Abstract
Previous work from our laboratory has shown that modifying cell surface glycosylation with either a Clostridium perfringens-derived sialidase (CP-Siase), or an O-linked glycoprotein endopeptidase (OSGE) can enhance the function of CD4 T cells from both young and old mice at multiple levels. Here we have re-assessed the effect of age on CD8 T-cell function, and examined the outcome of enzymatic treatment with CP-Siase and OSGE on its different aspects. Pre-treatment of CD8 T cells with either CP-Siase or OSGE led to a significant increase in anti-CD3-mediated Ca2+ response in both young and old mice. Pre-treated CD8 T cells from both age groups also displayed a significant increase in activation-induced CD69 and CD25 expression, and produced significantly higher amounts of interleukin-2 and interferon-γ in comparison to their untreated counterparts. Furthermore, pretreatment with either enzyme enhanced granzyme B expression in CD8 T cells, and increased their cytolytic activity in vitro. These data support the notion that glycosylated surface proteins hinder CD8 T-cell activation and function in both young and old mice, and raise the possibility of significantly improving CD8 T cell function in older individuals through enzymatic alteration of surface glycoproteins.
Keywords: rodent, ageing, TCR, signal transduction, glycosylation
Introduction
The immune system undergoes substantial change with age in both humans and animals. These changes are complex in nature and can include alterations in both the strength and quality of the immune response.1 Studies of the adaptive response have suggested age-dependent alterations in the generative, selective, homeostatic, and signal-transducing elements of the system.2–5 The most consistent and dramatic of these effects are on the T-cell compartment. CD4 T-cell responses of aged donors are typically slower and of lower amplitude than those of their young counterparts. A decline in the induction of the Ca2+ response,6 activation of protein kinase pathway,7 production of cytokines and induced cytokine receptors,8 and activation of the genes necessary for cell cycle entry9 are all well documented. Recent findings from our laboratory suggest that many of the shortcomings in CD4 T-cell activation might be caused by defects in cytoskeletal reorganization that precede the discrimination of agonist from antagonist peptides by the T-cell receptor (TCR)10 in conjunction with alterations in T-cell surface glycosylation patterns.11
T-cell development and differentiation are accompanied by changes in cell surface N- and O-linked glycans, as well as by alterations in glycoprotein sialylation.12 These changes might regulate the T-cell response through a direct effect on the intrinsic properties of specific proteins, or by modulating the binding of a disparate set of cell surface proteins to a specific carbohydrate moiety. Specific proteases and glycosidases serve as an important tool in studying the structural and functional characteristics of cell surface glycoproteins. In recent work, our laboratory has made use of two enzymes in particular: a sialidase from Clostridium perfringens (CP-Siase)13 and the O-sialoglycoprotein endopeptidase (OSGE) from Pasteurella haemolytica.14 CP-Siase cleaves terminal sialic acids linked via an α(2,3), α(2,6) or α(2,8) binding to N- or O-glycosidic-bound oligosaccharide chains of glycoproteins.15 On the T-cell surface, terminal sialic acid residues predominantly bind to galactose through α(2,3) and less frequently through α(2,6) links. A number of laboratories have documented the importance of the α(2,3) bond in CD8 T-cell homeostasis and in CD8–major histocompatibility complex (MHC) class I interactions16,17 and that of α(2,6) in CD45 clustering, phosphatase modulation and T-cell death.18 OSGE is a neutral metalloprotease that cleaves the protein backbone of O-glycosylated proteins on serine and threonine residues.14 CD34, CD43, CD44 and CD45 are proven targets of this enzyme.
We have recently shown that treatment of CD4 T cells with either CP-Siase or OSGE can enhance multiple aspects of CD4 T-cell function, leading to similar levels of function in young and old mice.11,19,20 More specifically, treatment of CD4 T cells from aged mice with OSGE can restore early agonist-independent and subsequent agonist-dependent stages of synapse formation. Furthermore, pretreatment with either CP-Siase or OSGE enhances the CD3-mediated Ca2+ response in CD4 T cells and their subsets; increases the induced expression of the early activation markers CD69 and CD25; and heightens the CD3/CD28-mediated production of interleukin-2 (IL-2) and interferon-γ (IFN-γ) in both age groups.
In an effort to extend our previous findings, in the present study we have examined the effects of CP-Siase and OSGE on CD8 T-cell function in young and old mice.
Materials and methods
Mice
Specific pathogen-free male C57BL/6 J and (BALB/cJ ×C57BL/6 J) F1 (CB6F1) mice were purchased from the National Institute of Aging contract colonies. The mice were considered young at 4–8 months of age, and old at 18–24 months of age. They were kept under specific pathogen-free (SPF) conditions at the University of Michigan Medical School Animal facilities, and given free access to food and water. Sentinel animals were examined quarterly for serological evidence of viral infection. All such tests were negative during the course of these studies. Mice with splenomegaly or macroscopically visible tumours at the time of sacrifice were not used in these experiments.
