Abstract
CD8+ T cells play a crucial role in protective immunity to viruses and tumours. Antiviral CD8+ T cells are initially activated by professional antigen presenting cells (pAPCs) that are directly infected by viruses (direct-priming) or following uptake of exogenous antigen transferred from virus-infected or tumour cells (cross-priming). In order to efficiently target each of these antigen-processing pathways during vaccine design, it is necessary to delineate the properties of the natural substrates for either of these antigen-processing pathways. In this study, we utilized a novel T-cell receptor (TCR) transgenic mouse to examine the requirement for both antigen synthesis and synthesis of other cellular factors during direct or cross-priming. We found that direct presentation required ongoing synthesis of antigen, but that cross-priming favoured long-lived antigens and did not require ongoing antigen production. Even after prolonged blockade of protein synthesis in the donor cell, cross-priming was unaffected. In contrast, direct-presentation was almost undetectable in the absence of antigen neosynthesis and required ongoing protein synthesis. This suggests that the direct- and cross-priming pathways may utilize differing pools of antigen, an observation that has far-reaching implications for the rational design of vaccines aimed at the generation of protective CD8+ T cells.
Keywords: antigen presentation, antigen processing, cytotoxic T cells, major histocompatibility complex, transgenic mice
Introduction
CD8+ T cells are vital components in the protective immune response directed against pathogens, such as viruses or intracellular bacteria, or against tumours. CD8+ T cells recognize eight to 11 residue peptides in complex with major histocompatibility complex (MHC) class I molecules. Induction of a primary CD8+ T cell-mediated response requires that peptides derived from intracellular pathogens or tumours be presented on MHC class I molecules on the surface of professional antigen-presenting cells (pAPCs), particularly dendritic cells, because only these cells are capable of providing the costimulatory signals necessary to induce proliferation in naïve T cells.1 Naïve CD8+ T cells can be triggered by viral peptides that are generated from two spatially distinct sources. The pAPC itself may be infected with virus, in which case peptides are generated from endogenous viral proteins in a process known as direct-presentation.2 Alternatively, an uninfected pAPC may acquire antigen from exogenous sources, presumably from non-pAPCs that are infected with virus, a process known as cross-priming.3,4 The extent to which each of these mechanisms contributes to the induction of primary CD8+ T-cell responses is unknown.5
Extensive in vitro studies have revealed the complex molecular interactions, proteolytic degradation pathways and vesicular trafficking events required for the presentation of endogenously synthesized antigen by the direct-presentation pathway. The major source of antigenic peptides in the direct-presentation pathway is the degradation of defective ribosomal products (DRiPs).6–8 DRiPs are newly synthesized polypeptides unable to reach their native state, presumably because of mistranslations, truncations, improper folding or improper post-translational modifications. DRiPs comprise 30% or more of the pool of synthesized proteins, but are degraded shortly after synthesis and can only be visualized under conditions in which their degradation is inhibited.7 A requirement for neosynthesis of antigen has been demonstrated in direct-presentation.9
We have previously demonstrated that long-lived, rather than rapidly degraded, proteins are preferential substrates for cross-priming in vivo.10 This is in marked contrast to direct presentation, where the majority of peptide–MHC complexes are derived from DRiPs7–9 and where enhanced degradation of antigen can increase presentation.11 Molecular chaperones, such as heat shock proteins, that have been implicated in cross-priming are known to stabilize misfolded proteins that normally have a short half-life.12 However, the requirement for the involvement of chaperones during cross-priming remains controversial.13,14
In this study, we examine the requirements for antigen or other protein synthesis in the donor cell for cross-priming in vivo. We demonstrate that antigen neosynthesis is not required in the antigen donor cell, further implicating stable proteins, or proteins stabilized for extended periods, as the antigenic substrate for cross priming. Even after prolonged blockade of protein synthesis in the donor cell, cross priming was unaffected. In contrast, direct presentation was greatly enhanced by the neosynthesis of antigen and required ongoing protein synthesis. This suggests that the direct- and cross-priming pathways may utilize different pools of antigen, an observation that has far-reaching implications for the rational design of vaccines aimed at the generation of protective CD8+ T cells.
Materials and methods
Animals
OT-1 TCR RAG1−/− transgenic mice15,16 were obtained from the National Institute of Allergy and Infectious Diseases (NIAID) Exchange Program (Line 4175; NIAID, Bethesda, MD). B6.SJL-Ptprca/BoAiTac mice were purchased from Taconic Farms (Germantown, NY) and bred with OT-1 TCR mice to produce OT-1.SJL offspring that were used for all adoptive transfer experiments. Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were maintained in specified pathogen-free conditions at Penn State Hershey Medical Center under the Institutional Animal Care and Use Committee guidelines.
Development of Bg1 TCR transgenic mice
Transgenic mice were produced with TCR cassette vectors generously provided by Dr Diane Mathis (Brigham and Women's Hospital, Boston, MA). RNA was isolated from a β-galactosidase (β-gal)96–103-specific CD8+ T-cell clone, generated by limiting dilution, using silica matrix columns (Qiagen, Valencia, CA). Known TCR α and β constant region sequences were used to perform 5′ rapid amplification of cDNA ends (Invitrogen, Carlsbad, CA), and TCR sequences were then cloned into pCR4TOPO TA cloning sequencing vectors (Invitrogen). The TCR α and β transcripts were sequenced using an ABI Prism (Perkin-Elmer, Wellesley, MA), and these sequences were compared with available sequences to develop genomic cloning polymerase chain reaction (PCR) primers based upon the method previously described.17 Briefly, these cloning primers provide amplification of the variable domains consisting of 10–20 bp upstream of the start codon through 200–300 bp of intronic sequence downstream of the junctional regions, thereby preserving splice donor/acceptor sites. The α and β genomic variable domains were PCR-amplified (Perkin-Elmer) and TA-cloned into a sequencing vector (Invitrogen). The genomic variable domains were sequenced (Vα1/JαTA13/Cα and Vβ7S1/Jβ2S4/Cβ2) and subcloned into the TCR cassette vectors. The α and β cassette vectors were coinjected into fertilized C57BL/6 embryos (SAIC, Frederick, MD) and founders were obtained. The resulting mice, named Bg1, were maintained as heterozygotes, as a high rate of lymphoma in homozygotes reduced their life span. Heterozygotes were bred to B6.SJL mice as above and transgene expression was monitored by staining of blood cells with anti-Vβ7 (Clone TR310) and analysis by flow cytometry as outlined below.
