Abstract
Interleukin-10 (IL-10) is a potent anti-inflammatory cytokine that suppresses the production of tumour necrosis factor-α (TNF-α) by monocytes and macrophages. Suppressor of cytokine signalling-3 (SOCS3), a negative regulator of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, is induced following IL-10 exposure but recent studies in mice suggest that SOCS3 only targets gp-130-dependent signal transduction pathways. Understanding the signalling pathways responsible for IL-10-mediated effects in primary human monocytes is relevant to human inflammatory disease and necessary for the identification of potential therapeutic targets. An adenoviral transfection system was used to express different levels of SOCS3 (quantified experimentally with its tag green fluorescent protein (GFP)) with the aim of investigating the role of SOCS3 in LPS-induced and IL-10-mediated suppression of TNF-α production by non-transformed human monocytes. SOCS3 over-expression had no effect on TNF-α mRNA levels induced by LPS or LPS plus IL-10, or on IL-10 phosphorylation of STAT3, STAT1 and ERK1/2. When data from all donors were combined, adenoviral overexpression of SOCS3 significantly reversed the suppressive effects of IL-10 on LPS-induced TNF-α production after 2 hr. However, there was a direct correlation between mean GFP intensity (extent of viral infection) and extent of reversal of IL-10's inhibitory effects. Physiological levels of SOCS3 detected in IL-10-exposed human monocytes had no effect on LPS-induced TNF-α production. Although overexpression of SOCS3 to supraphysiological levels transiently antagonized the regulatory properties of IL-10 by a post-transcriptional mechanism, these findings suggest that under pathological conditions SOCS3 does not control LPS-activation or the anti-inflammatory properties of IL-10 in primary human monocytes.
Keywords: human monocytes, SOCS3, TNF-α, adenovirus, IL-10
Introduction
Interleukin-10 (IL-10) was first identified as a ‘cytokine synthesis inhibitory factor’ produced by the murine T helper type 2 (Th2) subset of CD4+ T lymphocytes, that suppressed interferon-γ (IFN-γ) production by Th1 cells.1 IL-10 is now recognized as a pleiotropic molecule that inhibits the production of pro-inflammatory cytokines by activated monocytes including the two most important cytokines driving chronic inflammation, tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). The first evidence that IL-10 is a potent immunosuppressive molecule in vivo came from studies showing that IL-10 knock-out mice developed chronic enterocolitis,2,3 which was reversed following administration of IL-10.2 IL-10 deficiencies have been reported in a number of inflammatory diseases such as inflammatory bowel disease and psoriasis. Clinical trials have shown that IL-10 administration to patients with rheumatoid arthritis or inflammatory bowel disease is of limited use [for review see 4], and highlight the need to study signalling processes in human cells. Clearly, the development of new therapeutic strategies for inflammatory diseases depends upon a better understanding of the molecular mechanisms contributing to the anti-inflammatory effects of molecules such as IL-10.
Many studies have examined the anti-inflammatory properties of IL-10 but with conflicting results. This may reflect the use of murine macrophages or immortalized cell lines versus human monocytes [for review see 5]. For example, p38 activity is required for IL-10 regulation of TNF-α production in murine macrophages6 but not human macrophages.7 Disparate results may also reflect the use of overexpression systems or compensatory mechanisms in knock-out mice. Studies in human monocytes suggest that new protein synthesis is required for regulation of lipopolysaccharide (LPS)-induced TNF-α by IL-10 and have led to a search by array for newly transcribed genes in IL-10-treated cells.8–10
The suppressor of cytokine signalling (SOCS) proteins are negative regulators of cytokine production. They are induced on cytokine stimulation and block further signalling in a classic feedback loop by targeting signalling intermediates for degradation. Several SOCS proteins prevent sustained cytokine signalling by binding to cytokine receptor chains or their associated Janus kinases (JAKs) or by preventing STAT (signal transducers and activators of transcription) recruitment to the receptor [for review see 11]. SOCS3 expression is enhanced within 30–60 min of exposure of human monocytes to IL-10, using Western blot and gene array analysis.10,12–14
Induction of SOCS3 expression by IL-10 raises two questions. First, is SOCS3 part of the mechanism by which IL-10 controls TNF-α production, and second, is SOCS3 part of a regulatory mechanism to limit the anti-inflammatory properties of IL-10? These questions are not new but the answers to date have been contradictory.15–20 To address the first question, a recent study using retroviral transfection of SOCS3 into murine SOCS3–/– macrophage cell lines showed that SOCS3 was necessary for the early but not late regulation of TNF-α production by IL-10.20 SOCS3 was also essential for IL-10 inhibition of LPS-stimulated production of inducible nitric oxide synthase protein and nitric oxide.20 In contrast, recent studies with SOCS3–/– mice suggest that SOCS3 targets only gp130-dependent signal transduction pathways.16–18 In an additional study of murine RAW cells transfected with a SOCS3-lentiviral vector, LPS-induced TNF-α production was not altered.19 Further roles for SOCS3 have been described. For example, tyrosine-phosphorylated SOCS3 can bind p120 RasGAP and activate Ras and thus maintain activation of extracellular signal-related kinase (ERK).21 It has also been reported that SOCS3 is recruited to the phosphorylated granulocyte colony-stimulating factor receptor and can modulate its signal transduction.22
Until now a functional role for SOCS3 in IL-10 regulation of TNF-α production in LPS-stimulated human monocytes has not been reported, principally due to the difficulties in efficiently transfecting human primary monocytes. In this study we have used an adenoviral transfection system to express SOCS3 for 24 hr in human monocytes prior to exposure to LPS and IL-10. In different monocyte preparations, the level of SOCS3 expression varied as measured by detection of the cotransfectant, green fluorescent protein (GFP), and allowed a titration of the effects of SOCS3. No evidence to suggest that SOCS3 was part of IL-10's inhibitory pathway was obtained; SOCS3 did not affect LPS-induced TNF-α production nor enhance the suppression of LPS-induced TNF-α by low-dose IL-10. In contrast, expression of SOCS3 significantly antagonized the inhibition of TNF-α production by optimal concentrations of IL-10 at 2 hr but only in those cell populations expressing the highest levels of GFP. As these levels were significantly greater than those detected in IL-10-treated cells, we conclude that SOCS3 only at supraphysiological levels can reduce the suppressive effects of IL-10 on LPS-induced TNF-α production.