Antibodies, cell line and reagents
Antibodies against CD3 (clone 145–2C11), CD4 (clone GK1.5), CD8α (clone 53-6.7), CD19 (clone 1D3), CD25 (clone 7D4), CD28 (clone 37.51), CD44 (clone IM7), CD69 (clone H1.2F3), Fc RIII/II (clone 2.4G2), immunoglobulin M (IgM; clone R6-60.2), as well as Cytofix/Cytoperm, and Perm/Wash buffers were obtained from BD Biosciences (San Diego, CA). Anti-granzyme B (clone 16G6) was from eBioscience (San Diego, CA). P815, a DBA/2-derived (H-2d) mast cell line, was bought from the American Type Culture Collection (Manassas, VA). Indo-1 AM was from Molecular Probes (Eugene, OR). IL-2 and IFN-γ enzyme-linked immunosorbent assay (ELISA) kits, and recombinant mouse IL-2 were purchased from BioSource International (Camarillo, CA). CP-Siase and OSGE were from Roche (Indianapolis, IN), and CedarLane (Hornby, Ontario, Canada), respectively. 51Cr (specific activity >200 Ci/g Cr) was acquired from MP Biomedicals (Irvine, CA).
Enzyme treatment
Cells (5 × 106/ml in Hanks' balanced salt solution (HBSS)) were treated with either CP-Siase (100 mU/ml) or OSGE (50 µg/ml) for 60 min at 37°, and then washed with HBSS to remove the enzyme prior to the use of cells in any experiment. Control cells were similarly incubated in HBSS but without added enzyme.
Measurement of cytosolic Ca2+
Cytosolic Ca2+ measurement was performed as previously described.20 Cells were loaded with Indo-1 AM (2·5 µm) at room temperature for 30 min, washed and then stained with anti-CD8 and anti-CD44 antibodies. The Ca2+ mobilization assay was conducted at 37° on a FACSVantage flow-cytometer using FACSDiva software (BD Biosciences, San Jose, CA). Baseline levels were collected for 20 s, after which the cells were stimulated with 5 µg/ml of non-cross-linked anti-CD3. Each assay was performed for a 5 min period. The acquired data were analysed using FlowJo's kinetic platform (TreeStar, Ashland, OR). Each histogram displays the mean of the violet : blue ratio as a function of time with smoothing for moving average.
Measurement of cytokine levels
Splenic T cells were purified as previously described.21 Purified T cells were labelled with anti-CD4 antibody at 4° for 30 min, washed and then incubated with anti-rat IgG-coated magnetic beads to obtain CD8 T cells through negative selection. The CD8 T cells (either untreated or pretreated with CP-Siase or OSGE) were stimulated with 1 µg/ml each of non-cross-linked anti-CD3 and anti-CD28 for 6 hr in complete RPMI-1640 at 37° in a 5% CO2 atmosphere. The supernatants were collected at the end of the stimulation period, and stored at −70° until further analysis. For each sample, IL-2 and IFN-γ levels were quantified using ELISA kits according to the manufacturer's instructions. The detection limits of the kits were ∼8 pg/ml for IL-2, and ∼1 pg/ml for IFN-γ, respectively.
Induction and measurement of cytotoxic T lymphocyte (CTL) activity
Induction and measurement of cytotoxic activity were carried out as previously described22 with certain modifications. Splenocytes from young CB6F1 mice (H-2b,d) were used as stimulators after exposure to 2000 rads of γ-irradiation, whereas splenocytes from young and old C57BL/6 mice (H-2b) were used as responders. The stimulator and responder cells were coincubated for 7 days. The harvested cells were labelled with anti-CD4, anti-CD19 and anti-IgM antibodies at 4° for 30 min, washed and then incubated with anti-rat IgG-coated magnetic beads to obtain CD8 T cells through negative selection. The CD8 T cells were either left untreated as controls, or exposed to CP-Siase or OSGE. Afterwards, untreated and enzyme-treated CD8 T cells from individual young and old responders were incubated in triplicate wells with 5 × 103 51Cr-labelled P815 (H-2d) target cells for 4 hr at effector to target ratios of 30 : 1, 10 : 1, 3 : 1, and 1 : 1. To determine spontaneous and maximal 51Cr release from the targets, the P815 cells were incubated with complete RPMI-1640 alone or with complete medium containing 1% sodium dodecyl sulphate, respectively. At the end of the incubation period, the supernatants were harvested and counted on a γ counter. The corrected percent lysis for each concentration of effector cells was calculated, using the mean c.p.m. for each replicate of wells: corrected percentage lysis = 100 × (sample 51Cr release −spontaneous 51Cr release)/(maximal 51Cr release − spontaneous 51Cr release).
