Abstract
In this study, we investigated how pregnancy influences cytokine production in response to stimulation of the innate and the adaptive immune system, respectively. Peripheral blood mononuclear cells (PBMCs) from allergic (n = 44) and non-allergic (n = 36) women were collected at three time-points: during the third trimester, at delivery and at a non-pregnant state 2 years after delivery. The production of interleukin-1β (IL-1β), IL-6, IL-10 and IL-12 was measured by enzyme-linked immunosorbent assay (ELISA) or enzyme-linked immunospot assay (ELISPOT). The spontaneous cytokine production, and the response following stimulation with agents that primarily activate the adaptive part of the immune system [phytohaemagglutinin (PHA), allergen extracts from cat and birch], or lipopolysaccharide (LPS) that activate innate immunity was measured in vitro. There was a significantly higher spontaneous in vitro production of IL-1β, IL-6 and IL-10 by PBMCs during pregnancy than 2 years after pregnancy, and this was not affected by the allergic status of the women. Conversely, in PHA-stimulated cell cultures there was a lower production of IL-10 and IL-12 during pregnancy than 2 years after pregnancy. LPS-induced IL-6 levels were significantly lower in PBMCs obtained during pregnancy than at 2 years after pregnancy. In addition, we made the interesting observation that in allergic women total immunoglobulin E (IgE) levels were significantly lower 2 years after pregnancy compared to the levels during pregnancy. Taken together, our results indicate that while atopic allergy in women does not have a substantial effect on cytokine production, pregnancy has an obvious effect on the immune system in terms of cytokine production as well as on the total IgE levels.
Keywords: allergy, cytokines, IgE, LPS, pregnancy
Introduction
The cytokine environment plays a major role in determining the outcome of immune responses and has been related to susceptibility and severity of a number of maladies, including allergic diseases. During the last decade, numerous studies have investigated the involvement of T-helper (Th)1- and Th2-type cytokines in allergic disorders.1–3 While the Th2-type cytokine interleukin (IL)-4 is over-expressed in allergic individuals compared to non-allergic subjects, Th1-type cytokines such as interferon (IFN)-γ are reduced.4–6
Recently the importance of the innate part of the immune system in allergic conditions has been recognized.7,8 A well-studied agent that activates the innate immune system is lipopolysaccharide (LPS). CD14, a receptor expressed by monocytes and macrophages, recognizes and binds LPS.9 Toll-like receptors (TLR) are an important part of the innate immune system and the first line of defence against invading pathogens (reviewed in Pasare and Medzhitov10). TLR4 acts together with membrane-bound or soluble CD14 (sCD14) to initiate the cellular response to LPS.11,12
TLR2 and TLR4 polymorphisms seem to be related to asthma severity and to the susceptibility of developing allergies.13–15 Differences in CD14 expression have been correlated with allergic disease in some studies,16,17 but not in others.18,19 The idea that innate immune cells and receptors are important for allergic responses is supported by the discussion regarding microbial exposure and allergy prevalence, the so-called hygiene hypothesis (reviewed in Von Hertzen and Haahtela20). Simplified, this theory suggests that allergic disease might be a result of microbial deprivation, particularly early in life. Although this theory is not undisputable,21 a substantial number of studies support it.22–24 These reports describe an association between exposure to infections and microbes early in life, in particular to LPS, and a reduced occurrence of allergic diseases.
The Th1/Th2 balance during pregnancy is intricate and has been debated over the last decade. Until recently, a Th2-type of immunity was considered to be mandatory for a successful pregnancy. Combining old and newer studies indicate that both Th1- and Th2-type cytokines, in a dynamic interplay, are important for reproductive success (reviewed in Chaouat et al.25and Wilczynski26). During pregnancy, immune responses belonging to the adaptive part are suppressed while the innate branch of the maternal immune system is activated.27,28 As an example, monocytes have been shown to be primed to produce IL-12 during pregnancy.29 Further, granulocytes increase in numbers (reviewed in Luppi30) and both monocytes and granulocytes enhance their phagocytic capacity.31
We were interested in examining how atopic allergy influences the response to stimulation of the innate branch of the immune system. This was achieved by comparing the expression of IL-1β, IL-6, IL-10 and IL-12 in LPS-stimulated peripheral blood mononuclear cells (PBMCs) from allergic and non-allergic women. Secondly, we wanted to examine the responses obtained when cells were stimulated with agents that activate the adaptive immune system. The PBMCs were stimulated with cat extract, birch allergen extract and phytohaemagglutinin (PHA), a plant-derived glycoprotein that acts as a potent lymphocyte mitogen.
In addition, we wanted to determine whether pregnancy affects cytokine responses differently in allergic and non-allergic women. Diseases associated with altered cytokine responses such as atopic allergy could influence the intrauterine environment and also, eventually, the immune system of the growing fetus. We therefore measured cytokine production at two time-points during pregnancy as well as 2 years after pregnancy in both allergic and non-allergic women.