Materials and methods
Materials
Recombinant human macrophage colony-stimulating factor (M-CSF) and IL-10 were purchased from ProSpec-Tany TechnoGene (Rehovot, Israel), IL-6 was purchased from Bender MedSystems GmbH (Vienna, Austria), RPMI-1640, gentamicin and l-glutamine from Invitrogen Life Technologies (Mount Waverley, Victoria, Australia), Dulbecco's modified Eagle's medium (DMEM) from Sigma (St Louis, MO) and Hank's balanced salt solution and fetal calf serum (FCS) from ThermoTrace (Melbourne, Victoria, Australia). Anti-phospho-STAT3 (Tyr 705), antiphospho-STAT1 (Tyr 701) and anti-phospho-ERK1/2 antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA), anti-STAT3 (H-190) and anti-SOCS3 (M-20) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), anti-ERK2 (K-23) antibody was obtained from Sigma and anti-β-tubulin from Abcam (Cambridge, UK). Horseradish peroxidase-conjugated rabbit-anti-mouse and donkey-anti-goat secondary antibodies were obtained from Rockland Immunochemicals (Gilbertsville, PA).
Generation of SOCS3 expressing adenovirus
A human SOCS3-containing construct was kindly donated by Prof. N. Nicola and Dr T. Wilson (Walter and Eliza Hall Institute of Medical Research, Australia). SOCS3 cDNA was amplified using gene specific primers designed to generate an N-terminal hemagglutinin (HA) tag and restriction sites SalI and NotI at the 5′-and 3′ cDNA ends, respectively. Primer sequences were, for the SOCS3 forward primer, GCGGTCGACATGTACCCATACGACGTCCCAGATTACGCTATGGTCACCCACAGCAAG, and for the SOCS3 reverse primer, GATGCGGCCGCTTAAAGCGGGGC. Following amplification and purification, HA-SOCS3 cDNA was subcloned into a GFP expressing adenoviral transfer vector, pCMV-Adtrack (Qbiogene Inc., Carlsbad, CA). Positive clones were selected using kanamycin selective medium and confirmed by restriction digest and sequence analysis. pCMV-AdTrack constructs containing no insert (GFP alone) and SOCS3 were linearized by digesting with PacI and transformed into BJ5183 AD-1 cells (Stratagene, La Jolla, CA) containing pAdEasy-1 (Qbiogene Inc.). Recombinants containing SOCS3 and GFP were selected on the basis of colony size and confirmed by restriction digest and sequence analysis. pAdEasy-1 constructs containing SOCS3 and GFP were transfected into complementing HEK 293 cells using Lipofectamine 2000 (Invitrogen Life Technologies). HEK 293 cells were purchased from BD Clontech (Palo Alto, CA) and were maintained in DMEM supplemented with 5% FCS, 5 µg/ml gentamicin, 2 mm l-glutamine and 1 mm sodium pyruvate and incubated at 37° in 5% CO2. Recombinant viruses were purified from both the HEK 293 cell lysate and culture supernatant using a modified chromatography-based purification system, based on Clontech's BD Adeno-X virus purification kit (BD Clontech).
Isolation of primary human monocytes and adenoviral infection
Human monocytes were purified to ∼85% by centrifugal elutriation (Beckman JE-6B, Beckman, Palo Alto, CA) of mononuclear cells isolated from buffy coats on density gradients (Lymphoprep, Axis-Shield, Oslo, Norway). Buffy coats were kindly provided by the Australian Red Cross Blood Service, Perth, Australia. Monocytes were cultured in RPMI-1640 medium containing 2 mm glutamine, 50 µm 2-mercaptoethanol, 5 µg/ml gentamicin and 2 mm 3′-(N-Morpholino-propanesulfonic acid). After overnight incubation in Teflon pots (Savillex, Minnetonka, MN) containing RPMI supplemented with 10% FCS and 25 ng/ml M-CSF (to induce αvβ5 expression), the cells were harvested and plated at a density of 0·5 × 106 cells/100 µl in polypropylene culture tubes (Minisorb, Nunc, Roskilde, Denmark). AdV-GFP (control virus) or AdV-SOCS3 were added at a multiplicity of infection (MOI) of 50 unless otherwise indicated and centrifuged at 1000 g for 60 min at 37° as previously described.23 An additional 400 µl of culture medium was added to each tube before incubation for 24 hr at 37° prior to stimulation with LPS (500 ng/ml), IL-10 (10 ng/ml) or LPS with IL-10.
Determination of infection efficiency by flow cytometry
Cells incubated with AdV-GFP (empty vector control), AdV-SOCS3 or left uninfected were harvested by centrifugation 24 hr after AdV-infection and washed once in phosphate-buffered saline (PBS) containing 0·2% bovine serum albumin (BSA) and 0·02% sodium azide. Cells were resuspended in fluorescence-activated cell sorting (FACS) fixative (1% formaldehyde in PBS) and stored at 4° until flow analysis could be performed. CD14 expression confirmed that all larger cells defined by forward and side scatter were monocytes. Infection efficiency was determined as the percentage of GFP-positive cells. The amount of virus per cell, estimated by GFP expression, was assessed as mean fluorescence intensity (MFI) by flow cytometry (FACS Calibur, BD Biosciences, San Jose, CA) and expression levels analysed using FlowJo software (version 4.6.1).