Detection of granzyme B
Splenic T cells (either untreated or pretreated with CP-Siase or OSGE) were either stimulated with cross-linked anti-CD3 (5 µg/ml) and anti-CD28 (2 µg/ml) for 6 hr, or cultured with 100 IU of recombinant IL-2 for 72 hr, in complete RPMI-1640 at 37° in a 5% CO2 atmosphere. Intracellular staining was conducted as previously described.23 Briefly, at the end of each period, the collected T cells were washed, preblocked with anti-Fc RIII/II for 5 min, and then labelled with anti-CD44 and anti-CD8 antibodies. Afterwards, the cells were fixed overnight in Cytofix/Cytoperm buffer, washed with Perm/Wash buffer, and then labelled with anti-granzyme B antibody.
Statistical analyses
In the case of Ca2+ assays, the peak increase above baseline was calculated for CD8 T cells and their subsets in every sample, with the data then analysed using Friedman anova. Post-hoc analysis was done using Wilcoxon matched pairs tests. In all other cases, the data were analysed using repeated measures anova. Post-hoc analyses were done using paired t-tests.
Results
Pre-treatment of CD8 T cells with either CP-Siase or OSGE leads to enhanced anti-CD3-mediated Ca2+ response
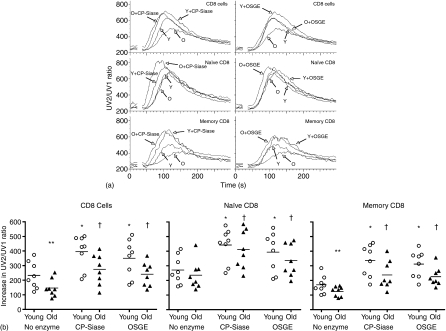
We have previously shown that both CP-Siase19 and OSGE20 can enhance the anti-CD3-induced Ca2+ response in CD4 T cells of both young and old mice. In this study, we examined the effect of the enzymes on CD8 T cells and their subsets. Figure 1(a) shows the outcome of one of eight experiments conducted, where anti-CD3 is used to induce a transient increase in cytosolic Ca2+. In the absence of enzyme treatment, the CD8 T-cell response was significantly higher in the young than in the old (P = 0·02). Naive CD8 T cells (CD8 CD44lo) in each age group had a significantly higher response to anti-CD3 stimulation than their memory (CD8 CD44hi) counterparts (P = 0·007 for both young and old). Ageing had a significant effect on the Ca2+ response of the memory CD8 T cells (P = 0·02), but not on the naive CD8 T-cell subset (P = 0·38). Pre-treatment with either CP-Siase or OSGE significantly enhanced the anti-CD3-induced Ca2+ response in CD8 T cells from both young and old mice. This was true for CD8 T cells as a whole, as well as for their naive and memory subsets (P = 0·007 for each comparison; Fig. 1b).
Figure 1.
Effects of CP-Siase and OSGE on the Ca2+ response. (a) CD8 T-cell Ca2+ responses were induced by non-cross-linked anti-CD3 in young (Y) and old (O) mice, either with or without pretreatment by CP-Siase (left panels) or OSGE (right panels). Top panels: CD8 T cells. Middle panels: Naive CD8 T cells, i.e. gated for low expression of CD44. Bottom panels: Memory CD8 T cells, i.e. gated for high expression of CD44. The break in each line shows where stimulatory antibody was added after 20 s of baseline data collection. The panels illustrate one experiment of the eight used in the statistical analysis. (b) The increase in response above baseline for all the experiments conducted. Left panel: CD8 T cells, middle panel: naive CD8 T cells, right panel: memory CD8 T cells. The symbols * and † indicate statistically significant difference with untreated young or old cells, respectively. **Denotes statistically significant difference between young and old.
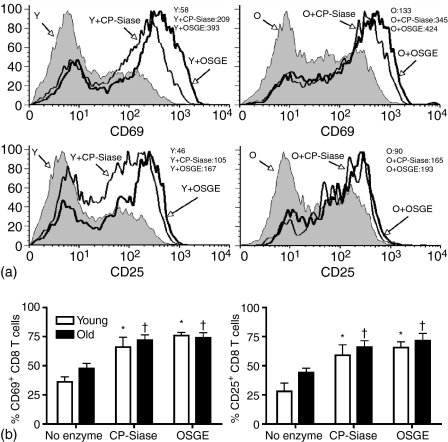
Pre-treatment of CD8 T cells with either CP-Siase or OSGE increases the anti-CD3/CD28-induced expression of CD69 and CD25
T cells from young and old mice were either left untreated or were treated with CP-Siase or OSGE, and then stimulated with non-cross-linked anti-CD3 (0·5 µg/ml) and anti-CD28 (0·5 µg/ml) antibodies. CD69 and CD25 expression levels on CD8 T cells were examined after 6 and 24 hr of stimulation, respectively (Fig. 2). In the absence of enzyme treatment, CD8 T cells from young and old mice showed no significant difference in the induced expression of CD69 (P = 0·14) or CD25 (P =0·14). Pre-treatment of the CD8 T cells with CP-Siase led to an increase in the induced expression of CD69 and CD25 in both young (CD69: P = 0·004; CD25: P = 0·03) and old (CD69: P = 0·0001; CD25: P = 0·002) mice. Pre-treatment with OSGE also augmented the induced expression of the two markers in both young (CD69: P = 0·0001; CD25: P = 0·0006) and old (CD69: P = 0·0001; CD25: P = 0·008) age groups. Neither CP-Siase nor OSGE induced the expression of CD69 or CD25 in the absence of anti-CD3/CD28 stimulation (data not shown).
