Abstract
Oral administration of a certain dose of antigen can generally induce immunological tolerance against the same antigen. In this study, we showed the temporal appearance of ovalbumin (OVA) antigens in both portal and peripheral blood of mice after the oral administration of OVA. Furthermore, we detected 45 000 MW OVA in mouse serum 30 min after the oral administration of OVA. Based on this observation, we examined whether the injection of intact OVA into the portal or peripheral vein induces immunological tolerance against OVA. We found that the intravenous injection of intact OVA did not induce immunological tolerance but rather enhanced OVA-specific antibody production in some subclasses, suggesting that OVA antigens via the gastrointestinal tract but not intact OVA may contribute to establish immunological tolerance against OVA. Therefore, we examined the effects of digesting intact OVA in the gastrointestinal tract on the induction of oral tolerance. When mice were orally administered or injected into various gastrointestinal organs, such as the stomach, duodenum, ileum, or colon and boosted with intact OVA, OVA-specific antibody production and delayed-type hypersensitivity (DTH) response were significantly enhanced in mice injected into the ileum or colon, compared with orally administered mice. These results suggest that although macromolecular OVA antigens are detected after oral administration of OVA in tolerant-mouse serum, injection of intact OVA cannot contribute to tolerance induction. Therefore, some modification of macromolecular OVA in the gastrointestinal tract and ingestion may be essential for oral tolerance induction.
Keywords: gastrointestinal ingestion, macromolecular antigen, oral tolerance, ovalbumin
Introduction
Although the gastrointestinal tract is incessantly exposed to dietary antigens and commensal micro-organisms, the antigens are not only eliminated, but immunological unresponsiveness to the antigens is also acquired. When an antigen is orally administered to animals, antigen-specific immune responses are suppressed after systemic immunization of the antigen, and this phenomenon is called oral tolerance.1,2 The development of food hypersensitivity is related to the failure of oral tolerance induction.3 Food allergy is categorized as class 1 food allergy, which might result from a breach in oral tolerance to foods, or class 2 food allergy, which might result from sensitization to respiratory allergens or other sensitization not via gastrointestinal mucosa.4,5 Class 1 food allergy typically occurs with food proteins, such as eggs or peanuts that are generally stable in digestion, in infants or children.5 In typical class 2 food allergy, immunoglobulin E (IgE) antibody against respiratory allergens such as pollens recognizes homologous epitopes in food proteins of some fruits or vegetables.5 In particular, to elucidate the pathogenesis of class 1 food allergy, it is fundamental to clarify mechanisms in the development and failure of oral tolerance. Moreover, the constructive induction of immunological suppression by oral tolerance is expected to contribute to prevent allergy6 or autoimmune diseases7,8 in which antigen-specific immune responses are pathologically enhanced.
When a dietary protein antigen is ingested, it is treated by digestive enzymes in the stomach and small intestine. Generated amino acids and small peptides are absorbed via the small intestinal lumen, and enter the portal vein through capillary vessels in the small intestine.9 However, an antigen that escapes digestion can also enter the body via the intestinal surface. Microfold cells (M cells) over Peyer's patches (PPs) of the intestines take up soluble macromolecule proteins10–12 as well as viruses13–15 and bacteria.16–18 After uptake via M cells, the antigens are processed and presented by dendritic cells (DCs) in PPs.19 In addition, DCs under intestinal epithelia send dendrites between epithelial cells and directly acquire antigens over epithelial cells.20 PPs are shown to be inductive sites for oral tolerance where T cells secreting regulatory cytokines, including interleukin (IL)-1021 and transforming growth factor-β (TGF-β)22 are induced; however, it is reported that oral tolerance can be induced in mice lacking PPs and mesenteric lymph nodes (MLNs).23 On the other hand, the liver is shown to be crucial to tolerance induction because the intraportal injection of allogeneic donor cells,24,25 eggs of a parasite26 or insoluble protein27 induces immunological tolerance against the corresponding antigen.
Ovalbumin (OVA) from chicken eggs is a dietary protein antigen that frequently causes food allergy.28,29 After the oral administration of intact OVA, OVA antigens are known to be detected in peripheral blood and are suggested to contribute to the induction of immunological tolerance against OVA.30–32 In this study, we attempted to examine OVA antigens in both portal and peripheral blood after the oral administration of OVA and tried to induce tolerance by intraportal and intravenous injection of intact OVA. Furthermore, to investigate the effects of digestion in the gastrointestinal tract on oral tolerance induction, intact OVA molecules were directly injected into the gastrointestinal tract and then the induction profile of tolerance against OVA was assessed.
