Abstract
Prostaglandin E2 (PGE2), a major lipid derived from the metabolism of arachidonic acid, is an environmentally bioactive substance produced by inflammatory processes and acts as a cAMP up-regulator that plays an important role in immune responses. It has been reported that PGE2 has the ability to inhibit the production of interleukin-12 by myeloid dendritic cells (MDCs) and macrophages, and then induce preferential T helper type 2 (Th2) cell responses. However, little is known of the function of PGE2 for plasmacytoid dendritic cells (PDCs), which may contribute to the innate and adaptive immune response to viral infection, allergy and autoimmune diseases. In the present study, we compared the biological effect of PGE2 on human PDCs and MDCs. PGE2 caused the death of PDCs but MDCs survived. Furthermore, we found that, whereas PGE2 inhibited interferon-α production by PDCs in response to virus or cytosine–phosphate–guanosine, it inhibited interelukin-12 production by MDCs in response to lipopolysaccharide (LPS) or poly(I:C). Although both virus-stimulated PDCs and LPS-stimulated MDCs preferentially induced the development of interferon-γ-producing Th1 cells, pretreatment with PGE2 led both DC subsets to attenuate their Th1-inducing capacity. These findings suggest that PGE2 represents a negative regulator on not only MDCs but also PDCs.
Keywords: cytokine, inflammation, plasmacytoid dendritic cells, prostaglandin E2, type I interferons
Introduction
Dendritic cells (DCs) are key initiators of priming immune responses.1 Recent progress in understanding DC activity to produce cytokines has revealed that myeloid DCs (MDCs) and plasmacytoid DCs (PDCs) show different susceptibilities to products derived from pathogens, depending on their toll-like-receptor (TLR) expression profiles.2,3 MDCs have some capacity to produce interleukin-12 (IL-12) in response to the microbial stimuli through TLR2, -3, -4 and -8 and, thereby, to induce T helper type 1 (Th1) development.3,4 On the other hand, PDCs, which are also referred to as type I interferon-producing cells (IFN-α/β/ω/λ),5,6 are the key effectors in innate immunity because of their capacity to produce exceptional amounts of type I IFNs against microbial infection, particularly viral infection through TLR7 and -9.3,6,7 In contrast to MDCs, human PDCs appear to have less capacity to produce IL-12 in response to viruses or TLR ligands.3,8 PDC-derived type I IFNs also participate in the T-cell-priming as Th1-inducing cytokines.9,10 Thus, in dictating Th cell responses, each type of DC has a distinct cytokine-dependent machinery.10
Prostanoids, a major class of eicosanoids, are important mediators that contribute to the inflammatory process caused by tissue injury or antigen-mediated immune responses.11 Prostaglandin E2 (PGE2) is one of the best known and most well-studied prostanoids. PGE2 (which is an environmentally bioactive substance produced by inflammatory processes and mainly released from activated mast cells, keratinocytes, or macrophages) increases vascular permeability along with several vasoactive elements such as histamine, bradykinin, or nitric oxide, thereby resulting in the onset of oedema, flare and hyperalgia at the local inflammatory sites.12 In addition, PGE2 was found to enhance MDC maturation that was dependent on tumour necrosis factor-α (TNF-α) but to inhibit the release of proinflammatory cytokines and IL-12 production from MDCs and macrophages activated with lipopolysaccharide (LPS).13–16 However, little is known about the function of PGE2 on PDCs.
Here, we have analysed the effects of PGE2 on the survival and cytokine production in PDCs. We found that, while PGE2 sustained the survival of MDCs, it negatively regulated PDC survival. Moreover, PGE2 inhibited the IFN-α production from PDCs upon viral stimulation and then suppressed the ability of PDCs to induce IFN-γ-producing Th1 cell development.
