Abstract
Previous work has shown that the function of mouse CD4+ T cells can be augmented by an enzyme, O-sialoglycoprotein endopeptidase (OSGE), which cleaves surface CD43, suggesting the idea that the high levels of glycosylated CD43 found on T cells from aged mice may contribute to immune senescence. New results now show that OSGE improves T-cell function even in mice lacking CD43, showing that other glycoproteins must contribute to the OSGE effect on function. Evaluation of other enzymes found two whose ability to stimulate CD4 activation was higher in aged than in young T cells. One of these, PNGase F, is a glycosidase specific for N-linked glycans, and the other, ST-Siase(2,3) from Salmonella typhimurium, is specific for α2,3-linked terminal sialic acid residues. Parallel lectin-binding experiments showed that removal of α2,3-linked sialic acid residues vulnerable to PNGase F and ST-Siase(2,3) was also greater in old than in young T cells. The preferential ability of PNGase F and ST-Siase(2,3) to improve the function of T cells from aged mice may involve cleavage of glycoproteins containing α2,3-linked sialic acid residues on N-linked or O-linked glycans or both.
Keywords: ageing, immunosenescence, signal transduction, cellular activation, glycosylation
Introduction
The age-related decline in immune function affects both the innate (macrophage activation, dendritic cell migration, and Toll-like receptor-mediated activation);1,2 and adaptive (T- and B-cell activation and germinal centre formation)3–5 immune responses. There is, however, some evidence that many of these defects are secondary to the age-related decline in T-cell function;6,7. Age-dependent defects in the T-cell compartment are well documented and thought to reflect both changes in T-cell subset composition, and defects in the activation processes within the peripheral T-cell pool.8,9 These defects lead to a decline in lymphocyte proliferation10 and deregulation in the production of cytokines, including interleukin-2 (IL-2).11 Evidence suggests that these functional age-related defects are the result of upstream transcriptional flaws caused by earlier signalling deficiencies that take place shortly after activation.3,12
Recent work has shown that the age-related decline in T-cell receptor (TCR) signalling may be due to defects in the very earliest stages of T-cell activation, involving reorganization of the cytoskeleton and formation of the immunological synapse.12 Studies focusing on the response of TCR-transgenic CD4+ cells to antagonist peptide sequences have shown that aged T cells exhibit defects in cytoskeletal rearrangement that contribute to synapse formation but precede peptide recognition by the TCR.12
Age-dependent cytoskeletal defects also lead to a failure, in aged CD4+ cells, to remove CD43 from the site of T cell/antigen-presenting cell (APC) interaction.13 CD43, a large and heavily glycosylated molecule, has been estimated to cover 28% of the T-cell surface. Its extracellular domain is approximately 45 nm in length, making it both the most abundant and the largest protein on the surface of the T cell.14 In some model systems, CD43 seems to act as a negative regulator in T-cell adhesion and activation.15–17 Data from our laboratory have illustrated that there is an age-dependent increase in specific glycosyl determinants (S7 and 1B11) on the CD43 molecule, and that high level expression of S7 and 1B11 is seen preferentially on a T-cell subset, marked by P-glycoprotein, that is known to be anergic to most forms of stimulation.13 In addition, we have shown that age-dependent defects in synapse formation in old CD4+ T cells can be corrected by treating the cells with O-sialoglycoprotein endopeptidase (OSGE), which cleaves the peptide backbone of O-linked glycoproteins, including CD43.13 Pre-treatment of the T cells with OSGE without stimulation did not result in cellular activation.13,18 Collectively, these observations suggest the hypothesis that the proteolytic cleavage of glycosylated CD43 molecules by OSGE is responsible for augmentation of cytokine production and calcium response in young T cells, and for the restoration of these signals and synapse formation by CD4+ cells of aged mice.
Our initial studies of OSGE included tests using another enzyme, the sialidase from Vibrio cholerae (VC-Siase), to see if the effects of OSGE might be due to the elimination of terminal sialic acid residues, and showed that VC-Siase had no effect on synapse formation by young or old T cells.13 A follow-up study, however, showed that a second sialidase from Clostridium perfringens (CP-Siase), did improve T-cell function in cells from both young and old mice.19 Like OSGE, pretreatment of the T cells with CP-Siase without stimulation did not result in cellular activation.19 Both VC-Siase and CP-Siase can cleave both α2,3- and α2,6-linked terminal sialic acid residues from a wide range of proteins. The difference in their functional effects was our point of departure for using glycosidases of narrower specificity to potentially shed light on the basis for age-related T-cell activation defects and the mechanism by which glycoprotein-specific hydrolases can augment function in both young and old T cells.
Materials and methods
Mice
CD43 knockout (KO) and CD43 wild type (WT) mice, on the C57BL/6 background, were a gift from Dr Anne Sperling (University of Chicago). Specific pathogen-free male (BALB/cJ × C57BL/6 J)F1 (CB6F1) mice were purchased from the National Aging Institute contract colonies. Mice were considered young at 3–6 months of age, and old at 20–26 months of age. All CD43 KO mice and their WT controls were used when young. Mice were given free access to food and water. Sentinel animals were examined quarterly for serological evidence of viral infection; all such tests were negative during the course of these studies. Mice with splenomegaly or macroscopically visible tumours at the time of death were not used for experiments.
