Abstract
Epicutaneous immunization is a potential novel technique for topical vaccine delivery. It targets the immunologically rich milieu of the skin while having the advantage of being a non-invasive immunization procedure. By disrupting the stratum corneum of the epidermis a natural adjuvant effect can be achieved through activation of resident Langerhans cells. This negates the normal need for co-application of noxious adjuvants. Epicutaneous immunization on barrier-disrupted skin induces potent antigen-specific systemic immunity with a strong T helper type 2 (Th2) bias. We show here that epicutaneous immunization enhances the vigour of a subsequent T-cell response to the same antigen. The induced systemic Th2 response prevents the development of Th1 responses induced through injection of antigen in complete Freund's adjuvant (CFA). Prior epicutaneous immunization results in reduced production of antigen-specific interferon-γ and immunoglobulin G2a (IgG2a) and enhanced interleukin-4, IgG1 and IgE responses to immunization with CFA. Moreover, epicutaneous immunization converts an established Th1 response to a Th2 response, as demonstrated by the specific reduction of interferon-γ and IgG2a and the enhancement of interleukin-4 and IgE. This Th2 dominance of epicutaneous immunization may have direct therapeutic application as an immune-modulating procedure in Th1-dominant diseases such as autoimmune rheumatoid arthritis, type 1 diabetes, Hashimoto's thyroiditis and multiple sclerosis.
Keywords: autoimmunity, epicutaneous immunization, skin, Th1/Th2 cells, vaccination
Introduction
The route of antigen administration is a key determinant in the development of active immunity or immunological tolerance. The microenvironmental milieu of the specific tissue at the time of administration also plays a pivotal role in determining the immunological outcome of an antigen encounter. In recent years, it has become clear that application of antigen onto bare skin induces potent systemic and mucosal immunity in an antigen-specific manner.1–3 The effectiveness of such epicutaneous immunization and the ways in which epidermal Langerhans cells and the immune system of the skin deal with antigens and promote active immunity make the skin a potential and attractive non-invasive route for vaccine delivery. Indeed, there has recently been considerable interest in exploiting the immune system of the skin for needle-free vaccine delivery and strategies for ‘transcutaneous immunization’ are being developed.4,5 For induction of good immune responses, these new strategies depend on the use of strong non-specific adjuvants. Co-administration of cholera toxin, heat-labile enterotoxin of Escherichia coli and CpG oligodeoxynucleotides onto the skin is commonly used for ‘transcutaneous immunization’.5–8 However, the inclusion of such toxins is generally not acceptable for human vaccines and alternatives are being sought. We have recently shown that a natural adjuvant effect can be achieved by simply disrupting the stratum corneum of the epidermis before topical antigen application.1 This activates the resting epidermal Langerhans cells and transforms them into vigorous T-cell stimulators. Application of antigen onto this activated epidermis represents a form of in situ dendritic cell immunotherapy and this ultimately results in potent systemic immunity without the use of adjuvants or toxins.
Epicutaneous immunization, without adjuvants, on barrier-disrupted skin induces potent and strongly T helper type 2 (Th2)-biased immunity. High levels of antigen-specific immunoglobulin G1 (IgG1) and IgE and strong production of interleukin-4 (IL-4) but low or no interferon-γ (IFN-γ) and IgG2a demonstrate the Th2 nature of the immune response following epicutaneous antigen application.1,9 The epidermal micromilieu appears to be particularly well suited to the induction of Th2 immunity. While potent Th2 responses are induced following epicutaneous antigen delivery, injection of antigen into the dermis promotes Th1-type responses.1 Epicutaneous exposure to antigen has also been shown to be the most potent route for induction of IgE.10 In addition, epicutaneous immunization can affect immune responses to a secondary antigen exposure at distant sites such as the gut-associated lymphoid tissue and the lung. Epicutaneous immunization induces active antigen-specific immunity in the gut and specifically enhances Th2 responses following oral antigen11 and inhalation of antigen.12
In this study, the potential of epicutaneous antigen immunization to modify immune responses to subsequently encountered antigens and to modify established antigen-specific immune responses is further investigated. We report that epicutaneous immunization interferes with the development of systemic Th1-type immune responses induced through injection of antigen in complete Freund's adjuvant (CFA). Prevention of Th1 responses only occurs for the epicutaneously applied antigen, and is therefore antigen-specific. Moreover, epicutaneous immunization converts an established antigen-specific Th1 response to a Th2 immune response. These results show that skin-induced immune responses can modify systemic responses to the same antigen. That the Th2 response induced by epicutaneous immunization is dominant over Th1 responses suggests that this simple route of antigen delivery may be a potential beneficial therapeutic tool in Th1-type autoimmune diseases such as rheumatoid arthritis, type 1 diabetes, Hashimoto's thyroiditis and multiple sclerosis.