Materials and methods
Media and reagents
RPMI-1640 supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 100 ng/ml streptomycin and heat-inactivated 10% fetal calf serum (Irvine Scientific, Santa Ana, CA) was used for cell cultures throughout the experiments. PGE2 (Sigma, St Louis, MO) was dissolved in anhydrous ethanol and used at a final concentration of 10−9 to 10−5 m. Ethanol was diluted in parallel to serve as vehicle control. Recombinant human cytokines, granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml) and IL-3 (10 ng/ml) were purchased from Pepro Tech EC (London, UK). LPS (Salmonella typhimurium) (1 μg/ml) was purchased from Sigma. Ultraviolet-irradiated Sendai virus (SeV) (HVJ: Cantell strain, provided by Sumitomo Pharmaceuticals, Ehime, Japan) was used at 5 haemagglutinating units/ml.17 Poly(I:C) (25 μg/ml) and cytosine–phosphate–guanosine-oligodeoxynucleotides (CpG-ODNs) 2216 (5 μm) were purchased from Invivogen (San Diego, CA).
Isolation of blood DC subsets
Peripheral blood DC subsets (MDCs and PDCs) were isolated according to the modified protocol, as described previously.18,19 Briefly, the DC-enriched population (CD4+ CD3− CD14− cells) was obtained from peripheral blood mononuclear cells by negative and subsequent positive immunoselections. The CD11c+ lineage− DR+ cells (as MDCs) and CD11c− lineage− DR+ cells (as PDCs) were sorted by an EPICS ALTRA® flow cytometer (Coulter Corp., Hialeah, FL) by using phycoerythrin (PE)-labelled anti-CD11c (Becton Dickinson, Sunnyvale, CA), a mixture of fluorescein isothiocyanate (FITC)-labelled monoclonal antibodies (mAbs) against lineage markers, CD3 (Exalpha, Boston, MA), CD14 (Exalpha), CD15 (Becton Dickinson), and CD56 (Becton Dickinson), and phycoerythrin–cynin 5.1 (PC5)-labelled human leucocyte antigen-DR (ImmunoTech, Marseille, France). The purity of each cell was 97% or greater.
Analysis of cultured DCs
The CD11c+ MDCs were cultured with medium alone, GM-CSF, LPS, or poly(I:C), while PDCs were cultured with IL-3, SeV, or CpG-2216 in 96-well, round-bottom tissue culture plates at 5 × 104 cells in 200 μl medium per well for 24 hr or 72 hr. PGE2 or vehicle was added into these cultures. In the viability assay, viable cells were counted after culture by trypan blue staining. After 24 hr of culture, the production of cytokines in the culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA) after 24 hr of culture (kits for IL-12 p40 + p70, IL-10 and TNF-α were purchased from Endogen, Rockford, IL, and that for IFN-α was obtained from PBL Biomedical Laboratories, Piscataway, NJ). Intracellular cytokine staining in PDCs was performed after 8 hr of culture with SeV alone or SeV plus PGE2 (10−6 m). Brefeldin A (10 μg/ml; Sigma) was added during the last 2 hr. After stimulation, cells were fixed and permeabilized using the FIX and PERM kit (CALTAG, Burlingame, CA) and then stained with FITC-labelled anti-IFN-α2 mAb (Chromaprobe, Maryland Heights, MO) and PE-labelled anti-BDCA4 mAb (Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany). Dead cells were excluded on the basis of side- and forward-scatter characteristics.
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted from PDCs that had been exposed to SeV with or without PGE2 (10−6 m) for 8 hr using the Qiagen RNase mini protcol and was converted to cDNA using oligo-dT, random hexamers and SuperScript II RT (Invitrogen, Carlsbad, CA). The temperature profiles of the PCR were as follows: an initial denaturation step at 94° for 5 min followed by 30 cycles at 94° for 1 min, 55° for 1 min, and 72° for 30 seconds and then a final elongation step at 72° for 7 min. The sequences of primers were as follows: IFNA2, forward: 5′-GTACTGCAGAATCTCTCTTTTCTCCTG-3′, reverse: 5′-GTGTCTAGATCTGACAACCTCCCAGGCACA-3′; and β-actin, forward: 5′-CTGGAACGGTGAAGGTGACA-3′ and reverse: 5′AAGGGACTTCCTGTAACAATGCA-3′.