Antibodies and reagents
Antibodies to mouse anti-CD3ε (clone 145-2C11) and anti-CD28 (clone 37.51) were produced in our laboratory. All monoclonal antibodies for flow cytometry were purchased from BD Biosciences (San Diego, CA). The lectins were purchased from E-Y Laboratories (San Mateo, CA). OSGE was purchased from Cedarlane Laboratories (Hornby, Ontario, Canada). CP-Siase and VC-Siase were purchased from Roche (Indianapolis, IN). Recombinant Salmonella typhimurium sialidase [ST-Siase(2,3)], peptide-N-glycosidase F (PNGase F) and endoglycosidase H (Endo H) expressed in Escherichia coli were purchased from New England Biolabs (Beverly, MA). Recombinant sialidase L and ceramide-glycase, both from Macrobdella decora, were purchased from V-Laboratories (Covington, LA). Endoglycoceramidase II was purchased from Takara Bio (Shiga, Japan).
Cell preparation, enzymatic treatment, and CD69 and CD25 flow cytometric analysis
T cells and CD4+ T cells were obtained as previously described.20 Flow cytometric analysis of a typical preparation showed it to be 90–95% positive for the population of interest. The purified T cells or CD4+ T cells (1 × 106 cells/ml in Hanks' balanced salt solution (HBSS)) were incubated with 50 µg/ml of OSGE (1 hr), 100 mU/ml of CP-Siase (or the control enzymes endoglycoceramidase I, Macrobdella decora ceramide-glycase or VC-Siase) (1 hr), 1500 U/ml of PNGase F (or the control enzyme Endo H) (12 hr) or 250 U/ml of ST-Siase(2,3) (12 hr) or at 37°. Cells were stimulated with anti-CD3 (αCD3) and the expression of CD69 and CD25 was examined by flow cytometry as previously described.13 Quantification was done using the mean fluorescence intensity (MFI).
Measurement of cytosolic Ca2+
Cytosolic Ca2+ measurement was performed as previously described.18 Splenic T cells (either untreated or pretreated with OSGE), were loaded with Indo-1 AM (2·5 µm; Molecular Probes, Eugene, OR) at room temperature for 30 min, washed and then stained with an anti-CD4 (clone RM4-5) or anti-CD8 (clone 53-6.7) antibody. The calcium mobilization assay was conducted at 37° on a FACSVantage flow cytometer using FACSDiva software (BD Biosciences, San Jose, CA). Baseline levels were collected for 20 s, after which the cells were stimulated with 5 µg/ml of non-cross-linked anti-CD3 (clone 145-2C11). Each assay was performed for a 5-min period. The acquired data were analysed using FlowJo's kinetic platform (TreeStar, Ahsland, OR). Each histogram displays the mean of the violet/blue ratio as a function of time with smoothing for moving average.
Cytokine enzyme-linked immunosorbent assay (ELISA)
Purified CD4+ T cells were stimulated for 6 hr in culture with αCD3 at 1 µg/ml or with αCD3 plus αCD28 at 1 µg/ml each. Supernatants were collected and stored at −70° until cytokine analysis. IL-2, IL-4 and interferon-γ (IFN-γ) were quantified using commercial enzyme-linked immunosorbent assay kits (BioSource International, Camarillo, CA) according to the manufacturer's protocols. The minimum detectable level was <8 pg/ml for IL-2, <1 pg/ml for IFN-γ, and <5 pg/ml for IL-4.
Lectin binding assays to cell surface proteins and flow cytometry
Lectin binding and CD4+ T-cell surface marker expression were analysed by flow cytometry using three-colour staining assays as previously described.19 Purified T cells were incubated with antibodies against CD4 (Cy-Chrome labelled) for 30 min in HBSS (containing 0·2% bovine serum albumin and 0·1%NaN3) on ice, washed twice, and incubated with fluoroscein isothiocyanate (FITC)-conjugated lectins at 1 µg/ml for 30 min at room temperature, washed twice, and analysed by flow cytometry.
Statistical analysis
In the case of Ca2+ assays, the peak increase above baseline was calculated for CD4+ and CD8+ T cells, with the data then subjected to a two-tailed Mann–Whitney test. Cytokine data were analysed using a Friedman anova, with post hoc analysis done using Wilcoxon matched pairs tests. CD69, CD25 and lectin binding data were analysed using a two-way anova. Post-hoc analysis was done using Dunnett's 2-sided multiple comparison test. Ageing comparisons (young versus old) were analysed using paired t-tests. In order to evaluate if a treatment had a differential effect on a group (either WT versus KO or young versus old), the absolute change of each mouse in the group was calculated after treatment and the groups were compared using paired t-tests. Comparisons were deemed significant when P = 0·05.
Results
OSGE enhances Ca2+ responses of CD4+ and CD8+ T cells even in CD43 KO mice
We have previously shown18 that OSGE cleaves the O-linked glycoprotein CD43 and increases αCD3-induced Ca2+ responses in CD4+ T cells of both young and old mice. To determine if the cleavage of CD43 is required for this effect, we evaluated the Ca2+ response of CD43 KO mice and their littermate controls. Figure 1 shows a representative experiment. Pre-treatment of the T cells with OSGE without stimulation did not result in calcium influx. Untreated cells from WT and CD43 KO mice mount similar responses (CD4+: P = 0·48; CD8+: P = 0·34). Pre-treatment with OSGE significantly enhanced the αCD3-induced Ca2+ response in CD4+ as well as CD8+ T cells from both WT (CD4+: P = 0·02; CD8+: P = 0·05) and CD43 KO (CD4+: P = 0·02; CD8+: P = 0·02) mice, showing that the effect of OSGE on cells from young mice does not require CD43.
Figure 1.
Effect of OSGE on the calcium response. CD4+ (top panel) and CD8+ (bottom panel) T cell Ca2+ responses were induced by non-cross-linked anti-CD3 in WT and CD43 KO mice, with or without pretreatment by OSGE. The break in each line shows where stimulatory antibodies were added after 20 s of baseline data collection. The graphs are representative of the data obtained from four WT and four CD43 KO mice from four independent experiments.