Materials and methods
Study subjects and diagnosis of atopy
Pregnant women were recruited from maternity wards in Stockholm, Sweden. They are part of a larger prospective study including 281 infants and their parents. The focus on the influence of heredity on cord blood mononuclear cells (CBMC) cytokine profiles from this group has been published elsewhere.2,8 The women were classified as allergic (n = 44) or non-allergic (n = 36) (Table 1) based on their clinical history (allergic bronchial asthma and/or allergic rhinoconjunctivitis towards animal dander and/or towards pollen), together with skin prick test (SPT) results. The same nurse performed SPT according to the manufacturer's recommendation (ALK, Copenhagen, Denmark) against the following inhalant allergens: cat, dog, Dermatophagoides farinae, birch, timothy, horse, rabbit and mugwort (Soluprick 10 histamine equivalent prick test). Histamine chloride (10 mg/ml) served as the positive control and the allergen diluent as the negative control. The SPT was considered positive if the weal diameter after 15 min was 3 mm.
Table 1.
Demographic data of allergic and non-allergic women
| Allergic mothers1 (n = 44) | Non-allergic mothers (n = 36) | P-value | |
|---|---|---|---|
| Maternal age at delivery (years) | 31(21–39)3 | 31 (23–40)3 | NS |
| Total IgE in third trimester (kU/l)2 | 107 (7–1327)3 | 10 (2–211)3 | P < 0·001 |
| Total IgE 2 years after delivery (kU/l)2 | 89* (7–1197)3 | 10 (2–327)3 | P < 0·001 |
The diagnosis of allergy was based on the women's own statement of allergic disease confirmed with a positive SPT (ALK, Copenhagen, Denmark) result.
Total serum IgE levels were analysed with Pharmacia CAP System IgE FEIA (Pharmacia & Upjohn Diagnostics AB). The detection limit was > 2 kU/l.
Median (range).
NS = not significant (Mann–Whitney U-test).
P < 0·01 in comparison with samples taken in the third trimester.
All pregnancies were term pregnancies (> 37 weeks), and there were no differences in the age of the mothers in the two groups (Table 1). Approval from the Human Ethics Committee at Huddinge University Hospital, Stockholm, Sweden was granted. All families gave their informed consent to the study.
Samples
Peripheral blood samples were collected from the same women at three time-points: during the third trimester of pregnancy, at delivery and at a non-pregnant state 2 years postpartum.
Determination of total plasma IgE levels
Total immunoglobulin E (IgE) levels were analysed with the Pharmacia CAP System IgE FEIA (detection limit 2 kU/l, Pharmacia Diagnostics AB, Uppsala, Sweden).
Separation of PBMCs
Maternal venous blood was obtained in heparinized Vacutainer tubes. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque (Pharmacia-Upjohn, Stockholm, Sweden) gradient centrifugation. The PBMCs were diluted with 50% heat-inactivated fetal calf serum (FCS) (Hyclone Laboratories Inc., Logan, UT) and 50% culture medium containing RPMI-1640 (Life Technologies, Täby, Sweden) supplemented with penicillin–streptomycin (100 IU/ml), l-glutamine (2 mm) (Merck, Darmstadt, Germany). Before freezing, the sample was diluted 1:2 in 10% heat-inactivated FCS and 20% dimethylsulphoxide (DMSO). Isolated cells were frozen gradually 1°/min to −70° in a freezing container (Nalgene Cryo 1°, Nalge Company, Rochester, NY) and the samples were stored at −150° for further analysis. There were no differences in time between sampling and freezing of the isolated PBMCs among the two groups.
Enumeration of IL-10- and IL-12-producing cells by enzyme-linked immunospot assay (ELISPOT)
The numbers of IL-10- and IL-12-producing PBMCs were measured by the ELISPOT method described previously,8 but with some differences, as explained in the following section. There was no prestimulation of cells before adding them to the plates. After plating the cells they were stimulated with LPS and incubated for 24 hr; for the other stimuli, cells were incubated for 40 hr. The following stimuli were used for all individuals, regardless of the results of the SPT; LPS 0·5 ng/ml, PHA 1 µg/ml (Orion Diagnostics, Trosa, Sweden), birch and cat allergen extracts (both Aquagen-SQ 8 × 1000 SQ-U/ml, birch equivalent to 0·984 µg/ml and cat equivalent to 1·168 µg/ml, ALK, Hörsholm, Denmark). The following monoclonal antibodies (mAbs) were used; IL-10 (9D7) and IL-12 (IL-12-I and IL-12-II) (Mabtech, Stockholm). The cells were counted using an image analysis system (Autoimmun Diagnostika GmbH, Straßberg, Germany) at Mabtech, Stockholm. To avoid differences in results due to interassay variations, all results are presented as a ratio of stimulated cells/unstimulated cells.