DC–T-cell coculture
CD4+ CD45RA+ naive T cells (purity > 98%) were isolated from peripheral blood mononuclear cells using CD4+ T-cell isolation Kit II (Miltenyi Biotec) followed by cell sorting (CD4+ CD45RA+ CD45RO− fraction). Each blood DC subset preincubated with the various stimuli in the presence of PGE2 (10−6 m) or vehicle for 24 hr was washed three times and then cocultured with allogeneic CD4+ naive T cells (105 cells/well, DC : T-cell ratio 1 : 5) for 7 days in 96-well round-bottom tissue culture plates.
Cytokine quantification of T cells
The primed T cells were collected and washed. For intracellular cytokine staining, the cells were restimulated with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (2 μg/ml) for 6 hr, and brefeldin A (10 μg/ml) was added during the last 2 hr (all from Sigma). The cells were stained with PE-labelled anti-IL-4 (Becton Dickinson) or with PE-labelled anti-IL-10 (Becton-Dickinson) plus FITC-labelled anti-IFN-γ mAbs (Becton Dickinson), using a FIX and PERM kit. For detection of cytokines in culture supernatants, the T cells were restimulated with plate-bound anti-CD3 (OKT3, 5 μg/ml) and soluble anti-CD28 (CD28.2, 1 μg/ml) at a concentration of 1 × 106/ml for 48 hr. Cytokine productions in the culture supernatants were determined by ELISA (kits for IL-4, IL-10, and IFN-γ were purchased from Endogen).
Statistical analysis
The paired Student's t-test was used for statistical analysis with a StatView statistical program (Abacus Concepts, Inc., Berkeley, CA). Differences were considered significant when P < 0·05.
Results
PGE2 differentially acts on the viability of PDCs and MDCs
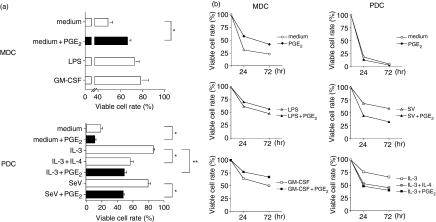
To investigate the biological effect of PGE2 on PDCs, we first comparatively evaluated the survival rate of PDCs and MDCs during the 24–72 h of culture in the presence of PGE2. Just as for GM-CSF and LPS, PGE2 was found to maintain the survival of MDCs [Fig. 1a(upper panel), b(left columns)]. This result is supported by recent reports showing that PGE2 promotes the survival of human monocyte-derived DCs and mouse bone-marrow-derived DCs.20,21 As has been previously shown, PDCs rapidly died when cultured in medium alone2,9,19 but viability was improved by the addition of IL-3 or SeV [Fig. 1a(lower panel)]. Interestingly, PGE2 had a detrimental effect on PDC survival during the 3 days of culture even in the presence of IL-3 or SeV, because IL-4 tends to kill PDCs [Fig. 1b(right columns)]22.
Figure 1.
Effect of PGE2 on the viability of DC subsets. In the presence or absence of PGE2 (10−6 m), MDCs were cultured with medium alone, LPS, and GM-CSF and PDCs were cultured with IL-3, IL-3 plus IL-4, and SeV for 24 hr and 72 hr. The numbers of viable cells in each DC subset was evaluated using trypan blue dye-exclusion test. (a) The data represent the means ± SEM of five independent experiments at 24 hr. Statistical significance is indicated using paired Student's t-test (*P < 0·05, **P < 0·005). (b) Data represent one of five independent experiments.
