Abstract
The genetic region of difference 1 (RD1) in Mycobacterium tuberculosis has recently been hypothesized to encode for proteins that are cytotoxic to the host cell in nature. We demonstrate here that while M. tuberculosis grew progressively in the lungs of gene disrupted mice (GKO) unable to produce interferon-γ (IFN-γ), similar mice infected instead with M. bovis bacillus Calmette–Guérin (BCG) reproducibly exhibited an obvious slowing of the disease after about 20 days. Closer examination of BCG-infected GKO mice showed a florid granulomatous inflammation in the lungs, whereas similar mice infected with M. tuberculosis exhibited wholesale progressive necrosis. In the BCG-infected GKO mice large numbers of activated effector T cells, some strongly positive for the cytokine tumour necrosis factor, as well as activated natural killer cells accumulated in the lungs. To further test the hypothesis that the differences observed were directly associated with the loss of the RD1 region, it was then shown that a mutant of M. tuberculosis lacking RD1 grew progressively in both normal and GKO mice but failed to induce any degree of necrosis in either animal despite reaching similar levels in the lungs. However, when mice were infected with this mutant, in which the RD1 region had been restored by complementation, wholesale necrosis of the lungs again occurred. These data support the hypothesis that proteins encoded in the RD1 region are a major cause of necrosis and contribute significantly to the pathogenesis of the disease.
Keywords: IFN-γ knockout mice, lung, RD1 gene region, tuberculosis
Introduction
Infection caused by Mycobacterium tuberculosis causes over 8 million new cases each year, with 2–3 million deaths.1,2 The vaccine, M. bovis bacillus Calmette–Guérin (BCG), appears to generate protective immunity in children but this wanes with age and individuals reaching adulthood are largely unprotected.3
For many years the actual differences between M. tuberculosis and BCG were unknown; more recently genetic analysis revealed that BCG lacked a group of genes (Rv3971 to Rv3879) including genes for the proteins ESAT-6 and CFP-10, which was designated the region of difference 1, or RD1.4–7 The precise action of these proteins, which are made by the bacterium very early during its growth in culture, has remained unclear but recent data in vitro8 support the hypothesis that these molecules contribute to cell necrosis.
Data presented in the current studies described here support this idea further. They show that M. tuberculosis that expresses RD1 causes dramatic necrosis in the lungs of mice unable to make interferon-γ (IFN-γ), whereas BCG, which lacks RD1, does not. In fact, the cellular and granulomatous response in the lungs of BCG-infected gene knockout (GKO) mice was not unlike that in normal immunocompetent animals. When mice were infected with a mutant M. tuberculosis lacking RD1 the infection grew progressively in the lungs but caused no apparent necrosis, but when this mutant was re-complemented with the RD1 locus the ability to cause necrosis was restored. These data indicate therefore that proteins encoded within RD1 cause necrosis of host cells, either directly or indirectly, under in vivo conditions. Moreover, the growth of the test organisms in the GKO mice suggests that an IFN-γ-independent mechanism is present and is responsible for a modest degree of host resistance.
Materials and methods
Animals
Specific pathogen-free female, 8–10-week-old, C57BL/6 and C57BL/6 IFN-γ GKO mice were purchased from Jackson Laboratories, Bar Harbor, ME. All mice were maintained under barrier conditions with sterile mouse chow and water ad libitum. The specific pathogen-free nature of the mouse colonies was demonstrated by testing sentinel animals, which were shown to be negative for 12 known mouse pathogens. All experimental procedures were approved by the Colorado State University Animal Care and Use Committee.