Materials and methods
Mice
BALB/c mice were bred and maintained on a special diet free of peanut, ovalbumin (OVA), soy milk and cows' milk. They were kept under specific pathogen-free conditions and provided with water ad libitum. Female mice aged 6–8 weeks were used in this study in accordance with Home Office regulations under the Animals (Scientific Procedures) Act 1986.
Protein antigens
Partly de-fatted peanut flour was obtained from the Golden Peanut Company (Alpharetta, GA). A concentrated peanut protein extract was prepared as described previously.1 In brief, the flour was defatted with hexane five times and then extracted with 0·1 m NH4HCO3. The supernatant was removed and precipitated with 60% (NH4)2SO4 for 2 hr at 4°. The precipitate was centrifuged, dissolved in a minimum volume of phosphate-buffered saline (PBS) and dialysed extensively against PBS. This method uniformly yielded 85–90% pure peanut protein. Peanut protein was biotinylated using standard methods.13 OVA grade V was purchased from Sigma (Gillingham, UK).
Immunizations
For epicutaneous immunization, the stratum corneum was removed from both sides of the earlobe by application and removal of cellophane tape (Scotch™; 3M, Cergy-Pontoise Cedex, France) five to eight times. Twenty-four hours later, 25 μl of peanut protein in PBS (4 mg/ml) was applied to both sides of the earlobe with a cotton bud. An estimated maximum of 100 μg of protein was deposited on the ear by this technique. The application of peanut protein to the skin was repeated on the next two consecutive days. Control animals had PBS without antigen applied to stripped skin in an identical manner or had no skin procedure at all.
Three weeks after epicutaneous immunization, all animals were immunized subcutaneously at the tail-base with 100 μg peanut protein emulsified 1 : 1 in CFA. After a further 3 weeks, animals were challenged in the footpad with 100 μg peanut protein in PBS and delayed-type hypersenstivity (DTH) responses were measured. One week later, para-aortic lymph nodes (PLN), spleen and serum were collected for analysis.
To control for antigen-specificity, some animals were tail-base immunized with both 100 μg peanut protein and 100 μg OVA in CFA 3 weeks after the epicutaneous immunization. After a further 3 weeks, PLN, spleen and serum were collected and analysed for peanut/OVA responses.
Alternatively, animals were first tail-base immunized with 100 μg peanut protein in CFA and then 3 weeks later were epicutaneously immunized with peanut protein as described above. After a further 3 weeks, animals were challenged in the footpad with 100 μg peanut protein in PBS and DTH responses were measured. One week later, cervical lymph nodes (CLN), spleen and serum were collected for analysis.
Tables indicating the order of the experimental procedures in the different in vivo experiments are provided with the figures.
DTH response
To elicit a DTH response, mice were challenged 3 weeks after the last epicutaneous or subcutaneous immunization by injection of 100 μg peanut protein in PBS into the left hind footpad. Net footpad swelling was measured using a microcalliper (Mitutoyo, Siwa, Japan) 24 hr after challenge. Mice were killed 1 week after measurement of the footpad.
T-cell proliferation and cytokine production
Spleen and lymph node cell suspensions were obtained by mechanical disaggregation, and 2 × 105 cells were cultured in 96-well flat-bottom plates in a total volume of 200 μl RPMI-1640 medium supplemented with 10% fetal calf serum, 50 μm 2-mercaptoethanol and 5 μg/ml gentamycin. Peanut protein was added at concentrations ranging from 5 to 450 μg/ml. Control responses to an irrelevant antigen (OVA or bovine serum albumin) at 50 μg/ml or concanavalin A at 1 μg/ml were also determined. Cultures were incubated at 37° for 90 hr and pulsed with 1 μCi of [3H]thymidine (Amersham Pharmacia, Little Chalfont, UK) for the last 16 hr. Cells were harvested and thymidine incorporation was determined by liquid scintillation counting on a Trilux MicroBeta machine (Wallac, Turku, Finland). Supernatants were assayed by enzyme-linked immunosorbent assay for IL-4, IL-10 and IFN-γ using antibodies from PharMingen (San Diego, CA), according to the manufacturer's protocol. Recombinant mouse IL-4, IL-10 and IFN-γ from PharMingen were used as standards. The detection limit of the assays was 5 pg/ml for IL-4 and 40 pg/ml for IL-10 and IFN-γ.