Viruses
Recombinant vaccinia viruses (rVACVs) expressing ovalbumin (OVA),18 β-gal19 and Kb20 were a kind gift from Drs Jon Yewdell and Jack Bennink (Laboratory of Viral Diseases, NIAID, Bethesda, MD). Mice were immunized with 1 × 107 plaque-forming units (PFU) rVACV intraperitoneally (i.p.) or intravenously (i.v.). Where VACV was used to express H2-Kb in L4·2, cells were infected with a multiplicity of infection (MOI) = 10 of rVACV-Kb, and incubated for 30 min with gentle agitation every 5 min. After 30 min of infection, medium containing 40 µg/ml cytosine 1-β-D-arabinofuranoside (AraC) (Sigma, St Louis, MO) was added to infected cells to prevent transcription of β-gal, which is encoded by a late gene in rVACV. AraC was present throughout the duration of the experiment.
A Phoenix Ampho packaging cell line producing a retrovirus expressing the murine MHC class I molecule H2-Kb was a kind gift from Dr Peter Cresswell (Department of Immunobiology, Yale University, New Haven, CT).21
Cells
All media were purchased from Invitrogen. The 293 Tetracycline-Regulated Expression (T-REx) cell line stably expressing the tetracycline (tet) repressor was purchased from Invitrogen and cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM) containing 10% fetal calf serum, 1% penicillin-streptomycin, 2 mm glutamine and 5 µg/ml blasticidin.
L4·2 cells (H2-Kb negative) were created as follows. pcDNA4/TO/lacZ, a plasmid containing the bacterial gene encoding β-galactosidase (LacZ) gene fused to the tetracycline operator and the selectable zeocin resistance gene, was purchased from Invitrogen. 293 T-REx cells (60% confluent) were transfected with 1 µg of purified plasmid DNA using lipofectamine as per the manufacturer's instructions (Invitrogen). Stably transfected cells were selected by zeocin (300 µg/ml) resistance over the course of 3 weeks. Clonal populations were then isolated by limiting dilution and screened for LacZ activity after induction with 1 μg/ml tetracycline. Clonal populations were maintained in DMEM containing 10% fetal calf serum, 1% penicillin-streptomycin, 2 mm glutamine, 5 µg/ml blasticidin and 300 μg/ml zeocin.
L4·2mKb cells were created from L4·2 cells. L4·2 cells were transduced with a retrovirus expressing the murine MHC class I molecule H2-Kb. Briefly, 5 × 105 L4·2 cells were plated in a 100 mm plate and then infected with the retrovirus in the presence of 4 μg/ml polybrene (Fisher, Fairlawn, NJ) for 3 hr. Additional medium was then added and cells were cultured for 48 hr. Retrovirally transduced cells were selected with 0·5 mg/ml geneticin (Invitrogen) for 3 weeks. L4·2mKb cells were then stained with the anti-Kb antibody HB176 and sorted using a MoFlo (Cytomation, Fort Collins, CO) to further select cells expressing Kb. Kb-expressing cells were maintained in DMEM containing 10% fetal calf serum, 1% penicillin-streptomycin, 2 mm glutamine, 5 μg/ml blasticidin, 300μg/ml zeocin and 0·5 mg/ml geneticin.
β2m−/− STBKM-1 cells (H2-Kb negative)10 and WT3 (H2-Kb positive)22 cells were cultured in DMEM containing 5% fetal calf serum, 1% penicillin-streptomycin and 2 mm glutamine. B3Z cells were cultured in RPMI containing 5% fetal calf serum, 1% penicillin-streptomycin, 2 mm glutamine and 1% non-essential amino acids.
Single cell LacZ assay
L4·2 cells were treated with 1 μg/ml tetracycline in culture media for 4 hr and β-gal expression was assayed using the chromogenic substrate 5-bromo-4-chloro-3-indolyl β-D galactopyranoside (X-gal). For staining, cells were harvested using versene, washed once in phosphate-buffered saline (PBS) and fixed with 2% paraformaldehyde/0·2% gluteraldehyde for 10 min at room temperature. Cells were washed again in PBS and then overlaid with a solution containing 1 mg/ml X-gal, 5 mm potassium ferrocyanide, 5 mm potassium ferricyanide and 2 mm MgCl2 in PBS and incubated overnight at 37°. Cells were counted using a hemocytometer to determine the number displaying LacZ activity. A minimum of 1000 cells were counted for each of the triplicate samples.23
Bulk LacZ assay
L4·2 cells were harvested and treated with 1 μg/ml tetracycline in Iscove's modified Dulbecco's medium (IMDM) containing 10% fetal calf serum, 1% penicillin-streptomycin, 2 mm glutamine and 5 μg/ml blasticidin at 37° for the times indicated. In instances where tetracycline was removed, L4·2 cells were harvested and treated as above for 4 hr. L4·2 cells were then washed twice in IMDM. For times under 24 hr, cells were resuspended in IMDM, transferred to a fresh tube and placed back on the rotator until the end-point. For times over 24 hr, cells were resuspended in culture media, plated into dishes to prevent the cell death observed when rotated for longer than 24 hr, and then harvested 1 hr prior to the end-point. Before being returned to dishes, cells were enumerated to allow determination of cell division over the time–course of the experiment. Tetracycline-treated cells rarely divided; after 8 days in culture, less than half of the cells had divided once. At the end-point, cells were harvested, washed twice in PBS, and plated at 1 × 105 cells/well in a flat-bottomed 96-well plate (Corning, Inc., Corning, NY). Ten-fold dilutions were then performed. Cells were lysed by addition of 200 μl of buffer containing 100 mmβ-mercaptoethanol, 9 mm MgCl2, 0·125% IGEPAL (Sigma) and 0·15 mm chlorophenol red β-galactoside (CPRG; Calbiochem, San Diego, CA) in PBS. After 10–20 min of incubation at 37°, 50 μl of stop buffer [300 mm glycine and 15 mm ethylenediaminetetraacetic acid (EDTA) in water] was added to each well, and absorption was read using a Dynex MRX 96-well plate-reader (Dynex, Chantilly, VA) at 595 nm with a reference filter of 630 nm.
