Abstract
Diminished neonatal antibody responses following infection or immunization may stem in part from intrinsic characteristics of neonatal B cells. In this study, we used B-cell subset sorting combined with gene expression assays to investigate major differences in the expression of host genes in neonatal and adult naïve B cells. We discovered significantly reduced expression of the interleukin (IL)-4 receptor alpha chain and reduced IL-4-induced signalling in neonatal B cells. Neonatal naïve B cells were susceptible to more rapid and more profound levels of apoptosis when cultured in vitro. They also exhibited a limited response to IL-4 treatment compared with adult cells. The expression level of the IL-13 receptor alpha 1 chain, a key component of the IL-13 receptor/IL-4 type II receptor, and the response to IL-13 treatment for protection against apoptosis in neonatal B cells were similar to those of the adult B cells. These studies suggest a possible mechanism underlying the limited magnitude and durability of neonatal antibody responses.
Keywords: B cells, chemokines, interleukins, human studies, neonatal
Introduction
Limited primary antibody responses following infection or immunization in neonates have been described frequently; however, the molecular basis of this limitation is not well understood.1–4 Identification of the underlying mechanisms of developmental regulation of immunity early in life could facilitate the development of improved strategies to vaccinate neonates.
The deficiencies observed in neonatal antibody production and quality could stem from intrinsic features of the function of neonatal B cells, or from immaturity in T helper or antigen-presenting cells.5–8 In mice, phenotypic and functional differences between neonatal and adult B cells have been reported over the last decade.9–11 However, studies of human neonatal B cell function using cells in the peripheral circulation reveal few of the ‘defects’ that are usually associated with neonatal splenic B cells in mice.12 It is noteworthy that previous studies compared human neonatal B cells, which are mainly naïve B cells, with adult B cells, which are approximately 40% memory B cells.13 Molecular characterization of mixed populations of adult naïve and memory B cells complicates interpretation of age-related differences in comparison with neonatal B cells. In contrast, neonatal and adult comparative studies in mice are less affected by the contamination of memory B-cell properties, as only about 5% of B cells are memory cells in adult mice.
Apoptosis is an integral aspect of B-cell development and homeostasis, and it is regulated by the engagement of antigen, costimulatory and cytokine receptors.14,15 It is well documented that IL-4 is a potent antiapoptotic cytokine for B cells.16–18 In fact, recent studies comparing human naïve and memory B cells demonstrated a unique pattern of IL-4 signalling in human naïve B cells. Expression of IL-4 receptor alpha chain (IL-4Rα) is significantly higher in human naïve B cells than in germinal centre/memory B cells.19–21 Although signal transducer and activator of transcription 6 (STAT6) does not appear to be required for the antiapoptotic effects of IL-4 in resting or activated T cells,22,23 the rapid phosphorylation of STAT6 is critical to the IL-4-induced molecular events that regulate B-cell survival.24,25 STAT6 has been shown to be a critical signalling molecule for IL-4-mediated protection of primary B cells from passive or Fas-induced cell death in in vivo studies of STAT6-deficient or insulin receptor substrate 2 (IRS-2)-deficient mice.26 IL-13, a cytokine that exhibits overlapping intracellular signalling pathways with IL-4, also plays an important role in B-cell survival, proliferation and differentiation.21,27–29
The IL-4 receptor complex exists in three possible conformations, only two of which have been conclusively demonstrated.30–33 In T and natural killer (NK) cells, the receptor is composed of two chains, the IL-4Rα chain and the gamma common (γc) chain which is also shared by other interleukin receptors. In colon cancer, renal cell cancer and brain tumour cells, the IL-4Rα chain pairs with the IL-13Rα1 chain. It is proposed that, in B cells, IL-4 signals through both types of receptor.34
In the present study, we used gene expression studies to determine that the level of IL-4Rα chain expression differed significantly between neonatal and adult naïve B cells. We characterized a reduced level of IL-4 signalling in neonatal B cells by investigating the phosphorylation of STAT6 in neonatal or adult naïve B cells with IL-4 treatment and by monitoring protection from apoptosis following IL-4 treatment in vitro. We further evaluated the expression of IL-13 receptor alpha 1 (IL-13Rα1), a key component of the IL-13 receptor/type II IL-4 receptor,30,35 and the response to IL-13 for protection against apoptosis in neonatal naïve B cells.
Materials and methods
Antibodies and reagents
We used the following antibodies and reagents: anti-human CD19-PE-Cy7, anti-human IgD-PE, anti-human CD27-APC, anti-human CD3-APC-Cy7, anti-human CD14-APC-Cy7 and anti-human IL-4Rα-PE (Beckton Dickinson, San Jose, CA) for B-cell staining and sorting; anti-human-STAT6 (pY641)-Alexa 488 (Beckton Dickinson) for detection of phosphorylation of Stat6, and human recombinant IL-4, IL-10 or IL-13 for B-cell culture (Pierce Biotechnology, Rockford, IL).
Subjects
Human cord blood samples (n = 20) containing 15 ml of heparinized blood were obtained from full-term healthy deliveries in the Vanderbilt University Medical Center labour and delivery ward. Peripheral blood samples (n = 18) from healthy adults, aged 20–40 years, were used for comparative purposes. All samples were obtained following informed consent under approval from the Vanderbilt University Medical Center Institutional Review Board.