Antibody responses
At the end of each experiment, mice were bled by cardiac puncture and sera were prepared for specific antibody determinations. For IgG, IgG1 and IgG2a antibodies, 96-well Maxisorb plates (Nunc, Roskilde, Denmark) were coated with peanut protein at 250 μg/ml in carbonate–bicarbonate buffer at 4° over night. Optimally diluted sera (100 μl in PBS) were added and the plates were incubated at 37° for 90 min. After washing, alkaline-phosphatase-conjugated polyclonal goat anti-mouse IgG Fc (Sigma), rat monoclonal antibody to mouse IgG1 (Zymed, San Francisco, CA) or rat monoclonal antibody to IgG2a (PharMingen) was added for 1 hr at 37°. The alkaline phosphatase substrate pNPP (Sigma) was then added and absorbance was measured at 405 nm. Antigen-specific IgE was measured by an IgE capture method. Sera to be tested were added to Maxisorb microtitre plate wells coated with 1 μg/ml of rat monoclonal anti-mouse IgE (PharMingen). Biotinylated peanut protein was then added at a concentration of 100 μg/ml and incubated for 2 hr at 37°. After washing, alkaline phosphatase streptavidin (PharMingen) was added for 1 hr followed by pNPP substrate.
Statistical evaluation
The statistical significance of differences between experimental groups with regard to DTH responses and serum antibody levels was determined using two-tailed Student's t-test for unpaired data. Statistical significance of T-cell proliferation differences between experimental groups over the entire dose–response curve was determined by a two-tailed t-test on the slope of straight-line fits to the data, equivalent to a one-way anova on two groups. If the slopes were not significantly different, the intercepts were compared using a two-tailed t-test, which was equivalent to an analysis of covariance on two groups.14 Differences were regarded as significant when P < 0·05.
Results
Epicutaneous immunization enhances and modifies responses obtained by immunization with CFA
Application of protein antigen on barrier-disrupted skin (24 hr after removal of the stratum corneum by tape-stripping) gives rise to potent systemic immune responses. Immune responses following such epicutaneous immunization are strongly Th2-biased with high levels of antigen-specific IgG1 and IgE and strong production of IL-4 but with little or no IgG2a and IFN-γ produced as described previously.1,11 To investigate the persistence of this Th2 immunity and the potential for epicutaneous antigen immunization to modify responses to subsequently encountered antigen, mice were epicutaneously immunized with peanut protein and 3 weeks later were immunized subcutaneously with peanut protein in CFA (experimental groups and procedures are listed in the inset table Fig. 1).
Figure 1.
Epicutaneous immunization enhances T-cell responses to subsequent immunization with CFA. DTH responses (a) were measured 24 hr after single peanut protein challenge in the footpad and are expressed as mean increment of footpad swelling + 1 SEM (n = 7). T cells taken from PLN draining the peanut/CFA immunization site 7 weeks after the initial epicutaneous immunization were cultured for 90 hr with peanut protein or control antigen (▪) and proliferation was determined by [3H]thymidine incorporation (b) Results are expressed as mean c.p.m. ± 1 SEM. Background proliferation when no antigen was present has been subtracted. All groups responded with similar c.p.m. when stimulated with Con A. Experimental groups and order of procedures are listed in the inset table.