Materials and methods
Mice
Female BALB/c, C57BL/6 or BDF1 mice were used between the ages of 6 and 12 weeks. Mice were purchased from Charles River (Tokyo, Japan) or Sankyo Labo Service Co. (Shizuoka, Japan) and maintained in a specific pathogen-free environment.
Oral administration of OVA
OVA, chicken egg, grade V (Sigma, St. Louis, MO) were dissolved in sterilized phosphate-buffered saline (PBS, pH 7·4). Mice were orally administered with 250 ng, 250 µg, 2·5 mg, 25 mg or 250 mg of OVA once. As a control, mice were orally treated with PBS in the same way. In some experiments, mice were orally administered with 1 or 100 mg of OVA or the same dose of OVA plus 10 µg of cholera toxin (CT; List Biological Laboratory Inc., Campbell, CA) once weekly for 4 weeks.
Intraportal or intravenous injection of OVA
Intraportal injection was performed as described previously.27 Mice were anaesthetized and underwent an abdominal operation. Filtered 2·5 mg or 250 mg of OVA in 250 µl of 0·03% trypan blue-PBS was injected into the portal vein using a 29G needle-tipped syringe. As a control, filtered 0·03% trypan-blue PBS was injected in the same way. In this case, OVA solution was coloured by adding trypan blue to confirm that it was really injected into the liver through the portal vein. After the injection, bleeding from the portal vein was stopped with thrombin (Mochida Pharmaceutical Co., LTD, Tokyo, Japan) and then the peritoneum and skin were sutured.
For intravenous injection, mice were anaesthetized and injected with filtered 2·5 mg or 250 mg of OVA in 250 µl of PBS into the tail vein. As a control, PBS was injected in the same way.
Injection of OVA into the digestive tract
Mice were anaesthetized and underwent an abdominal operation. They were injected with 25 mg of OVA in 250 µl of PBS into the stomach, duodenum, ileum or colon using a 29G needle-tipped syringe, respectively, and then the peritoneum and skin were sutured.
Intraperitoneal immunization of OVA
Mice were intraperitoneally (i.p) injected with 50 µg of OVA and 4 mg of alum, Al(OH)3, in 0·5 ml of PBS. Two weeks later, the second immunization was performed in the same manner. In some experiments, a third boost was performed by i.p. injection of 0·5 mg of OVA in 0·5 ml of PBS.
Gut content collection
The stomach and small intestine were removed from mice and washed with PBS. Supernatants were collected from the wash fluid and stored frozen at −80° until assay.
Collection of portal or peripheral plasma and faecal samples
For portal blood collection, mice were anaesthetized and underwent an abdominal operation. Portal blood was collected from the portal vein using a 24G catheter and heparinized capillary tubes, and then the peritoneum and skin were sutured. Peripheral blood was collected from anaesthetized mice using heparinized capillary tubes. The blood was centrifuged for 10 min at 6000 g, and plasma was collected and stored frozen at −80° until assay.
Faecal extracts were prepared by the method described previously.33 Fresh faeces were collected and weighed, and PBS containing 0·01% sodium azide was added to the faeces (100 mg/ml). The faeces in PBS were homogenized by continuous shaking for 10 min with a Vortex, and centrifuged for 10 min at 12 000 g at 4°. Supernatants were collected and stored frozen at −80° until assay.
Enzyme-linked immunosorbent assay (ELISA)
OVA antigen levels in the gut contents or the plasma and anti-OVA antibody levels in the plasma or the faecal extracts were determined by ELISA as described previously.34,35 For the assay of OVA antigen levels, 96-well flat-bottomed microtitre plates were coated with rabbit anti-OVA IgG (Rockland, Gilbertsville, PA) in carbonate buffer (pH 9·6) at 4° overnight. Wells were blocked with 1% bovine serum albumin (BSA) in PBS at 37° for 1 hr. Gut contents or plasma samples diluted appropriately in PBS were added to the wells in duplicate, and incubated at 37° for 1 hr. Biotinylated anti-OVA IgG (Rockland) was added to the wells and incubated at 37° for 1 hr. Horseradish peroxidase-conjugated streptavidin (Caltag Laboratory, Burlingame, CA) was then added and incubated at 37° for 30 min. Enzyme reaction was performed with 1 mm 2,2′-azino-bis diammonium salt (ABTS, Sigma) in sodium citrate buffer (pH 5·4) in the presence of 0·01% H2O2. The reaction was interrupted by the addition of 2 mm NaN3 in PBS, and absorbance was measured at 415 nm. To draw a standard curve, various quantities of OVA were added to the part of the plate coated with anti-OVA IgG and blocked. The wells were added to biotinylated anti-OVA IgG and coloured in the same manner as the sample wells. For the assay of anti-OVA immunoglobulin, plates were coated with OVA (100 µl of 1 mg/ml) in carbonate buffer. After blocking, diluted plasma or faecal extract samples were added to the wells and incubated. Biotinylated anti-mouse immunoglobulins (Amersham Life Science, Amersham, UK), IgA (Sigma), IgG1, IgG2a, IgG2b, IgM or IgE (BD PharMingen, San Diego, CA) were added to the wells and incubated. Horseradish peroxidase-conjugated streptavidin was then added and incubated. Enzyme reaction was performed with ABTS, and absorbance was measured at 415 nm. To draw a standard curve, part of the assay plate was coated with various quantities of purified mouse IgG1, IgG2a (BD PharMingen) or IgA (ICN/Cappel, Aurora, OH). After blocking, biotinylated antimouse IgG1, IgG2a or IgA were added to the wells and coloured in the same manner as the sample wells.