OSGE enhances cytokine production by CD4+ T cells from young CD43 KO mice
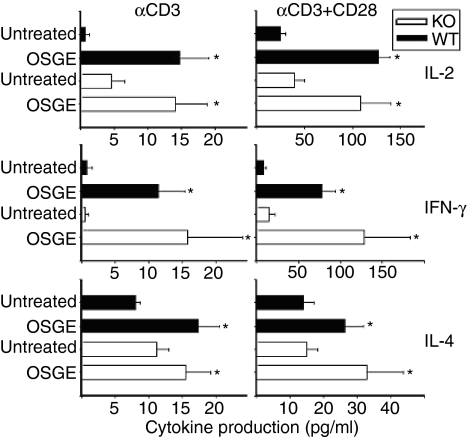
Our previous data have shown that OSGE enhances the production of IL-2 and IFN-γ by CD4+ T cells from young and old mice.18 To determine if these effects of OSGE are mediated through cleavage of CD43 alone, we evaluated the production of IL-2, IFN-γ, and IL-4 by CD4+ T cells from CD43 KO mice in responses stimulated by soluble αCD3 (CD3s; left panels of Fig. 2) or by CD3s plus αCD28 (right panels). Cytokine secretion was measured by ELISA 6 hr after stimulation. The CD43 KO and CD43 WT mice were on the C57BL/6 background, while previous experiments were performed on CB6F1 mice.18 In our hands, CD4+ T cells from C57BL/6 mice produced lower amounts of cytokines as compared to those from CB6F1 mice. Pre-treatment of the T cells with OSGE without stimulation did not result in the production of any cytokines (data not shown). In no case did untreated cells from the CD43 KO mice make significantly more cytokine than untreated control cells. In all cases, treatment with OSGE led to significant increases (P < 0·05) in cytokine production by both WT and CD43 KO cells. OSGE did not have a differential effect on WT or CD43 KO mice, except in the case of IL-4 after CD3s stimulation, where OSGE had a greater effect (P = 0·05) on T cells from WT mice. These data, like the calcium results, suggest that the stimulatory effect of OSGE on young CD4+ cells does not depend upon cleavage of CD43.
Figure 2.
OSGE enhances IL-2, IFN-γ, and IL-4 production in WT and CD43 KO mice. CD4+ T cells (106) /ml from WT (dark bars) and CD43 KO (light bars) mice were either treated with OSGE or left untreated, and stimulated with αCD3, or αCD3 plus αCD28 (αCD3 + αCD28). The supernatants were collected after 6 h of stimulation and analysed for IL-2 (top two panels), IFN-γ (middle two panels), and IL-4 (bottom two panels) by ELISA. The bars represent the mean ± SEM from six WT and six CD43 KO mice from six independent experiments. Asterisks (*) indicate significant difference with untreated mice of the same group (WT or CD43 KO).
Treatment with OSGE can enhance the expression of early activation markers in both WT and CD43 KO mice
We also evaluated the effects of OSGE on the expression of CD69 at 6 hr, and CD25 at 24 hr after activation in both CD4+ and CD8+ T cells. The results are shown in Fig. 3. Pre-treatment of the T cells with OSGE without stimulation did not result in the expression of CD69 or CD25 (data not shown). The CD69 data showed that OSGE led to a significant enhancement (P < 0·01) in both CD4+ and CD8+ T cells of both CD43 KO and WT mice. There were no significant differences between responses of WT and CD43 KO mice, either in untreated or in OSGE-treated cells. Although modestly significant (P = 0·01) differences were seen between WT and KO cells in the effect of OSGE, these differences are quite small, and the expression of CD69 on OSGE-treated cells was essentially equivalent. For CD25 expression, measured at a later time point, the effects of OSGE were consistent but less dramatic than in the CD69 data, and reached statistical significance for CD4+ responses in WT mice and CD8+ responses in CD43 KO mice (P < 0·02 in each case) but not for the other comparisons (P < 0·1) shown in Fig. 3. There were no differences in OSGE effect on CD25 expression in either CD4+ or CD8+ cells.
Figure 3.
OSGE enhances early activation marker expression in CD43 KO mice. T cells from WT (dark bars) and CD43 KO (light bars) mice were treated with OSGE or left untreated, and then stimulated with αCD3. Gated CD4+ or CD8+ T cells were examined for CD69 (top two panels) expression after 6 hr of stimulation, and for CD25 (bottom two panels) 24 hr after stimulation. The bars represent the mean ± SEM from six WT and six CD43 KO mice from six independent experiments. Asterisks (*) indicate significant difference with untreated mice of the same group (WT or CD43 KO).