Isolation of circulating naïve B cells from blood
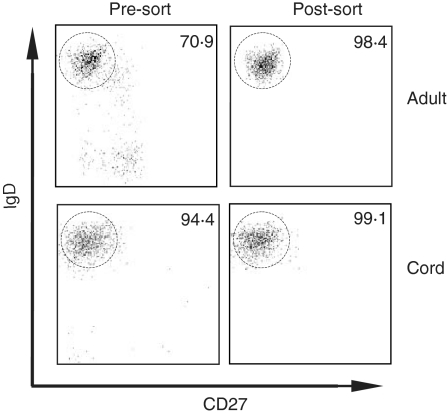
Peripheral blood mononuclear cells (PBMCs) were isolated from cord or adult blood samples by Ficoll-Hypaque (Sigma Aldrich, St Louis, MO) density gradient centrifugation, then stained for 30 min at 4° in the dark using fluorescent conjugated mouse anti-human antibodies, including anti-CD19-PE-Cy7, anti-IgD-PE, anti-CD27-APC and anti-CD3/CD14-APC-Cy7 (Beckton Dickinson). Cells were processed immediately for flow cytometric analysis and cell sorting using a FACSAria cytometer (Beckton Dickinson). After each sorting experiment, a portion of the sorted sample was analysed to determine the post-sort purity. All sorted [CD19+ immunoglobulin D (IgD)+CD27−] naïve B-cell samples exhibited > 95% purity. Data analysis was performed using FlowJo software, version 6·1 or above (Tree Star, Inc., Ashland, OR). Representative sorting data for cord and adult blood samples are shown in Fig. 1.
Figure 1.
Representative data from flow cytometric analyses demonstrating that human naïve B cells were isolated from adult and cord blood samples with high purity. The numbers indicate the percentage of cells that were of naïve phenotype (IgD+ CD27–) as indicated by the circled gate. All cells plotted were gated for CD19+. All post-sort samples contained > 99% CD19+ B cells.
RNA extraction from fluorescence-activated cell sorter (FACS)-isolated naïve B cells
Total RNA samples were isolated from sorted B cells using the RNeasy Total RNA Isolation Kit (Qiagen, Valencia, CA). The concentration and quality of the RNA samples were determined using a ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Microarray experiments
Microarray experiments were carried out using Human Genome U133 plus 2 gene chips and the Affymetrix microarray system (Affymetrix Inc., Santa Clara, CA). Briefly, reverse transcription was performed using an oligo-dT primer. Each resulting cDNA served as template to amplify 50–100 copies of biotin-labelled cRNA using T7 RNA polymerase. Fragmentation of cRNA samples was achieved by heating at 94°. Fragmented cRNA samples then were hybridized to Human Genome U133 plus 2 gene chips. Hybridized biotin-labelled cRNA was stained with strepavidin-phycoerythrin (PE), and the chips were scanned in a confocal laser scanner.
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assays for gene expression
Real-time RT-PCR assays for the transcript level of individual genes, including IL-4Rα, IL-13Rα1 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH; a housekeeping gene control target), were performed on total RNA samples extracted from neonatal or adult naïve B cells using commercial reagents [High Capacity cDNA Archive Kit (ABI, Foster City, CA), Omnimix HS beads (Takara-Cepheid, Sunnyvale, CA) and Assays-on-Demand™ Gene Expression predesigned primer and probe sets for genes of interest (ABI)]. The Smart Cycler II thermocycler (Cepheid, Sunnyvale, CA) was used as the real-time PCR amplification and analysis system. The relative expression level of genes of interest was determined by normalization to that of GAPDH. Calculation of the relative level of gene expression was performed using the 2−ΔΔCTmethod.36
Immunostaining of IL-4Rα protein
Polychromatic flow cytometry assays for the protein expression level of IL-4Rα were carried out using PE-conjugated mouse anti-human IL-4Rα antibody in combination with anti-CD19-PE-Cy7, anti-IgD-FITC, anti-CD27-APC and anti-CD3/CD14-APC-Cy7 (Beckton Dickinson). T cells (CD3+) and monocytes (CD14+) were excluded from the analysis. The relative level of IL-4Rα protein expression in neonatal or adult naïve B cells was evaluated using FlowJo software.
Detection of phosphorylation of STAT6 in human naïve B cells using flow cytometric staining
After sorting by FACS, human neonatal or adult naïve B cells were kept on ice for 1 hr prior to stimulation at a density of 1 × 106 cells/ml. Cells were then incubated at 37° for 1 hr with or without human recombinant IL-4 (20 ng/ml), IL-4 (100 ng/ml), IL-13 (100 ng/ml), or IL-10 (100 ng/ml). STAT6 phosphorylation in naïve B cells was detected and quantified by flow cytometry following intracellular staining using the phospho-specific antibody anti-human STAT6 (pY641)-Alexa 488 (Beckton Dickinson).
In vitro B-cell culture and flow cytometric analysis of apoptosis
FACS-purified neonatal or adult naïve B cells were cultured at a density of 100 000 cells per 200-µl volume per well of 96-well round-bottom culture plates in RPMI media supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine, 2·5 µg/ml amphotericin B, 60 µg/ml tylosin, 50 µg/ml gentamicin and 50 µm 2-mercaptoethanol. Human recombinant IL-4 (20 ng/ml) or IL-13 (50 ng/ml) (Pierce Biotechnology) was added to different culture wells. Untreated B-cell culture wells were set up in parallel. All samples were tested in triplicate in each assay. In vitro cultured neonatal or adult naïve B cells that were untreated or IL-4- and/or IL-13-treated were collected at 0, 20, 44 or 72 hr after initiation of culture. Cells were then stained with Annexin V-FITC (Beckton Dickinson) and 7-AAD (Molecular Probe, Eugene, OR). Apoptotic cells were distinguished by relative fluorescence for these markers using an LSR II flow cytometer (Beckton Dickinson). For experiments using neutralizing antibody against IL-4Rα, FACS-purified adult naïve B cells were preincubated with 100 ng/ml, 500 ng/ml or 2 µg/ml mouse anti-human IL4-Rα monoclonal antibody (R & D Systems Inc, Minneapolis, MN) for 1 hr at the same density and in the same culture media as described above prior to IL-4 treatment.
Statistical analysis
For the statistical analysis, P-values were obtained using a paired Student's t-test for all data except for the in vitro cell culture and apoptosis measurement data, in which repeated measures analysis of variance (ANOVA) was applied. Results were considered statistically significant when P-values were < 0·05.