Measurement of IL-1β and IL-6 by enzyme-linked immunosorbent assay (ELISA)
LPS (0·05 ng/ml)-stimulated and -unstimulated cells were incubated at 37° for 24 hr with 5% CO2. The cell-free supernatants were collected and stored at −20°. Costar enzyme immunoassay/radioimmunoassay (EIA/RIA) 3590 plates were coated overnight at 4° with 50 µl/well of capture mAbs [IL-1β (10219) and IL-6 (13A5) Mabtech AB, Stockholm, Sweden]. After washing, the plates were incubated with 100 µl/well of 0·5% bovine serum albumin (BSA). Fifty µl of the culture supernatants from the stimulation procedure or standard dilutions were added to duplicate wells and incubated. The standards (NIBSC, Hertfordshire, UK) used were IL-1β (86/680) and IL-6 (89/548). After washing, 50 µl/well of 1 µg/ml biotinylated mAbs (Mabtech AB) were added. Biotinylated mAbs specific for IL-1β (HB10222) and IL-6 (39C3) were used. Colour was developed at room temperature with p-nitrophenyl phosphate (Sigma Diagnostics, St Louis, MO), and the absorbance was measured at 405 nm with a precision microplate reader (SOFTmax alias, Molecular Devices, kinetic microplate reader, Sunnyvale, CA). To avoid differences in results due to interassay variations, all results are presented as a ratio of stimulated cells/unstimulated cells.
Statistical analysis
The statistica version 6·1 software package was used for statistical analysis. The Mann–Whitney U-test was used to compare total IgE levels and cytokine responses between groups of subjects with different atopic status. The Wilcoxon matched-pairs test was used to compare differences in parameters between the same woman at different time-points: during the third trimester of pregnancy, at delivery and 2 years postpartum. As there were no statistically significant differences at any point in cytokine production between the two time-points during pregnancy, in the third trimester and at delivery, all statistics comparing the pregnant state versus the non-pregnant state were based on the sample taken during the third trimester. Differences were considered significant if P < 0·05.
Results
Responses to microbial stimuli do not differ between allergic and non-allergic women
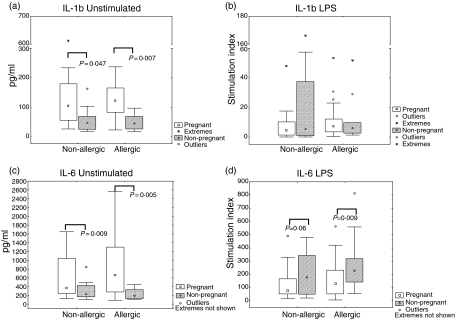
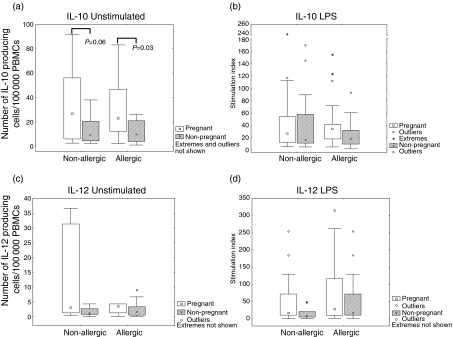
To assess the role of the innate immune system with regard to allergy we looked at the production of IL-1β, IL-6, IL-10 and IL-12 in response to LPS, a substance that activates the innate immune system. Receptors involved in recognition of microbial stimuli such as LPS have been shown to be linked to susceptibility to developing allergy.14–17 However, the results of our study showed no statistically significant differences in IL-1β(Fig. 1b), IL-6 (Fig. 1d), IL-10 (2b) or IL-12 (2d) production between allergic and non-allergic subjects upon stimulation of PBMCs with LPS at any of the time-points evaluated. For the spontaneous secretion of IL-1β (Fig. 1a), IL-6 (Fig. 1c), IL-10 (Fig. 2a) or IL-12 (Fig. 2c) we could not observe any statistically significant differences between allergic and non-allergic subjects, either during pregnancy or 2 years after delivery.
Figure 1.
Cytokine production by peripheral blood mononuclear cells (PBMCs) from allergic and non-allergic women in response to stimulation of the innate immune system [lipopolysaccharide (LPS)], at pregnancy and 2 years after measured by enzyme-linked immunosorbent assay (ELISA). Results are expressed as the ratio between stimulated PBMCs and unstimulated PBMCs (stimulation index). (a) IL-1β production by unstimulated cells. (b) IL-1β production by LPS (0·05 ng/ml)-stimulated cells (c) IL-6 production by unstimulated cells. (d) IL-6 production by LPS (0·05 ng/ml)-stimulated cells. ○, outliers. *, extreme outliers. Boxes cover the middle 50% of the data values, between the 25th and 75th percentiles, the central square being the median. Lines extend out to nonoutlier max and nonoutlier min.
Figure 2.