Figure 2.
CP-Siase and OSGE enhance the expression of early activation markers on CD8 T cells of young and old mice. T cells from young and old mice were either left untreated or treated with CP-Siase or OSGE, and then stimulated with non-cross-linked anti-CD3 and anti-CD28. Gated CD8 T cells were examined for CD69 expression after 6 hr, and for CD25 expression after 24 hr of stimulation. (a) Histograms of CD69 (upper panels) and CD25 (lower panels) expression in one young (Y) and one old (O) mouse. Mean fluorescence intensity (MFI) of the traces are shown at the top right corner of each panel. (b) Bar graphs of the percentage of CD8 T cells expressing CD69 (left panel) and CD25 (right panel). The bars represent the mean ± SEM of six young and six old mice for CD69, and five young and five old mice for CD25. The symbols * and † indicate statistically significant difference with untreated young or old, respectively. Unstimulated CD8 T cells (whether untreated or enzyme-treated) expressed <2% CD69 or CD25 in either age group.
Pre-treatment of CD8 T cells with either CP-Siase or OSGE causes higher production of IL-2 and IFN-γ after anti-CD3/CD28 stimulation
To study the effect of enzyme treatment on cytokine secretion by CD8 T cells, CD8 T cells (either untreated or pretreated with CP-Siase or OSGE) were stimulated with non-cross-linked anti-CD3 and anti-CD28 for 6 hr. The concentrations of IL-2 and IFN-γ in each supernatant were then determined by ELISA (Fig. 3). Untreated CD8 T cells from young and old mice secreted similar amounts of IL-2 (P = 0·4) after anti-CD3/CD28 activation. In contrast, CD8 T cells from old mice produced significantly higher amounts of IFN-γ than their young counterparts (P = 0·05). Pre-treatment of the cells with CP-Siase caused an increased secretion of both cytokines in young (IL-2: P = 0·007; IFN-γ: P = 0·01) and old (IL-2: P = 0·01; IFN-γ: P = 0·006) mice. Pre-treatment with OSGE also augmented the production of both cytokines in the young (IL-2: P = 0·03; IFN-γ: P = 0·01) and old (IL-2: P = 0·001; IFN-γ: P = 0·009) age groups.
Figure 3.
CP-Siase and OSGE increase the secretion of IL-2 and IFN-γ by CD8 T cells of young and old mice. CD8 T cells from young (light bars) and old (dark bars) mice were either left untreated or treated with CP-Siase or OSGE, and then stimulated with non-cross-linked anti-CD3 and anti-CD28 for 6 hr. The collected supernatants were analysed for IL-2 (left panel), and IFN-γ (right panel) content by ELISA. The bars represent the mean ± SEM of five young and five old mice for IL-2, and six young and six old mice for IFN-γ. The symbols * and † indicate statistically significant difference with untreated young or old, respectively. **Denotes statistically significant difference between young and old. IL-2 and IFN-γ were undetectable in supernatants from unstimulated CD8 T cells (whether untreated or enzyme-treated) of either age group.
Both CP-Siase and OSGE pretreatment enhance CD8 T-cell mediated cytotoxicity
The influence of ageing on the generation of cytotoxic T cells in vitro is a matter of contention. Assays performed with bulk splenic cultures have led to reports of either an increase or a decrease in cytotoxic activity with age.24,25 Fractionation studies have shown that any decline with age is at least partly due to the lack of adequate T-cell help.26,27 Furthermore, the question of whether CTL, when generated, show an age-dependent decline in lytic function remains unsettled.28,29 To address the issue, splenocytes from young and old C57BL/6 mice were cultured with irradiated splenocytes from young CB6F1 mice to induce the differentiation of H-2d-specific cytotoxic effectors. CD8 T cells were then purified by negative selection. The purified cells were either left untreated or were treated with CP-Siase or OSGE, and then incubated with 51Cr-labelled P815 cells for 4 hr over a range of effector: target ratios. Figure 4 (upper panels) shows the outcome of this effector cell titration in one of the six experiments conducted. CD8 T cells from both young and old mice display the highest percent lysis at the 30 : 1 effector : target ratio, with a linear decline in lytic activity at lower ratios. Figure 4 (lower panel) displays the percent lysis obtained at the 30 : 1 ratio in each experiment. Statistical analysis of this data set showed that, in the absence of enzymatic treatment, CD8 T cells from young donors were less effective than those from old donors at lysing target cells (P = 0·01). Furthermore, both CP-Siase and OSGE increased the percentage of target cell lysis by CD8 T cells derived from young (CP-Siase: P = 0·002; OSGE: P = 0·01) and old (CP-Siase: P = 0·0005; OSGE: P = 0·002) mice.