Western blot analysis
After isolation and overnight culture with M-CSF, monocytes were isolated and infected with AdV-GFP, AdV-SOCS3 or left uninfected (no virus) for 24 hr. They were then stimulated with 10 ng/ml IL-10 and/or 500 ng/ml LPS for 0–120 min. Cells were harvested by centrifugation and monocytes lysed in protein lysis buffer (10 mm Tris, 50 mm NaCl, 5 mm ethylenediaminetetra-acetic acid, 1% Triton-X-100, pH 7·6) supplemented with 5 mm sodium fluoride, 10 mm sodium molybdate, 1 mm sodium pyrophosphate, 2 mm sodium orthovanadate and 1 × protease inhibitors (complete mini, protease inhibitor cocktail tablets, Roche, Penzberg, Germany). Approximately, 7·5 µg of protein lysate was resolved per lane of a 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membrane (Pall Scientific, MI). Membranes were blocked for at least 1 h in 5% skim milk in Tris-buffered saline (TBS)/0·05% Tween-20 (block buffer) followed by 2 h incubation with primary antibodies diluted in block buffer or 5% BSA in TBS/0·05% Tween-20 according to the manufacturer's guidelines. Following four sequential 5 min washes in TBS/0·05% Tween-20 (TBS/T), membranes were incubated with HRP-conjugated anti-rabbit immunoglobulin G (IgG) or anti-goat IgG secondary antibodies diluted in block buffer. Bound secondary antibody was detected using chemiluminescence (Roche Diagnostics) and visualized using CL-XPosure film (Pierce, Rockford, IL).
Real-time polymerase chain reaction (PCR) Analysis of TNF-α mRNA
Total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies) from approximately 0·5 × 106 monocytes. Total RNA was reverse transcribed to cDNA using omniscript II reverse transcriptase (Qiagen, Clifton Hill, Victoria, Australia) and oligo-dT primers (Promega, Madison, WI) in the presence of RNase Inhibitor (Perkin Elmer, Boston, MA). Single stranded cDNA was diluted 1 : 5 and real-time PCR performed using the QuantiTect SYBR green PCR kit (Qiagen). Intron-spanning gene-specific primers were designed to recognize TNF-α and UBE2D2 (Ubiquitin conjugating enzyme E2D2) transcript.
TNF-α-forward: GGCAGTCAGATCATCTTCTCGA;
TNF-α-reverse: TCAGCTTGAGGGTTTGCTACAA;
UBE2D2-forward: ATGGCAGCATTTGTCTTGATATTCTAC;
UBE2D2-reverse: TGGATTGGGATCACACAACAGA.
PCR was performed using ABI-PRISM 7900HT (Applied Biosystems, Foster City, CA). PCR conditions were initial denaturation at 95° for 15 min followed by 40 cycles of 95° for 15 s, then 60° for 1 min. Melting curve analysis was used to assess the specificity of the PCR. Copy numbers were determined by 10-fold serial dilutions of plasmid standards and normalized to the reference gene UBE2D2.24
Assay of TNF-α
TNF-α was assayed in culture supernatants using the human TNF-α enzyme-linked immunosorbent assay assay kit (BD OptEIA, BD Biosciences, San Diego, CA) and the DELFIA assay system (Perkin Elmer). For each experiment samples were assayed in triplicate. The assays were sensitive to levels >10 pg/ml. For each donor, the mean level of TNF-α detected in triplicate cultures incubated with LPS plus IL-10 was calculated as a percentage of the mean level induced by LPS.
Statistical analysis
All values have been expressed as mean ± SEM. Significance of the results has been evaluated using one-way anova or a paired Student's t-test. A P-value of <0·05 was considered significant.
Results
SOCS3 is over-expressed in primary human monocytes using adenoviral transfection
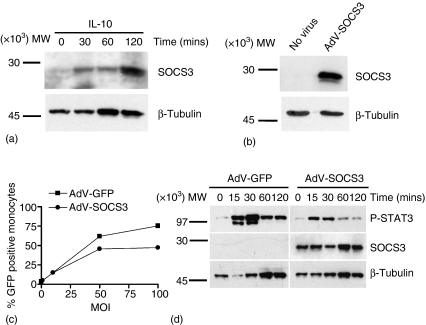
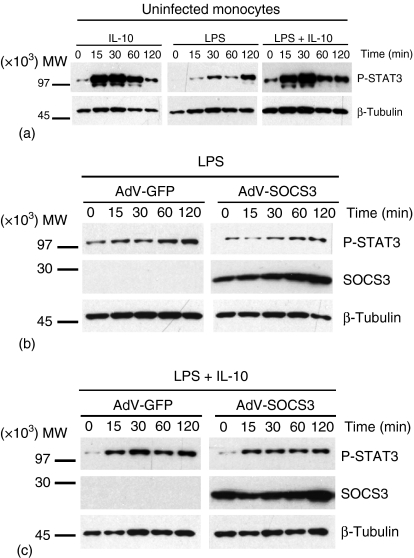
As endogenous SOCS3 protein expression is up-regulated in human peripheral blood monocytes at 30 min following stimulation with IL-10 (Fig. 1a using 20 µg lysate/lane),12–14 the role of SOCS3 in the regulation of IL-10-mediated suppression of LPS-induced TNF-α production was investigated. Monocytes were infected with AdV encoding SOCS3 (AdV-SOCS3) at MOI 50 for 24 hr. High levels of SOCS3 expression were detected using Western blot analysis in monocytes infected with AdV-SOCS3, while endogenous SOCS3 protein expression remained below the level of detection in uninfected monocytes (Fig. 1b). AdV-GFP and AdV-SOCS3 viruses were titrated in monocytes and maximal infection efficiency after 24 hr was achieved at MOI 50 (Fig. 1c). Therefore, all subsequent experiments were performed using the adenoviral vectors at MOI 50. Flow cytometry revealed infection efficiencies after 24 hr at MOI 50 of 55 ± 5% (mean ± SEM, n = 10 experiments) for infection with AdV-GFP and 53 ± 3% for monocytes infected with AdV-SOCS3, respectively. SOCS3 regulates IL-6/gp130-mediated signal transduction. The ability of AdV-SOCS3 to reduce IL-6-mediated STAT3 activation was determined using Western blot analysis (Fig. 1d). AdV-SOCS3 and AdV-GFP-infected monocytes were stimulated with IL-6 (20 ng/ml) and the effect of SOCS3 overexpression on STAT3 phosphorylation determined over a 2 hr time course. In AdV-GFP-infected monocytes IL-6 stimulation rapidly induced STAT3 phosphorylation at 15 min which decreased at 60 and 120 min. IL-6-induced STAT3 phosphorylation was reduced in AdV-SOCS3-infected cells compared those infected with AdV-GFP. In this experiment an infection efficiency of 63% was obtained for AdV-SOCS3.