Epicutaneous immunization significantly enhanced the vigour of the T-cell response to a subsequent peanut/CFA immunization. DTH responses to peanut protein were larger in epicutaneously immunized animals (group 2) compared to control animals epicutaneously immunized with saline (group 1, P = 0·030) or receiving no skin procedure (group 3, P = 0·003) before peanut/CFA immunization (Fig. 1a). Dose-dependent proliferation of T cells from PLN was obtained in response to peanut protein, but not to control antigen, in all groups 8 weeks after the epicutaneous immunization (Fig. 1b). The proliferative response was however, significantly greater in animals epicutaneously immunized with peanut protein before the peanut/CFA immunization (Fig. 1b group 2, P < 0·01). Moreover, the pattern of cytokines produced by these PLN cells was the inverse to the pattern of cytokines produced by PLN cells from control animals (Fig. 2a). The prior epicutaneous immunization enhanced levels of antigen-driven IL-4 and decreased levels of IFN-γ and IL-10 (group 2, Fig. 2a) compared to control responses to CFA immunization (group 1 and 3). Similar proliferation and cytokine patterns were obtained using splenic T cells (data not shown). In addition, analysis of peanut-specific antibody responses showed that epicutaneous immunization with peanut protein significantly enhanced levels of IgG1 and IgE following peanut/CFA immunization (Fig. 2b). In contrast, levels of specific IgG2a were reduced compared to controls. This Th2-dominant antibody pattern in animals epicutaneously immunized before CFA immunization was consistent with the cytokine response and suggests that the epicutaneous immunization biased responses to antigen in CFA from Th1- to Th2-type immunity.
Figure 2.
Epicutaneous immunization interferes with development of Th1-type responses. Production of IL-4, IFN-γ and IL-10 (a) by PLN T cells collected 7 weeks after the initial epicutaneous immunization were determined after 4–7 days reactivation with peanut protein in vitro. No cytokines were produced in response to OVA as a control antigen or when cells were not stimulated. Peanut-specific IgG, IgG1, IgG2a and IgE were measured in serum 7 weeks after the initial epicutaneous immunization (4 weeks after CFA immunization) (b) Serum samples were diluted 1 : 1000 for IgG and IgG1, 1 : 200 for IgG2a and 1 : 10 for IgE prior to analysis. Each bar represent mean antibody level + 1 SEM (n = 7). Experimental groups and order of procedures as in the inset table Fig. 1.
Epicutaneous immunization modifies immune responses in an antigen-specific manner
To examine whether the immune-modulating effect of epicutaneous immunization was antigen-specific, animals were epicutaneously immunized with peanut protein, or saline as control, and 3 weeks later were immunized with peanut protein and chicken egg OVA in CFA (experimental groups and procedures are listed in the inset table Fig. 3). Responses to both peanut protein and OVA were subsequently analysed.
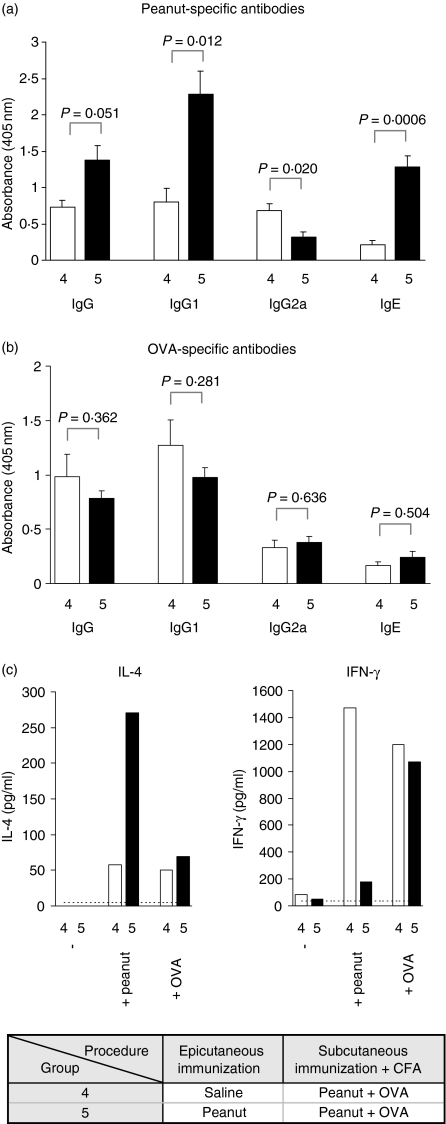
Figure 3.