Figure 4.
CP-Siase and OSGE pretreatment enhance CD8 T cell-mediated cytotoxicity. Young and old CD8 T cells purified after incubation with irradiated stimulator cells were either left untreated or were treated with CP-Siase or OSGE. They were then incubated with 5000 51Cr-labelled P815 target cells in triplicate at effector to target ratios of 30 : 1, 10 : 1, 3 : 1, and 1 : 1 for 4 hr. The upper panels show the corrected percent lysis of target cells by CD8 T cells from one young (upper left) and one old (upper right) mouse at all the examined effector : target ratios. The lower panel shows the corrected percent lysis of target cells at the 30 : 1 ratio for all the experiments conducted. The symbols * and † indicate statistically significant difference with untreated young or old, respectively. **Denotes statistically significant difference between young and old.
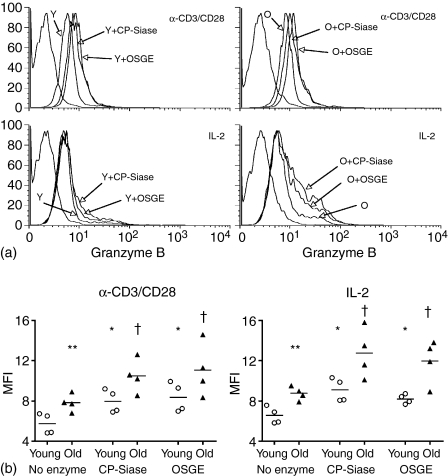
CP-Siase and OSGE enhance anti-CD3/CD28- and IL-2-induced granzyme B expression in CD8 T cells
Granzyme B is a caspase-like serine protease that is released by cytotoxic lymphocytes during their interaction with target cells, and might contribute to targeted cell death through inducing DNA fragmentation or BH3 interacting domain death agonist (BID)-mediated release of cytochrome C from mitochondria.30 To examine the effects of CP-Siase and OSGE on granzyme B expression, splenic T cells (either untreated or pretreated with CP-Siase or OSGE) were stimulated with cross-linked anti-CD3 and anti-CD28 for 6 hr. The cells were then stained for surface CD8, and for intracellular granzyme B. Up-regulation of CD44 during the 6 hr activation period precluded the use of this marker to distinguish between naive and memory CD8 T cells. Figure 5(a) shows the outcome of one of four experiments conducted. In the absence of enzyme treatment, CD8 T cells from old donors had significantly higher expression of granzyme B than those from the young (P = 0·01). Pre-treatment with either CP-Siase or OSGE led to an increase in activation-induced granzyme B expression in CD8 T cells of both the young (CP-Siase: P = 0·0009; OSGE: P = 0·002) and old (CP-Siase: P =0·007; OSGE: 0·03) age groups (Fig. 5a, upper panels, and Fig. 5b, left panel).
Figure 5.
CP-Siase and OSGE pretreatment increase CD3/CD28- and IL-2-induced granzyme B expression in CD8 T cells. (a) T cells from young (Y) and old (O) mice were either left untreated or were treated with CP-Siase or OSGE, and then stimulated with cross-linked anti-CD3 and anti-CD28 for 6 hr (upper panels), or incubated with 100 IU of recombinant IL-2 for 72 hr (lower panels). For each set of samples, gated CD8 T cells were examined for granzyme B expression at the end of the incubation period. The graphs illustrate one experiment of the four used in the statistical analysis. The far left trace in each panel is the isotype control. (b) Mean fluorescence intensities (MFI) of granzyme B expression for all experiments conducted. The symbols * and † indicate statistically significant difference with untreated young or old, respectively. **Denotes statistically significant difference between young and old.