Aerosol infection with M. tuberculosis and M. bovis BCG
The M. tuberculosis strains Erdman and H37Rv, originally obtained from the Trudeau Institute (Saranac Lake, NY), were grown in Proskauer–Beck liquid medium containing 0·05% Tween-80 to mid-log phase and then frozen in aliquots at −70° until needed. These two strains are of equal virulence for mice. Mycobacterium bovis BCG Connaught was grown for 18–21 days in Middlebrook 7H9 broth supplemented with 10% albumin, dextrose and NaCl (ADC) and 0·2% glycerol, following the manufacturer's instructions (Difco Laboratories, Detroit, MI). Mutants of M. tuberculosis lacking RD1 or re-complemented were generated as previously described.8 Mice were infected following previously described procedures.9,10 Briefly, bacterial stocks were diluted in 5 ml of sterile distilled water to 2 × 106 colony-forming units/ml and placed in a nebulizer attached to an airborne infection system (Glass-Col, Terre Haute, IN). Mice were exposed to 40 min of aerosol during which time approximately 100–200 bacteria were deposited in the lungs of each animal. Bacterial load was determined by plating left lung lobe homogenates onto nutrient 7H11 agar supplemented with oleic acid-dextrose complex medium (OADC). Colonies were enumerated after 21 days incubation at 37°.
Lung cell digestion
Mice were killed by CO2 asphyxiation and the pulmonary cavity was opened. The lung was cleared of blood by perfusion through the pulmonary artery with 10 ml ice-cold phosphate-buffered saline (PBS) containing 50 U/ml of heparin (Sigma, St Louis, MO). Lungs were aseptically removed, teased apart and treated with a solution of deoxyribonuclease IV (DNAse) (Sigma Chemical, 30 μg/ml) and collagenase XI (Sigma Chemical, 0·7 mg/ml) for 45 min at 37°. To obtain a single cell suspension, organs were gently passed through cell strainers (Becton Dickinson, Labware, Lincoln Park, NJ). Remaining erythrocytes were lysed with Gey's solution (0·15 m NH4Cl, 10 mm KHCO3) and cells were washed with Dulbecco's minimum essential medium. Total cell numbers were determined using a Neubauer chamber.
Flow cytometric analysis of cell surface markers
Cells were labelled with the following specific antibodies; fluorescein isothiocyanate anti-CD44 (clone IM7), anti-CD45RB (clone 16A), anti-CD3 (145-2C11), anti-CD69 (clone H1.2F3), and anti-CD4 (clone H129.19); and allophycocyanin (APC) anti-NK1.1 (clone PK136), or anti-CD62L (clone MEL-14). All antibodies were purchased from BD Pharmingen (San Jose, CA) and were used at 0·2 μg/106 cells in PBS containing 10% mouse serum and 0·1% sodium azide for 30 min at 4°. After washing with PBS containing 0·1% sodium azide, the cells were analysed on a Becton Dickinson FACScalibur and data were analysed using CellQuest software (Becton Dickinson Immmunocytometry Systems, San Jose, CA). Cells were gated on lymphocytes or macrophages based on characteristic forward and side scatter profiles. Individual cell populations were identified according to their presence of specific fluorescently labelled antibody. All the analyses were performed with an acquisition of at least 100 000 events.
Intracellular TNF-α staining by flow cytometry
Measurement of intracellular tumour necrosis factor-α (TNF-α) was conducted by preincubating lung cells with a solution containing monensin (3 μm), anti-CD3/anti-CD28 (2 μg/ml) for 4 hr at 37°, 5%CO2. The cells were then stained with APC anti-CD4, and peridin-chlorophyll-protein (PerCP) anti-CD8 for 30 min, washed with PBS containing 0·1% sodium azide, fixed and permeabilized with Perm Fix/Perm Wash (BD Pharmingen), and then stained for intracellular phycoerythrin anti-TNF-α or phycoerythrin rat immunoglobulin G1 isotype control (BD Pharmingen) for a further 30 min. The antibodies were diluted in PBS containing 0·1% sodium azide and 10% normal mouse serum (Serotec, Raleigh, NC).
Histological analysis
At each time-point, the posterior right lung lobes were aseptically removed from each of the dead mice and infused with 10% neutral buffered formalin. These were stored in 10% neutral buffered formalin before routine microscopic processing. All tissues were stained with haematoxylin & eosin. Tissues were coded and evaluated in a randomized fashion and were assigned to distinct histological groups.