PGE2 inhibits IFN-α production from PDCs
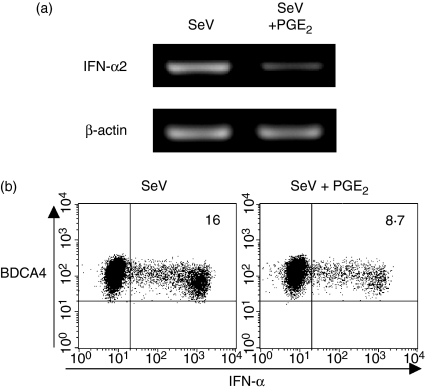
Innate and adaptive immune responses are regulated mainly by DC-derived cytokines.2,23 Therefore, we next examined the effects of PGE2 on the capacity of PDCs to produce DC-derived cytokines. We found that PGE2 inhibited the production of IFN-α by PDCs in response to SeV or CpG 2216 in a dose-dependent fashion [Fig. 2a(upper panels)]; this was also confirmed at mRNA level by a RT-PCR (Fig. 3a). To further investigate whether PGE2 inhibits the induction of IFN-α expression simply by killing PDCs, we analysed the intracellular IFN-α expression in viable PDCs after exposure to PGE2. Eight hours after activation by SeV, 16% of PDCs produced IFN-α. In this setting, PGE2 showed an inhibitory effect on the production of IFN-α even in viable PDCs (from 16% to 8·7%) (Fig. 3b). PGE2 also inhibited the LPS-induced or poly(I:C)-induced IL-12 production [Fig. 2b(upper panels)] but augmented the IL-10 production [Fig. 2b(middle panels)] from MDCs in a dose-dependent fashion. These findings were consistent with previous observations.14,24,25 Furthermore, PGE2 inhibited the TNF-α production by both PDCs and MDCs during activation [Fig. 2a,b(lower panels)]. These findings suggest that PGE2 has a negative regulatory function in the production by DCs of proinflammatory cytokines that activate innate and adaptive immune responses.
Figure 2.
Effect of PGE2 on cytokine production by DC subsets. PDCs. (a) and MDCs (b) were cultured with the indicated stimuli in the absence or presence of different doses of PGE2 (10−9 to 10−5 m) for 24 hr. Cytokine production in supernatant of each DC subset was measured by ELISA. Data represent one of three independent experiments.
Figure 3.
IFN-α expression in PDCs in response to SeV with or without PGE2 by RT-PCR and intracellular staining. PDCs were cultured for 8 hr with SeV plus vehicle or SeV plus PGE2 (10−6 m). (a) IFN-α2 mRNA expression in PDCs was analysed by RT-PCR. (b) IFN-α staining in PDCs was performed intracellularly by flow cytometry. Percentages of IFN-α-producing PDCs are indicated. Viable PDCs were analysed by exclusion of dead cells on the basis of side- and forward-scatter characteristics. Data represent one of three experiments.
PGE2 modulates DC-mediated T-cell responses
Both IL-12 and type 1 IFN are thought to be the Th1-driving cytokines by MDCs and PDCs, respectively,9,26,27 and their ability to produce IL-12 and type 1 IFN is a key property of adaptive immune responses. Therefore, we next investigated how PGE2 affects DC-mediated Th cell responses. Naive CD4+ T cells were cultured for 7 days with allogeneic PDCs precultured with IL-3, SeV, or SeV plus PGE2 and with allogeneic MDCs precultured with medium alone, LPS, or LPS plus PGE2. As shown in Fig. 4(a), CD4+ T cells primed by LPS-stimulated MDCs (LPS-MDCs) produced large amounts of the IFN-γ and low amounts of IL-4 and IL-10, whereas CD4+ T cells primed by SeV-stimulated PDCs (SeV-PDCs) produced large amounts of the IFN-γ and IL-10 and low amounts of IL-4, which is consistent with previous findings.9 We found that PGE2 pretreatment made LPS-MDCs and SeV-PDCs lose their ability to induce IFN-γ-producing Th1 cell development. The ability of PGE2 to inhibit the generation of IFN-γ-producing Th1 cells in DC-mediated Th cell responses was confirmed by ELISA (Fig. 4b). This experiment further demonstrated that PGE2 represents a negative regulator for DC-mediated Th1 cell development.
Figure 4.
Effect of PGE2 on DC-mediated Th cell polarization. Naive CD4+ T cells were cocultured for 7 days with MDCs preincubated with medium, LPS, LPS + PGE2 (10−6 m) or PDCs preincubated with IL-3, SeV, SeV + PGE2 (10−6 m). Production of IFN-γ, IL-4 and IL-10 by T cells was analysed intracellularly by flow cytometry (a) and measured in supernatants by ELISA (b). The percentages of the respective cytokine-producing T cells are indicated in each dot blot profile in (a). Data represent the means ± SEM of three independent experiments. Statistical significance is indicated using paired Student's t-test (*P < 0·05).