Concurrently, splenic T cells from the same mice (either untreated or pretreated with CP-Siase or OSGE) were incubated with IL-2 for 72 hr in the absence of anti-CD3/CD28 stimulation. They were then labelled for surface CD8, and for intracellular granzyme B. Once again, in the absence of enzyme treatment, CD8 T cells from old donors had a more pronounced expression of granzyme B than their young counterparts (P = 0·0009). Furthermore, pretreatment with the enzymes enhanced the IL-2-induced expression of granzyme B in CD8 T cells of both young (CP-Siase: P = 0·0009; OSGE: P = 0·008) and old (CP-Siase: P = 0·02; OSGE: P = 0·02) animals (Fig. 5a, lower panels, and Fig. 5b, right panel).
Discussion
Our previous work has shown that pretreatment of CD4 T cells with either CP-Siase or OSGE can enhance TCR-mediated CD4 T-cell function at multiple levels.11,19,20 The present study demonstrates that pretreatment of CD8 T cells with either of these enzymes enhances the CD3-mediated Ca2+ response in CD8 T cells and their subsets; augments the induced expression of CD69 and CD25; heightens the CD3/CD28-mediated production of IL-2 and IFN-γ; enhances CD3/CD28- and IL-2-induced granzyme B expression; and increases CD8 T-cell cytolytic activity in vitro in both young and old mice. These findings indicate that the enhancing effects of CP-Siase and OSGE on T-cell function are not limited to CD4 T cells, but pertain to CD8 T cells as well.
Our laboratory31 and others32 have previously shown an age effect on the CD3-mediated Ca2+ response in CD8 T cells and their memory subset, and that memory CD8 T cells mount a significantly lower Ca2+ response than their naive counterparts. The present study confirms these findings. Unlike CD4 T cells11,19 CD8 T cells showed no significant ageing effect on the induced expression of either CD69 or CD25. As for cytokine production, measurement of cytokine content in activated CD8 T-cell supernatants showed a significant age effect on IFN-γ production, in concordance with previous findings,33 but not for IL-2. The higher production of IFN-γ is plausibly caused by the higher proportion of memory T cells in the CD8 pools of older donors. Furthermore, in agreement with previous reports34 intracellular staining documented a significant increase in the percentage of IL-2- and IFN-γ-producing CD8 T cells with age (data not shown). All these aspects of CD8 T-cell function were enhanced by enzymatic treatment in both age groups.
The CD3-mediated Ca2+ response is an early activation event, which precedes the development of a mature synapse and is initiated within seconds of the formation of TCR microclusters.35 In contrast, the expression of early activation markers and production of cytokines follow the generation of a stable synapse. Furthermore, although the expression of CD69 and CD25 depend on the activity of a limited number of transcription factors,36,37 the production of cytokines requires sustained TCR engagement and the integrated effect of numerous waves of gene activation.38 The enhancement of all these functions by CP-Siase and OSGE suggests that, in CD8 T cells, cell surface glycoproteins might act to inhibit both early and late aspects of the activation process.
Ca2+ influx, expression of early activation markers, and cytokine production are shared manifestations of activation between CD4 and CD8 T cells, whereas the generation of cytotoxic effectors and expression of granzyme B are specialized characteristics of the CD8 T-cell subset. Cytotoxic T lymphocytes (CTL) use two distinct cytolytic pathways in vitro: the Fas ligand/Fas pathway, and the granule exocytosis pathway.39 In the latter, granzyme B entry into the target cell will cause caspase activation and apoptosis either directly or through mitochondrial damage.30 The enhancement of CD8 T-cell mediated cytotoxicity in vitro, and the increase in CD3/CD28- and IL-2-induced granzyme B expression in CD8 T cells after treatment with either CP-Siase or OSGE shows that the two enzymes augment specialized CD8 T-cell functions as well as those aspects of activation that are shared by CD4 and CD8 T cells.
The present study gives further credence to a model5 in which T cells from young and old mice contain specific polysaccharide moieties on their surface that can hinder maximal T-cell signalling and function, and where ageing leads to further changes in the levels or functional effects of these glycoproteins. In this model, CP-Siase- and OSGE-mediated cleavage of these determinants would leave T cells from young and old donors with similar patterns of surface glycosylation, and thus with comparable functional ability, as now evidenced by our data on both CD411,19,20 and CD8 T cells.
The increase with age in the expression of glycan-dependent CD43 determinants on anergic CD4 T cells, together with the age-related decline in the exclusion of CD43 from the immunological synapse, had suggested that cleavage of CD43 by CP-Siase and OSGE might underlie the enhancing effects of the enzymes on T-cell function.11,19,20 Nonetheless, our recent work on young CD43 knockout mice proves that the enhancing effects of CP-Siase and OSGE on CD4 and CD8 T-cell function are even present in CD43-deficient mice.40 This suggests that, at least in young animals, CP-Siase- and OSGE-sensitive molecules other than CD43 contribute to the inhibition of T-cell function.