Cytokine production by peripheral blood mononuclear cells (PBMCs) from allergic and nonallergic women in response to stimulation of the innate immune system, at pregnancy and 2 years after measured by enzyme-linked immunospot assay (ELISPOT). Results are expressed as the ratio between stimulated PBMCs and unstimulated PBMCs (stimulation index). (a) Interleukin-10 (IL-10) production by unstimulated cells. (b) IL-10 production by lipopolysaccharide (LPS) (0·05 ng/ml)-stimulated cells. (c) IL-12 production by unstimulated cells. (d) IL-12 production by LPS (0·05 ng/ml)-stimulated cells. ○, outliers; *, extreme outliers. Boxes cover the middle 50% of the data values, between the 25th and 75th percentiles, the central square being the median. Lines extend out to non-outlier maximum and non-outlier minimum.
Responses to T cell stimuli do not differ between allergic and non-allergic women
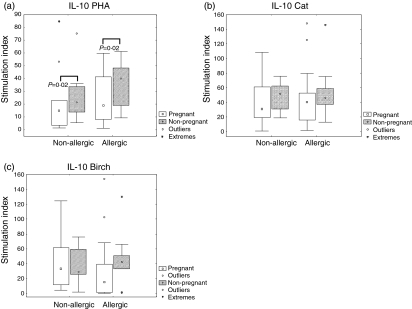
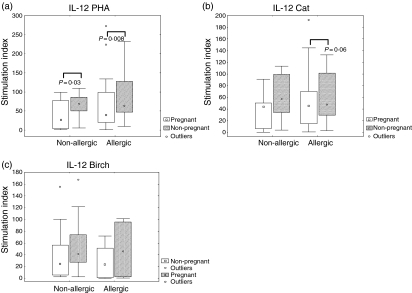
In order to explore how allergic disease influences the above-mentioned cytokine patterns in response to T cell stimuli we investigated the production of IL-10 and IL-12 in PBMCs before and after stimulation with PHA, cat allergen extract and birch allergen extract. There were no differences after in vitro stimulation with PHA (Figs 3a and 4a), cat (Figs 3b and 4b) or birch (Figs 3c and 4c) between allergic and non-allergic subjects, either at pregnancy or 2 years after pregnancy.
Figure 3.
Interleukin-10 (IL-10) production by peripheral blood mononuclear cells (PBMCs) from allergic and non-allergic women in response to stimulation of the adaptive immune system, at pregnancy and 2 years after measured by enzyme-linked immunospot assay (ELISPOT). Results are expressed as the ratio between stimulated PBMCs and unstimulated PBMCs (stimulation index). (a) Phytohaemagglutinin (PHA) (1 µg/ml)-stimulated cells. (b) Cat allergen (8 kU/ml)-stimulated cells. (c) Birch allergen (8 kU/ml)-stimulated cells. ○, outliers; *extreme outliers. Boxes cover the middle 50% of the data values, between the 25th and 75th percentiles, the central square being the median. Lines extend out to non-outlier maximum and non-outlier minimum.
Figure 4.
Interleukin-12 (IL-12) production by peripheral blood mononuclear cells (PBMCs) from allergic and non-allergic women in response to stimulation of the adaptive immune system, at pregnancy and 2 years after measured by enzyme-linked immunospot assay (ELISPOT). Results are expressed as the ratio between stimulated PBMCs and unstimulated PBMCs (stimulation index). (a) Phytohaemagglutinin (PHA) (1 µg/ml)-stimulated cells. (b) Cat allergen (8 kU/ml)-stimulated cells. (c) Birch allergen (8 kU/ml)-stimulated cells. ○, outliers. Boxes cover the middle 50% of the data values, between the 25th and 75th percentiles, the central square being the median. Lines extend out to non-outlier maximum and non-outlier minimum.
Pregnancy influences cytokine responses regardless of allergic status
As several innate and proinflammatory components of the immune system are induced during pregnancy,27–31 we evaluated IL-1β, IL-6, IL-10 and IL-12 cytokine responses in women during pregnancy and 2 years after delivery. There were no statistically significant differences in cytokine secretion from the same individual between the two time-points, late pregnancy and delivery, either for unstimulated or for stimulated cells, regardless of allergic status (data not shown). The value obtained during late pregnancy was used in further comparisons with the non-pregnant state 2 years after delivery. The spontaneous production of IL-1β (Fig. 1a), IL-6 (Fig. 1c) and IL-10 (Fig. 2a) was significantly higher during pregnancy than 2 years after delivery in allergic women. For the non-allergic women the spontaneous production of IL-1β (Fig. 1a), IL-6 (Fig. 1c) and IL-10 (Fig. 2a) was also higher during the pregnant state versus the non-pregnant state, although it did not reach statistical significance for IL-1β (P = 0·14) and for IL-10 (P = 0·06). When comparing cytokine responses to LPS from the same women at pregnancy with the responses 2 years after delivery, IL-6 was shown to be increased for allergic women as well as for the non-allergic women 2 years after delivery, although the P-value for non-allergic women did not reach statistical significance (P = 0·06) (Fig. 1d). No differences in IL-1β (Fig. 1b), IL-10 (Fig. 2b) or IL-12 (Fig. 2d) production in response to LPS were detected when comparing the pregnant state of the women versus the non-pregnant state. However, IL-10 and IL-12 both showed a trend of higher responses during pregnancy than 2 years after delivery.