In vivo cross-priming
β2-microglobulin knockout (STBKM-1) cells, which were unable to present the H2-Kb-restricted OVA257–264 or β-gal96–103 peptides derived from endogenously synthesized proteins (data not shown), were harvested and treated with 25 μg/ml emetine (Sigma) or cycloheximide (Calbiochem). Cells were kept in suspension by gentle continuous rotation for the duration of treatment, a maximum of 5 hr. At the end-point, a suspension of 2–4 × 106 cells in PBS containing 10mm MgCl2 and 1 mg/ml OVA (Worthington Chemicals, Lakewood, NJ) was electroporated at 0·25 kV in disposable cuvettes using a Bio-Rad gene pulser (Bio-Rad, Hercules, CA). Cells were incubated on ice for 10 min immediately before and after electroporation and then washed three times before being subjected to 20 000 rads of gamma-irradiation. After irradiation, cells were washed once and counted prior to injection. Each batch of OVA used was screened for the presence of contaminating antigenic peptide by incubating with fixed APCs and assaying for presentation to an OVA-specific CD8+ T-cell hybridoma (Reis e Sousa & Germain24 and data not shown). Only batches of OVA with undetectable levels of contaminating peptide were used in our studies. The concentration of OVA was chosen after titration to determine the lowest concentration that generated an antigen-specific CD8+ T-cell response following immunization in vivo (data not shown).
Purification and adoptive transfer of TCR transgenic T cells
Splenocyte populations were obtained from OT-I.SJL TCR transgenic mice or Bg1.SJL TCR transgenic mice as follows. Lymph nodes (popliteal, inguinal, brachial, axillary and superficial cervical) and spleen were removed, homogenized and centrifuged over lymphocyte separation medium (LSM; Cambrex, Walkersville, MD) to yield mononuclear cells. OT-I.SJL or Bg1.SJL cells were washed in PBS, then incubated with 5 μm CFDA-SE (Molecular Probes, Eugene, OR) for 10 min at 37°, and washed twice in IMDM prior to injection. Between 5 × 106 and 1 × 107 cells were injected into recipients.
Isolation and in vitro culture of TCR transgenic T cells
Splenocytes (1 × 107) from Bg1.SJL mice were plated with 5 × 105 of a gamma-irradiated cell line, E22, that stably expresses β-gal. On day 3 after culture set-up, cell debris was removed by centrifugation over LSM and then cells were cultured for an additional 3 days before use. Cultures were either used on day 6 or restimulated with gamma-irradiated E22 cells on day 7 if cultured for longer.
In vitro direct presentation
WT3 cells were treated with either 2·5 μg/ml emetine or 25 μg/ml cycloheximide. Cells were kept in suspension by gentle rotation for the duration of treatment. WT3 cells were then electroporated as described above, with the following exceptions. WT3 cells were electroporated at 0·45 kV in the presence of 10 mg/ml OVA. Following electroporation, cells were washed twice and plated at 1 × 105 to 5 × 105 cells/well depending on the conditions examined. WT3 cells treated with cycloheximide were then incubated in the presence of cycloheximide for an additional 5 hr before being washed twice and then fixed or not as indicated. WT3 cells were fixed for 10 min at room temperature in 1% paraformaldehyde (Sigma). Fixed cells were washed twice in PBS, treated with 0·2 m glycine (Sigma) and then washed twice more before the addition of B3Z cells to test for antigen presentation in vitro.
Intracellular cytokine staining
L4·2mKb or L4·2 cells (5 × 104 cells/well) infected with r.VACV-Kb were plated with 1 × 105 β-gal96–103-specific CD8+ T cells and cocultured for 2 hr before the addition of 10 μg/ml Brefeldin A (Sigma) and then cultured for an additional 4 hr prior to antibody staining.
Flow cytometric analysis
All antibodies were purchased from BD Biosciences (San Jose, CA), and flow cytometry analysis was performed on a FACSCanto or FACScan (BD Biosciences). Data were analysed using FlowJo software (Treestar, San Carlos, CA). Cells were incubated in 2·4G2 (anti-CD16, -CD32) supernatant containing 10% mouse serum (Sigma) for 20 min on ice to block Fc receptor-mediated uptake of antibody. For analysis of cell division in vivo, spleens were harvested from two mice per group and homogenized and the cells were pooled. Mononuclear cells were isolated by centrifuging over LSM and harvesting the cells at the LSM/medium interface, then stained with anti-CD45·1-PE (clone A20) and anti-CD8-PECy5 (clone 53-6·7). Cells were washed three times prior to data capture. Only CD45·1, CD8 double positive cells were analysed for CFDA-SE staining.
For analysis of cytokine production, CD8+ T cells were stained with anti-CD8α-PE-Cy5 (clone 53-6·7) for 60 min on ice, washed and then fixed in 1% paraformaldehyde for 10 min at 25°. Lymphocytes were then stained with anti-mouse interferon (IFN)-γ-fluorescein isothiocyanate (FITC) (XMG1·2) in Fc block containing 0·5% saponin and 10% mouse serum for 60 min on ice. Data are expressed as the percentage of CD8+ T cells that produce IFN-γ.
Results
To address whether neosynthesis of proteins was required for donation of antigen during cross priming in vivo, it was necessary to control both the initiation and the cessation of antigen synthesis. In order to achieve this, we expressed β-gal, a model antigen, driven by an inducible promoter. β-gal was expressed in 293A cells transfected with the tet repressor, which produce undetectable levels of protein in the absence of tetracycline. A similar study examining the requirement for neosynthesis of antigen for direct presentation utilized nucleoprotein (NP) from lymphocytic choriomeningitis virus (LCMV) as an antigen. In contrast to NP, our model antigen, β-gal, is an enzyme that can be detected at very low quantities. We super-transfected 293A cells already expressing the tet repressor with the β-gal-expressing plasmid and characterized expression as follows.