Immunoprecipitation and immunoblotting
For immunoprecipitation, 0·1 mg of rabbit anti-OVA IgG (Rockland) was incubated with 20 µl of protein-G coupled sepharose (Sigma) at 4° on an orbital shaker overnight. After washing with PBS three times, the treated sepharose was incubated with 1 ml of mouse serum or 100 ng of OVA mixed with 1 ml of untreated mouse serum at 4° on an orbital shaker overnight. After washing with PBS twice and 0·05 m Tris buffer once, the sepharose was resuspended in 20 µl of sample buffer (Invitrogen, Carlsbad, CA) including sample reducing agent (Invitrogen) and heated at 70° for 10 min. The supernatants were collected and diluted fivefold with the sample buffer. Five µl of the diluted samples were loaded on 4–12% Bis-Tris gel (Invitrogen). Proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Invitrogen). The membranes were blocked in 1% BSA 0·1% Tween-20 PBS and incubated with rabbit anti-OVA polyclonal IgG (Rockland) at 4° overnight. This was followed by incubation with peroxidase-conjugated anti-rabbit IgG (Seikagaku Corporation, Tokyo, Japan). The tetramethyl benzidine (TMB) substrate kit for peroxidase (Vector Labotarories, Inc., Burlingame, CA) was used for detection.
OVA-specific T cell proliferation
The spleens or MLNs were removed and crushed in RPMI-1640 medium (Sigma). Red blood cells in spleen cells were depleted by cell lysis. Single spleen cells or MLN cells were suspended in complete T-cell medium (CTM) composed of RPMI-1640 medium supplemented with 2 mm l-glutamine, 1 mm sodium pyruvate, 0·1 mm nonessential amino acid, a mixture of vitamins, 1 mm HEPES, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µm 2-mercaptoethanol, and 10% heat-inactivated fetal calf serum (FCS). OVA-specific T-cell proliferation was analysed by the modified method described previously.36 Spleen or MLN T cells were taken using a nylon wool column and a single cell suspension was prepared in the CTM. The spleen or MLN T cells (5 × 105) were cultured with 0, 111, 333 or 1000 mg/ml of OVA in the presence of 2·5 × 105 of irradiated (3000 rad) spleen cells from naive C57BL/6 mice in 96-well flat-bottomed culture plates at 37° in 5% CO2 for 4 days. During the last 18 hr of the 4-day culture, 0·5 μCi of tritiated [3H]thymidine was added to each well. The plates were harvested and counted using a β counter (1450 Microbeta Trilux; Wallac, Gaithersburg, MD).
Cytokine analysis
Levels of IL-4 or IL-2 in the spleen T-cell culture supernatants were analysed by an IL-4-dependent cell line, CT.4S cells (kindly gifted by Prof. William E. Paul, National Institutes of Health, Bethesda, MD), or an IL-2-dependent cell line, CTLL-2 cells (American Type Culture Collection, ATCC, Manassas, VA). T-cell culture supernatants were collected on day 3 and stored frozen at −80° until assay. CT.4S or CTLL-2 cells (5 × 103) were cultured in the presence of the supernatant in 96-well flat-bottomed culture plates at 37° in 5% CO2 for 3 or 2 days, respectively. To draw a standard curve, CT.4S or CTLL-2 cells were cultured with various quantities of recombinant mouse IL-4 (rIL-4) or rIL-2 (Genzyme, Cambridge, MA), respectively. During the last 18 hr of incubation, 0·5 μCi of [3H]thymidine was added to each well. The plates were harvested and counted using the β counter.