Inhibition of CD69 and CD25 expression by 2,3-linked sialic acid residues and N-linked oligosaccharides in CD4+ T cells of old and young mice
The studies of CD43 KO mice were motivated by our hypothesis18 that CD43 cleavage by OSGE was largely responsible for the ability of this enzyme to improve multiple aspects of function in CD4+ T cells of both young and old mice. The evidence that OSGE has strong effects on T-cell function even in CD43 KO mice prompted a search for other glycoprotein-specific enzymes that might have similar functional effects, perhaps by alteration of an overlapping set of surface macromolecules. Figure 4 shows the results with two such enzymes, ST-Siase(2,3) and PNGase F, using CD69 and CD25 expression as indices of CD4+ and CD8+ T cell function. ST-Siase(2,3) is a sialidase isolated from Salmonella typhimurium, and is specific for 2,3-linked Gal/GalNAc groups. Peptide-N-Glycosidase F (PNGase F) is an amidase that hydrolyses the links between the innermost GlcNAc and asparagine residues of oligosaccharides from N-linked glycoproteins. Pre-treatment of the T cells with ST-Siase(2,3) or PNGase F without stimulation did not result in the expression of CD69 or CD25 (data not shown). The CD69 data (top panels) show, as expected, a small but significant age-dependent decline in CD69 expression after 6 hr of stimulation in both CD4+ (P = 0·001) and CD8+ (P = 0·006) cells. We have shown previously,13,19 that OSGE or CP-Siase treatment of CD4+ T cells from young and old mice results in a significant increase in CD69 expression after stimulation. The results in Fig. 4 confirm this for CD4+ cells and show similar changes for CD8+ T cells (both P < 0·05 for both cell types). After treatment with either enzyme, responses of T cells from young and old donors were statistically indistinguishable in the case of both CD4+ and CD8+ cells. In agreement with our previous results19 treatment of the T cells with endoglycoceramidase I or Macrobdella decora ceramide-glycase (enzymes that remove sialic acid residues and polysaccharide structures from surface glycolipids), and with VC-Siase (another 2,3 and 2,6 sialidase, similar to CP-Siase) did not result in enhanced expression of CD69 or CD25 (data not shown), suggesting that the negative regulation of the TCR response is dependent upon specific sialic acid residues present on specific glycoproteins.
Figure 4.
ST-Siase(2,3) and PNGase F enhance early activation marker expression in CD4+ and CD8+ T cells from young and old mice. T cells from young (dark bars) and old (light bars) mice were treated with OSGE, CP-Siase, ST-Siase(2,3) or PNGase F, or left untreated, and then stimulated with αCD3. Gated CD4+ or CD8+ T cells were examined for CD69 (top panels) expression after 6 hr of stimulation, and for CD25 (bottom panels) 24 hr after stimulation. The bars represent the mean ± SEM from six young and six old mice from six independent experiments. Asterisks (*) indicate significant difference with untreated mice of the same age group (young or old). (a) and (b) indicate significant effects of age.
Exposure to ST-Siase(2,3) induced a significant increase (P < 0·05) in the ability to express CD69 in both CD4+ and CD8+ cells of old mice (Fig. 4, top panels); the responses of cells from young donors were not enhanced to a significant extent by this enzyme. The effect of ST-Siase(2,3) was significantly stronger in old T cells than in cells from young donors for both CD4+ (P = 0·01) and CD8+ (P = 0·008) subsets. Treatment with PNGase F also improved CD69 expression in both CD4+ and CD8+ cells from both young and old mice. After treatment with either enzyme, responses of T cells from aged and young donors were statistically indistinguishable. However, treatment with Endo H, another amidase which cleaves within the chitobiose core of high mannose and some hybrid oligosaccharides from N-linked glycoproteins, had no effect on CD69 expression in either CD4+ or CD8+ cells from young or old donors (data not shown). These results suggest that specific N-glycosylated moieties on surface glycoproteins are responsible for the inhibition of TCR signal.
Similar results were found in the analysis of CD25 expression after 24 hr of αCD3 stimulation (Fig. 4, bottom panels). There was a significant age-dependent decline in CD25 expression after activation (P = 0·001 for CD4+ cells, P = 0·01 for CD8+). Treatment with ST-Siase(2,3) or PNGase F also produced significant enhancement of CD25 expression in CD4+ and CD8+ T cells from young and old mice (P < 0·05 for both subsets). For CD4+ cells, both enzymes had a larger effect in old T cells than in young ones (ST-Siase(2,3) P = 0·04; PNGase F P = 0·03). For CD8+ cells, the effects were also larger in cells from old mice, but the age effect did not reach statistical significance (ST-Siase(2,3) P = 0·06, PNGase F (P = 0·09).
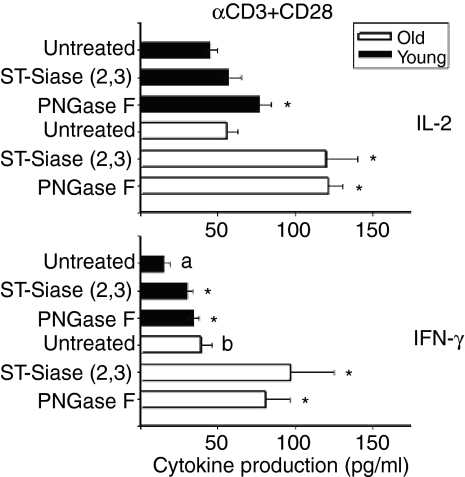
ST-Siase(2,3) and PNGase F enhance production of IL-2 and IFN-γ
Figure 5 shows the outcome of experiments in which CD4+ T cells were treated with these enzymes and then tested for the production of cytokines after 6 h of stimulation. Pre-treatment of the T cells with ST-Siase(2,3) or PNGase F without stimulation did not result in cytokine production (data not shown). Treating CD4+ cells from young mice with ST-Siase(2,3) had no significant effect on IL-2 production (Fig. 5, top panel), but this enzyme did lead to a significant (P = 0·03) enhancement of IL-2 production by T cells of old mice. PNGase F also increased IL-2 production from both young and old mice (P < 0·03). The enhancements observed after PNGase F treatment were significantly greater in the T cells from the aged mice as compared to their young counterparts (ST-Siase(2,3) P = 0·01; PNGase F P = 0·02). Production of IFN-γ, a cytokine whose production by mouse CD4+ increases with age, was also augmented by both ST-Siase(2,3) and PNGase F in both young and old animals (Fig. 5, bottom; P < 0·03 for each effect). There was a suggestion that ST-Siase(2,3) and PNGase F enhanced IFN-γ production to a greater extent in old than in young mice, but the differences were not statistically significant: the responses to ST-Siase(2,3) were 3·6-fold higher in old mice (P = 0·14) and the responses to PNGase F were 2·1-fold higher (P = 0·07).