Studies of CD8 ligand binding have shown that mature CD8 T cells bind less avidly to ligands than their thymic precursors. This is in part caused by differential sialylation of CD8β by ST3Gal-I sialyltransferase over the course of development.16,17 Some might therefore argue that the effects examined in this work could result from the cleavage of CD8 itself by CP-Siase or OSGE. This is quite unlikely because the enzymes have similar effects on CD4 T-cell function, and in contrast to CP-Siase and OSGE, the sialidase used in an earlier study does not improve the Ca2+ response in mature CD8 T cells.17 Lastly, the functional responses examined in the present report are triggered by ligation of CD3, with or without CD28, and do not require the binding of CD8 to MHC determinants.
Additional work will be required to identify the specific T-cell surface glycoproteins responsible for hindering optimal T-cell signalling, and to determine which ones display a susceptibility to enzymatic digestion that parallels the enzymes' ability to enhance T-cell function. New data on these points might help us develop novel approaches to the restoration of immune function in the elderly.
Acknowledgments
The authors would like to thank Dr Gonzalo Garcia for advice and comments, and the staff of the University of Michigan Flow-Cytometry Core Facility for their technical expertise. This work was supported by NIH grants AG024824, AG19619, and AG00114.
Abbreviations
- CP-Siase
Clostridium perfringens-derived sialidase
- OSGE
O-sialoglycoprotein endopeptidase
References
- 1.Hodes RJ. Aging and the immune system. Curr Opin Immunol. 2005;17(5):455–6. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on early B- and T-cell development. Immunol Rev. 2005;205:7–17. doi: 10.1111/j.0105-2896.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 3.Cancro MP. B cells and aging: gauging the interplay of generative, selective, and homeostatic events. Immunol Rev. 2005;205:48–59. doi: 10.1111/j.0105-2896.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 4.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–5. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Curr Opin Immunol. 2005;17:486–91. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Grossmann A, Maggio-Price L, Jinneman JC, Rabinovitch PS. Influence of aging on intracellular free calcium and proliferation of mouse T-cell subsets from various lymphoid organs. Cell Immunol. 1991;135:118–31. doi: 10.1016/0008-8749(91)90259-e. [DOI] [PubMed] [Google Scholar]
- 7.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 8.Thoman ML, Weigle WO. Lymphokines and aging: interleukin-2 production and activity in aged animals. J Immunol. 1981;127:2102–6. [PubMed] [Google Scholar]
- 9.Buckler AJ, Vie H, Sonenshein GE, Miller RA. Defective T lymphocytes in old mice. Diminished production of mature c-fmyc RNA after mitogen exposure not attributable to alterations in transcription or RNA stability. J Immunol. 1988;140:2442–6. [PubMed] [Google Scholar]
- 10.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–7. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 11.Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33:3464–72. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- 12.Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–10. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy JT, Jourdian GW, Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965;240:3501–6. [PubMed] [Google Scholar]
- 14.Sutherland DR, Abdullah KM, Cyopick P, Mellors A. Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. J Immunol. 1992;148:1458–64. [PubMed] [Google Scholar]
- 15.Bouwstra JB, Deyl CM, Vliegenthart JF. Purification and kinetic properties of sialidase from Clostridium perfringens. Biol Chem Hoppe Seyler. 1987;368:269–75. doi: 10.1515/bchm3.1987.368.1.269. [DOI] [PubMed] [Google Scholar]
- 16.Moody AM, Chui D, Reche PA, Priatel JJ, Marth JD, Reinherz EL. Developmentally regulated glycosylation of the CD8alphabeta coreceptor stalk modulates ligand binding. Cell. 2001;107:501–12. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- 17.Starr TK, Daniels MA, Lucido MM, Jameson SC, Hogquist KA. Thymocyte sensitivity and supramolecular activation cluster formation are developmentally regulated: a partial role for sialylation. J Immunol. 2003;171:4512–20. doi: 10.4049/jimmunol.171.9.4512. [DOI] [PubMed] [Google Scholar]
- 18.Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–75. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- 19.Garcia GG, Berger SB, Sadighi Akha AA, Miller RA. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 2005;35:622–31. doi: 10.1002/eji.200425538. [DOI] [PubMed] [Google Scholar]