Immunohistochemical analysis
The anterior and middle right lung lobes from each mouse were aseptically removed and infused with 30% OCT (Tissue-Tek, Inc., Torrane, CA) in PBS through the trachea. After removing the lungs from the pulmonary cavity, they were embedded in OCT, frozen in a bath of liquid nitrogen for a few seconds and then stored at −70°. Serial sections from each lung were cut at a thickness of 5–7 μm on a cryostat (Leica, CM 1850), employing the tape transfer system (Instrumedics), fixed in cold acetone for 10 min and air-dried. Blocking of endogenous peroxidase was performed by incubating the tissue for 5–10 min at room temperature with peroxidase block (Daco, Carpinteria, CA). Anti-CD4 and anti-CD8α (BD-Pharmingen) antibodies at 25 μg/ml were added and the slides were incubated at room temperature for 30 min. Control sections were incubated with isotype controls. All sections were washed three times in PBS and incubated with the secondary detection antibody goat F(ab′)2 anti-rat IgG conjugated to horseradish peroxidase (Biosource International, Camarillo, CA). Finally, the reaction was developed using diaminobenzine (Ventana Medical Systems, Tucsun, AR) as substrate. Sections were counterstained with Meyer's haematoxylin and covered with crystal/mount (Biomed, Foster City, CA).
Statistics
Statistical significance was determined using the Student's t-test.
Results
Course of infections in normal and IFN-γ gene knockout mice
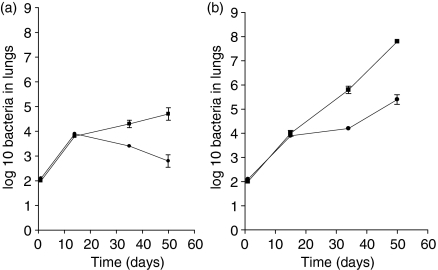
To compare the bacterial load and bacterial clearance in the lung between wild-type mice and GKO mice, mice were infected by aerosol exposure with M. tuberculosis or M. bovis BCG and the colony-fomring units in the lungs were determined against time. Figure 1 shows the growth of M. tuberculosis and BCG in the left lung lobe of normal immunocompetent mice after exposure to aerosol infection. As expected, the M. tuberculosis infection gradually assumed a chronic state, whereas after some brief growth in the lungs BCG began to be slowly cleared. To determine if there was a difference between the ability of BCG and M. tuberculosis to grow in the lungs of GKO mice (with a disrupted IFN-γ gene) the experiment was repeated using these animals. As shown, both infections grew at the same rate for about the first 20 days, but thereafter while the M. tuberculosis infection continued to grow progressively in the GKO mice, the BCG infection slowed and from that point on exhibited a bacterial load about 1 log lower. This event was found to be reproducible in three separate experiments. Although the majority of M. tuberculosis-infected mice died before 60 days post infection, GKO mice that were aerosol-infected with M. bovis BCG did not show major signs of deleterious disease until at least 20 days later.
Figure 1.
IFN-γ knockout mice control BCG infection in the lung. (a) Growth kinetics in the lungs of C57BL/6 mice infected with M. tuberculosis (▪) or M. bovis BCG (•). (b) Growth kinetics in the lungs of GKO mice infected with M. tuberculosis or M. bovis BCG (symbols as above). Data represent the mean ±SEM from six mice per time-point. The results are representative of three independent experiments.
Lesions induced by BCG in the lungs of GKO mice are devoid of necrosis
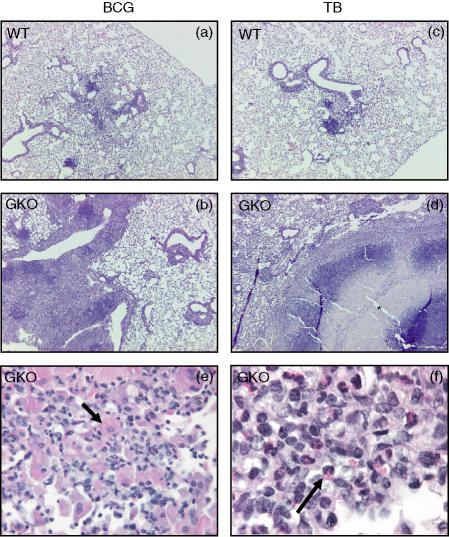
As a starting point to explain the differences between the two infection models lungs were fixed and examined histologically. Representative sections are shown in Fig. 2. In the wild-type animals granulomatous lesions induced by the two infections were similar, consisting by day 62 of the study of regions of granulomatous inflammation dominated by macrophages and lymphocytes. Lesions induced in the GKO mice were very large in comparison to those in the wild-type group. GKO mice infected with BCG exhibited lesions characterized by an abundance of large epithelioid macrophages with a fibrillar hypereosinophilic cytoplasm. In addition, the presence of large numbers of eosinophils was also very consistent and striking, as previously shown.11 In GKO mice infected with M. tuberculosis the predominant feature was lesion necrosis resulting in destruction of the pulmonary parenchyma. As we have shown elsewhere,12 necrosis is first evident about 25–30 days into the course of pulmonary infection in GKO mice, and is progressive.