Selection of β-gal-expressing clones
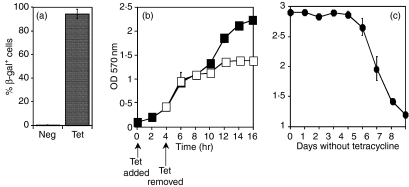
Transfected cells were treated with tetracycline and stained with the β-gal substrate X-gal to visualize inducible expression of the transgene. A positively staining colony was selected, designated L4·2, and used for all subsequent experiments. More than 90% of L4·2 cells expressed β-gal after 4 hr of tetracycline induction, and less than 0·1% of the cells expressed β-gal in the absence of tetracycline treatment (Fig. 1a).
Figure 1.
Characterization of β-galactosidase (β-gal) in L4·2 cells. L4·2 cells were treated with 1 μg/ml tetracycline (Tet) for 4 hr, and the percentage of cells producing β-gal (a), the time-point after tetracycline withdrawal at which new synthesis of β-gal ceased (b), and the length of time β-gal persisted in the cell after treatment was withdrawn (c) were measured. (b) Cells were cultured in the presence of tetracycline for either 4 hr (open squares) or for the entire duration of the experiment (closed squares). β-gal was quantified by 5-bromo-4-chloro-3-indolyl b-D galactopyranoside (X-gal) (a) or chlorophenol red β galactoside (CPRG) staining (b, c). Error bars indicate the standard error of the mean. Each panel represents three to five experiments. OD, optical density; Neg, untreated.
Characterization of β-gal expression upon tetracycline induction
L4·2 cells were treated with tetracycline for 4 hr and then cultured in the absence of tetracycline to determine both the time-point at which new synthesis of β-gal ceased (Fig. 1b) and the time for which β-gal lingered in the cells after withdrawal of treatment (Fig. 1c). β-gal production was assayed in bulk L4·2 cultures, after normalizing cell numbers from each time-point, using the choromogenic β-gal substrate CPRG. As shown in Fig. 1(b), new protein synthesis of β-gal ceased 8 hr after the withdrawal of tetracycline, as after this time-point the intracellular levels of β-gal did not increase. The failure to increase β-gal levels is probably the result of a shutdown of antigen synthesis, but it could also be the result of decreased antigen production concurrent with proteolysis of enzymatically active β-gal. β-gal generally has a half-life in the order of days.25,26 To determine whether antigen degradation could account for the reduced β-gal levels in L4·2 cells 8 hr after withdrawal of tetracycline, we examined the reduction in levels of β-gal over a number of days. Levels of β-gal remained constant in L4·2 cells for an additional 5 days before a decrease could be observed, indicating that degradation is not responsible for the flattening of the β-gal production early after tetracycline withdrawal. Indeed, β-gal is still detectable by CPRG staining 8 days after tetracycline has been withdrawn from the culture.
Characterization of Bg1 TCR transgenic mice
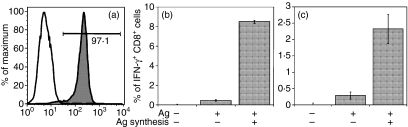
In order to measure cross priming of antigen in vivo in a controlled manner without the need to account for potentially confounding expansion and contraction of naïve CD8+ T cells, we used mice expressing transgenes encoding the α and β chains of a T-cell receptor that is specific for a peptide derived from β-gal. The receptor was cloned from a previously characterized CD8+ T-cell clone and recognizes residues 96–103 (sequence DAPITNYV) in the context of H2-Kb. The majority (> 85%) of CD8+ T cells from the resulting transgenic mice (named Bg1) expressed the transgene, as measured by expression of the Vβ7 chain (Fig. 2a), whereas in wild-type littermates less than 5% of CD8+ T cells expressed the receptor (Fig. 2b).
Figure 2.
Characterization of Bg1 T-cell receptor (TCR) transgenic mice. Splenocytes from Bg1 (a) or C57BL/6 (b) mice were stained with antibodies to CD8 and Vβ7 and CD8+ cells were analysed for Vβ7 expression by flow cytometry. Splenocytes from Bg1 mice were restimulated with cells transfected with β-galactosidase (β-gal) for 6 days then tested for their ability to produce interferon (IFN)-γ in response to titrated doses of β-gal96–103 peptide (c). Proliferation (d–g) and effector function (h) of adoptively transferred CFDA-SE-labelled Bg1 (Vβ7+) CD8+ T cells 3 days after mock immunization (d; Con in h) or immunization with recombinant vaccinia virus (rVACV) expressing full-length β-gal (e; FL in h) or ER-targeted β-gal96–103 (f; MG in h), or Western Reserve VACV that does not express the β-gal peptide (g; Irr in h) are shown. (h) Splenocytes were incubated with (open bars) or without (closed bars) β-gal96–103 peptide.
Although expression of the specific Vβ chain is a good indication of T-cell receptor expression, it was necessary to characterize the antigen specificity of CD8+ T cells from these mice in vitro and in vivo. Splenocytes from the Bg1 mice were restimulated in vitro with E22 cells that express β-gal19 and 6 days later tested for their ability to produce IFN-γ in response to titrated amounts of β-gal96–103 peptide. As shown in Fig. 2(c), the cells demonstrated half maximal activation at levels of peptide between 10−9 and 10−10 m peptide. Bg1 splenocytes did not produce IFN-γ in response to any other peptides tested (data not shown). To ensure antigen specificity was also applicable in vivo, Bg1 splenocytes were labelled with CFDA-SE and adoptively transferred into wild-type recipients that were then infected with recombinant vaccinia viruses (rVACVs). Significant proliferation of CD8+ T cells could be detected 3 days after infection with rVACV expressing either full-length β-gal (Fig. 2e) or an endoplasmic reticulum (ER)-targeted version of the minimal β-gal96–103 peptide (Fig. 2f). No significant proliferation was detected in the absence of infection (Fig. 2d) or upon infection with rVACV that did not express β-gal (Fig. 2g). Bg1 CD8+ T cells also acquired effector activity, as measured by production of IFN-γ in response to β-gal96–103 peptide 6 days after immunization with rVACVs as above (Fig. 2h). No effector activity was detected in the absence of infection or after infection with VACV that does not express β-gal or the β-gal96–103 peptide.
