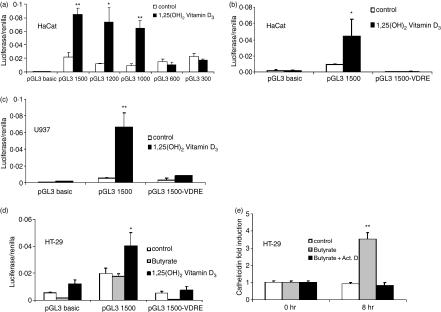
Figure 6.
CAMP promoter activity in keratinocytes, colon cells and monocytes. Fragments of the 5′UTR of the human cathelicidin gene CAMP were cloned into a luciferase reporter plasmid. (a) HaCat keratinocytes were transfected with different fragments of the CAMP promoter and stimulated with 1,25(OH)2 VD3 (100 nm). After 24 hr cells were harvested and luciferase activity assayed. Only fragments containing the VDRE at −619 bp to −633 bp relative to the translation start site showed increased transcriptional activity after stimulation. HaCat keratinocytes (b) and U937 monocytes (c) were transfected with promoter constructs containing an intact (pGL3 1500) or deleted VDRE (pGL3 1500-VDRE), stimulated with 1,25(OH)2 VD3 and subsequently luciferase activity measured. Deletion of the VDRE completely blocked VD3-induced transcriptional activity in both cell types. (d) HT-29 colon cells were transfected with pGL3 1500 and pGL3 1500-VDRE construct and stimulated with butyrate (2 mm) or 1,25(OH)2 VD3. Vitamin D3 increased transcriptional activity in HT-29 cells but did not increase mRNA abundance (Fig. 1), while butyrate had no effect on transcriptional activity of the pGL3 1500 construct. (e) To investigate if butyrate increases cathelicidin expression through a transcriptional mechanism, HT-29 cells were stimulated in the presence of Actinomycin D and cathelicidin evaluated by real-time PCR after 8 hr. Despite an inability to CAMP promoter activity, butyrate induced cathelicidin mRNA expression in colon cells was blocked by inhibition of mRNA transcription by Actinomycin D. All data shown are means (± SD) of single experiments performed in triplicates and are representative of at least three independent experiments. (*P < 0·05; **P < 0·01; Student's t-test).