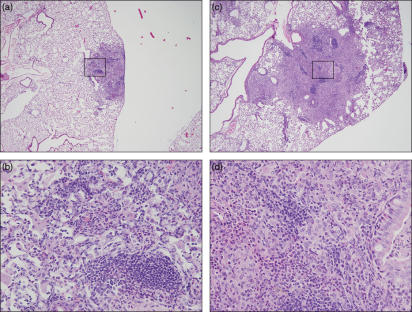
Figure 2.
Mycobacterium tuberculosis induces necrosis in GKO mice while BCG infection confers a florid inflammatory response without necrosis. Mice were aerosol infected with M. bovis BCG or M. tuberculosis (TB). (a, c) Histological pattern seen in wild-type mice (WT), (b, d) histological pattern in GKO mice (100 ×), (e, f) higher magnification (400 ×) of the inflammatory cells characteristic of the lesion seen in (b); arrow in (e) indicates large epithelioid macrophages with a fibrillar hypereosinophilic cytoplasm, arrow in (f) indicates eosinophils; asterisk indicates necrosis. The examples shown here are from mice harvested at 62 days post infection. Haematoxylin & eosin stain. The lesions are representative of one out of three independent experiments with similar results.
In the absence of necrosis lymphocytes and macrophages accumulate in BCG-induced lesions
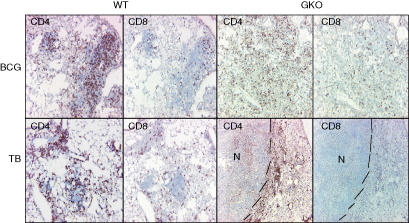
IFN-γ is known to be a potent activator for macrophages and in response those cells secrete the cytokines and chemokines necessary for CD4 and CD8 cell migration and activation. Since it appeared that large numbers of mononuclear cells were accumulating in the lesions of GKO mice infected with BCG, we used immunohistochemistry to identify them. As shown in Fig. 3, BCG-infected GKO mice had influxes of CD4 and CD8 cells fairly similar to those seen in wild-type mice, with perhaps the only difference being that the CD4 staining pattern was less aggregated and more diffuse in GKO mice.
Figure 3.
Diffuse distribution of CD4+ and CD8+ T cells in GKO BCG-infected mice. Mice were infected with M. bovis BCG or M. tuberculosis and lungs were collected at 62 days post infection. Frozen sections of lung tissue were prepared and stained for CD4 or CD8 using monoclonal antibodies. Lesions from wild-type mice (WT) and IFN-γ knockout mice (GKO) are shown. The top panel represents mice infected with BCG while the bottom panel represents the lesions found among the M. tuberculosis-infected mice. The dashed bars were added to delineate the borders of the necrotic lesions. Note the diffuse distribution of CD4 and CD8 cells in GKO mice infected with BCG when compared to wild-type mice. CD4 and CD8 cells could be detected in M. tuberculosis-infected GKO mice, but only beyond the necrotic areas (delimited by dashed lines).
In GKO mice infected with M. tuberculosis, previous studies have shown wholesale destruction of the mononuclear response,12,13 and this was confirmed here in that virtually no staining for CD4 and CD8 cells was positive, except for a few cells beyond the rim of the necrotic lesion.