Results
Reduced messenger RNA expression level of IL-4Rα in neonatal naïve B cells
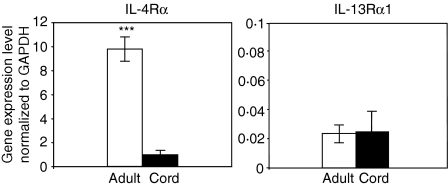
Total RNA samples prepared from purified naïve B cells of human neonatal cord blood or adult blood specimens were used for Affymetrix microarray studies. The IL-4Rα gene was found to be expressed differentially at a lower level in all four of the neonatal naïve B-cell samples. The IL-4Rα signal in neonatal naïve B cells detected by Affymetrix microarray was approximately 75% reduced compared with the IL-4Rα signal of adult naïve B cells, with a signal of 1562 ± 553 intensity units [mean ± standard deviation (SD)] in neonatal B cells versus 4433 ± 1728 in adult B cells (P < 0·05). To further confirm the microarray data, real-time RT-PCR experiments were performed on RNA samples from six additional subjects for each age group. Lower IL-4Rα expression was detected in all six neonatal naïve B-cell RNA samples compared with the six adult RNA samples (Fig. 2). The mRNA level of IL-4Rα in neonatal naïve B cells determined by real-time RT-PCR was 90 ± 4·3% (mean ± SD) reduced compared with that in adult naïve B cells (P < 0·001).
Figure 2.
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) revealed a lower level of transcription of interleukin (IL)-4Rα in neonatal naïve B cells compared with adult naïve B cells. Real-time RT-PCR experiments were performed on six neonatal and six adult naïve B-cell RNA samples. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference gene for individual samples. Lower IL-4Rα expression was detected in each of the six neonatal naïve B-cell RNA samples compared with adult RNA samples (***P < 0·001, paired Student's t-test). Similar levels of IL-13Rα1 expression were detected among the adult and neonatal naïve B-cell samples.
Similar messenger RNA expression levels of IL-13Rα1 in adult and neonatal naïve B cells
Expression of IL-13Rα1 was evaluated using real-time RT-PCR. Similar levels of expression were found in all six neonatal naïve B-cell RNA samples compared with the six adult RNA samples (Fig. 2). Interestingly, IL-13Rα1 gene expression in human naïve B cells from each age group was much lower than that of IL-4Rα expression.
Immunostaining of naïve B cells showed a lower protein level of IL-4Rα in neonatal naïve B cells
To evaluate whether IL-4Rα protein expression was significantly lower in neonatal naïve B cells compared with adult cells, flow cytometric analysis of cell surface IL-4Rα protein on naïve B cells was performed on eight neonatal naïve B-cell and eight adult naïve B-cell samples. Reduced expression of IL-4Rα protein was found in each sample of neonatal naïve B cells, as shown in Fig. 3. The level of IL-4Rα protein expression indicated by mean fluorescence intensity of the anti-IL-4Rα-PE staining in neonatal naïve B cells was reduced by 71·1 ± 15% (mean ± SD) compared with the IL-4Rα level in adult naïve B cells (P < 0·01).
Figure 3.
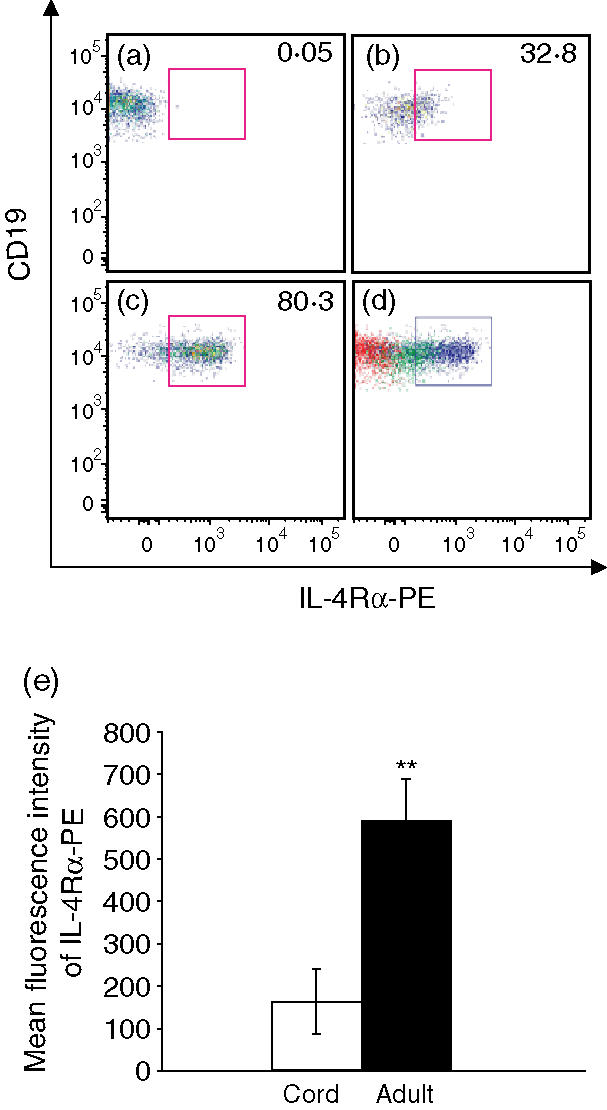
Fluorescence immunostaining of naïve B cells showed a lower protein level of interleukin (IL)-4Rα in neonatal naïve B cells. Lower IL-4Rα expression was indicated by both a reduced percentage of IL-4Rα-positive naïve B cells and reduced fluorescence intensity of the IL-4Rα-PE staining of the naïve B cells. (a) A fluorescence minus one (FMO) control sample was used to analyse cells stained with all of the fluorescence-conjugated antibodies except anti-human IL-4Rα-PE, to establish an appropriate gate for IL-4Rα-PE-positive cells. (b) A cord blood naïve B-cell sample. (c) An adult naïve B-cell sample. (d) An overlay of each dot plot (a, b, and c); FMO control (red), cord naïve B cells (green), and adult naïve B cells (blue). (e) Summary of mean fluorescence intensity data for IL-4Rα-PE in immunostained neonatal and adult naïve B cells. The results shown are the average of eight independent experiments and are expressed as the mean ± standard deviation (**P < 0·01, paired Student's t-test).