Figure 5.
ST-Siase(2,3) and PNGase F enhance cytokine expression in CD4+ T cells from young and old mice. 106 CD4+ T cells/ml from young (dark bars) and old (light bars) mice were either treated with ST-Siase(2,3) or PNGase F, or left untreated, and then stimulated with αCD3 plus αCD28. The supernatants were collected after 6 hr of stimulation and analysed for IL-2 (top panel), and IFN-γ (bottom panel) by ELISA. The bars represent mean ± SEM from six young and six old mice from six independent experiments. Asterisks (*) indicate significant difference with untreated mice of the same age group (young or old). Letters (a) and (b) indicate significant effects of age.
Lectin binding analysis after treatment with ST-Siase(2,3) and PNGase F
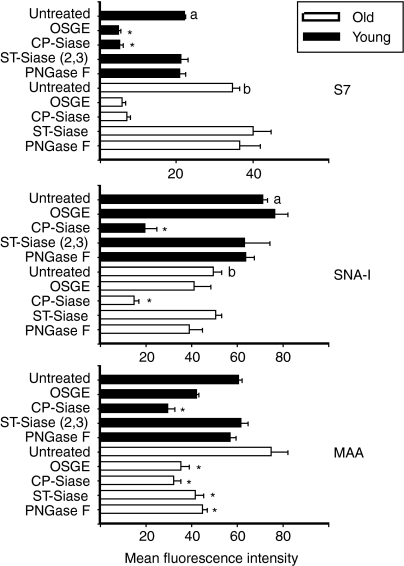
To learn more about the cell surface glycoproteins whose cleavage by OSGE, CP-Siase, ST-Siase(2,3) or PNGase F improve T-cell function, we evaluated the binding of antibodies and lectins to CD4+ cells before and after enzyme exposure using flow cytometry. Figure 6 shows these results for both young and old mice. The S7 antibody recognizes a sialylated polysaccharide determinant found on the CD43 molecule on all resting T lymphocytes. Confirming our previously published data13 we noted (top panel) an age-dependent increase in the mean fluorescence intensity of the S7 determinant (P < 0·001). Both OSGE and CP-Siase caused significant reductions in S7 levels on CD4+ cells from young or old mice (P < 0·05). ST-Siase(2,3) and PNGase F, however, did not diminish S7 binding, even at concentrations that do augment several aspects of CD4+ T-cell function. These data suggest that removal of the S7 determinant of CD43 is not required for enzyme-mediated enhancement of CD4+ T cell function.
Figure 6.
Analysis of S7 and lectin binding in CD4+ T cells from young and old donors. T cells from young (dark bars) and old (light bars) mice were treated with the indicated enzymes and then incubated with αCD4 and with either FITC-S7 (top panel), SNA-I (middle panel), or MAA (bottom panel). Gated CD4+ cells were then analysed by flow cytometry. Bars represent the mean fluorescence intensity ± SEM of 10 young and 10 old mice from 10 independent experiments. Asterisks (*) indicate significant difference with untreated mice of the same age group (young or old). Letters (a) and (b) indicate significant effects of age.
Sambucus nigra type-1 (SNA-I) lectin specifically recognizes α(2,6) linkages of sialic acid to galactose. The middle panel of Fig. 6 shows, in good agreement with our previous results19 a significant age-related decline in SNA-I binding to the surface of CD4+ T cells (P < 0·001). This decline is independent of the naïve to memory shift that takes place with ageing (data not shown19). As expected, the positive control (CP-Siase) drastically lowered SNA-I binding in CD4+ cells from young and old animals. Treatment with ST-Siase(2,3) OSGE or PNGase F, in contrast, did not change SNA-I binding to CD4+ cells from young and old mice. The results suggest that the enhancement of T cell function by these three enzymes are not due to changes in the α(2,6)-Siase content of surface glycoproteins.
The Maackia amurensis lectin (MAA) recognizes 2,3-linked sialic acid residues.19 As is shown in Fig. 6 (bottom panel), MAA binding to CD4+ cells increases slightly with age; the change is about 25%, but is not statistically significant (P = 0·08) in the current study. Separate analyses found that the age-dependent increase in MAA binding affected both naïve and memory subsets (data not shown). Figure 6 shows that CP-Siase significantly reduced MAA binding in young and in old CD4+ T cells. In contrast, OSGE, ST-Siase(2,3) and PNGase F diminished MAA binding sites in CD4+ cells of old mice only; these three enzymes did not alter MAA binding in cells derived from young mice. This age-specific difference in the biochemical effects of ST-Siase(2,3) and PNGase F suggests that the functional effects of these enzymes may depend on removal of specific 2,3-linked terminal sialic acid residues rather than on a global change in cell surface sialic acid content, and suggest that the specific residues whose removal augments function may be more prevalent on old than on young CD4+ cells.
Discussion
Our previous work has shown that OSGE-mediated proteolytic cleavage of the external domains of O-linked sialoglycoproteins restores the ability of CD4+ T cells from aged donors to form immunological synapses. OSGE also enhances the ability of CD4+ T cells from young and old mice to mobilize calcium, express early activation markers and secrete various cytokines after stimulation.13,18,19 We also found similar enhancements in T-cell function using a sialidase from Clostridium perfringens (CP-Siase) that removes terminal sialic acid residues, but did not see functional changes in T cells treated with other modulators of sialic acid residues including VC-Siase, or the glycolipid-specific enzymes endoglycoceramidase I or Macrobdella decora ceramide-glycase.19 These results suggested a model in which T cells from both young and old animals contain specific polysaccharide moieties on the cell surface that can inhibit T-cell signalling and function, and in which ageing leads to further changes in the amount or relative levels of these inhibitory glycoproteins. In this model, treatment with OSGE and CP-Siase is postulated to remove some of these polysaccharide determinants, leaving the T cells from young and old donors with similar patterns of glycosylation on their surface and similar levels of function.