Cellular influx in GKO mice infected with BCG
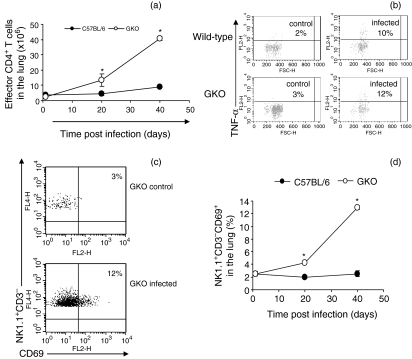
As a result of the observation that lymphocytes increased in numbers in the lungs of GKO mice infected with BCG, we isolated these cells and analysed them by flow cytometry. As shown in Fig. 4, large numbers of CD4 T cells in the lungs of GKO mice expressed a CD44hi CD62Llo CD45RBlo activated/effector phenotype. In the absence of IFN-γ, we questioned whether these activated CD4 cells were secreting TNF-α or IL-4 in response to the infection. In mice infected with M. bovis BCG there were similar numbers of CD4 cells positive for TNF-α in the lungs of both wild-type and GKO mice. We did not detect CD4 cells producing IL-4 (data not shown). In addition, lung NK cells were in an activated state, shown by the expression of CD69, and were increased in number in the GKO mice. Numbers of alveolar and dendritic macrophages in the lungs of control and GKO mice infected with BCG were similar (data not shown).
Figure 4.
BCG infection confers early activation/migration of effector CD4+ T cells and natural killer (NK) cells in the lung of infected GKO mice. GKO and wild-type mice were infected with M. bovis BCG. Lung single cell suspensions were characterized by flow cytometry. CD4+ cells were gated and analysed for surface molecule characteristics of the effector cell (CD44high CD62Llow CD45RBlow) phenotype in (a). (b) CD4+ cells were gated and analysed for the expression of TNF-α after 4 hr of incubation with anti-CD3/CD28 and monensin. (c,d) NK cells (NK1.1+, CD3–) were gated and analysed for early activating factor expression (CD69). In (c) the dot plot shows the activation of NK cells after M. bovis infection of GKO mice at 40 days post infection. (d) Kinetics of CD69+ NK cells in the lung of infected mice during 40 days post infection. Data shown are the mean ± SEM of six mice per time-point/group. Three independent experiments were performed with similar results. *Statistical differences between wild-type and GKO infected mice, P < 0·05.
A mutant strain of M. tuberculosis lacking RD1 grows progressively but does not induce necrosis in the lungs
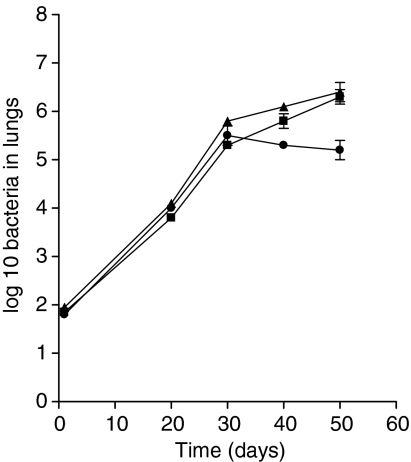
As shown in Fig. 5 the RD1 deleted mutant of M. tuberculosis grew progressively in both normal and GKO mice, with the immunocompetent mice showing evidence of containment after about day 30 of the infection. In GKO mice infected with the RD1 re-complemented mutant, the course of the infection was similar to that of the wild-type infection. At the pathological level (Fig. 6) lesions on day 40 in the C57BL/6 mice were primarily mild and dominated by lymphocytes. Lesions seen at day 50 were similar, with tightly aggregated lymphocytes and lower numbers of peri-lesional and intralesional epithelioid macrophages. This included some vacuolated foamy macrophages, and the occasional multinucleated giant cell. In the GKO mice on day 40 the inflammatory cells in lesions were mostly lymphocytes, with fewer numbers of macrophages and neutrophils. There were subpleural lesions, in which alveoli had filled with macrophages. Aggregates of neutrophils and smooth muscle hypertrophy of adjacent arterioles were also evident. By day 50 (Fig. 6) there were significantly fewer lymphocytes and larger numbers of macrophages and multinucleated giant cells. Some of the latter had elongated and possessed cytoplasmic cholesterol granules. There were multiple aggregates of neutrophils in clusters throughout the lesions. In addition to large lesions there were numerous small lesions in early stages of development indicating widespread dissemination of the infection. Importantly, in all sections taken on days 40 or 50 for analysis there was no significant necrosis or fibrosis.