Figure 1.
SOCS3 expression in primary human monocytes in response to IL-10 and AdV-SOCS3. (a) Human monocytes were exposed to IL-10 (10 ng/ml) for 0–120 min and endogenous SOCS3 expression analysed by Western blot (20 µg lysate/lane). (b) Whole cell lysates were prepared from monocytes infected with AdV-SOCS3 at MOI 50 for 24 hr or left uninfected (No virus) and SOCS3 expression determined using an anti-SOCS3 antibody (7·5 µg lysate/lane). Protein loading in (a) and (b) was visualized using an anti-β-tubulin antibody. (c) AdV-SOCS3 and AdV-GFP (empty vector control) viruses were titrated in monocytes and the percentage GFP-positive cells measured by flow cytometry 24 hr following infection with the virus at MOI 0, 10, 50 and 100. These data are from one of two experiments performed in duplicate. (d) Monocytes were infected with AdV-GFP or AdV-SOCS3 at MOI 50 for 24 hr before being stimulated with IL-6 (20 ng/ml) for 0–120 min. Western blot analysis was performed (7·5 µg lysate/lane) and the kinetics of STAT3 phosphorylation determined. Overexpression of SOCS3 by AdV-SOCS3-infected cells was confirmed using an anti-SOCS3 antibody. Protein loading was visualized using anti-β-tubulin. The blots shown are representative of two independent experiments using monocytes harvested from different donors.
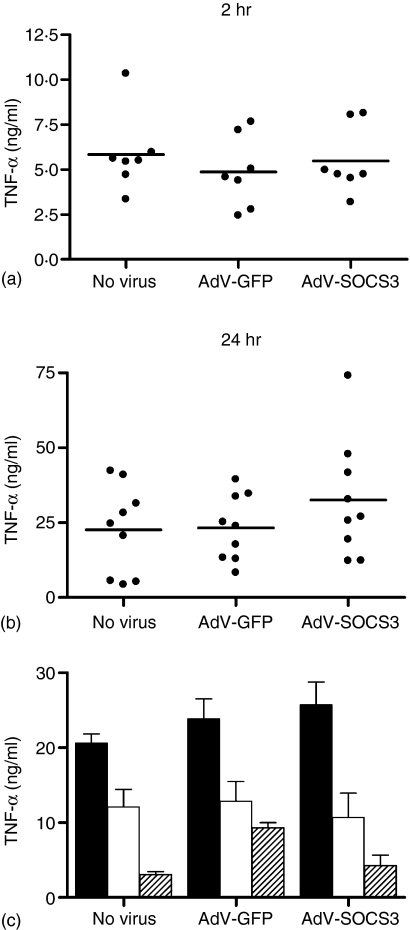
SOCS3 does not regulate TNF-α production following LPS stimulation
Infection with AdV-GFP or AdV-SOCS3 at MOI 50 had no effect on LPS-induced TNF-α production after 2 and 24 hr demonstrating that SOCS3 over-expression does not heighten the activation state of monocytes, nor does it antagonize the effects of LPS (Fig. 2a, Fig. b). IL-10 suppression of LPS-induced TNF-α production was dose dependent with maximal suppression (80%) after 24 hr detected following treatment with 10 ng/ml IL-10 (Fig. 2c). No increase in the suppressive effects of 1 or 10 ng/ml IL-10 on TNF-α production was observed in AdV-SOCS3-infected monocytes compared with uninfected and AdV-GFP-infected cells (Fig. 2c).
Figure 2.
AdV infection does not modulate the LPS response. Monocytes were infected with AdV-GFP or AdV-SOCS3 at MOI 50 or left uninfected for 24 hr prior to stimulation with 500 ng/ml LPS. (a) LPS-induced TNF-α levels in culture supernatants after 2 hr (n = 7 donors), and (b) 24 hr (n = 9 donors) following LPS stimulation. Each data point represents the mean LPS-induced TNF-α (ng/ml) for a single donor, assayed in triplicate. The mean TNF-α level is shown by the horizontal line. (c) Uninfected, AdV-GFP- and AdV-SOCS3-infected monocytes were stimulated for 24 hr with LPS alone (solid bars) or with LPS in the presence of 1 ng/ml (open bars) or 10 ng/ml IL-10 (shaded bars).