Several lines of circumstantial evidence suggested that OSGE-mediated cleavage of CD43 was a major component of the OSGE effect. CD43, also called sialophorin, is a large glycoprotein and is expressed at high levels on the T-cell surface.21 Most published data suggest that CD43 is a negative regulator of T-cell activation, but its function is still somewhat controversial. It has been shown that the presence of CD43 interferes with T-cell adhesion,16,17,22 perhaps through interfering with leucocyte function-associated antigen-1 binding to intracellular adhesion molecule-1, and treatment of cells with a neuraminidase decreases this anti-adhesion effect.15 CD43 has also been shown to negatively regulate rolling in T cells23 and T-cell homing.22 In some studies, T cells from CD43 KO mice have shown a marked increase in their in vitro ability to proliferate in response to several forms of stimulation, both TCR-mediated and receptor-independent,16,24In vivo analyses using CD43 KO mice illustrated that these mice were hyper-responsive to keyhole-limpet haemocyanin (KLH) immunization and a delayed-type hypersensitivity (DTH) response to trimellitic anhydride (TMA), showed increased antibody titres following immunization with sheep red blood cells and displayed an augmented antiviral cytotoxic T cell response.16,24 These negative regulatory effects exerted by CD43 are suspected to be the result of steric hindrance or electrostatic repulsion mediated by the large extracellular domain of CD43,25 but there is also evidence showing that the intracellular portion of CD43 can negatively regulate T-cell function.26
In contrast to these reports, other work has shown that CD43 plays a positive role in T-cell activation by facilitating the emigration of leucocytes into tissues23 and by acting as a costimulator of T-cell activation,27,28 particularly in memory cells.29 Another study reported that T cells from CD43 KO animals displayed no substantial or consistent hyper-responsive pattern to stimulation.30 Our own data finds no evidence that T cells from CD43 KO animals are hyper-responsive when compared to WT controls, although we note that our protocols use antibodies rather than antigen-bearing APCs as the means of stimulation.
Our own work13 had shown an age-dependent increase in the S7 and 1B11 determinants of CD43, a decline with age in the relocation of CD43 away from the synapse after T-cell activation, and that cells with high expression of S7 and 1B11 were in an anergic subset marked by the expression of P-glycoprotein. Taken together, these data suggested that functional defects of T cells from old donors might reflect the inhibitory effects of over-glycosylated CD43 left within the synapse as a result of cytoskeletal defects.
Our new data show that OSGE augments the function of both CD4+ and CD8+ T cells of young CD43 KO mice. We did note that the effect of OSGE on CD69 expression was greater in WT than in CD43 KO cells, suggesting that CD43 may have effects on AP-1 mediated events, such as expression of CD69,31 with less influence over other events such as calcium influx and cytokine production. Our work shows that CD43 does not play a major role in inhibition of T cell function in young mice, although it is possible that CD43 might contribute to inhibition of function in T cells from aged animals.
What OSGE-sensitive surface glycoprotein(s), then, inhibit the function of T cells from young mice? CD45 is one obvious possibility. CD45 is, like CD43, a large, abundant, and heavily O-glycosylated molecule shown to exert both positive32 and negative33,34 effects in T-cell activation. The smallest isoform of CD45, CD45RO, has a relatively strong association with CD4-p56lck and the TCR as compared to larger isoforms, and promotes enhanced phosphorylation of cellular activation proteins including Vav, as well as IL-2 production.35,36 Unpublished data from our laboratory have shown that OSGE is able to cleave the larger isoforms of the CD45 molecule, while leaving the CD45RO isoform intact, suggesting that OSGE may function through this mechanism.
In addition to its glycosylation, CD45 is known to bear large amounts of sialic acid. Previous work from our lab has shown a large age-related increase in the amount of Siaα(2,3)Gal/GalNAc on CD45 with age.19 Sialic acid residues on the surface of CD45 may inhibit its interaction with CD4 and thus diminish T-cell activation. Removal of these inhibitory sialic acid residues from CD45 using CP-Siase, might promote CD45–CD4 interaction, leading to enhanced signalling and function.
Sialic acid residues of T-cell surface glycoproteins have been shown to regulate the assembly of the TCR complex37 and T cell apoptosis38 as well as the initial interactions between T cells and APCs.39,40 Two varieties of terminal sialic acid residues are found on T-cell glycoproteins, one containing an α2,3 linkage to an underlying galactose or N-acetyl-galactosamine residue (Siaα(2,3)Gal/GalNAc), and the other involving an α2,6 glycosidic linkage (Siaα(2,6)Gal/GalNAc). Little is known about the distribution of these two forms of sialic acid residues among T-cell surface glycoproteins, the effects of age on these moieties, or the roles each may play in modulating early events in T-cell activation. Our new data, using ST-Siase(2,3) suggest that Siaα(2,3)Gal/GalNAc residues, on proteins still to be determined, inhibit expression of CD69 and CD25, as well as production of IL-2 and IFN-γ by CD4+ T cells from old animals. Interestingly, ST-Siase(2,3) is significantly less effective in augmenting CD69 expression and cytokine production in T cells from young mice (Figs 4 and 5). These results suggest that age may increase the proportion of functionally relevant Siaα(2,3) links susceptible to cleavage, and indeed the MAA results of Fig. 6 show that ST-Siase(2,3) is able to diminish MAA binding sites in CD4+ T cells of old but not of young mice. We do not know in what surface molecules those Siaα(2,3) links are located, but the enhancement of the biological activity of CD4+ T cells by treatment with PNGase F and not Endo H suggests that some of these may be attached to specific N-linked glycans. Consistent with this idea, we note that removal of MAA-binding sites by PNGase F is significantly higher on old than on young CD4+ cells (Fig. 6, bottom).