Figure 5.
Growth of an M. tuberculosis H37Rv mutant lacking the RD1 gene region in GKO mice (▪) or in normal C57BL/6 mice (•), compared to the growth of the RD1 re-complemented mutant in GKO mice (▴). Data are given as mean values ± SEM for five mice.
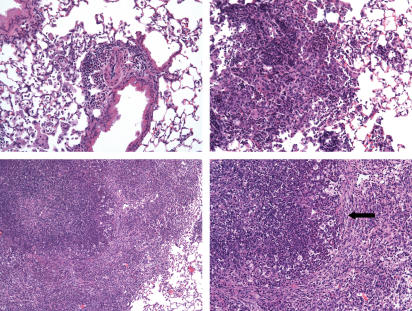
Figure 6.
Histological appearance of fixed lung tissues from C57BL/6 (a, b) and GKO mice (c, d) 50 days after aerosol infection with M. tuberculosis RD1 gene KO mutant. In both groups lesions were perivascular and peribronchiolar. In the C57BL/6 mice lesions were focal and compact composed of small mature lymphocytes (L) mixed with macrophages (M) and occasional neutrophils (arrows). Lesions in GKO mice were usually larger and multifocal, containing intra-alveolar accumulations of macrophages, compact foci of lymphocytes, and neutrophils. Some multinucleated giant cells were seen. Haematoxylin & eosin staining; (a, c) 40 ×; (b, d) 400 × magnification.
As shown in Fig. 7, infection of mice with the RD1 re-complemented RD1-KO mutant restored the ability of the organism to cause severe lung tissue necrosis. In these mice the lungs were severely consolidated by large fields of necrotic tissue. As above, in lungs of mice infected with the RD1-KO mutant there was no evidence of necrosis despite an identical bacterial load at that time.
Figure 7.
Comparison of lungs tissues harvested from GKO mice 34 days after infection with the RD1-KO mutant (top panels) or this mutant re-complemented with RD1 (bottom panels). The top left (magnification 100 ×) shows periarteriolar infiltrates of mild mixed inflammatory cells that are composed of primarily lymphocytes and fewer neutrophils. The peribronchiolar alveoli have similar infiltrates in addition to multiple multinucleated giant cells. The top right panel (200 ×) shows sheets of lymphocytes, macrophages and fewer granulocytes with no evidence of necrosis. In comparison, the bottom left panel (100 ×) shows necrosis and mixed inflammation in which neutrophils are a predominant cell type, whereas the bottom right (200 ×) shows increased fibrous connective tissue forming an incomplete capsule (arrow) that delineates necrosis, and neutrophilic inflammation from more mixed mononuclear cell infiltrate.
Discussion
The results of this study show that whereas both M. tuberculosis and BCG infections grow at the same rate in GKO mice over the first 10–20 days, after this point the BCG infection was slowed and the bacterial load became at least 10-fold lower. As the two infections progressed the histology of the lungs was characterized by severe necrosis in the GKO mice infected with M. tuberculosis, whereas this was completely absent in mice infected with BCG. In these latter mice CD4 and CD8 T cells continued to increase in numbers in the lungs, while in M. tuberculosis-infected mice, cell numbers declined and could only be detected by immunohistochemistry on the periphery of necrotic lesions.
An established major difference between M. tuberculosis and BCG is the lack of the RD1 region in the latter, a region that encodes for proteins implicated in in vitro studies using lung cell lines as cytotoxic.8 To test this directly we repeated our studies using an M. tuberculosis mutant lacking RD1 and were able to demonstrate that despite progressive growth of this organism no evidence of necrosis in the lungs was observed. When the RD1 region was restored, necrosis was again observed. This clearly implicates the proteins present in the RD1 region in this process. This could be a direct effect, or secondary to further interactions between the bacterial proteins and the host cell.