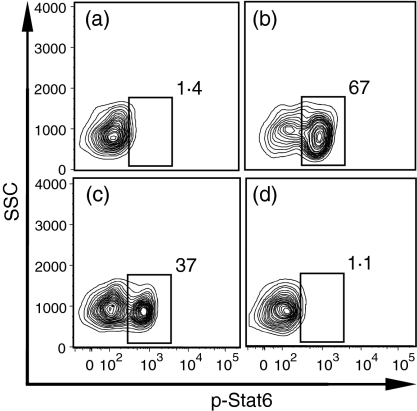
IL-4 or IL-13 induced high levels of phosphorylation of STAT6 in adult naïve B cells, while IL-10 did not
As both mRNA and protein levels of the IL-4Rα were reduced significantly in neonatal naïve B cells, we hypothesized that signalling in neonatal B cells in response to IL-4 would be reduced. We first investigated the downstream signal transduction events induced by IL-4 or IL-13 treatment in human adult naïve B-cell samples, using a flow cytometric technique. Data from a representative experiment are shown in Fig. 4. Without treatment, only 1·1 ± 0·4% (mean ± SD) of the FACS-purified adult naïve B cells were p-STAT6 positive. After 100 ng/ml IL-4 treatment for 1 hr, 62 ± 6·8% of the adult naïve B cells became p-STAT6 positive. Treatment with 100 ng/ml IL-13 for 1 hr generated 35 ± 2·5% p-STAT6-positive naïve B cells. IL-10, a cytokine that signals through the Janus Kinase (JAK)-STAT3 pathway, as expected, did not induce phosphorylation of STAT6 in adult naïve B cells, with only 1 ± 0·2% cells positive for p-STAT6.
Figure 4.
Interleukin (IL)-4 or IL-13 induced high levels of phosphorylation of signal transducer and activator of transcription 6 (STAT6) in adult naïve B cells. Fluorescence-activated cell sorter (FACS)-purified adult naïve B cells were either untreated (a) or treated with 100 ng/ml of IL-4 (b), IL-13 (c) or IL-10 (d), respectively, for 1 hr. P-STAT6-positive cells were gated, and the percentages of positive cells are indicated. The data are representative of five independent experiments with similar results.
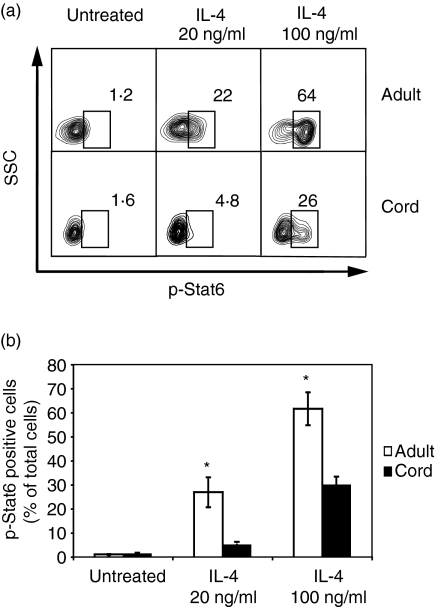
Lower phosphorylation of STAT6 in neonatal naïve B cells following treatment with IL-4
We next sought to test the hypothesis that lower expression of IL-4Rα in neonatal naïve B cells leads to a lower magnitude of IL-4-induced signalling in those cells. We compared the phosphorylation of STAT6 in six neonatal naïve B-cell samples with that in six adult naïve B-cell samples. Two different concentrations of IL-4, 20 or 100 ng/ml, were used followed by measurement of phosphorylation of STAT6 at 1 hr in the FACS-purified naïve B cells and compared with untreated controls. As indicated by a representative experiment shown in Fig. 5, neonatal naïve B cells showed less phosphorylation of STAT6 than adult cells for both concentrations of IL-4 treatment, with 5 ± 1·2% (mean ± SD) versus 27 ± 6·2% at 20 ng/ml (P < 0·05), and 30 ± 3·5% versus 62 ± 6·8% at 100 ng/ml (P < 0·05).
Figure 5.
Lower phosphorylation of signal transducer and activator of transcription 6 (STAT6) was observed in neonatal naïve B cells following treatment with interleukin (IL)-4. Fluorescence-activated cell sorter (FACS)-purified adult and cord blood naïve B cells were either untreated or treated with 20 or 100 ng/ml of IL-4, followed by measurement of phosphorylation of Stat6 at 1 hr. (a) Flow cytometric analyses of one representative experiment are shown. P-STAT6-positive cells were gated, and the percentages are indicated. (b) Data of six independent experiments with similar results are presented and are expressed as the mean ± standard deviation (*P < 0·05). Different treatments of cultured naïve B cells are shown on the x-axis, and the percentages of p-STAT6-positive cells in adult or cord blood naïve B cells are shown on the y-axis.