Requirement for neosynthesis of antigen for direct presentation
In order to measure direct-presentation of antigen, it was necessary to express an MHC class I molecule in the L4·2 cells for which a determinant derived from β-gal had been mapped. L4·2 cells were retrovirally transduced to express the murine MHC class I molecule H2-Kb (Fig. 3a) to generate L4·2mKb cells. L4·2mKb cells were also tested to verify that the kinetics of β-gal expression after tetracycline induction remained unchanged (data not shown).
Figure 3.
Antigen synthesis is required for direct-presentation. L4·2mKb cells were evaluated for their ability to express Kb after flow cytometric sorting of Kb-expressing cells by MoFlo (a). The closed peak indicates L4·2mKb cells, and the open peak indicates L4·2 cells. (b) Untreated L4·2mKb cells or 1 μg/ml tetracycline-treated cells; tetracycline was withdrawn immediately prior to or 24 hr prior to coculture with β-galactosidase (β-gal)96–103-specific CD8+ T cells cultured from Bg1.SJL mice. CD8+ cells were then evaluated for interferon (IFN)-γ production. (c) Same as (b), except that L4·2 cells were used and infected with recombinant vaccinia virus (rVACV)-Kb 2 hr prior to coculture with CD8+ cells. Each panel is representative of three experiments. Error bars indicate the standard error of the mean.
The requirement for neosynthesis of antigen for direct-presentation was examined by measuring activation of β-gal96–103-specific CD8+ T cells by L4·2mKb cells. L4·2mKb cells in which β-gal production had recently been induced by tetracycline treatment, or L4·2mKb cells that had been treated with tetracycline for 4 hr but then cultured in the absence of tetracycline for 24 hr, were used to stimulate CD8+ T cells (Fig. 3b). L4·2mKb cells that were no longer synthesizing β-gal (24 hr after tetracycline treatment) had equivalent levels of intracellular β-gal to those recently treated with tetracycline (data not shown). Activation of CD8+ T cells was measured by induction of IFN-γ production. L4·2mKb cells that were no longer synthesizing β-gal triggered few Bg1.SJL cells to produce IFN-γ, with activation marginally above background. In contrast, L4·2mKb cells that had recently been treated with tetracycline, and so were still synthesizing β-gal, were able to stimulate much greater numbers of Bg1.SJL CD8+ T cells to produce IFN-γ. Thus, neosynthesis of antigen greatly enhances the efficiency of direct-presentation. Similar results were obtained when L4·2 cells infected with rVACV-Kb for 2 hr to induce expression of H2-Kb were used (Fig. 3c) to stimulate CD8+ T cells. β-gal-specific CD8+ T cells were not activated under any conditions in the absence of mKb expression (data not shown).
Requirement for new synthesis of antigen in cross priming in vivo
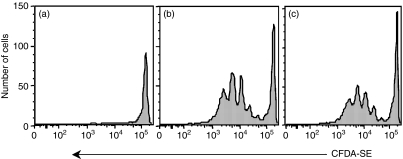
As L4·2 cells do not present β-gal96–103 in the absence of mKb expression they are ideally suited for use as antigen donor cells with which to study the requirement for new protein synthesis during cross-priming in vivo. Splenocytes were harvested from Bg1.SJL mice, labelled with CFDA-SE, and adoptively transferred into wild-type C57BL/6 recipient mice. These mice were then immunized with L4·2 cells that had either been recently treated with tetracycline or treated with tetracycline 24 hr previously. Mice immunized with L4·2 cells that had not been treated with tetracycline were included as a negative control group. Seventy-two hours after immunization, Bg1 CD8+ T-cell proliferation was measured by dilution of CFDA-SE fluorescence. Comparable Bg1 proliferation was seen from L4·2 cells treated with tetracycline, whether it was withdrawn just prior to injection or 24 hr prior to injection (Figs 4b and c). No proliferation was observed in response to untreated L4·2 cells (Fig. 4a). Thus, in contrast to direct-presentation, where active synthesis of protein was required for efficient presentation (Fig. 3), new synthesis of antigen was not required for cross priming in vivo (Fig. 4).
Figure 4.
Antigen synthesis is not required for antigen donation during cross-priming in vivo. B6 mice that had received an adoptive transfer of CFDA-SE-labelled Bg1.SJL cells were immunized with untreated L4·2 cells (a), or 1 μg/ml tetracycline-treated L4·2 cells (b, c) where tetracycline treatment was withdrawn immediately before injection (b) or 24 hr before injection (c). Three days later CD8+, CD45·1+ cells were evaluated for proliferation by dilution of CFDA-SE fluorescence. Each panel is representative of four experiments.
We have demonstrated that antigen synthesis is not required for donation of antigen during cross-priming in vivo. However, it is possible that synthesis of other cellular proteins is required for donation of antigen, perhaps via association of newly synthesized cellular proteins with antigen. Thus, we sought to examine the requirement for cellular protein synthesis in both direct- and cross-presentation.