- 20.Berger SB, Sadighi Akha AA, Miller RA. A glycoprotein endopeptidase enhances calcium influx and cytokine production by CD4+ T cells of old and young mice. Int Immunol. 2005;17:983–91. doi: 10.1093/intimm/dxh279. [DOI] [PubMed] [Google Scholar]
- 21.Garcia GG, Miller RA. Differential tyrosine phosphorylation of zeta chain dimers in mouse CD4 T lymphocytes: effect of age. Cell Immunol. 1997;175:51–7. doi: 10.1006/cimm.1996.1040. [DOI] [PubMed] [Google Scholar]
- 22.Simpson E, Chandler P. Analysis of cytotoxic T cell responses. In: Weir DM, editor. Handbook of Experimental Immunology. 4. Vol. 2. Oxford: Blackwell Scientific Publications; 1986. [Google Scholar]
- 23.Afanasyeva M, Wang Y, Kaya Z, Stafford EA, Dohmen KM, Sadighi Akha AA, Rose NR. Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-gamma-independent pathway. Circulation. 2001;104:3145–51. doi: 10.1161/hc5001.100629. [DOI] [PubMed] [Google Scholar]
- 24.Saxena RK, Saxena QB, Adler WH. Lectin-induced cytotoxic activity in spleen cells from young and old mice. Age-related changes in types of effector cells, lymphokine production and response. Immunology. 1988;64:457–61. [PMC free article] [PubMed] [Google Scholar]
- 25.Bender BS, Johnson MP, Small PA. Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology. 1991;72:514–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Miller RA, Stutman O. Decline, in aging mice, of the anti-2,4,6-trinitrophenyl (TNP) cytotoxic T cell response attributable to loss of Lyt-2-, interleukin 2-producing helper cell function. Eur J Immunol. 1981;11:751–6. doi: 10.1002/eji.1830111004. [DOI] [PubMed] [Google Scholar]
- 27.Nordin AA, Collins GD. Limiting dilution analysis of alloreactive cytotoxic precursor cells in aging mice. J Immunol. 1983;131:2215–8. [PubMed] [Google Scholar]
- 28.Bloom ET, Kubota LF, Kawakami K. Age-related decline in the lethal hit but not the binding stage of cytotoxic T-cell activity in mice. Cell Immunol. 1988;114:440–6. doi: 10.1016/0008-8749(88)90335-8. [DOI] [PubMed] [Google Scholar]
- 29.Gottesman SRS, Edington J. Proliferative and cytotoxic immune functions in aging mice. V. Deficiency in generation of cytotoxic cells with normal lytic function per cell as demonstrated by the single cell conjugation assay. Aging: Immunol Infect Dis. 1990;2:19–29. [Google Scholar]
- 30.Trapani JA, Sutton VR, Granzyme B. Pro-apoptotic, antiviral and antitumor functions. Curr Opin Immunol. 2003;15:533–43. doi: 10.1016/s0952-7915(03)00107-9. [DOI] [PubMed] [Google Scholar]
- 31.Philosophe B, Miller RA. Diminished calcium signal generation in subsets of T lymphocytes that predominate in old mice. J Gerontol. 1990;45:B87–93. doi: 10.1093/geronj/45.3.b87. [DOI] [PubMed] [Google Scholar]
- 32.Grossmann A, Ledbetter JA, Rabinovitch PS. Aging-related deficiency in intracellular calcium response to anti-CD3 or concanavalin A in murine T-cell subsets. J Gerontol. 1990;45:B81–6. doi: 10.1093/geronj/45.3.b81. [DOI] [PubMed] [Google Scholar]
- 33.Engwerda CR, Fox BS, Handwerger BS. Cytokine production by T lymphocytes from young and aged mice. J Immunol. 1996;156:3621–30. [PubMed] [Google Scholar]
- 34.Mu XY, Thoman ML. The age-dependent cytokine production by murine CD8+ T cells as determined by four-color flow cytometry analysis. J Gerontol Biol Sci Med Sci. 1999;54:B116–23. doi: 10.1093/gerona/54.3.b116. [DOI] [PubMed] [Google Scholar]
- 35.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–6. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castellanos MC, Lopez-Giral S, Lopez-Cabrera M, de Landazuri MO. Multiple cis-acting elements regulate the expression of the early T cell activation antigen CD69. Eur J Immunol. 2002;32:3108–17. doi: 10.1002/1521-4141(200211)32:11<3108::AID-IMMU3108>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 37.John S, Reeves RB, Lin JX, Child R, Leiden JM, Thompson CB, Leonard WJ. Regulation of cell-type-specific interleukin-2 receptor alpha-chain gene expression. potential role of physical interactions between Elf-1, HMG-I (Y), and NF-kappa B family proteins. Mol Cell Biol. 1995;15:1786–96. doi: 10.1128/mcb.15.3.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwashima M. Kinetic perspectives of T cell antigen receptor signaling. A two-tier model for T cell full activation. Immunol Rev. 2003;191:196–210. doi: 10.1034/j.1600-065x.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 39.Henkart PA, Catalfamo M. CD8+ effector cells. Adv Immunol. 2004;83:233–52. doi: 10.1016/S0065-2776(04)83007-4. [DOI] [PubMed] [Google Scholar]
- 40.Berger SB, Sadighi Akha AA, Miller RA, Garcia GG. CD43-independent augmentation of mouse T-cell function by glycoprotein cleaving enzymes. Immunology. 2006 doi: 10.1111/j.1365-2567.2006.02419.x. doi: 10.1111/j.1365-2567.2006.02419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]