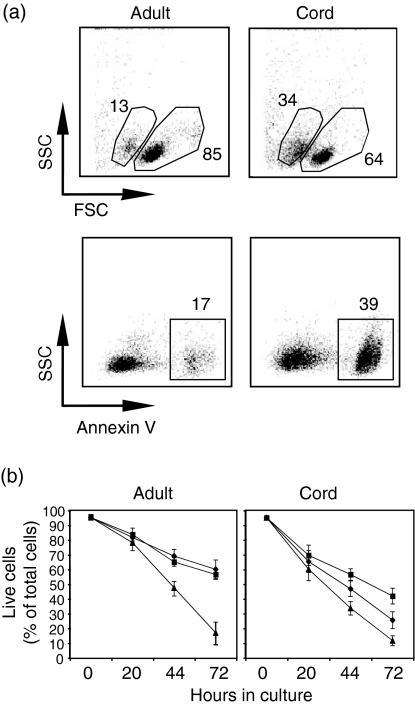
Cultured neonatal naïve B cells underwent apoptosis more rapidly than adult naïve B cells, and showed limited response to IL-4 treatment
Neonatal naïve B cells underwent rapid apoptosis when cultured in vitro in medium lacking cytokines (Fig. 6). We examined six neonatal and six adult naïve B-cell samples following IL-4 or IL-13 treatment. At 20 hr post culture, 60 ± 7·7% (mean ± SD) of neonatal naïve B cells were live cells, indicated by the AnnexinV– 7-AAD– phenotype. In contrast, 78 ± 5·2% of adult naïve B cells were live cells. By 44 hr post culture, 34 ± 4·6% of neonatal naïve B cells were live cells, compared with 48 ± 6·2% of adult naïve B cells. Addition of IL-4 or IL-13 reduced the level of adult B-cell apoptosis by approximately 45 or 42% at 44 hr and 62 or 55% at 72 hr post culture, respectively, in a similar manner. In contrast, IL-13 was more potent than IL-4 in rescuing neonatal naïve B cells, with a reduction in apoptosis of approximately 46% at 44 hr and 41% at 72 hr, while IL-4 reduced the level of apoptosis in neonatal naïve B cells only by approximately 22% at 44 hr and 18% at 72 hr (P < 0·001).
Figure 6.
In vitro cultured neonatal B cells underwent apoptosis more rapidly than adult naïve B cells did, and showed a limited response to interleukin (IL)-4 treatment. (a) Flow cytometric analyses of untreated adult or neonatal naïve B cells at 20 hr post culture. The cells were examined for apoptosis by flow cytometry using forward/side scatter characteristics, Annexin V and 7-AAD staining. The data are representative of six independent experiments with similar results. (b) Neonatal or adult naïve B cells were cultured in media alone (triangles) or with the addition of either IL-4 (20 ng/ml) (diamonds) or IL-13 (50 ng/ml) (squares). After 20, 44 or 72 hr in culture, the cells were examined for apoptosis by flow cytometry. The percentage of live cells, as a percentage of the total number of cells, at various time-points is shown. The data shown are from six independent experiments and are expressed as the mean ± standard deviation.
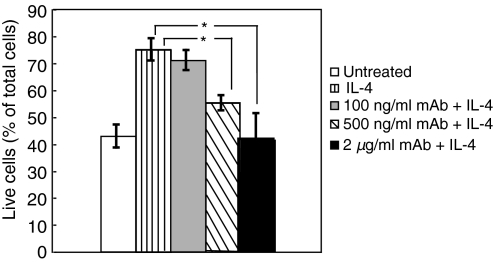
Neutralizing cell surface IL-4Rα-mediated bioactivity reduced adult naïve B-cell responses to IL-4 protection against apoptosis
Bioactivity of IL-4 in cells depends not only on the concentration of IL-4, but also on the number of available IL-4 receptors present on the cell surface. To confirm the specific role of level of IL-4Rα expression in determining the level of response to IL-4 treatment in human naïve B cells, we used a monoclonal antibody that binds to IL4Rα and neutralizes cell surface IL4-Rα-mediated bioactivity. Pre-incubation of adult naïve B cells with varying concentrations of the neutralizing monoclonal antibody against IL-4Rα reduced the response of such cells to IL-4 protection against apoptosis in a dose-dependent manner. As indicated in Fig. 7, the response to IL-4 protection against apoptosis in cells pretreated with 500 ng/ml of neutralizing antibody was reduced compared with the response of IL-4-treated B cells without neutralizing antibody (54·8 ± 2·7% live cells as a percentage of the total in samples pretreated with 500 ng/ml of antibody, versus 74·5 ± 4·0% in samples without antibody treatment at 44 hr post culture). Cells pretreated with 2 µg/ml of antibody (41·5 ± 9·5% live cells as a percentage of the total) showed a complete inhibition of the IL-4 response (P < 0·05).
Figure 7.
Neutralizing antibody against interleukin (IL)-4Rα-mediated bioactivity reduced the response to IL-4 protection against apoptosis in a dose-dependent manner. The data presented summarize five independent experiments and are shown as the mean ± standard deviation. Adult naïve B cells were either untreated or pretreated with 100 ng/ml, 500 ng/ml or 2 µg/ml of mouse anti-human IL-4α monoclonal antibody prior to treatment with 20 ng/ml of IL-4 in culture. After 44 hr, the cells were examined for apoptosis by flow cytometry. The percentage of live cells, as a percentage of the total number of cells, is shown (*P < 0·05).
Discussion
In this study, we found reduced levels of IL-4Rα expression with consequently reduced IL-4 signalling in human neonatal cord blood B cells. Significant reductions in IL-4Rα expression at both mRNA and protein levels were observed in neonatal B cells. The lower expression of IL-4Rα in neonatal naïve B cells suggested an attenuated level of IL-4-mediated signalling in neonatal B cells. As a consequence, neonatal B cells appear to be less amenable to IL-4-mediated rescue from apoptosis.
It was important to compare B cells of comparable (naïve) phenotype in order to identify the age-related effect. Recent studies have facilitated a more detailed characterization of human peripheral naïve and memory B cells.13,37,38 CD27 was identified as a reliable marker for peripheral blood memory B cells based on the fact that CD27-expressing peripheral B cells, including unclass-switched IgD+ CD27+ B cells, carry mutated antibody genes, a hallmark for memory B cells. With better definition of naïve and memory B cells, we were able to compare only naïve B cells from cord blood and adult blood samples in this study, focusing on the B cells that are responsible for primary antibody responses.