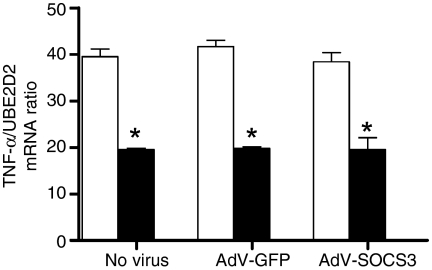
AdV-SOCS3 does not regulate the effects of LPS or LPS + IL-10 on TNF-α mRNA levels in monocytes
The mechanism of regulation by IL-10 on LPS-induced TNF-α production was determined using real time PCR (Fig. 3). Monocytes were stimulated with LPS or LPS + IL-10 and TNF-α mRNA levels measured 1 hr following stimulation. There was a significant increase (P < 0·05) in LPS-induced TNF-α transcript levels at 1 hr, which was reduced by a mean of 49% for cells cotreated with LPS and IL-10. This demonstrated that IL-10-mediated effects were at least partially regulated at the transcriptional level. TNF-α mRNA levels at time 0 were negligible for cells incubated with No Virus, AdV-GFP or AdV-SOCS3. Furthermore, AdV-GFP and AdV-SOCS3 infection of monocytes had no effect on IL-10 control of TNF-α mRNA levels (Fig. 3) and suggests that if SOCS3 regulates TNF-α production in LPS-stimulated monocytes, it is via a post-transcriptional mechanism.
Figure 3.
AdV-SOCS3 does not regulate TNF-α mRNA induced by LPS or LPS + IL-10. Total RNA was isolated from uninfected, AdV-GFP- and AdV-SOCS3-infected monocytes following treatment with 500 ng/ml LPS (open bars) or LPS in the presence of 10 ng/ml IL-10 (solid bars) for 60 min. TNF-α mRNA levels were measured using real time PCR and normalized to the housekeeping gene UBE2D2 (Ubiquitin conjugating enzyme E2D2). Values are the mean ± SEM of three replicates. Statistical significance was calculated using a one-way anova (*P < 0·05). The data are representative of two experiments from individual donors. TNF-α mRNA at time 0 was negligible on this scale for uninfected cells and AdV-GFP and AdV-SOCS3-infected monocytes (data not shown).
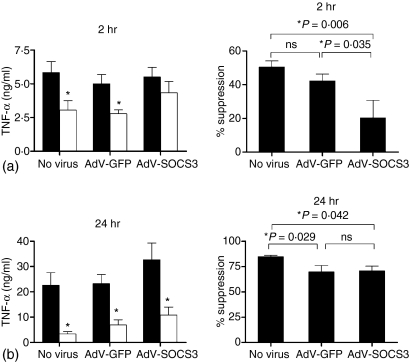
AdV-SOCS3 infection limits IL-10 suppression of LPS-induced TNF-α production at 2 hr poststimulation
IL-10 suppressed both LPS-induced TNF-α mRNA levels (Fig. 3) and TNF-α production at 2 hr (Fig. 4a). The role of SOCS3 in the suppression of LPS-induced TNF-α production by human monocytes was investigated. Monocytes were infected for 24 hr with AdV-SOCS3, AdV-GFP or left untreated (no virus control) and then stimulated with LPS, in the presence or absence of IL-10. TNF-α levels were assayed in the culture supernatants after 2 and 24 hr. After 2 hr, a significant reduction in LPS-induced TNF-α levels was observed for untreated and AdV-GFP-infected cells, by 52 ± 3% and 42 ± 4%, respectively (mean percentage suppression ± SEM, n = 7). In comparison, in AdV-SOCS3-infected monocytes, IL-10 did not significantly suppress LPS-induced TNF-α production at 2 hr. There was a significant difference in the regulatory properties of IL-10 between monocytes infected with AdV-GFP and AdV-SOCS3 (P = 0·035, Fig. 4a). At 24 hr poststimulation, over-expression of SOCS3 had no effect on IL-10 suppression of TNF-α production, with IL-10 suppressing LPS-induced TNF-α by 83 ± 2%, 68 ± 5% and 68 ± 6% for uninfected, AdV-GFP- and AdV-SOCS3-infected monocytes, respectively (Fig. 4b).
Figure 4.
SOCS3 regulates IL-10 suppression of LPS-induced TNF-α production at 2 hr. Monocytes were left uninfected or infected with either AdV-GFP or AdV-SOC3 for 24 h prior to stimulation with LPS (solid bars) and LPS + IL-10 (open bars). TNF-α levels were measured in culture supernatants at (a) 2 hr and (b) 24 hr following stimulation. Data are represented as the TNF-α levels (ng/ml) as well as the mean percentage suppression by IL-10 of LPS-induced TNF-α. Values are the mean ± SEM of seven experiments for 2 hr analysis or nine experiments for 24 hr. Statistical significance was calculated using a one-way anova (*P < 0·05, ns = not significant).
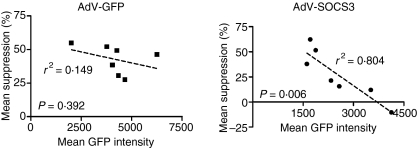
Regulation of IL-10-mediated suppression by AdV-SOCS3 infection at 2 hr depends on magnitude of infection
To determine whether the level of AdV-SOCS3 infection per cell had any influence on IL-10 mediated effects, the mean suppression (%) by IL-10 of LPS-induced TNF-α production was plotted against mean fluorescence intensity (mean GFP intensity of Fig. 5). A correlation (r2 =0·804) with a significance of P = 0·006 between the level of AdV-SOCS3 infection per cell and the percentage reduction by IL-10 of LPS-induced TNF-α production was observed. No significant correlation was identified for AdV-GFP-infected cells. These data suggest that the amount of SOCS3 expressed per cell inversely determines the magnitude of the decrease by IL-10 of LPS-induced TNF-α production at 2 hr.
Figure 5.
SOCS3-regulation of IL-10 activity depends on the level of SOCS3 expression per cell. Monocytes were infected with AdV-GFP and AdV-SOCS3 (MOI 50) for 24 hr and the GFP-mean fluorescence intensity (MFI) determined using flow cytometry. The mean percentage suppression in TNF-α production observed 2 hr after cotreatment with LPS and IL-10 was plotted against MFI. Each graphed value represents one experiment (n = 3 within each experiment).