IL-10 (Fig. 3a) and IL-12 (Fig. 4a) secretion in response to PHA both showed the same pattern of significantly lower production during pregnancy than 2 years after delivery. The pattern of lower responses during pregnancy than 2 years after delivery was also seen for IL-10 or IL-12 secretion in response to allergen extracts (Figs 3b, c, 4b, c), even though none of them reached statistical significance.
Taken together, these data suggest that there is a pronounced influence of pregnancy on spontaneous cytokine expression, as well as for the IL-10 and IL-12 responses to the T cell stimuli PHA, and to some extent for innate stimuli (with regard to IL-6 production). We could also conclude that the responses are constant during the later stages of pregnancy.
IgE levels in plasma from allergic women are significantly lower 2 years after delivery
To explore further the immunological modulation that pregnancy seemed to have on the women in this study, we compared the total IgE levels 2 years after delivery with the value from samples taken in the third trimester. Total plasma IgE levels differed significantly between allergic and non-allergic women (P < 0·001, Table 1) for samples collected at both time-points. Interestingly, the total IgE levels in allergic women were significantly (P < 0·01) lower 2 years after delivery in comparison with the levels during pregnancy.
Discussion
We have demonstrated recently that children with allergic mothers seem to have a reduced capacity to respond to microbial stimuli at birth compared to children with non-allergic mothers.32 According to that hypothesis, individuals being less well equipped to respond to microbial stimuli would therefore run a higher risk of developing allergic diseases.
In this study, we demonstrate that IL-1β, IL-6, IL-10 and IL-12 production in PBMCs from adult women are not influenced by allergic status, either for unstimulated cells or for cells stimulated with microbial stimuli (LPS). In contrast to studies showing that differences in receptors recognizing microbial products influence the likelihood of developing allergic diseases,13–17 we could not prove any differences regarding cytokine production between allergic and non-allergic individuals. Having differences in receptor expression between allergic and non-allergic individuals does not mean arbitrarily that the final response (here cytokines) has to differ. There might be different compensation mechanisms involved, making it hard to relate different genetic markers to an actual difference in cytokine production.
As for the stimulation of the innate immune system (LPS), we could neither find a difference between allergic and non-allergic individuals in terms of IL-10 nor IL-12 production when stimulating the adaptive immune system (PHA and allergen extracts). Looking at allergy from a Th1/Th2 perspective, allergic individuals are supposed to produce less IL-12 than non-allergic individuals,33 thereby skewing the immune system towards a Th2 phenotype, characteristic for allergic diseases. We could not detect such differences in IL-12 production, either for spontaneous production or for production in response to different stimuli. Our group has previously shown a relationship between sensitization at 2 years of age and having lower numbers of IL-12-producing cells at birth (CBMC).8 As we did not detect any differences regarding IL-12 production attributed to allergic status in this study, it could be speculated that the impaired IL-12 production at birth is not related strongly to IL-12 production later in adult life. This highlights the importance of not regarding allergy as a strict Th2 phenomenon, as the cytokine pattern seems to be influenced by other factors such as age. Further, we cannot exclude an LPS contamination of the allergen extracts used in this study, as this is not measured routinely by the manufacturer. A possible explanation for the absence of differences between allergic and non-allergic individuals in response to allergen extracts could be that differences are masked by the response to an eventual LPS contamination.
During pregnancy there is a profound activation of the innate branch of the immune system.27,28 In this study we were also interested in comparing the cytokine pattern in women during pregnancy with the cytokine pattern 2 years after delivery, in both allergic and non-allergic individuals. The elevated spontaneous innate cytokine levels (IL-1β, IL-6) during pregnancy are in line with previous publications illustrating the activated innate immune components during pregnancy (reviewed in Luppi30). In line with the theory of a suppressed adaptive immunity during pregnancy we found that PHA, a T cell stimulus, induced lower production of IL-10 and IL-12 during pregnancy than 2 years after delivery. As IL-10 and IL-12 are produced by cells of both the innate and the adaptive immune systems, the lower IL-10 and IL-12 production in response to PHA would be attributed most probably to diminished production from T and B cells, respectively.
The fact that LPS had a greater effect on IL-6 production 2 years after delivery than during pregnancy is perhaps an unexpected result as LPS acts on the innate cells, and the innate immunity during pregnancy is supposed to be up-regulated. It is possible that the woman's immune system is modulated by pregnancy, and that she has an increased ability to respond to microbial stimuli due to the fact that she has given birth 2 years earlier. Also, as the results from stimulation of PBMCs in this study are presented as a stimulation index, the fact that there is a higher spontaneous production of, for example, IL-6 during pregnancy is, of course, influencing the fact that the index is lower during pregnancy than after delivery.