Epicutaneous immunization interferes with Th1-type responses in an antigen-specific manner. Peanut-specific (a) and OVA-specific (b) IgG, IgG1, IgG2a and IgE were measured in serum 7 weeks after the initial epicutaneous immunization (4 weeks after CFA immunization). Serum samples were diluted 1 : 1000 for IgG and IgG1, 1 : 200 for IgG2a and 1 : 10 for IgE before analysis. Results are expressed as mean antibody level + 1 SEM (n = 6 for group 4, n = 7 for group 5). Antigen-driven production of IL-4 and IFN-γ (c) by PLN T cells collected 7 weeks after the initial epicutaneous immunization were determined by ELISA after 96 hr stimulation with peanut protein or OVA in vitro. Experimental groups and order of procedures are listed in the inset table.
As expected from previous findings (Fig. 2), epicutaneous immunization with peanut protein biased responses to the subsequent peanut/CFA immunization from Th1 to Th2. Levels of peanut-specific IgG1 and IgE were significantly enhanced while levels of peanut-IgG2a were reduced (Fig. 3a, group 5). In addition, levels of peanut protein-driven production of IL-4 were enhanced and IFN-γ were markedly reduced compared to controls (Fig. 3c, group 5). This was not the case for OVA. There was no difference in the levels of OVA-specific antibodies between animals epicutaneously immunized with peanut protein or saline (Fig. 3b). Neither was there a difference between the two groups in the levels of OVA-driven production of IL-4 and IFN-γ. Both groups produced low amounts of IL-4 and high amounts of IFN-γ in response to OVA (Fig. 3c). These data suggest that the modifying effect of epicutaneous immunization on subsequent Th1-inducing immunizations is antigen-specific.
Epicutaneous immunization converts an established antigen-specific Th1- to a Th2-type response
To investigate whether epicutaneous immunization could modify an established antigen-specific Th1-type immune response, mice were immunized with peanut protein in CFA first and then 3 weeks later were epicutaneously immunized with peanut protein or saline as control (experimental groups and procedures are listed in the inset table Fig. 4).
Figure 4.
Epicutaneous immunization boosts T-cell responses to previous CFA immunization. DTH responses (a) were measured 24 hr after single peanut protein challenge in the footpad and are expressed as mean increment of footpad swelling + 1 SEM (n = 5 for group 6, n = 6 for group 7). Splenic T cells collected 7 weeks after the initial CFA immunization (4 weeks after epicutaneous immunization) were cultured for 90 hr with peanut protein or control antigen (▮) and proliferation was determined by [3H]thymidine incorporation (b) Results are expressed as mean c.p.m. ± 1 SEM. Background proliferation when no antigen was present has been subtracted. All groups responded with similar c.p.m. when stimulated with Con A. Experimental groups and order of procedures are listed in the inset table.
Animals epicutaneously immunized with peanut protein after the peanut/CFA immunization (group 7) responded vigorously to footpad recall immunization with footpad swellings of 37 ± 0·4 mm. These high DTH responses were enhanced, although not significantly (P = 0·063), compared to controls (Fig. 4a). Splenic T cells from animals epicutaneously immunized with peanut protein after the CFA immunization also showed significantly greater antigen-specific proliferation compared to controls (Fig. 4b, group 7). A similar proliferation pattern was seen with PLN cells. A further enhanced proliferation was measured in cultures of CLN T cells (P < 0·001); however, cells from this site in control animals proliferated poorly (data not shown).
Epicutaneous immunization not only enhanced the potency of the ongoing immune response but analysis of cytokine and antibody responses showed that it also changed the nature of the established peanut-specific immune response (Fig. 5). Splenic (and PLN) cells from control animals with an established immune response to peanut protein produced large amounts of IFN-γ and IL-10, but little IL-4 when re-stimulated with peanut protein (Fig. 5a, group 6). In contrast, animals epicutaneously immunized with peanut protein after the peanut/CFA immunization showed reduced levels of IFN-γ but enhanced levels of IL-4 upon re-stimulation (Fig. 5a, group 7). There was no difference between the experimental groups in the levels of secreted IL-10. A modulating effect of epicutaneous immunization on the established CFA-induced immune response was also evident when levels of peanut-specific antibodies were analysed. Epicutaneous immunization significantly reduced the level of IgG2a while the level of IgE was significantly increased compared to control animals (Fig. 5b, group 7). No difference in the levels of specific IgG and IgG1 was detected between the two experimental groups. Taken together, these results suggest that epicutaneous immunization modified an established antigen-specific immune response by specifically decreasing Th1-type responses and enhancing Th2-type responses.
Figure 5.