SOCS3 regulates IL-10 suppression of LPS-induced TNF-α production by a STAT3-independent mechanism
To determine if excessive SOCS3 expression, even if at supraphysiological levels, gave insight into regulatory processes, IL-10-mediated suppression of LPS-induced TNF-α production at 2 hr was investigated. The blots shown (Figs 6 and 7) are representative of three donors. Lysates from monocytes infected with AdV-GFP and AdV-SOCS3 (MOI 50) with mean infection efficiencies of 68 ± 4% and 63 ± 1%, respectively (mean ± SEM, n = 3) are shown. For this experiment, a mean suppression in TNF-α production of 15% was detected which was significantly lower than the suppression observed in AdV-GFP-infected (30%) and uninfected cells (50%, P < 0·05). In this experiment the mean fluorescence intensity for GFP was 4353 and 2582 for AdV-GFP- and AdV-SOCS3-infected monocytes, respectively (Fig. 6b).
Figure 6.
SOCS3-regulation of IL-10 activity occurs via a STAT3-independent mechanism. (a) Uninfected monocytes were exposed to 10 ng/ml IL-10, 500 ng/ml LPS or LPS with IL-10 for 0–120 min. (b) Monocytes were infected with AdV-GFP or AdV-SOCS3 at MOI 50 for 24 hr before being stimulated with LPS, or (c) LPS + IL-10 for 0–120 min. Western blot analysis was performed (7·5 µg lysate/lane) and the kinetics of STAT3 phosphorylation determined. Over-expression of SOCS3 by AdV-SOCS3-infected cells was confirmed using an anti-SOCS3 antibody. Protein loading was visualized using anti-β-tubulin. The blots shown are representative of three independent experiments using monocytes harvested from different donors.
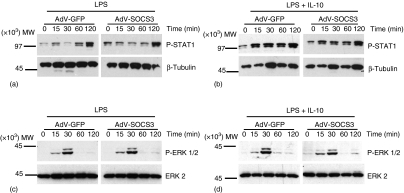
Figure 7.
SOCS3-regulation of IL-10 activity does not occur via STAT1 or ERK1/2 MAPK pathway. Monocytes were infected with AdV-GFP or AdV-SOCS3 at MOI 50 for 24 hr and stimulated with LPS (a and c) or LPS + 10 ng/ml IL-10 (b and d) for 0–120 min and total cell lysates prepared. Phosphorylation of STAT1 (a and b) and ERK1/2 (c and d) was determined using Western blot analysis (7·5 µg lysate/lane). Membranes were also probed with anti-β-tubulin to visualize protein loading. Alternatively, membranes were stripped and re-probed for the detection of total ERK2.
The effects of AdV-GFP and AdV-SOCS3 infection were determined on LPS-induced STAT3 activation. As observed for uninfected monocytes, LPS-induced STAT3 phosphorylation increased with time in cells infected with AdV-GFP. LPS-mediated phosphorylation of STAT3 was not affected by SOCS3 over-expression and suggests that SOCS3 does not alter LPS-induced STAT3-mediated signal transduction (Fig. 6b). Similarly, SOCS3 over-expression did not modulate STAT3 phosphorylation following incubation with LPS and IL-10. Consistent with the expression pattern observed in uninfected monocytes, a rapid increase in STAT3 phosphorylation was observed in AdV-GFP- and AdV-SOCS3-infected monocytes and these high levels of STAT3 activation were maintained throughout the 2 hr time course. High levels of SOCS3 protein expression were observed throughout the experiment (Fig. 6b, c; 7·5 µg lysate/lane). Thus, it is unlikely that SOCS3, even at excessively high levels, can regulate IL-10 suppression of LPS-induced TNF-α production by negative regulation of STAT3 activation.
Early regulation of IL-10-mediated anti-inflammatory effects by SOCS3 does not occur via STAT1 or p42/p44 ERK
In addition to activating STAT3, IL-10 activates STAT1 in monocytes.25 Using lysates from the experiment described above, the effect of SOCS3 over-expression on STAT1 activation was determined by Western blot analysis. LPS-induced STAT1 phosphorylation increased over 120 min (Fig. 7a). STAT1 phosphorylation was rapidly induced and was maintained at high levels 2 hr following costimulation with LPS and IL-10 (Fig. 7b). There was no difference in STAT1 phosphorylation induced by LPS or LPS + IL-10 between monocytes infected with AdV-GFP or AdV-SOCS3 suggesting that SOCS3 over-expression does not affect STAT1 activation. In addition, the effect of SOCS3 over-expression on the activation of p42/p44 ERK was determined. Infection with AdV-SOCS3 had no effect on the level of ERK 1/2 phosphorylation induced by LPS and LPS + IL-10 (Fig. 7c, d), similar to the results obtained for STAT3 and STAT1, suggesting that IL-10-mediated effects are unlikely to be mediated through an ERK1/2 mitogen-activated protein kinase (MAPK)-directed pathway.
Discussion
The molecular mechanisms responsible for IL-10-mediated anti-inflammatory effects remain poorly understood. While a number of studies have suggested that IL-10-mediated effects on TNF-α production may be regulated at a transcriptional level7,8,13,26,27 others favour a post-transcriptional mechanism of regulation of TNF-α.6,7,28 In a recent study, transcriptional control of TNF by IL-10 was shown by replacement of the 3′ AU-rich region with a-3′ untranslated region from the stable gene GAPDH.8 Many studies suggest the actions of IL-10 are indirect with a requirement for de novo protein synthesis.8–10,26 Conflicting results of an involvement of SOCS3 may reflect differences in the species and cell types used, as well as artefact introduced by over- and underexpression systems. Variability may also arise because SOCS3 protein is very unstable;21 when SOCS3 was transfected into SOCS3–/– murine macrophage cell lines20 it was necessary to pretreat cells with a proteasome inhibitor to prevent SOCS3 protein degradation prior to exposure to IL-10 for 2–4 hr.