A study from 2002 showed that IL-12 production by PBMCs was significantly lower in healthy pregnant women than in non-pregnant women.34 Another study showed that IL-12 production in response to LPS was enhanced during pregnancy.29 We did not find any statistically significant alterations in IL-12 production during pregnancy, either in unstimulated or in stimulated cells. The enhancement of IL-12 production in response to LPS in Sacks et al.'s study was based on intracellular measurements and our results are based on extracellular measurements, which could have influenced the results.
Recent studies have shown that the more children women have, the less likely the women are to be atopic.35–37 As the mother's immune system during pregnancy influences the immune system of the fetus, this would imply that children with older siblings are less likely to develop allergies. That maternal but not paternal total Ig E levels correlate with cord blood (CB) total IgE38,39 also indicates that maternal factors, placental factors or both have an impact on perinatal allergic sensitization.
In this study, we found significantly (P < 0·01, n = 44) lower total IgE levels in allergic women 2 years after delivery in comparison with samples taken during pregnancy and at delivery. In contrast to earlier studies performed where the total IgE levels are compared between mothers with different parity status, or in CB of babies with different birth order,38,40 in this study we could follow the same women at different time-points. It is therefore of great interest to find that even 2 years after giving birth there seems to be a profound effect on total IgE levels in the allergic women. In all these studies, including ours, differences in IgE levels have been attributed only to total IgE and not to specific IgE. It would be of great interest to see if the specific IgE levels follow our results of lower total IgE values after delivery.
Several studies have shown that having older siblings is protective against the development of allergic diseases (reviewed in Von Hertzen and Haahtela20). This was first believed to be due to factors taking place during the child's infancy, such as an increase in childhood infections. However, Karmaus et al. could show in a study from 2001 that IgE in cord blood is reduced with increasing birth order, indicating that the sibling effect may already have its origin in utero.38
The decreased IgE levels observed 2 years after delivery in this study are also in concordance with results showing that children born after interpregnancy intervals of less than 2 years were far less likely to have positive SPT compared with children of women who had had no previous pregnancies, or the women that had longer interpregnancy intervals.41 The immunological changes imposed by pregnancy might still have a strong effect after 2 years as seen in our study, and a follow-up of the same women could have shown how long-lasting is the effect of pregnancy. Interestingly, however, in the context of the so-called sibling effect, recent studies have pointed out the fact that total IgE levels in children <5 years should be interpreted with caution as they correlate poorly with total IgE levels later in life,42 but also that having older siblings delays the onset of IgE sensitization but may not prevent IgE sensitization per se.43 To prove that the differences in total IgE and cytokine production between the pregnant and non-pregnant state really are the effects of immunomodulation due to pregnancy, the ideal scenario would, of course, have been to also evaluate the women prior to their pregnancies. As the women were enrolled in the study when visiting the maternity ward this was not feasible. To exclude the possibility that age is a confounding factor for the decreasing total IgE values, we divided the women into three age groups. If age was a confounding factor the older women would have lower total IgE values than women in the lowest age group at both time-points. However, the group with the oldest women had the highest total IgE at both time-points measured. Further, total IgE is decreased after pregnancy regardless of age (our unpublished observation).
Cells were collected at three time-points: in the third trimester, at delivery and at a non-pregnant state 2 years after delivery. The process of labour involves the release of proinflammatory cytokines and inflammatory mediators.44 In order to exclude any possible influence of labour in our results and analyses, we chose to include only third-trimester samples in comparison with the non-pregnant samples.
In conclusion, our results show that cytokine responses to PHA, allergen extracts (cat and birch) and LPS in adult women are not influenced by the allergic status. In addition, we show that pregnancy seems to have a substantial effect on the cytokine levels, regardless of allergic status. Our finding that women have lower total IgE levels 2 years after pregnancy than in the third trimester also shows that the immune system seems to be modulated by pregnancy, and that the effect is long-lasting.
Acknowledgments
We thank Monica Nordlund and Anna Stina Ander for assistance, Pharmacia Diagnostics AB for supply of reagents for plasma analysis, Lennart Larsson for providence of LPS, and Jan-Olov Persson for advice on statistical analysis. We would also like to thank the families who participated in the study. This work was supported financially by grants from the Swedish Asthma and Allergy Association's Research Foundation, the Swedish Research Council K2004-74X-15042-01 A, 74XD-15160 and 74 PD-15159, the memory of King Oscar II's and Queen Sophia's Golden Anniversary, the Swedish Medical Society, the Hesselman's Foundation, Golje's Foundation and Åhléns Foundation.