Epicutaneous immunization converts an established antigen-specific Th1- to a Th2 response. Production of IL-4, IFN-γ and IL-10 (a) by splenic T cells collected 7 weeks after the initial CFA immunization were determined after 4–7 days reactivation with peanut protein in vitro. No cytokines were produced in response to OVA as a control antigen or when cells were not stimulated. Peanut-specific IgG, IgG1, IgG2a and IgE were measured in serum 7 weeks after the initial CFA immunization (4 weeks after epicutaneous immunization) (b) Serum samples were diluted 1 : 1000 for IgG and IgG1, 1 : 200 for IgG2a and 1 : 10 for IgE prior to analysis. Each bar represent mean antibody level + 1 SEM (n = 6–7). Experimental groups and order of procedures as in the inset table Fig. 4.
Discussion
In this study, we asked whether the Th2 dominance of epicutaneous immunization could interfere with the development of Th1-mediated responses and even modify established Th1 responses. Our results show that immune responses induced by epicutaneous immunization are potent and persistent and can both enhance and modify the nature of subsequent immune responses to the same antigen, including strong adjuvant-driven responses. The systemic Th2 immune response elicited by epicutaneous immunization interfered with the development of subsequent Th1-type responses obtained by subcutaneous immunization with CFA. Strong T-cell responses induced by epicutaneous immunization were demonstrated in PLN draining the CFA immunization site and in the spleen 8 weeks after the initial epicutaneous immunization. This potent response was not merely acting in synergy with the adjuvant-driven response, because the epicutaneous immunization predisposed animals to reduce production of IFN-γ and IgG2a and enhance IL-4, IgG1 and IgE production in response to the subsequent CFA immunization (Fig. 2), suggesting an immunological switch from Th1 to Th2. The prevention of the development of a Th1 immune response was specific to the epicutaneously applied antigen because injection of a control antigen in CFA into the same animals induced normal Th1-type immunity (Fig. 3).
The Th2 dominance of the responses to epicutaneous immunization makes it a potentially useful immune-modulating procedure in Th1-type autoimmune diseases such as rheumatoid arthritis, type 1 diabetes, Hashimoto's thyroiditis and multiple sclerosis. Autoimmune diseases such as these result from dysregulated immune responses to self-antigens and are in many cases driven by Th1 autoreactive T cells. The paradoxical application of self-antigens to prevent or delay the onset of disease has been validated in many animal models of autoimmune disease.15,16 Oral or nasal application of autoantigens has been the predominant mode of autoimmune therapy with the aim of inducing antigen-specific systemic hyporesponsiveness or ‘mucosal tolerance’. Such mucosally delivered autoantigens have been demonstrated to be efficacious in preventing autoimmunity in a range of animal models but have, however, proven less successful in treating established disease and reproducible clinical efficacy in human autoimmune disease has still not been demonstrated.15,16 Experimentally, it is well established that it is difficult to alter an ongoing immune response through mucosal delivery of antigens,17,18 which may partly explain the disappointing results of initial human disease trials. Although the ultimate goal is to re-establish tolerance in the autoimmune patient, using ‘mucosal tolerance’ may not prove powerful enough, and a different strategy inducing active immunity may be necessary.
Confirming the idea that epicutaneous delivery of autoantigens may interfere with the development of inflammatory Th1-type autoimmune diseases, a recent study showed that epicutaneous administration of autoantigenic myelin basic protein (MBP) protected transgenic mice from induced experimental allergic encephalomyelitis (EAE).19 In this study MBP peptides were applied twice on an occlusive patch for 2 weeks on shaved back skin, and EAE was subsequently induced by subcutaneous immunization with the immunodominant peptide of MBP in CFA. The disease resistance was mediated by CD4+ suppressor T cells but was not dependent on CD4+ CD25+ T cells.19 The therapeutic potential of epicutaneous autoantigen administration was not determined. However, if treatment of human disease is to become a therapeutic reality the ability to modulate an ongoing immune response and re-establish immunological balance or tolerance is crucial. The present study in principle shows that this may also be possible through epicutaneous immunization on barrier-disrupted skin. Animals with established antigen-specific Th1-type immunity responded to epicutaneous immunization by reducing levels of specific IFN-γ and IgG2a and enhancing levels of IL-4 and IgE (Fig. 5). This suggests that epicutaneously driven immune responses can induce systemic immune deviation from Th1 to Th2. It may be possible to further reduce Th1 and enhance Th2 responses by repeating the epicutaneous immunization, which in this study was given only once. The possibility of using in situ Langerhans cell activation and epicutaneous delivery of antigens as therapy for Th1-type autoimmune diseases is currently being investigated in our laboratories using models of rheumatoid arthritis and type 1 diabetes.