It is clear that human non-transformed TNF-α-producing monocytes must be studied to have relevance to the treatment of human inflammatory conditions. In this study, an early SOCS3-dependent mechanism was detected by which the regulatory properties of IL-10 were curtailed. This time-related difference in the mechanism of action of IL-10 was similar to the findings of Williams and colleagues who, using a similar adenoviral transfection system and a dominant negative form of STAT3, reported an early transient STAT3-independent mechanism of IL-10 regulation of TNF-α in human monocyte-derived macrophages.13 Further, studies of SOCS3 retrovirally transfected into SOCS3–/– macrophage lines defined SOCS3 as an important mediator of IL-10 inhibition of macrophage activation after 2 but not 4 hr.20 To evaluate the possibility that our overexpression system had introduced non-physiological conditions, the extent of SOCS3 overexpression was estimated by measurement of the expression of the cotransfectant GFP. This correlation demonstrated that the effects of SOCS3 were significant at only the highest levels of coexpressed GFP. In Fig. 1(a), it was necessary to use approximately 20 µg lysate/lane for detection of IL-10-induced SOCS3. In the AdV-SOCS3-infected cells, only 7·5 µg lysate/lane was consistently required and suggests that even in the cell populations with lower levels of GFP expression, the levels of SOCS3 expressed were significantly higher than detected under physiological conditions. Further, a recent report suggested that the physiological levels of SOCS3 in murine cells are insufficient to stem pro-inflammatory signalling under pathogenic circumstances.29 Thus, the investigators used a recombinant cell-penetrating form of exogenous SOCS3 which could protect mice from the lethal effects of staphylococcal enterotoxin B and LPS by reducing production of inflammatory cytokines.29
In the present study an adenoviral transfection system, previously optimized in our laboratory23 to overcome difficulties associated with transfecting primary human monocytes, was used to overexpress SOCS3. Using this method we consistently obtained 50–60% monocyte infection 24 hr after exposure to the adenoviral vectors. Real-time PCR performed 1 hr after LPS stimulation clearly demonstrated that IL-10-mediated effects on TNF-α were regulated, at least partially, at the transcriptional level in human monocytes. In SOCS3-reconstituted SOCS3–/– murine macrophage lines, SOCS3 was necessary for early IL-10 inhibition of TNF-α mRNA expression.20 In our study, AdV-SOCS3 had no effect on TNF-α mRNA levels.
LPS can induce SOCS3 in human monocytes,12 and in mouse J774 macrophages stable transfection of SOCS3 reduced LPS-induced TNF-α.15 There are two pathways for LPS activation of TNF-α production, notably an early Myd88-dependent pathway and a late Myd88-independent pathway, the latter acting via induction of IFN-α/β response genes and involving STAT1 activation. A recent study of SOCS proteins transfected into murine RAW 264.7 cells concluded that induction of SOCS3 by Toll-like receptor (TLR) stimulation was strictly dependent on MyD88.30 Further, they found no inhibitory effect of SOCS1, SOCS2, SOCS3 or CIS on TNF-α production. SOCS3 in murine macrophages is a weak regulator of the indirect pathway of signalling from TLR.30 Thus, in our study with human cells it was important to demonstrate that AdV-SOCS3, and also AdV-GFP, were not interfering with the LPS activation process. No significant difference was observed in the level of LPS-induced TNF-α production by AdV-SOCS3-infected monocytes when compared with uninfected or AdV-GFP-infected monocytes suggesting that over-expression of the adenoviral constructs had no effect on the activation state of the cells. Similarly, over-expression of the adenoviruses had no inductive effect on TNF-α mRNA levels, measured by real-time PCR. In comparison with SOCS3, there has been stronger evidence that SOCS1 can regulate responses to LPS by a mechanism regulated by IFN-α/β.31–33
Changes in the phosphorylation of the STAT family of transcription factors, in response to IL-10 stimulation, have been previously reported.12,13,25,34 Sustained STAT3 activation induces SOCS3 expression and it has been speculated that SOCS3 regulates cytokine-induced STAT3 activation via a negative feedback loop.12 Our observations, consistent with previously published findings, demonstrated enhanced STAT3 phosphorylation upon IL-10 (maximal at 15–30 min) and LPS (not maximal until 120 min or later) stimulation in human monocytes. The prolonged phosphorylation of STAT3 in response to IL-10 stimulation in the presence of LPS was not altered in monocytes over-expressing SOCS3. Similarly, the extent and kinetics of STAT1 phosphorylation in monocytes exposed to IL-10 for 2 hr were not altered by AdV-SOCS3 infection. IL-10 activation of ERK p42/p44 MAPK has been reported in human monocytes.12 It was possible that IL-10 regulates TNF-α production through ERK. However, ERK phosphorylation by LPS, with or without IL-10, was identical in cells infected with AdV-GFP or AdV-SOCS3. A direct relationship in macrophages, in response to IL-6 signalling through gp130, between the STAT3 pathway and signalling output from the ERK-MAPK pathway has been described.35 With no change in STAT3 phosphorylation with AdV-SOCS3 infection, it was therefore not surprising that no changes in ERK activation were observed with SOCS3 over-expression.
This is the first study to investigate a role for SOCS3 in the regulation by LPS and by IL-10 of TNF-α production in human primary monocytes. Previous studies with over-expression of SOCS3 in murine cell lines implicated a role for this molecule in the signalling machinery of IL-10.15,20 In contrast, studies with SOCS3–/– mice suggested no role for SOCS3 in the regulatory properties of IL-10.16,17 An overexpression study was used but the levels of SOCS3 were carefully monitored. As transient effects of SOCS3 were detected only in the cells with the highest levels of expressed SOCS3, we conclude that SOCS3 even at pathological levels is unable to provide a target for regulation of TNF-α production by activated monocytes/macrophages at inflammatory sites such as the rheumatoid arthritis joint. Thus, a SOCS3-dependent mechanism by which the regulatory properties of LPS and IL-10 are curtailed in human monocytes is not supported.