Abbreviations
- BSA
bovine serum albumin
- CB
cord blood
- CBMC
cord blood mononuclear cells
- DMSO
dimethyl sulphoxide
- FCS
fetal calf serum
- IFN
interferon
- IgE
immunoglobulin E
- IL
interleukin
- LPS
lipopolysaccharide
- PBMCs
peripheral blood mononuclear cells
- mAbs
monoclonal antibodies
- PHA
phytohemagglutinin
- sCD14
soluble CD14
- SPT
skin prick test
- Th
T-helper
- TLR
Toll-like receptor
References
- 1.Saito H, Asakura K, Ogasawara H, Watanabe M, Kataura A. Topical antigen provocation increases the number of immunoreactive IL-4-, IL-5- and IL-6-positive cells in the nasal mucosa of patients with perennial allergic rhinitis. Int Arch Allergy Immunol. 1997;114:81–5. doi: 10.1159/000237647. [DOI] [PubMed] [Google Scholar]
- 2.Gabrielsson S, Soderlund A, Nilsson C, Lilja G, Nordlund M, Troye-Blomberg M. Influence of atopic heredity on IL-4-, IL-12- and IFN-gamma-producing cells in in vitro activated cord blood mononuclear cells. Clin Exp Immunol. 2001;126:390–6. doi: 10.1046/j.1365-2249.2001.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez FD, Stern DA, Wright AL, Holberg CJ, Taussig LM, Halonen M. Association of interleukin-2 and interferon-gamma production by blood mononuclear cells in infancy with parental allergy skin tests and with subsequent development of atopy. J Allergy Clin Immunol. 1995;96:652–60. doi: 10.1016/s0091-6749(95)70264-4. [DOI] [PubMed] [Google Scholar]
- 5.Halonen M, Martinez FD. A deficient capacity to produce interferon-gamma: is it a risk for asthma and allergies? Clin Exp Allergy. 1997;27:1234–6. [PubMed] [Google Scholar]
- 6.Hoekstra MO, Hoekstra Y, De Reus D, Rutgers B, Gerritsen J, Kauffman HF. Interleukin-4, interferon-gamma and interleukin-5 in peripheral blood of children with moderate atopic asthma. Clin Exp Allergy. 1997;27:1254–60. [PubMed] [Google Scholar]
- 7.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson C, Larsson AK, Hoglind A, Gabrielsson S, Troye Blomberg M, Lilja G. Low numbers of interleukin-12-producing cord blood mononuclear cells and immunoglobulin E sensitization in early childhood. Clin Exp Allergy. 2004;34:373–80. doi: 10.1111/j.1365-2222.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- 9.Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–14. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 10.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [Review] [DOI] [PubMed] [Google Scholar]
- 11.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 13.Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, Nowak D, Martinez FD. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113:482–8. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- 14.Fagerås Böttcher M, Hmani-Aifa M, Lindström A, et al. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12 (p70) responses in Swedish children. J Allergy Clin Immunol. 2004;114:561–7. doi: 10.1016/j.jaci.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 15.Yang IA, Barton SJ, Rorke S, Cakebread JA, Keith TP, Clough JB, Holgate ST, Holloway JW. Toll-like receptor 4 polymorphism and severity of atopy in asthmatics. Genes Immun. 2004;5:41–5. doi: 10.1038/sj.gene.6364037. [DOI] [PubMed] [Google Scholar]
- 16.Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A polymorphism in the 5′ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol. 1999;20:976–83. doi: 10.1165/ajrcmb.20.5.3494. [DOI] [PubMed] [Google Scholar]
- 17.Litonjua AA, Belanger K, Celedon JC, et al. Polymorphisms in the 5′ region of the CD14 gene are associated with eczema in young children. J Allergy Clin Immunol. 2005;115:1056–62. doi: 10.1016/j.jaci.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Jones CA, Holloway JA, Popplewell EJ, Diaper ND, Holloway JW, Vance GH, Warner JA, Warner JO. Reduced soluble CD14 levels in amniotic fluid and breast milk are associated with the subsequent development of atopy, eczema, or both. J Allergy Clin Immunol. 2002;109:858–66. doi: 10.1067/mai.2002.123535. [DOI] [PubMed] [Google Scholar]
- 19.Holmlund U, Hoglind A, Larsson AK, Nilsson C, Sverremark Ekstrom E. CD14 and development of atopic disease at 2 years of age in children with atopic or non-atopic mothers. Clin Exp Allergy. 2003;33:455–63. doi: 10.1046/j.1365-2222.2003.01629.x. [DOI] [PubMed] [Google Scholar]
- 20.Von Hertzen LC, Haahtela T. Asthma and atopy – the price of affluence? Allergy. 2004;59:124–37. doi: 10.1046/j.1398-9995.2003.00433.x. [Review] [DOI] [PubMed] [Google Scholar]