An interesting regulatory pathway in human naïve and memory B cells is the IL-4 signalling cascade. IL-4 was first discovered as a comitogen and differentiation factor in B-cell responses.2,39,40 Later studies suggested that IL-4 acts as a survival factor and extends the life span of B cells.17,18 Recently, distinct responses of naïve and memory B cells to IL-4 treatment were documented.19–21 Wagner et al. discovered that resting naïve human tonsil B cells expressed significantly higher IL-4Rα on their cell surface than the combined germinal centre/memory B cells. They also demonstrated that the addition of IL-4 protected naïve B cells, but not germinal centre/memory B cells, from apoptosis when cultured in vitro.19 Given that IL-4Rα is expressed differentially in naïve and memory B cells, it is not surprising that previous studies using pooled adult B cells did not discover the low expression of this protein in neonatal B cells.
Compared with adult B cells, phosphorylation of STAT6 in neonatal naïve B cells was reduced following IL-4 treatment. These data suggest that IL-4-induced downstream signalling events that are critical for B-cell activation and proliferation in neonatal naïve B cells are reduced. STAT6 activation has also been demonstrated to be critical for the expression of class II MHC molecules in B cells. It is intriguing to speculate that, in vivo, during activation of B cells that requires cognate T-cell/B-cell help, reduced IL-4 signalling in neonatal B cells might result in reduced efficiency in up-regulation of class II MHC molecules needed to present antigen on the cell surface. CD23 is another key STAT6-responsive gene, and it is related to IgE production and the development of allergic diseases. The altered IL-4 signalling that we observed in cord blood B cells suggests that features of the cellular machinery related to the emergence of allergy in this population may be developmentally regulated. Future study of IL-4-mediated induction of CD23 and enhancement of IgE production in CD40L-stimulated neonatal B cells could be helpful to investigate the contribution of reduced expression of IL-4Rα to alteration of allergic status in healthy or allergy-prone neonates.
Neonatal naïve B cells demonstrated a limited response to IL-4 treatment against apoptosis compared with adult naïve B cells when cultured in vitro. Low expression of IL-4Rα on neonatal B cells correlated with the limited response towards IL-4-mediated rescue observed in these cells. It is noteworthy that, without treatment, neonatal B cells demonstrated greater susceptibility to apoptosis than adult B cells. An altered balance of pro-apoptotic and antiapoptotic proteins in neonatal B cells may cause this apoptosis-prone status.
Data from studies using immortalized human B-cell lines and human primary tonsillar B cells have suggested that IL-4 signals through two different types of receptor in B cells.34 The type II IL-4 receptor, composed of IL-4Rα and IL-13Rα1 chains, is also the only known functional IL-13 receptor. IL-13, a cytokine that has a similar role in B-cell proliferation and differentiation to IL-4, uses the type II IL-4 receptor to mediate its biological functions inside cells. It was possible theoretically that IL-13 signalling was altered as a result of the low expression of IL-4Rα in neonatal B cells. We found that level of expression of IL-13Rα1 mRNA in neonatal B cells was similar to that of adult B cells. In addition, neonatal B cells were able to respond to IL-13 treatment for protection against apoptosis to a similar extent compared with adult naive cells when cultured in vitro. These findings suggest that the IL-13R complex is intact and appears functional in neonatal B cells. The competent state of IL-13 signalling in neonatal B cells may in part compensate for the reduced IL-4 signalling capacity.
Another recent discovery is the characterization of circulating human transitional B cells. Sims et al. reported the identification of a small population of circulating human B cells with phenotype and function similar to those of mouse transitional type 1 (T1) B cells.41 These T1 cells were shown to be short-lived in culture and to have reduced capacity to enter the cell cycle when stimulated with IL-4/anti-IgM or anti-CD40L/anti-IgM. Interestingly, using flow cytometry-based immune surface staining, they demonstrated that IL-4Rα was expressed at a low level in the circulating T1 cells compared with mature naïve B cells. In addition, increased proportions of T1 B cells were found in cord blood compared with adult peripheral blood (13·6 ± 4·3 versus 2·2% ± 0·4). Our observations of low expression and reduced signalling of IL-4Rαand heightened susceptibility to apoptosis of cord blood naïve B cells support the hypothesis that was also suggested by previous studies, namely that human neonatal naïve B cells are intrinsically limited in their capacity to survive, proliferate and differentiate. These features may account for the lower humoral immune responses following primary viral infection or vaccination in neonates.
As reported previously, the level of IL-4Rα expression was increased transiently (up to 24 hr) in primary B cells during in vitro culture following IL-4 treatment, and was increased to a lesser extent when cells were stimulated with lipopolysaccharide (LPS), CD40L, or anti-B cell receptor antibodies.42–44 High levels of IL-4 theoretically could support the function of neonatal B cells in vivo by up-regulating IL-4R expression. Based on our in vitro studies, however, the transiently increased IL-4Rα expression in neonatal B cells in response to IL-4 treatment was not sufficient to overcome the intrinsically reduced levels of IL-4Rα expression and IL-4-mediated signalling. Therefore, although up-regulation of IL-4Rα did occur following IL-4 treatment, this response did not alter the survival status of these cells.
It is of interest to investigate whether signalling via CD40 and other cytokines could compensate for the limitation in IL-4 signalling in neonatal B cells. The CD154–CD40 interaction is important in triggering resting B cells to proliferate, to switch immunoglobulin class, and to up-regulate costimulatory molecules such as CD80/CD86. Interestingly, accumulated data from previous studies suggest that there is a limitation of the CD154–CD40 interaction in neonatal immune cells, probably related to features of the neonatal T cells. Decreased CD154 expression and a reduced up-regulation of CD154 upon activation by neonatal CD4+ T cells have been reported.45–47 In contrast, CD40 was shown to be expressed at similar levels on resting B cells from adults, young children or cord blood.48 Cord blood B cells showed comparable responses to CD40 ligation to those of the adult B cells.49 Because of the limitation of the CD154–CD40 interaction in neonatal T and B cells, the likelihood of signalling via this pathway to compensate for the limitation in IL-4 signalling in neonatal B cells in vivo is low.