Acknowledgments
We thank Dr Bruno Meloni for concentration of AdV from HEK293 culture supernatants. This work was supported by the National Health and Medical Research Council of Australia (#275546 to P.H.H.).
Abbreviations
- IL-10
interleukin-10
- TNF
tumour necrosis factor
- LPS
lipopolysaccharide
- SOCS
suppressor of cytokine signalling
- STAT
signal transducer and activator of transcription
- M-CSF
macrophage colony-stimulating factor
- HEK 293
human embryonic kidney 293
- BSA
bovine serum albumin
- MOI
multiplicity of infection
- MFI
mean fluorescence intensity
- MAPK
mitogen-activated protein kinase
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 3.Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10 (–/–) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829–33. doi: 10.1152/ajpgi.2000.278.6.G829. [DOI] [PubMed] [Google Scholar]
- 4.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy – review of a new approach. Pharmacol Rev. 2003;55:241–69. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 5.Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- 6.Kontoyiannis D, Kotlyarov A, Carballo E, et al. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001;20:3760–70. doi: 10.1093/emboj/20.14.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denys A, Udalova IA, Smith C, et al. Evidence for a dual mechanism for IL-10 suppression of TNF-alpha production that does not involve inhibition of p38 mitogen-activated protein kinase or NF-kappa B in primary human macrophages. J Immunol. 2002;168:4837–45. doi: 10.4049/jimmunol.168.10.4837. [DOI] [PubMed] [Google Scholar]
- 8.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA. 2005;102:8686–91. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 10.Antoniv TT, Park-Min KH, Ivashkiv LB. Kinetics of IL-10-induced gene expression in human macrophages. Immunobiology. 2005;210:87–95. doi: 10.1016/j.imbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–29. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 12.Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Muller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–72. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 13.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–76. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 14.Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, Larner AC, Finbloom DS. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–63. [PubMed] [Google Scholar]
- 15.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, fBazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404–11. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 16.Yasukawa H, Ohishi M, Mori H, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–6. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 17.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, Rutschman R, Murray PJ. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–50. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 18.Johnston JA, O'Shea JJ. Matching SOCS with function. Nat Immunol. 2003;4:507–9. doi: 10.1038/ni0603-507. [DOI] [PubMed] [Google Scholar]
- 19.Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, Akira S. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102:4123–9. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- 20.Qasimi P, Ming-Lum A, Ghanipour A, et al. Divergent mechanisms utilized by SOCS3 to mediate interleukin-10 inhibition of tumour necrosis factor α and nitric oxide production by macrophages. J Biol Chem. 2006;281:6316–24. doi: 10.1074/jbc.M508608200. [DOI] [PubMed] [Google Scholar]
- 21.Cacalano NA, Sanden D, Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3:460–5. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- 22.Hortner M, Nielsch U, Mayr LM, Johnston JA, Heinrich PC, Haan S. Suppressor of cytokine signaling-3 is recruited to the activated granulocyte-colony stimulating factor receptor and modulates its signal transduction. J Immunol. 2002;169:1219–27. doi: 10.4049/jimmunol.169.3.1219. [DOI] [PubMed] [Google Scholar]
- 23.Mayne GC, Borowicz RA, Greeneklee KV, Finlay-Jones JJ, Williams KA, Hart PH. Centrifugation facilitates transduction of green fluorescent protein in human monocytes and macrophages by adenovirus at low multiplicity of infection. J Immunol Methods. 2003;278:45–56. doi: 10.1016/s0022-1759(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 24.Hamalainen HK, Tubman JC, Vikman S, Kyrola T, Ylikoski E, Warrington JA, Lahesmaa R. Identification and validation of endogenous reference genes for expression profiling of T helper cell differentiation by quantitative real-time RT-PCR. Anal Biochem. 2001;299:63–70. doi: 10.1006/abio.2001.5369. [DOI] [PubMed] [Google Scholar]
- 25.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–90. [PubMed] [Google Scholar]
- 26.Song S, Ling-Hu H, Roebuck KA, Rabbi MF, Donnelly RP, Finnegan A. Interleukin-10 inhibits interferon-gamma-induced intercellular adhesion molecule-1 gene transcription in human monocytes. Blood. 1997;89:4461–9. [PubMed] [Google Scholar]
- 27.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994;153:811–6. [PubMed] [Google Scholar]
- 28.Kishore R, Tebo JM, Kolosov M, Hamilton TA. Cutting edge. clustered AU-rich elements are the target of IL-10-mediated mRNA destabilization in mouse macrophages. J Immunol. 1999;162:2457–61. [PubMed] [Google Scholar]
- 29.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11:892–8. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 30.Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem. 2004;279:54708–15. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- 31.Gingras S, Parganas E, de Pauw A, Ihle JN, Murray PJ. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of Toll-like receptor signaling. J Biol Chem. 2004;279:54702–7. doi: 10.1074/jbc.M411043200. [DOI] [PubMed] [Google Scholar]
- 32.Fenner JE, Starr R, Cornish AL, et al. Suppressor of cytokine signaling 1 regulated the immune response to infection by a unique inhibition of type 1 interferon activity. Nat Immunol. 2006;3:33–9. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 33.Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, Kishimoto T. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proc Natl Acad Sci USA. 2005;47:17089–94. doi: 10.1073/pnas.0508517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassatella MA, Gasperini S, Bovolenta C, et al. Interleukin-10 (IL-10) selectively enhances CIS3/SOCS3 mRNA expression in human neutrophils: evidence for an IL-10-induced pathway that is independent of STAT protein activation. Blood. 1999;94:2880–9. [PubMed] [Google Scholar]
- 35.Jenkins BJ, Roberts AW, Najdovska M, Grail D, Ernst M. The threshold of gp130-dependent STAT3 signaling is critical for normal regulation of hematopoiesis. Blood. 2005;105:3512–20. doi: 10.1182/blood-2004-09-3751. [DOI] [PubMed] [Google Scholar]