Requirement for cellular protein synthesis for direct-presentation
To examine whether association with newly synthesized cellular factors is required for antigen presentation, it is necessary to introduce antigen following the inhibition of protein synthesis. To address the requirement for cellular protein synthesis in direct-presentation, we treated H-2b WT3 cells with the protein synthesis inhibitor cycloheximide for 1 hr, and then electroporated the cells with 10 mg/ml OVA. OVA used in the experiment was free of contaminating peptide that could potentially confound interpretation of the results obtained. The effect of cycloheximide is readily reversible, so 5 hr after electroporation cells were fixed to prevent cellular protein synthesis after withdrawal of the inhibitor. Removal of the inhibitor was necessary in order to assay antigen presentation using the Kb-restricted OVA257-264-specific B3Z hybridoma. B3Z produce β-gal upon activation, so activation was measured by staining with the β-gal substrate X-gal. WT3 cells that were electroporated and fixed efficiently activated B3Z, but treatment of WT3 cells with cycloheximide completely ablated B3Z activation (Fig. 5a). Similar results were observed after treatment of WT3 cells with the irreversible protein synthesis inhibitor emetine (data not shown). Fixed WT3 cells that were either treated with cycloheximide or untreated efficiently presented exogenous peptide to B3Z (data not shown and Fig. 5a). To rule out the possibility that the inhibitory effect of cycloheximide was a non-specific toxic effect, we treated cells for 5 hr as above, and then removed the drug without fixing the APCs. After removing cycloheximide, WT3 cells recovered their ability to present peptide produced from electroporated OVA (Fig. 5b). Thus, specific blockade of protein synthesis prevented direct-presentation.
Figure 5.
Direct-presentation requires cellular protein synthesis. WT3 cells were treated with cycloheximide for 1 hr prior to electroporation with ovalbumin (OVA). Cells were then treated for an additional 5 hr with cycloheximide (CHX) before being washed (b) or fixed (a). Cells were then incubated overnight with B3Z cells and then stained with 5-bromo-4-chloro-3-indolyl β-D galactopyranoside (X-gal). Each panel is representative of four experiments. Fixed and unfixed cells are presented on different panels because the stimulator to responder ratio was different. The same number of stimulator cells were initially plated, but a greater number of cells were lost in the fixed wells than were lost in the unfixed wells. Error bars represent the standard error of the mean.
Requirement for cellular protein synthesis for cross-priming in vivo
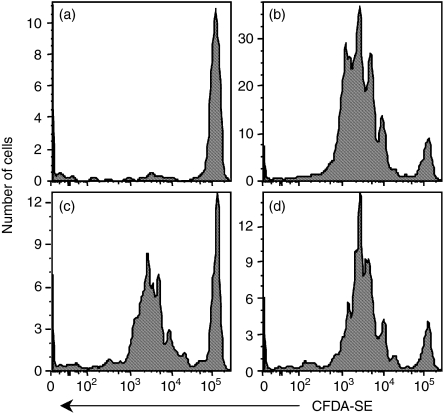
To assess the role of cellular protein synthesis in donation of antigen during cross-priming, we treated murine H2b cells lacking β2-microglobulin (STBKM-1) with the irreversible protein synthesis inhibitor emetine. STBKM-1 cells were incapable of presenting rVACV-encoded or electroporated OVA to the B3Z hybridoma, but presentation could be restored by infection with rVACV expressing β2m (data not shown). After treatment with emetine, cells were electroporated with 1 mg/ml OVA and introduced into recipient mice that had previously received CFDA-SE-labelled transgenic CD8+ T cells from OT-I mice specific for OVA257-264-Kb complexes. Under the conditions used, STBKM-1 cells that were incubated with OVA but not electroporated failed to induce proliferation of OT-1.SJL cells following immunization. In addition, STBKM-1 cells electroporated with concentrations of OVA lower than 1 mg/ml did not reproducibly induce proliferation of OT-1.SJL cells, indicating that under these conditions antigen was limiting (data not shown). Seventy-two hours after immunization with STBKM-1 cells, OT-I activation was measured by dilution of CFDA-SE fluorescence resulting from proliferation (Fig. 6b–d). Comparable OT-1.SJL proliferation was seen after immunization with STBKM-1 cells that were treated or untreated with emetine. Thus, protein synthesis was not required in the antigen donor cell for cross-priming to occur.
Figure 6.
Cross-priming in the absence of protein synthesis. Mice that had received an adoptive transfer of CFDA-SE-labelled OT1.SJL cells were immunized with β2m−/− cells that were mock electroporated (a) or electroporated in the presence of 1 mg/ml ovalbumin (OVA) (b–d). The cells were either untreated (b) or treated with the protein synthesis inhibitor emetine for 1 hr (c) or 5 hr (d) prior to electroporation. Each panel is representative of four experiments.
Discussion
The rational design of vaccines aimed at inducing protective CD8+ T cells requires a mechanistic understanding of the antigen processing and presentation pathways that generate MHC class I–peptide complexes in vivo. In this study, we set out to better characterize the mechanistic requirements for MHC class I-restricted presentation via the cross-priming pathway, and we contrast this to the requirements for presentation via the direct-presentation pathway. In particular, we examined the requirements for new antigen synthesis and protein synthesis in the antigen donor cell when antigen is presented via the cross-priming pathway, and in the APC when antigen is presented via the direct-presentation pathway.
By driving expression of an enzymatically active antigen, β-gal, with an inducible promoter, we demonstrated that ongoing synthesis of antigen is not required for cross-priming of naïve CD8+ T cells in vivo. In contrast, direct-presentation in vitro was greatly enhanced by neosynthesis of β-gal in the APCs and was almost undetectable in the absence of ongoing antigen synthesis. The latter finding is consistent with the findings of Khan et al.,9 although our system allows for detection of much lower levels of immunologically relevant antigen than the western blot analysis used in the previous study. Our observation, along with previously published studies,7,8 implicates DRiPs as the major source of antigenic peptides for direct-presentation. DRiPs are short-lived polypeptides that can only be visualized by inhibiting their rapid degradation shortly after synthesis. To date, it has not been possible to isolate single antigenic species of DRiPs, and it is unlikely that the measurement of enzymatically active β-gal in our experiments accounts for the presence of these molecules. However, it is clear from our data that, under conditions where direct-presentation is barely detectable, antigen is clearly available and sufficient for donation during cross priming in vivo. Thus, cross-priming can utilize pools of antigen that are not available for use by the direct-presentation pathway.