In summary, the data presented here unveil a novel and physiologically relevant characteristic of neonatal B cells, namely reduced expression of IL-4Rα. Reduced phosphorylation of STAT6 in IL-4-treated neonatal B cells correlated with the reduced expression level of IL-4Rα in these cells. Using an in vitro culture system, we demonstrated that neonatal B cells were less responsive to IL-4 apoptosis protection signalling than adult naïve B cells. These data suggest that low expression of IL-4Rα and reduced signalling in neonatal B cells via the IL-4 receptor complex may lead them to be more susceptible to cell death during antigen exposure, therefore limiting the antibody response following primary infection in neonates. These intrinsic differences in neonatal B cells may contribute to the observed reduced magnitude, quality and durability of the neonatal humoral immune response to infection or vaccination.
Acknowledgments
We thank all the volunteers who participated in this study for their generosity. We gratefully acknowledge the consultations of Dr Maurice Bondurant and Dr Mark Boothby.
Abbreviations
- FACS
fluorescence activated cell sorting
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IL-4Rα
interleukin-4 receptor alpha chain
- IL-13Rα1
interleukine-13 receptor alpha-1 chain
- IRS-2
insulin receptor substrate 2
- PBMCs
peripheral blood mononuclear cells
- STAT6
signal transducer and activator of transcription 6
- T1 B cell
transitional type 1 B cell
References
- 1.Marshall-Clarke S, Reen D, Tasker L, Hassan J. Neonatal immunity: how well has it grown up? Immunol Today. 2000;21:35–41. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- 2.Lawton AR. Ontogeny of B cells and pathogenesis of humoral immunodeficiencies. Clin Immunol Immunopathol. 1986;40:5–12. doi: 10.1016/0090-1229(86)90064-4. [DOI] [PubMed] [Google Scholar]
- 3.Andersson U, Bird G, Britton S. A sequential study of human B lymphocyte function from birth to two years of age. Acta Paediatr Scand. 1981;70:837–42. doi: 10.1111/j.1651-2227.1981.tb06236.x. [DOI] [PubMed] [Google Scholar]
- 4.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 5.Andersson U, Bird G, Britton S. Cellular mechanisms of restricted immunoglobulin formation in the human neonate. Eur J Immunol. 1980;10:888–94. doi: 10.1002/eji.1830101115. [DOI] [PubMed] [Google Scholar]
- 6.Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res. 2000;22:177–90. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- 7.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 8.Lu CY, Calamai EG, Unanue ER. A defect in the antigen-presenting function of macrophages from neonatal mice. Nature. 1979;282:327–9. doi: 10.1038/282327a0. [DOI] [PubMed] [Google Scholar]
- 9.King LB, Norvell A, Monroe JG. Antigen receptor-induced signal transduction imbalances associated with the negative selection of immature B cells. J Immunol. 1999;162:2655–62. [PubMed] [Google Scholar]
- 10.Marshall-Clarke S, Tasker L, Parkhouse RM. Immature B lymphocytes from adult bone marrow exhibit a selective defect in induced hyperexpression of major histocompatibility complex class II and fail to show B7.2 induction. Immunology. 2000;100:141–51. doi: 10.1046/j.1365-2567.2000.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benschop RJ, Brandl E, Chan AC, Cambier JC. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J Immunol. 2001;167:4172–9. doi: 10.4049/jimmunol.167.8.4172. [DOI] [PubMed] [Google Scholar]
- 12.Tasker L, Marshall-Clarke S. Functional responses of human neonatal B lymphocytes to antigen receptor cross-linking and CpG DNA. Clin Exp Immunol. 2003;134:409–19. doi: 10.1111/j.1365-2249.2003.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galibert L, Burdin N, Barthelemy C, et al. Negative selection of human germinal center B cells by prolonged BCR cross-linking. J Exp Med. 1996;183:2075–85. doi: 10.1084/jem.183.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galibert L, Burdin N, de Saint-Vis B, et al. CD40 and B cell antigen receptor dual triggering of resting B lymphocytes turns on a partial germinal center phenotype. J Exp Med. 1996;183:77–85. doi: 10.1084/jem.183.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgkin PD, Go NF, Cupp JE, Howard M. Interleukin-4 enhances anti-IgM stimulation of B cells by improving cell viability and by increasing the sensitivity of B cells to the anti-IgM signal. Cell Immunol. 1991;134:14–30. doi: 10.1016/0008-8749(91)90327-8. [DOI] [PubMed] [Google Scholar]
- 17.Illera VA, Perandones CE, Stunz LL, Mower DA, Jr, Ashman RF. Apoptosis in splenic B lymphocytes. Regulation by protein kinase C and IL-4. J Immunol. 1993;151:2965–73. [PubMed] [Google Scholar]
- 18.Parry SL, Hasbold J, Holman M, Klaus GG. Hypercross-linking surface IgM or IgD receptors on mature B cells induces apoptosis that is reversed by costimulation with IL-4 and anti-CD40. J Immunol. 1994;152:2821–9. [PubMed] [Google Scholar]
- 19.Wagner EF, Hanna N, Fast LD, et al. Novel diversity in IL-4-mediated responses in resting human naive B cells versus germinal center/memory B cells. J Immunol. 2000;165:5573–9. doi: 10.4049/jimmunol.165.10.5573. [DOI] [PubMed] [Google Scholar]
- 20.Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J Immunol. 2003;170:686–94. doi: 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- 21.Fecteau JF, Neron S. CD40 stimulation of human peripheral B lymphocytes: distinct response from naive and memory cells. J Immunol. 2003;171:4621–9. doi: 10.4049/jimmunol.171.9.4621. [DOI] [PubMed] [Google Scholar]