The route of antigen delivery is clearly important in determining the resulting immune response. It is generally thought that immunity is controlled by the lymphocytes of the adaptive immune system or by the dendritic cells, macrophages, etc. of the innate immune system. However, it may be more likely that the ultimate power to decide if and how to respond lies within the tissues. Different tissues appear to have different means of determining the effector class of an immune response.20 More attention needs to be aimed at understanding the normal mechanisms and types of immune responses in different tissues, as well as to understanding the responses in stressed/injured tissues. Such knowledge may enable us to manipulate immune responses that have ‘gone wrong’ in the tissues. Additionally, different tissues could be used as natural therapeutic tools in gaining a particular class of immune response. By using the in situ strength of the immune system, the use of toxins and non-specific adjuvants for vaccine delivery may not be needed.
Induction of natural immune responses through the epidermal tissue evidently leads to Th2-type immunity.1–3 How this epicutaneously induced response can modify subsequently induced and established antigen-specific immunity is not known. The mechanisms of antigen-induced immune modulation are dependent on the route of initial antigen delivery, but most frequently suggested are apoptosis, anergy, regulatory T cells and immune deviation. It has been suggested that epicutaneous immunization with autoantigenic MBP peptides may induce T cells with a suppressive function, which can transfer protection against EAE.19 The induction of a population of regulatory T cells through the skin is an intriguing possibility that requires further investigation. In our study, epicutaneous immunization induced very active immune responses and its ability to interfere with Th1-type immunity may suggest a mechanism of immune deviation. Whether the switch from a Th1 to a Th2 response represents induction of a new dominant antigen-specific Th2 population or an actual reversion of the Th cells' differentiation commitment is not possible to determine from the present data. Differentiation of Th1 and Th2 cells are strongly influenced by the presence of IFN-γ and IL-4, which control expression of the transcription factors T-bet21 and GATA-3.22 T-cell differentiation at a population level ultimately depends on the expression dynamics of T-bet and GATA-3. Through the mutually exclusive expression of these transcription factors and their regulation through IFN-γ and IL-4 it is theoretically possible to reverse Th cell commitment through manipulation of the cytokine microenvironment.23
While using epicutaneous immunization as a novel vaccine strategy shows definite potential, the dangers of therapeutic manipulation of the Th1/Th2 balance must be kept in mind. In directly utilizing the Th2-bias of epicutaneously induced responses in the treatment of autoimmunity, the possible induction of pathogenic IgE responses must be monitored carefully. The epicutaneous immunization presented in this study did not, however, rely on adjuvants for Th2 potency, and thus appears to have the advantage of initiating antigen-specific Th2 responses. The possibilities of pathogenic responses may be greater in treatments involving adjuvants that non-specifically direct responses towards Th1 or Th2; although, many of the experimentally successful allergy therapies for example have been associated with immune deviation from Th2 to Th1 responses, and do not appear to have resulted in autoimmune or otherwise aberrant Th1-mediated diseases.24 However, there is naturally a serious risk of oversimplifying the concepts, if disease is solely explained by polarization of Th1 versus Th2 effector cells. Nevertheless, re-establishing a balanced Th1/Th2 response to specific antigens may relieve symptoms of disease and ultimately re-establish healthy immune regulation and tolerance.
Acknowledgments
The work was funded and supported by the Food Standard Agency. Research at the Institute of Child Health and Great Ormond Street Hospital for Children National Health Service (NHS) Trust benefits from research and development funding received from the UK NHS Executive.