Discussion
Microenvironmental factors may instruct DC-mediated immune responses. In the present experiments, we studied the effects of PGE2, a major inflammatory mediator in the local environment, on the blood MDCs and PDCs. Although many publications describe the roles of PGE2on myeloid-lineage DCs in humans and mice,28,29 the function of PGE2 on PDCs has not been clarified. PDCs are found to infiltrate the inflamed lymphoid tissues, nasal allergic mucosa, rheumatoid arthritis synovium and skin lesions in systemic lupus erythematosus and psoriasis30–33. Therefore, PDCs may be influenced by PGE2 at the inflammatory site. We found here that PGE2 exerted an inhibitory effect on PDC survival and on the ability of PDCs to produce IFN-α and TNF-α. As a result of the pleiotropic effects of type I IFNs and TNF-α on various immune cells, PDCs promote the function of CD8+ T cells,34 natural killer cells,35 B cells,36 monocyte37 and myeloid DCs.38 Indeed, PDCs produce huge amounts of type I IFNs in response to viruses or nucleoside-based products; they therefore play an important role in antiviral immunity and are involved in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus.37 Therefore, PGE2 may play a pivotal role in regulating the antiviral immune response or autoimmune inflammation.
Several studies have shown that PGE2 has an inhibitory effect on IL-12 production by myeloid lineage DCs induced by several stimuli such as LPS, IFN-γ, or CD40 ligand.14,24,39 In the present study also, PGE2 was shown to have the ability to inhibit IL-12 production by MDCs in response to LPS or poly(I:C). However, considering the evidence that fully mature monocyte-derived DCs are resistant to these modulatory effects of PGE2 with regard to IL-12 production,40 PGE2 may control the Th1-inducing capacity of DCs in the immature sentinel state at the peripheral inflammatory sites. In this context, because PDCs are the sensors and prompt responders to microbial infections in the periphery,41 it is most likely that PGE2 suppresses inflammatory responses in the early phase by inhibiting PDC survival, the ability of PDCs to produce proinflammatory cytokines and their function to induce Th1 cell responses. It has been demonstrated that these effects of PGE2 on DCs operate through an intracellular cAMP-signalling pathway.13,38 Indeed, the other cAMP up-regulators, cholera toxin and histamine, suppress MDC-derived IL-12 and PDC-derived type I IFNs, respectively.42–45 Thus, PGE2 could inhibit the Th1-inducing capacity not only in MDCs but also in PDCs as a cAMP up-regulator.
Based on these findings, the physiological actions of PGE2 in DC-mediated adaptive immunity are speculated to be as follows. On encountering bacteria or a virus, an antigen-specific immunogenic Th1 response is induced by MDCs or PDCs through secretion of IL-12 or type I IFNs. PGE2 in the inflammatory milieu not only directly suppresses the T-cell production of IFN-γ39 but also may inhibit the extensive production of IL-12 or type I IFNs from DC subsets, thereby limiting the duration of Th1 responses. This may possibly be a negative-feedback mechanism in the phase of immunogenic responses.
In conclusion, PGE2 acts on the viability of blood DC subsets, and in particular inhibits PDC survival. Moreover, PGE2 suppresses the ability of PDCs to produce IFN-α and their Th1-inducing activity. Thus, PGE2 may control DC-mediated immune responses as a local inflammatory instruction.