- 22.Wurster AL, Withers DJ, Uchida T, White MF, Grusby MJ. Stat6 and IRS-2 cooperate in interleukin 4 (IL-4)-induced proliferation and differentiation but are dispensable for IL-4-dependent rescue from apoptosis. Mol Cell Biol. 2002;22:117–26. doi: 10.1128/MCB.22.1.117-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamorano J, Wang HY, Wang LM, Pierce JH, Keegan AD. IL-4 protects cells from apoptosis via the insulin receptor substrate pathway and a second independent signaling pathway. J Immunol. 1996;157:4926–34. [PubMed] [Google Scholar]
- 24.Clark EA, Shu GL, Luscher B, et al. Activation of human B cells. Comparison of the signal transduced by IL-4 to four different competence signals. J Immunol. 1989;143:3873–80. [PubMed] [Google Scholar]
- 25.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 26.Wurster AL, Rodgers VL, White MF, Rothstein TL, Grusby MJ. Interleukin-4-mediated protection of primary B cells from apoptosis through Stat6-dependent up-regulation of Bcl-xL. J Biol Chem. 2002;277:27169–75. doi: 10.1074/jbc.M201207200. [DOI] [PubMed] [Google Scholar]
- 27.Lomo J, Blomhoff HK, Jacobsen SE, Krajewski S, Reed JC, Smeland EB. Interleukin-13 in combination with CD40 ligand potently inhibits apoptosis in human B lymphocytes: upregulation of Bcl-xL and Mcl-1. Blood. 1997;89:4415–24. [PubMed] [Google Scholar]
- 28.McKenzie AN, Zurawski G. Interleukin-13: characterization and biologic properties. Cancer Treat Res. 1995;80:367–78. doi: 10.1007/978-1-4613-1241-3_15. [DOI] [PubMed] [Google Scholar]
- 29.Lai YH, Mosmann TR. Mouse IL-13 enhances antibody production in vivo and acts directly on B cells in vitro to increase survival and hence antibody production. J Immunol. 1999;162:78–87. [PubMed] [Google Scholar]
- 30.Zurawski SM, Chomarat P, Djossou O, et al. The primary binding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Biol Chem. 1995;270:13869–78. doi: 10.1074/jbc.270.23.13869. [DOI] [PubMed] [Google Scholar]
- 31.de Waal Malefyt R, Abrams JS, Zurawski SM, et al. Differential regulation of IL-13 and IL-4 production by human CD8+ and CD4+ Th0, Th1 and Th2 T cell clones and EBV-transformed B cells. Int Immunol. 1995;7:1405–16. doi: 10.1093/intimm/7.9.1405. [DOI] [PubMed] [Google Scholar]
- 32.Demaziere A, Leek R, Athanasou NA. Histological distribution of the interleukin-4 receptor (IL4R) within the normal and pathological synovium. Rev Rhum Mal Osteoartic. 1992;59:219–24. [PubMed] [Google Scholar]
- 33.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 34.Graber P, Gretener D, Herren S, et al. The distribution of IL-13 receptor alpha1 expression on B cells, T cells and monocytes and its regulation by IL-13 and IL-4. Eur J Immunol. 1998;28:4286–98. doi: 10.1002/(SICI)1521-4141(199812)28:12<4286::AID-IMMU4286>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 35.Izuhara K, Yang G, Miyajima A, Howard M, Harada N. Structure of the IL4 receptor and signal transduction mechanism of IL4. Res Immunol. 1993;144:584–90. doi: 10.1016/s0923-2494(05)80007-0. [DOI] [PubMed] [Google Scholar]
- 36.Schmittgen KJ, La TD. Analysis of relative gene expressiondata using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–6. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Agematsu K, Ochs HD, Sugane K. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin Immunol. 2003;108:128–37. doi: 10.1016/s1521-6616(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 39.Defrance T, Vanbervliet B, Aubry JP, et al. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987;139:1135–41. [PubMed] [Google Scholar]
- 40.Vitetta ES, Ohara J, Myers CD, Layton JE, Krammer PH, Paul WE. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985;162:1726–31. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–8. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohara J, Paul WE. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc Natl Acad Sci USA. 1988;85:8221–5. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renz H, Domenico J, Gelfand EW. IL-4-dependent up-regulation of IL-4 receptor expression in murine T and B cells. J Immunol. 1991;146:3049–55. [PubMed] [Google Scholar]
- 44.Siepmann K, Wohlleben G, Gray D. CD40-mediated regulation of interleukin-4 signaling pathways in B lymphocytes. Eur J Immunol. 1996;26:1544–52. doi: 10.1002/eji.1830260721. [DOI] [PubMed] [Google Scholar]
- 45.Jullien P, Cron RQ, Dabbagh K, et al. Decreased CD154 expression by neonatal CD4+ T cells is due to limitations in both proximal and distal events of T cell activation. Int Immunol. 2003;15:1461–72. doi: 10.1093/intimm/dxg145. [DOI] [PubMed] [Google Scholar]
- 46.Brugnoni D, Airo P, Graf D, et al. Ineffective expression of CD40 ligand on cord blood T cells may contribute to poor immunoglobulin production in the newborn. Eur J Immunol. 1994;24:1919–24. doi: 10.1002/eji.1830240831. [DOI] [PubMed] [Google Scholar]
- 47.Nonoyama S, Penix LA, Edwards CP, et al. Diminished expression of CD40 ligand by activated neonatal T cells. J Clin Invest. 1995;95:66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elliott SR, Roberton DM, Zola H, Macardle PJ. Expression of the costimulator molecules, CD40 and CD154, on lymphocytes from neonates and young children. Hum Immunol. 2000;61:378–88. doi: 10.1016/s0198-8859(99)00189-5. [DOI] [PubMed] [Google Scholar]
- 49.Han P, McDonald T, Hodge G. Potential immaturity of the T-cell and antigen-presenting cell interaction in cord blood with particular emphasis on the CD40-CD40 ligand costimulatory pathway. Immunology. 2004;113:26–34. doi: 10.1111/j.1365-2567.2004.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]