There is evidence from other laboratories that removing N-linked sugars can lower the threshold of T cell activation. T cells from Mgat5 KO mice are deficient in N-linked sugar branching and show a lowered threshold for TCR-dependent tyrosine phosphorylation, calcium mobilization, and T-cell proliferation in response to extrinsic stimuli.41 It has also been shown that CD28 costimulation is negatively regulated by N-linked carbohydrates.42 Thus although it is possible that the effects of PNGase F on T-cell function reflect removal of terminal sialic acid residues on specific N-linked glycans, it is also plausible that the treatment may mimic the broad changes seen in T cells of Mgat5 KO mice, or act through relieving N-linked sugar restraints on costimulatory molecules.
Our data, showing OSGE-mediated functional effects on CD43 KO mice and, conversely, functional effects of enzymes that do not alter expression of the S7 determinant of CD43 (Fig. 6), refute our original hypothesis attributing OSGE effects largely to cleavage of CD43. The SNA-I binding data also argue against the idea that Siaα(2,6)Gal/GalNAc determinants play a crucial role in dampening TCR signalling in CD4+ T cells from young and old mice. Our results do not eliminate the possibility that these determinants may participate in other T-cell functions; such as apoptosis38 or T cell–APC interactions.39
Additional experiments are under way to further elucidate which specific glycosylated molecules are involved in dampening T-cell signalling and function in young and old mice. Overall, our data further support the notion that glycosylation plays an essential role in modulating CD4+ T-cell signalling and function, and that an age-related dysregulation in the expression of negative regulatory glycosyl moieties, possibly including Siaα(2,3)Gal/GalNAc residues, contributes to immunosenescence. Understanding these phenomena may help us to explore new methods in restoring immune protection in the elderly.
Acknowledgments
The authors would like to thank Dr Anne Sperling for her kind gift of the CD43 KO and WT mice, and the University of Michigan Flow Cytometry Core Facility and Lynn Winkleman for their technical expertise. This work was supported by NIH grants AG19619, and AG00114.
Abbreviations
- CP-Siase
Clostridium perfringens-derived sialidase
- Endo H
Endogycosidase H
- OSGE
O-sialoglycoprotein endopeptidase
- PNGase F
Peptide-N-Glycosidase F
- ST-Siase(2,3)
Salmonella typhimurium-derived sialidase
- VC-Siase
Vibrio cholerae-derived sialidase
References
- 1.Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J Leukoc Biol. 1988;64:703–12. doi: 10.1002/jlb.64.6.703. [DOI] [PubMed] [Google Scholar]
- 2.Plackett TP, Boehmer ED. Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–9. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 3.Miller RA, Berger SB, Burke DT, Galecki A, Garcia GG, Harper JM, Sadighi Akha AA. T cells in aging mice: genetic, developmental, and biochemical analyses. Immunol Rev. 2005;205:94–103. doi: 10.1111/j.0105-2896.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 4.Riley RL, Blomberg BB, Frasca D. B cells, E2A, and aging. Immunol Rev. 2005;205:30–47. doi: 10.1111/j.0105-2896.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 5.Szakal AK, Aydar Y, Balogh P, Tew JG. Molecular interactions of FDCs with B cells in aging. Semin Immunol. 2002;14:267–74. doi: 10.1016/s1044-5323(02)00059-3. [DOI] [PubMed] [Google Scholar]
- 6.Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243–84. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- 7.Nikolich-Zugich J. T cell aging: naive but not young. J Exp Med. 2005;201:837–40. doi: 10.1084/jem.20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspinall R. Age-related changes in the function of T cells. Microsc Res Techn. 2003;62:508–13. doi: 10.1002/jemt.10412. [DOI] [PubMed] [Google Scholar]
- 9.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 10.Stoltzner G, Makinodan T. Age dependent decline in proliferation of lymphocytes. Adv Exp Med Biol. 1975;61:21–37. doi: 10.1007/978-1-4615-9032-3_2. [DOI] [PubMed] [Google Scholar]
- 11.Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–8. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021–7. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 13.Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33:3464–72. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- 14.Cullinan P, Sperling AI, Burkhardt JK. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol Rev. 2002;189:111–22. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- 15.Ardman B, Sikorski MA. Staunton DE. CD43 interferes with T-lymphocyte adhesion. Proc Natl Acad Sci USA. 1992;89:5001–5. doi: 10.1073/pnas.89.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manjunath N, Correa M, Ardman M, Ardman B. Negative regulation of T-cell adhesion and activation by CD43. Nature. 1995;377:535–8. doi: 10.1038/377535a0. [DOI] [PubMed] [Google Scholar]
- 17.Manjunath N, Johnson RS, Staunton DE, Ardman B. Targeted disruption of CD43 gene enhances T lymphocyte adhesion. J Immunol. 1993;151:1528–34. [PubMed] [Google Scholar]
- 18.Berger SB, Sadighi Akha AA, Miller RA. A Glycoprotein endopeptidase enhances calcium influx and cytokine production by CD4+ T cells of old and young mice. Int Immunol. 2005;17:983–91. doi: 10.1093/intimm/dxh279. [DOI] [PubMed] [Google Scholar]