Abbreviations
- CFA
complete Freund's adjuvant
- CLN
cervical lymph nodes
- DTH
delayed type hypersensitivity
- EAE
experimental allergic encephalomyelitis
- IFN-γ
interferon-γ
- IgG1
immunoglobulin 1
- IL-4
interleukin-4
- MBP
myelin basic protein
- OVA
ovalbumin
- PBS
phosphate-buffered saline
- PLN
para-aortic lymph node
- Th1
T helper type 1
References
- 1.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol. 2004;34:2100–9. doi: 10.1002/eji.200425196. [DOI] [PubMed] [Google Scholar]
- 2.Herrick CA, Xu L, McKenzie AN, Tigelaar RE, Bottomly K. IL-13 is necessary, not simply sufficient, for epicutaneously induced Th2 responses to soluble protein antigen. J Immunol. 2003;170:2488–95. doi: 10.4049/jimmunol.170.5.2488. [DOI] [PubMed] [Google Scholar]
- 3.Wang LF, Lin JY, Hsieh KH, Lin RH. Epicutaneous exposure of protein antigen induces a predominant Th2-like response with high IgE production in mice. J Immunol. 1996;156:4077–82. [PubMed] [Google Scholar]
- 4.Glenn GM, Taylor DN, Li X, Frankel S, Montemarano A, Alving CR. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med. 2000;6:1403–6. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 5.Partidos CD, Beignon AS, Briand JP, Muller S. Modulation of immune responses with transcutaneously deliverable adjuvants. Vaccine. 2004;22:2385–90. doi: 10.1016/j.vaccine.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 6.Beignon AS, Briand JP, Muller S, Partidos CD. Immunization onto bare skin with synthetic peptides: immunomodulation with a CpG-containing oligodeoxynucleotide and effective priming of influenza virus-specific CD4? T cells. Immunology. 2002;105:204–12. doi: 10.1046/j.0019-2805.2001.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beignon AS, Briand JP, Muller S, Partidos CD. Immunization onto bare skin with heat-labile enterotoxin of Escherichia coli enhances immune responses to coadministered protein and peptide antigens and protects mice against lethal toxin challenge. Immunology. 2001;102:344–51. doi: 10.1046/j.1365-2567.2001.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond SA, Walwender D, Alving CR, Glenn GM. Transcutaneous immunization: T cell responses and boosting of existing immunity. Vaccine. 2001;19:2701–7. doi: 10.1016/s0264-410x(00)00506-5. [DOI] [PubMed] [Google Scholar]
- 9.Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769–79. doi: 10.1002/(SICI)1521-4141(199803)28:03<769::AID-IMMU769>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Nelde A, Teufel M, Hahn C, Duschl A, Sebald W, Brocker EB, Grunewald SM. The impact of the route and frequency of antigen exposure on the IgE response in allergy. Int Arch Allergy Immunol. 2001;124:461–9. doi: 10.1159/000053781. [DOI] [PubMed] [Google Scholar]
- 11.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757–66. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 12.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochet O, Teillaud J, Sautès C. Immunological Techniques Made Easy. Chichester: John Wiley & Sons; 1998. [Google Scholar]
- 14.Armitage P. Statistical Methods in Medical Research. Oxford: Blackwell; 1971. [Google Scholar]
- 15.Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4:407–19. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- 16.Weiner HL. Current issues in the treatment of human diseases by mucosal tolerance. Ann NY Acad Sc. 2004;1029:211–24. doi: 10.1196/annals.1309.053. [DOI] [PubMed] [Google Scholar]
- 17.Moldoveanu Z, Oliver F, Mestecky J, Elson CO. Oral tolerance in humans: failure to suppress an existing immune response by oral antigen administration. Ann NY Acad Sci. 2004;1029:299–309. doi: 10.1196/annals.1309.051. [DOI] [PubMed] [Google Scholar]
- 18.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–81. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 19.Bynoe MS, Evans JT, Viret C, Janeway CA. Epicutaneous immunization with autoantigenic peptides induces T suppressor cells that prevent experimental allergic encephalomyelitis. Immunity. 2003;19:317–28. doi: 10.1016/s1074-7613(03)00239-5. [DOI] [PubMed] [Google Scholar]
- 20.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 21.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 22.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 23.Yates A, Callard R, Stark J. Combining cytokine signalling with T-bet and GATA-3 regulation in Th1 and Th2 differentiation: a model for cellular decision-making. J Theor Biol. 2004;231:181–96. doi: 10.1016/j.jtbi.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Burks W, Bannon G, Lehrer SB. Classic specific immunotherapy and new perspectives in specific immunotherapy for food allergy. Allergy. 2001;56(Suppl. 67):121–4. doi: 10.1034/j.1398-9995.2001.00935.x. [DOI] [PubMed] [Google Scholar]