Our data do not rule out a role for rapidly degraded antigens as substrates for cross-priming, although other studies have indicated that proteins with very short half-lives are poor substrates for cross-priming in vivo.10,27 It is possible that DRiPs may be stabilized for extended periods of time by molecular chaperones, proteins that have been implicated in the cross-priming process.28 Although treatment of donor cells with inhibitors of heat shock protein 90 (hsp90) can inhibit cross-priming in vitro,29 the pleiotropic effects of these inhibitors when used for extended periods of time prevent firm conclusions being reached from this study. The role of hsp90 and other molecular chaperones in the cross-priming process remains highly controversial.30,31 Post-proteasomal degradation intermediates, which are presumably short-lived polypeptides, have been implicated in cross-priming,32 and these products may bind to the chaperone TCP-l ring complex (TriC),33 reducing their rate of degradation. However, these products are available for direct-presentation, and so are not likely to be represented in the system used in our studies.
Our data reveal that, 16 hr after the cessation of the production of enzymatically active β-gal, antigen is still available for donation during cross-priming. Even allowing for the fact that production of DRiPs, which are undetectable in our system, may proceed for a period of time after the production of intact protein, stabilization by chaperones would be required for many hours prior to antigen donation. Chaperones typically associate with unfolded proteins to target their degradation34,35 or to assist with their folding into a functional conformation.36,37 Under these conditions chaperone–substrate interactions are transient and are unlikely to occur for the ≥16 hr we have examined here. Chaperones are known to associate constitutively with some substrates, such as the glucocorticoid receptor,38,39 but these associations appear to be highly specialized interactions. In contrast, the requirement that a broad repertoire of antigens be available during cross-priming would necessitate chaperone–antigen interactions that are both long-lived and promiscuous, at least on the part of the chaperone. Such promiscuous interactions would significantly reduce the efficacy of the housekeeping functions of chaperones, a very costly process for all cells to allow immune monitoring.
The observation that electroporated OVA protein is available for direct-presentation may appear to contrast with a requirement for neosynthesis of antigen in this process. However, a number of factors may allow the direct-presentation of electroporated OVA in this system. First, presentation of β-gal in the absence of ongoing synthesis is inefficient, but could potentially be overcome by the presence of large intracellular depots of antigen. When presentation of electroporated antigen was examined we used 10 mg/ml OVA, or 10 times the amount used for in vivo cross-priming experiments. Lower doses of OVA did not generate measurable activation of the highly sensitive B3Z hybridoma, indicating a requirement for high concentrations of antigen in this process. In addition to the large dose of antigen used, electroporation may alter cellular metabolism, or may physically allow access of antigen to compartments that it does not normally reach, allowing direct-presentation.
There have been a number of studies indicating that donor cell-encoded proteins40 or the phenotypic status of the donor cell41–43 plays a significant role in the transfer of antigen to donor cells. Cells may synthesize proteins that modulate cross-priming by increasing the uptake of antigen, at the level of either cellular clearance or antigen transfer to APCs, or by modulating the pAPC phenotype to enhance antigen presentation. To address this issue, we blocked protein synthesis and examined the effects upon direct-presentation and antigen donation during cross-presentation. In contrast to direct-presentation, which was completely blocked upon inhibition of protein synthesis, no effect of a blockade of protein synthesis was observed on donation of antigen during cross-priming. Thus, newly synthesized proteins are not essential for antigen transfer. This was the case even when antigen was added to donor cells 5 hr after protein synthesis was blocked, indicating that cellular proteins with a half-life of less than 5 hr are probably not involved in antigen donation.
The blockade of direct-presentation under conditions in which protein synthesis is prevented probably represents a requirement for neosynthesis of components of the MHC class I processing pathway. Such a system can be readily subverted during viral infection, as one of the earliest acts of many viruses following infection is the shut-off of host protein synthesis. Under the conditions used here, it is not possible to examine the requirements for protein synthesis in the presentation of peptides derived from DRiPs. In addition, components of the MHC class I processing pathway may be specialized to allow presentation from DRiPs in the absence of synthesis of other cellular proteins. However, it is clear that antigen donation during cross-priming in vivo has no such requirement for ongoing protein synthesis. In effect, antigen donation is a passive process that requires little or no participation by the donating cell. Indeed, we have preliminary data indicating that vesicular trafficking is not required in the donor cell during donation of virus-encoded antigen (data not shown). These findings strongly suggest that antigen donation is a process that is much less susceptible to evasion, modulation or other manipulation by viruses, particularly after the shut-down of host protein synthesis. The passive nature of this process may allow the generation of effective immune responses to viruses that efficiently shut down host protein synthesis effectively.
In summary, our data demonstrate two findings of relevance to the design of vaccines aimed at inducing protective CD8+ T cells. First, the cross-priming and direct-priming pathways utilize distinct pools of antigen. Thus, an ideal CD8+ T cell-mediated vaccine would utilize presentation by both pathways to achieve optimal efficacy. Secondly, we have demonstrated that antigen donation during cross-priming is a passive process that avoids modulation by viruses that actively shut down host protein synthesis in infected cells. Thus, cross-priming may account for a greater proportion of the CD8+ T cells specific for viruses that efficiently shut down host protein synthesis, and vaccines aimed at these pathogens may benefit from a preferential targeting of the cross-priming mechanism.
Acknowledgments
We thank Amanda Schell and Irene Reider for excellent technical assistance, Drs Jon Yewdell and Jack Bennink for the viruses expressing β-gal, and the members of the Norbury laboratory as well as Dr Emmy Truckenmiller and Dr Chris Nicchitta for critical review of the manuscript, and helpful comments and suggestions. We acknowledge the contributions of Nate Sheaffer of the Cell Science/Flow Cytometry Core Facility and Anne Stanley of the Macromolecular Core Facility of the Section of Research Resources, Penn State College of Medicine. This work was supported by National Institutes of Health Grant AI056094 to CCN and C06 RR-15428-01 to Penn State College of Medicine Department of Comparative Medicine.