- 19.Garcia GG, Berger SB, Sadighi Akha AA, Miller RA. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 2005;35:622–31. doi: 10.1002/eji.200425538. [DOI] [PubMed] [Google Scholar]
- 20.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–51. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 21.Sperling AI, Sedy JR, Manjunath N, Kupfer A, Ardman B, Burkhardt JK. Cutting edge. TCR signaling induces selective exclusion of CD43 from the T cell-antigen-presenting cell contact site. J Immunol. 1988;161:6459–62. [PubMed] [Google Scholar]
- 22.Stockton BM, Manjunath N, Ardman B, von Andrian UH. Negative regulation of T cell homing by CD43. Immunity. 1988;8:373–81. doi: 10.1016/s1074-7613(00)80542-7. [DOI] [PubMed] [Google Scholar]
- 23.Woodman RC, Johnston B, Hickey MJ, Teoh D, Reinhardt P, Poon BY, Kubes P. The Functional paradox of CD43 in leukocyte recruitment: a study using CD43-deficient mice. J Exp Med. 1998;188:2181–6. doi: 10.1084/jem.188.11.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurman EC, Walker J, Jayaraman S, Manjunath N, Ardman B, Green JM. Regulation of in vitro and in vivo T cell activation by CD43. Int Immunol. 1998;10:691–701. doi: 10.1093/intimm/10.5.691. [DOI] [PubMed] [Google Scholar]
- 25.Ostberg JR, Barth RK, Frelinger JG. The Roman god Janus. A paradigm for the function of CD43. Immunol Today. 1998;19:546–50. doi: 10.1016/s0167-5699(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 26.Tong J, Allenspach EJ, Takahashi SM, Mody PD, Park C, Burkhardt JK, Sperling AI. CD43 regulation of T cell activation is not through steric inhibition of T cell–APC interactions but through an intracellular mechanism. J Exp Med. 2004;199:1277–83. doi: 10.1084/jem.20021602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperling AI, Green JM, Mosley RL, Smith PL, DiPaolo RJ, Klein JR, Bluestone JA, Thompson CB. CD43 is a murine T cell costimulatory receptor that functions independently of CD28. J Exp Med. 1995;182:139–46. doi: 10.1084/jem.182.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onami TM, Harrington LE, Williams MA, et al. Dynamic regulation of T cell immunity by CD43. J Immunol. 2002;168:6022–31. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 29.Kyoizumi S, Ohara T, Kusunoki Y, Hayashi T, Koyama K, Tsuyama N. Expression characteristics and stimulatory functions of CD43 in human CD4+ memory T cells: analysis using a monoclonal antibody to CD43 that has a novel lineage specificity. J Immunol. 2004;172:7246–53. doi: 10.4049/jimmunol.172.12.7246. [DOI] [PubMed] [Google Scholar]
- 30.Carlow DA, Corbel SY, Ziltener HJ. Absence of CD43 fails to alter T cell development and responsiveness. J Immunol. 2001;166:256–61. doi: 10.4049/jimmunol.166.1.256. [DOI] [PubMed] [Google Scholar]
- 31.Iwashima M. Kinetic perspectives of T cell antigen receptor signaling. A two-tier model for T cell full activation. Immunol Rev. 2003;191:196–210. doi: 10.1034/j.1600-065x.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 32.Trowbridge IS, Thomas ML. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 33.D'Oro U, Ashwell JD. Cutting edge. The CD45 tyrosine phosphatase is an inhibitor of lck activity in thymocytes. J Immunol. 1999;162:1879–83. [PubMed] [Google Scholar]
- 34.Irie-Sasaki J, Sasaki T, Matsumoto W, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–54. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 35.Dornan S, Sebestyen Z, Gamble J, et al. Differential Association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem. 2002;277:1912–8. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 36.McKenney DW, Onodera H, Gorman L, Mimura T, Rothstein DM. Distinct isoforms of the CD45 protein-tyrosine phosphatase differentially regulate interleukin 2 secretion and activation signal pathways involving Vav in T cells. J Biol Chem. 1995;270:24949–54. doi: 10.1074/jbc.270.42.24949. [DOI] [PubMed] [Google Scholar]
- 37.Moody AM, North SJ, Reinhold B, et al. Sialic acid capping of CD8beta core 1-O-glycans controls thymocyte-major histocompatibility complex class I interaction. J Biol Chem. 2003;278:7240–6. doi: 10.1074/jbc.M210468200. [DOI] [PubMed] [Google Scholar]
- 38.Priatel JJ, Chui D, Hiraoka N, et al. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 2000;12:273–83. doi: 10.1016/s1074-7613(00)80180-6. [DOI] [PubMed] [Google Scholar]
- 39.Pappu BP, Shrikant PA. Alteration of cell surface sialylation regulates antigen-induced naive CD8+ T cell responses. J Immunol. 2004;173:275–84. doi: 10.4049/jimmunol.173.1.275. [DOI] [PubMed] [Google Scholar]
- 40.Moody AM, Chui D, Reche PA, Priatel JJ, Marth JD, Reinherz EL. Developmentally regulated glycosylation of the CD8αβ coreceptor stalk modulates ligand binding. Cell. 2001;107:501–12. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- 41.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–9. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 42.Ma BY, Mikolajczak SA, Yoshida T, Yoshida R, Kelvin DJ, Ochi A. CD28 T cell costimulatory receptor function is negatively regulated by N-linked carbohydrates. Biochem Biophys Res Commun. 2004;317:60–7. doi: 10.1016/j.bbrc.2004.03.012. [DOI] [PubMed] [Google Scholar]