- 21.Platts-Mills TA, Erwin E, Heymann P, Woodfolk J. Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma? Allergy. 2005;60(Suppl. 79):25–31. doi: 10.1111/j.1398-9995.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 22.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–43. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 23.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 24.Riedler J, Braun-Fahrlander C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–33. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 25.Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004;134:93–119. doi: 10.1159/000074300. [Review] [DOI] [PubMed] [Google Scholar]
- 26.Wilczynski JR. Th1/Th2 cytokines balance – yin and yang of reproductive immunology. Eur J Obstet Gynecol Reprod Biol. 2005;122:136–43. doi: 10.1016/j.ejogrb.2005.03.008. [Review] [DOI] [PubMed] [Google Scholar]
- 27.Sachs G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999;20:114–8. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Iwatani Y, Kaneda T, Hidaka Y, Mitsuda N, Morimoto Y, Amino N. Changes in T, B, and NK lymphocyte subsets during and after normal pregnancy. Am J Reprod Immunol. 1997;37:368–77. doi: 10.1111/j.1600-0897.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 29.Sacks GP, Redman CW, Sargent IL. Monocytes are primed to produce the Th1 type cytokine IL-12 in normal human pregnancy: an intracellular flow cytometric analysis of peripheral blood mononuclear cells. Clin Exp Immunol. 2003;131:490–7. doi: 10.1046/j.1365-2249.2003.02082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21:3352–7. doi: 10.1016/s0264-410x(03)00331-1. [Review] [DOI] [PubMed] [Google Scholar]
- 31.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 32.Amoudruz P, Holmlund U, Malmstrom V, Trollmo C, Bremme K, Scheynius A, Sverremark-Ekstrom E. Neonatal immune responses to microbial stimuli: is there an influence of maternal allergy? J Allergy Clin Immunol. 2005;115:1304–10. doi: 10.1016/j.jaci.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 33.Reider N, Reider D, Ebner S, Holzmann S, Herold M, Fritsch P, Romani N. Dendritic cells contribute to the development of atopy by an insufficiency in IL-12 production. J Allergy Clin Immunol. 2002;109:89–95. doi: 10.1067/mai.2002.120556. [DOI] [PubMed] [Google Scholar]
- 34.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Reprod Immunol. 2002;47:91–7. doi: 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 35.Sunyer J, Anto JM, Harris J, Torrent M, Vall O, Cullinan P, Newman-Taylor A. Maternal atopy and parity. Asthma Multi-centre Infants Cohort Study (AMICS) Clin Exp Allergy. 2001;31:1352–5. doi: 10.1046/j.1365-2222.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- 36.Harris JM, White C, Moffat S, Mills P, Newman Taylor AJ, Cullinan P. New pregnancies and loss of allergy. Clin Exp Allergy. 2004;34:369–72. doi: 10.1111/j.1365-2222.2004.01905.x. [DOI] [PubMed] [Google Scholar]
- 37.Forastiere F, Sunyer J, Farchi S, et al. Number of offspring and maternal allergy. Allergy. 2005;60:510–4. doi: 10.1111/j.1398-9995.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 38.Karmaus W, Arshad H, Mattes J. Does the sibling effect have its origin in utero? Investigating birth order, cord blood immunoglobulin E concentration, and allergic sensitization at age 4 years. Am J Epidemiol. 2001;154:909–15. doi: 10.1093/aje/154.10.909. [DOI] [PubMed] [Google Scholar]
- 39.Liu CA, Wang CL, Chuang H, Ou CY, Hsu TY, Yang KD. Prenatal prediction of infant atopy by maternal but not paternal total IgE levels. J Allergy Clin Immunol. 2003;112:899–904. doi: 10.1016/j.jaci.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 40.Karmaus W, Arshad SH, Sadeghnejad A, Twiselton R. Does maternal immunoglobulin E decrease with increasing order of live offspring? Investigation into maternal immune tolerance. Clin Exp Allergy. 2004;34:853–9. doi: 10.1111/j.1365-2222.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 41.Wegienka G, London SJ, Johnson CC, Ownby DR. Interpregnancy interval might affect the risk of childhood atopy. J Allergy Clin Immunol. 2004;113:169–71. doi: 10.1016/j.jaci.2003.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nickel R, Illi S, Lau S, et al. Variability of total serum immunoglobulin E levels from birth to the age of 10 years. A prospective evaluation in a large birth cohort. German Multicenter Allergy Study. Clin Exp Allergy. 2005;35:619–23. doi: 10.1111/j.1365-2222.2005.02237.x. [DOI] [PubMed] [Google Scholar]
- 43.Turner SW, Palmer LJ, Gibson NA, Rye PJ, Goldblatt J, Landau LI, Le Souef PN. The effect of age on the relationship between birth order and immunoglobulin E sensitization. Clin Exp Allergy. 2005;35:630–4. doi: 10.1111/j.1365-2222.2005.02229.x. [DOI] [PubMed] [Google Scholar]
- 44.Molloy EJ, O'Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM, Webb DW, Watson RW. Labor induces a maternal inflammatory response syndrome. Am J Obstet Gynecol. 2004;190:448–55. doi: 10.1016/j.ajog.2003.08.027. [DOI] [PubMed] [Google Scholar]