Abbreviations
- β-gal
β-galactosidase
- NP
nucleoprotein
- CPRG
chlorophenol red β-galactoside
- rVACV
recombinant vaccinia virus
- DRiPs
defective ribosomal products
- TCR
T-cell receptor
- pAPC
professional antigen-presenting cell
References
- 1.Sigal LJ, Reiser H, Rock KL. The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J Immunol. 1998;161:2740–5. [PubMed] [Google Scholar]
- 2.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–71. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 3.Bevan MJ. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J Immunol. 1976;117:2233–8. [PubMed] [Google Scholar]
- 4.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–8. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norbury CC, Sigal LJ. Cross priming or direct priming: is that really the question ? Curr Opin Immunol. 2003;15:82–8. doi: 10.1016/s0952791502000031. [DOI] [PubMed] [Google Scholar]
- 6.Yewdell JW, Anton LC, Bennink JR. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J Immunol. 1996;157:1823–6. [PubMed] [Google Scholar]
- 7.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–4. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 8.Reits EA, Vos JC, Gromme M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404:774–8. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- 9.Khan S, de Giuli R, Schmidtke G, Bruns M, Buchmeier M, van den Broek M, Groettrup M. Cutting edge: neosynthesis is required for the presentation of a T cell epitope from a long-lived viral protein. J Immunol. 2001;167:4801–4. doi: 10.4049/jimmunol.167.9.4801. [DOI] [PubMed] [Google Scholar]
- 10.Norbury CC, Basta S, Donohue KB, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–21. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 11.Townsend A, Bastin J, Gould K, et al. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988;168:1211–24. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 13.Nicchitta CV. Re-evaluating the role of heat-shock protein–peptide interactions in tumour immunity. Nat Rev Immunol. 2003;3:427–32. doi: 10.1038/nri1089. [DOI] [PubMed] [Google Scholar]
- 14.Baker-LePain JC, Reed RC, Nicchitta CV. ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr Opin Immunol. 2003;15:89–94. doi: 10.1016/s0952791502000067. [DOI] [PubMed] [Google Scholar]
- 15.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 16.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 17.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Meth. 1995;180:273–80. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 18.Restifo NP, Bacik I, Irvine KR, et al. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Overwijk WW, Surman DR, Tsung K, Restifo NP. Identification of a Kb-restricted CTL epitope of beta-galactosidase: potential use in development of immunization protocols for ‘self’ antigens. Methods. 1997;12:117–23. doi: 10.1006/meth.1997.0461. [DOI] [PubMed] [Google Scholar]
- 20.Basta S, Chen W, Bennink JR, Yewdell JW. Inhibitory effects of cytomegalovirus proteins US2 and US11 point to contributions from direct priming and cross-priming in induction of vaccinia virus-specific CD8 (+) T cells. J Immunol. 2002;168:5403–8. doi: 10.4049/jimmunol.168.11.5403. [DOI] [PubMed] [Google Scholar]
- 21.Ackerman AL, Cresswell P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J Immunol. 2003;170:4178–88. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 22.Pretell J, Greenfield RS, Tevethia SS. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). V. In vitro demonstration of SV40 TrAg in SV40 infected nonpermissive mouse cells by the lymphocyte mediated cytotoxicity assay. Virology. 1979;97:32–41. doi: 10.1016/0042-6822(79)90370-2. [DOI] [PubMed] [Google Scholar]
- 23.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–76. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 24.Reis e Sousa C, Germain RN. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841–51. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–86. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 26.Smith RL, Geller AI, Escudero KW, Wilcox CL. Long-term expression in sensory neurons in tissue culture from herpes simplex virus type 1 (HSV-1) promoters in an HSV-1-derived vector. J Virol. 1995;69:4593–9. doi: 10.1128/jvi.69.8.4593-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolkers MC, Brouwenstijn N, Bakker AH, Toebes M, Schumacher TN. Antigen bias in T cell cross-priming. Science. 2004;304:1314–7. doi: 10.1126/science.1096268. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–94. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 29.Basta S, Stoessel R, Basler M, van den Broek M, Groettrup M. Cross-presentation of the long-lived lymphocytic choriomeningitis virus nucleoprotein does not require neosynthesis and is enhanced via heat shock proteins. J Immunol. 2005;175:796–805. doi: 10.4049/jimmunol.175.2.796. [DOI] [PubMed] [Google Scholar]
- 30.Shen L, Rock KL. Cellular protein is the source of cross-priming antigen in vivo. Proc Natl Acad Sci USA. 2004;101:3035–40. doi: 10.1073/pnas.0308345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–9. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 32.Serna A, Ramirez MC, Soukhanova A, Sigal LJ. Cutting edge: efficient MHC class I cross-presentation during early vaccinia infection requires the transfer of proteasomal intermediates between antigen donor and presenting cells. J Immunol. 2003;171:5668–72. doi: 10.4049/jimmunol.171.11.5668. [DOI] [PubMed] [Google Scholar]
- 33.Kunisawa J, Shastri N. The group II chaperonin TRiC protects proteolytic intermediates from degradation in the MHC class I antigen processing pathway. Mol Cell. 2003;12:565–76. doi: 10.1016/j.molcel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Molinari M, Galli C, Piccaluga V, Pieren M, Paganetti P. Sequential assistance of molecular chaperones and transient formation of covalent complexes during protein degradation from the ER. J Cell Biol. 2002;158:247–57. doi: 10.1083/jcb.200204122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004;16:343–9. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Ostermann J, Horwich AL, Neupert W, Hartl FU. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989;341:125–30. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- 37.Martin J, Langer T, Boteva R, Schramel A, Horwich AL, Hartl FU. Chaperonin-mediated protein folding at the surface of groEL through a ‘molten globule’-like intermediate. Nature. 1991;352:36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- 38.Dittmar KD, Demady DR, Stancato LF, Krishna P, Pratt WB. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J Biol Chem. 1997;272:21213–20. doi: 10.1074/jbc.272.34.21213. [DOI] [PubMed] [Google Scholar]
- 39.Dittmar KD, Pratt WB. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation. J Biol Chem. 1997;272:13047–54. doi: 10.1074/jbc.272.20.13047. [DOI] [PubMed] [Google Scholar]
- 40.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellone M, Iezzi G, Rovere P, et al. Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol. 1997;159:5391–9. [PubMed] [Google Scholar]
- 42.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I- restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 43.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]