Acknowledgments
We are grateful to Ms Mayumi Nakamura for her secretarial work and assistance in experiments.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–9. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 6.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–12. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. The specialization, kinetics, and repertoire of type I interferon responses by human plasmacytoid pre-dendritic cells. Blood. 2006;107:2423–31. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–26. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T, Liu YJ, Kadowaki N. Functional diversity and plasticity of human dendritic cell subsets. Int J Hematol. 2005;81:188–96. doi: 10.1532/IJH97.05012. [DOI] [PubMed] [Google Scholar]
- 11.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 12.Kubo S, Takahashi HK, Takei M, Iwagaki H, Yoshino T, Tanaka N, Mori S, Nishibori M. E-prostanoid (EP) 2/EP4 receptor-dependent maturation of human monocyte-derived dendritic cells and induction of helper T2 polarization. J Pharmacol Exp Ther. 2004;309:1213–20. doi: 10.1124/jpet.103.062646. [DOI] [PubMed] [Google Scholar]
- 13.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells. Synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–8. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 15.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 16.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–70. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uno K, Nakano K, Maruo N, et al. Determination of interferon-alpha-producing capacity in whole blood cultures from patients with various diseases and from healthy persons. J Interferon Cytokine Res. 1996;16:911–18. doi: 10.1089/jir.1996.16.911. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Inaba M, Inaba K, et al. A CD1a+/CD11c– subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 1999;163:1409–19. [PubMed] [Google Scholar]
- 19.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baratelli F, Krysan K, Heuze-Vourc'h N, et al. PGE2 confers survivin-dependent apoptosis resistance in human monocyte-derived dendritic cells. J Leukoc Biol. 2005;78:555–64. doi: 10.1189/jlb.1004569. [DOI] [PubMed] [Google Scholar]
- 21.Vassiliou E, Sharma V, Jing H, Sheibanie F, Ganea D. Prostaglandin E2 promotes the survival of bone marrow-derived dendritic cells. J Immunol. 2004;173:6955–64. doi: 10.4049/jimmunol.173.11.6955. [DOI] [PubMed] [Google Scholar]
- 22.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 23.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 24.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–9. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 25.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin E(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–63. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 26.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 27.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 28.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E2 regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 29.Harizi H, Juzan M, Grosset C, Rashedi M, Gualde N. Dendritic cells issued in vitro from bone marrow produce PGE2 that contributes to the immunomodulation induced by antigen-presenting cells. Cell Immunol. 2001;209:19–28. doi: 10.1006/cimm.2001.1785. [DOI] [PubMed] [Google Scholar]
- 30.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123 high) dendritic cells in human nasal allergy. J Immunol. 2000;165:4062–8. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- 31.Cavanagh LL, Boyce A, Smith L, Padmanabha J, Filgueira L, Pietschmann P, Thomas R. Rheumatoid arthritis synovium contains plasmacytoid dendritic cells. Arthritis Res Ther. 2005;7:R230–40. doi: 10.1186/ar1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronnblom L, Alm GV. Systemic lupus erythematosus and the type I interferon system. Arthritis Res Ther. 2003;5:68–75. doi: 10.1186/ar625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–43. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothenfusser S, Hornung V, Ayyoub M, et al. CpG-A and CpG-B oligonucleotides differentially enhance human peptide-specific primary and memory CD8+ T-cell responses in vitro. Blood. 2004;103:2162–9. doi: 10.1182/blood-2003-04-1091. [DOI] [PubMed] [Google Scholar]
- 35.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–34. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 36.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 37.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 38.Fonteneau JF, Larsson M, Beignon AS, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–32. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–9. [PubMed] [Google Scholar]
- 41.Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol. 2005;26:221–9. doi: 10.1007/s00281-004-0180-4. [DOI] [PubMed] [Google Scholar]
- 42.Lavelle EC, Jarnicki A, McNeela E, Armstrong ME, Higgins SC, Leavy O, Mills KH. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J Leukoc Biol. 2004;75:756–63. doi: 10.1189/jlb.1103534. [DOI] [PubMed] [Google Scholar]
- 43.van der Pouw Kraan TC, Snijders A, Boeije LC, de Groot ER, Alewijnse AE, Leurs R, Aarden LA. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J Clin Invest. 1998;102:1866–73. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panina-Bordignon P, Mazzeo D, Lucia PD, D'Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–19. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzoni A, Leifer CA, Mullen GE, Kennedy MN, Klinman DM, Segal DM. Cutting edge: histamine inhibits IFN-alpha release from plasmacytoid dendritic cells. J Immunol. 2003;170:2269–73. doi: 10.4049/jimmunol.170.5.2269. [DOI] [PubMed] [Google Scholar]