 25-OH VD3, which also induced cathelicidin expression in this study. In our experiments UVB did not induce cathelicidin expression in cultured keratinocytes. This is in contrast to the recent report by Mallbris et al. observing that UVB irradiation of healthy skin leads to induction of cathelicidin expression.34 In their study, UVB simultaneously induced VDR expression, suggesting a dual mechanism through which UVB might increase cathelicidin in the skin: by increasing 1,25(OH)2VD3 concentration and/or enhancing vitamin D responsiveness. Consideration of these observations as a whole supports the conclusion that the activity of factors which influence AMP expression is relevant to the cell microenvironment.
The pattern of cathelicidin expression in skin and colon tissues also suggests a correlation of cathelicidin abundance and the state of cellular differentiation.10–12 1,25(OH)2VD3 induces differentiation in keratinocytes,26 however, induction of differentiation is not sufficient to induce cathelicidin expression as other differentiation-inducing agents like calcium or retinoic acid did not affect cathelicidin abundance. This dissociation of cell differentiation and cathelicidin expression is in accordance with data reported earlier for colon cells and monocytes.11,18
Taken together, the observations here show that distinct mechanisms function to regulate the ability of epithelial cells to express cathelicidins or β-defensins. In some cases this response leads to a direct increase in the capacity of the cell to inhibit bacterial growth. This protective effect was observed subsequent to an increase in cathelicidin while no such activity could be seen with an increase in HBD-2. Care must be taken in drawing conclusions from this observation because the inability of HBD-2 to provide protection may be a consequence of the microbial challenge examined or the cell system used. Antimicrobial activity of cathelicidin and defensins is affected by test conditions such as salt concentrations or the presence of bicarbonate.35 It is noteworthy that cathelicidin was functional under these conditions while not permissive for antimicrobial activity mediated by HBD-2.
We demonstrate for the first time that selective pharmacological manipulation of AMP expression results in enhanced antimicrobial activity and that this can be accomplished through activation of elements that are not typical of pro-inflammatory signalling events. These observations suggest the potential for therapeutic approaches through the induction of AMP expression as an alternative in preventing or treating infections. As AMP expression is cell specific, the modulation of AMP expression may be approached in a targeted and tissue-specific manner without enhancing local inflammation.
Acknowledgments
We are grateful to John Carethers (Division of Gastroenterology, UCSD) for provision of HT-29 and FET colon cells and to Dr Norbert Fusening for providing HaCat cells. This work was supported by a VA Merit award and by National Institutes of Health grants NIH/NIAID HHSN26620040029C, ADB Contract #N01-AI-40029AI48176, AI052453, and AR45676 to R.L.G., and a grant from the Deutsche Akademie der Naturforscher Leopoldina to J.S. (BMBF-LPD 9901/8–119).
Abbreviations
- AMP
antimicrobial peptides
- EGF
epidermal growth factor
- HBD-1
human β-defensin-1
- IGF-1
insulin-like growth factor-1
- IL-1β
interleukin-1β
- NHEK
normal human epidermal keratinocytes
- 1,25(OH)2VD3
1,25-dihydroxy-vitamin D3
- PBS
phosphate-buffered saline
- SELDI-TOF
surface-enhanced laser desorption ionization time-of-flight
- VDR
vitamin D receptor
- VDRE
vitamin D responsive element
References
- 1.Gallo RL. Antimicrobial Peptides in Human Health and Disease. Norwich: U.K. Horizon Press; 2005. [Google Scholar]
- 2.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaiou M, Gallo RL. Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med. 2002;80:549–61. doi: 10.1007/s00109-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 4.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–7. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 5.Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–32. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen OE, Gram L, Johnsen AH, et al. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: a novel mechanism of generating antimicrobial peptides in vagina. J Biol Chem. 2003;278:28540–6. doi: 10.1074/jbc.M301608200. [DOI] [PubMed] [Google Scholar]
- 7.Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol. 2004;172:3070–7. doi: 10.4049/jimmunol.172.5.3070. [DOI] [PubMed] [Google Scholar]
- 8.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387(6636):861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 9.O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–24. [PubMed] [Google Scholar]
- 10.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953–63. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schauber J, Svanholm C, Termén S, et al. The expression of the cathelicidin LL-37 is modulated by short-chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:743–51. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorschner RA, Pestonjamasp VK, Tamakuwala S, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–7. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 13.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 14.Hase K, Murakami M, Iimura M, et al. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125:1613–25. doi: 10.1053/j.gastro.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Wehkamp J, Harder J, Weichenthal M, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–64. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson G. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180–5. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 17.Wang T-T, Nestel F, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 18.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 19.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–2. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol. 2003;170:5583–9. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 21.Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522–9. doi: 10.1111/j.0022-202X.2004.23234.x. [DOI] [PubMed] [Google Scholar]
- 22.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–81. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prinzen C, Muller U, Endres K, Fahrenholz F, Postina R. Genomic structure and functional characterization of the human ADAM10 promoter. Faseb J. 2005;19:1522–4. doi: 10.1096/fj.04-3619fje. [DOI] [PubMed] [Google Scholar]
- 24.Lee PH, Ohtake T, Zaiou M, Murakami M, Rudisill JA, Lin KH, Gallo RL. Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc Natl Acad Sci USA. 2005;102:3750–5. doi: 10.1073/pnas.0500268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peschel A, Jack RW, Otto M, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with 1-lysine. J Exp Med. 2001;193:1067–76. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–44. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 27.Pütsep K, Carlsson G, Boman H, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–9. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 28.Cole AM, Shi J, Ceccarelli A, Kim YH, Park A, Ganz T. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood. 2001;97:297–304. doi: 10.1182/blood.v97.1.297. [DOI] [PubMed] [Google Scholar]
- 29.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 30.Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol. 2005;174:4901–7. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 31.Morrison GM, Davidson DJ, Dorin JR. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 1999;442:112–16. doi: 10.1016/s0014-5793(98)01630-5. [DOI] [PubMed] [Google Scholar]
- 32.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414(6862):454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 33.Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994;35(1 Suppl.):S35–8. doi: 10.1136/gut.35.1_suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallbris L, Wiegleb Edstrom D, Sundblad L, Granath F, Stahle M. UVB upregulates the antimicrobial protein hCAP18 mRNA in human skin. J Invest Dermatol. 2005;125:1072–4. doi: 10.1111/j.0022-202X.2005.23872.x. [DOI] [PubMed] [Google Scholar]
- 35.Dorschner RA, Lopez-Garcia B, Peschel A, et al. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 2006;20:35–42. doi: 10.1096/fj.05-4406com. [DOI] [PubMed] [Google Scholar]