In addition, the generation of lung necrosis did not appear to be the result of bacterial load itself. In separate studies of BCG, M. tuberculosis and M. tuberculosis lacking RD1 all reached close to or exceeded 106 bacilli in the lungs, but only the intact M. tuberculosis strain induced necrosis. With the other two infections, lung sections were devoid of necrosis regardless of the point in the study when the lungs were harvested. This was then further illustrated when the mutant and the re-complemented mutant were compared, with necrosis only occurring in the bacilli containing RD1 despite the similar bacterial load in the lungs.
Although M. tuberculosis is a well-adapted intracellular bacterial parasite, there are two occasions in its life cycle when cell escape is potentially needed. The first is during the initial infection. How this occurs remains unknown, but we have suggested that spreading of infected alveolar macrophages across the surface of the alveolus is followed by escape of the bacterium across into the lung interstitium,14 an event that clearly would be facilitated by damage to the phagosomal membrane, the macrophage cell membrane and the lung cell membrane by the proteins encoded by RD1. The second occurs when it is in the interests of the bacterium to disseminate, perhaps triggered by nutrient starvation. It is interesting to note that ESAT-reactive T cells can be detected well into the chronic disease state when necrosis begins to occur.15
It is well established that the T helper type 1 pathway is essential to resistance to tuberculosis.16,17 The results here suggest that if the integrity of the lung tissues is sufficiently retained, then mechanisms other than IFN-γ can contribute to at least the partial containment of the BCG infection. T cells and macrophages were continuing to enter inflammatory sites in the lungs in the BCG-infected GKO mice, and while the CD4 cells appeared less organized or aggregated in the GKO mice this suggests that the chemokine network needed to attract these cells was still operational. Moreover, no overt differences in CD8 influx could be seen. In fact, overall, other than the absence of IFN-γ, many of the characteristic components of the granulomatous response seemed to still be present in the BCG-infected GKO mice. Having said that, an additional cellular component in the BCG infected mice was the presence of eosinophils, for reasons that are as yet unknown.
This study also demonstrates that if a suitably attenuated mycobacterial infection is given to GKO mice then this can allow insight into the underlying IFN-γ-independent mechanisms possessed by these mice and can provide a suitable animal model to investigate these further. In this regard, such mechanisms may be of even greater importance in humans infected with tuberculosis in whom it is very much less clear just how important IFN-γ is to control of the disease process.18–25 In addition, these findings indicate that BCG can induce secondary or underlying compensatory mechanisms which could be exploited in vaccine development.
A study by Cowley and Elkins26 is also consistent with our observations, by showing that GKO mice can express some degree of resistance to BCG and tuberculosis infection. Although these observations were based upon an in vitro-infected macrophage culture assay, they nevertheless provided data consistent with the hypothesis that CD4 cells could mediate resistance independently of IFN-γ.
IFN-γ is critical to macrophage activation, but not to recruitment, and the results of this study indicate that the granulomatous process itself has some intrinsic antimicrobial protection, albeit minor. Other cytokine and chemokine pathways remain intact also, including cytokines related to IFN-γ itself. However, to date, the relative importance of these remains unknown. The cytokine TNF-α is probably more responsible for the intact granuloma integrity27,28 and we have shown above that it is still made in normal levels by T cells in the infected GKO mice. The antimicrobial properties of this cytokine are well established.
In summary, these data support the growing hypothesis that the primary gene deletion in BCG, the RD1 gene region, encodes for molecules (CFP-10, ESAT-6) that are directly involved in a process leading to lung cell cytolysis;8 therefore this information would explain the lack of necrosis seen in the BCG-infected GKO mice despite their inability to clear the organism.
Acknowledgments
This work was supported by National Institutes of Health Public Health Service grants AI-40488, AI-45707, AI-95383 and HL-55967. S.A.F. was supported by a K08 award.
Abbreviations
- ADC
albumin/dextrose/NaCl
- BCG
bacillus Calmette–Guérin
- GKO
interferon-γ gene knockout mice
- IFN-γ
interferon-γ
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- RD1
genetic region of diffference 1
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Raviglione MC, Dye C, Schmidt S, Kochi A. Assessment of worldwide tuberculosis control. WHO Global Surveillance and Monitoring Project. Lancet. 1997;350:624–9. doi: 10.1016/s0140-6736(97)04146-9. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette–Guérin attenuation. J Infect Dis. 2003;187:117–23. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearns AM, Magee JG, Gennery A, Steward M, Graham C, Seiders PR, Freeman R. Rapid identification of Mycobacterium bovis BCG by the detection of the RD1 deletion using a multiplex PCR technique. Int J Tuberc Lung Dis. 1999;3:635–8. [PubMed] [Google Scholar]
- 6.Behr MA. Comparative genomics of BCG vaccines. Tuberculosis (Edinb) 2001;81:165–8. doi: 10.1054/tube.2000.0253. [DOI] [PubMed] [Google Scholar]
- 7.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 8.Hsu T, Hingley-Wilson SM, Chen B, et al. The primary mechanism of attenuation of bacillus Calmette–Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA. 2003;100:12420–5. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junqueira-Kipnis AP, Turner J, Gonzalez-Juarrero M, Turner OC, Orme IM. Stable T-cell population expressing an effector cell surface phenotype in the lungs of mice chronically infected with Mycobacterium tuberculosis. Infect Immun. 2004;72:570–5. doi: 10.1128/IAI.72.1.570-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, Turner J, Orme IM. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol. 2003;171:6039–45. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 11.Erb KJ, Kirman J, Delahunt B, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-BCG induces both Th1 and Th2 immune responses in the absence of interferon-gamma signalling. Eur Cytokine Netw. 1999;10:147–54. [PubMed] [Google Scholar]
- 12.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orme IM. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–7. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 15.Winslow GM, Roberts AD, Blackman MA, Woodland DL. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J Immunol. 2003;170:2046–52. doi: 10.4049/jimmunol.170.4.2046. [DOI] [PubMed] [Google Scholar]
- 16.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 17.Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–12. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 18.Steele J, Flint KC, Pozniak AL, Hudspith B, Johnson MM, Rook GA. Inhibition of virulent Mycobacterium tuberculosis by murine peritoneal macrophages and human alveolar lavage cells. The effects of lymphokines and recombinant gamma interferon. Tubercle. 1986;67:289–94. doi: 10.1016/0041-3879(86)90018-8. [DOI] [PubMed] [Google Scholar]
- 19.Silver RF, Li Q, Boom WH, Ellner JJ. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160:2408–17. [PubMed] [Google Scholar]
- 20.Rook GA, Steele J, Ainsworth M, Champion BR. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986;59:333–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Cappelli G, Volpe P, Sanduzzi A, Sacchi A, Colizzi V, Mariani F. Human macrophage gamma interferon decreases gene expression but not replication of Mycobacterium tuberculosis: analysis of the host-pathogen reciprocal influence on transcription in a comparison of strains H37Rv and CMT97. Infect Immun. 2001;69:7262–70. doi: 10.1128/IAI.69.12.7262-7270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brill KJ, Li Q, Larkin R, Canaday DH, Kaplan DR, Boom WH, Silver RF. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect Immun. 2001;69:1755–65. doi: 10.1128/IAI.69.3.1755-1765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraser DA, Bulat-Kardum L, Knezevic J, et al. Interferon-gamma receptor-1 gene polymorphism in tuberculosis patients from Croatia. Scand J Immunol. 2003;57:480–4. doi: 10.1046/j.1365-3083.2003.01253.x. [DOI] [PubMed] [Google Scholar]
- 24.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–5. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 25.Altare F, Lammas D, Revy P, et al. Inherited interleukin 12 deficiency in a child with bacille Calmette–Guérin and Salmonella enteritidis disseminated infection. J Clin Invest. 1998;102:2035–40. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowley SC, Elkins KL. CD4+ T cells mediate IFN-γ-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J Immunol. 2003;171:4689–99. doi: 10.4049/jimmunol.171.9.4689. [DOI] [PubMed] [Google Scholar]
- 27.Orme IM. Beyond BCG. the potential for a more effective TB vaccine. Mol Med Today. 1999;5:487–92. doi: 10.1016/s1357-4310(99)01594-4. [DOI] [PubMed] [Google Scholar]
- 28.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–11. [PubMed] [Google Scholar]