Delayed-type hypersensitivity (DTH) response
Mice were anaesthetized and intradermally injected 20 µg of OVA in 20 µl of saline into the right ears. As negative controls, saline was injected into the left ears. Ear thickness was measured using a dial thickness gauge (Ozaki MFG. Co., LTD. Tokyo, Japan) before and 24 hr after the injection. The swelling rate of the ear was calculated as follows: [(thickness of the ear 24 hr after challenge − thickness of the ear before challenge)/thickness of the ear before challenge] × 100%.
Statistical analysis
Student's t-test was used to determine the statistical significance of differences between groups. Data were considered significant at P < 0·05.
Results
Appearance of detectable OVA antigens in both portal and peripheral blood after oral administration of OVA
The oral administration of OVA induces oral tolerance against OVA, and it has been shown that OVA antigens are detected in peripheral blood after the oral administration of OVA.30,31 First, we attempted to detect OVA antigens in the digestive tract and in blood after absorption via the guts.
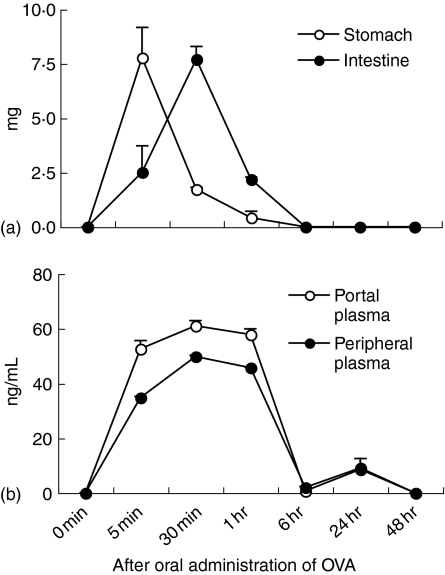
When 25 mg of OVA was orally administered to mice, many OVA antigens in the gastric contents and fewer OVA antigens in the small intestinal contents were detected at 5 min after oral administration (Fig. 1a). While the amount of detectable OVA antigens was reduced at 30 min in the stomach, they reversely increased in the small intestine at the same time. OVA antigens in the guts could not be detected at 6 hr and subsequently (Fig. 1a).
Figure 1.
Kinetics of OVA antigens detected in gut contents and plasma after oral administration of OVA. BALB/c mice were orally administered with 25 mg of OVA. At various times after oral administration, the stomach or small intestine contents were collected (a). Portal and peripheral blood was collected from mice at various times after oral administration (b). Levels of OVA antigens in the gut contents or plasma were assessed by ELISA. The data are expressed as the mean + standard error (SE) of three mice.
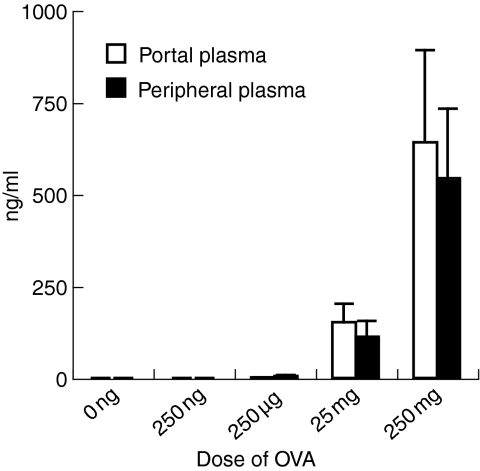
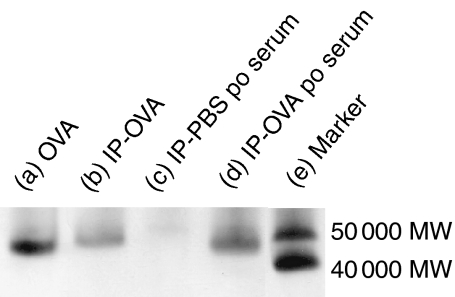
OVA antigens were observed in both portal and peripheral blood at 5 min after the oral administration of OVA (Fig. 1b). They reached a peak at 30 min and became undetected at 6 hr after oral administration (Fig. 1b). Thus, kinetics of OVA antigens in the portal and peripheral blood corresponded to that in the small intestinal contents. The amount of OVA antigens detected in the portal and peripheral blood was dependent on the dose of orally administered OVA (Fig. 2). Levels of OVA antigens in the portal blood were more than in the peripheral blood (Fig. 1b and Fig. 2). OVA antigens were observed in the blood from all strains used in this study, BALB/c, C57BL/6, and BDF1 mice (data not shown). To examine the molecular weights of OVA antigens in the serum, we performed immunoprecipitation and immunoblotting in serum samples using an anti-OVA polyclonal antibody. Surprisingly, 45 000 MW OVA was clearly detected in the serum 30 min after the oral administration of OVA (Fig. 3). In our immunoprecipitation and immunoblotting system using an anti-OVA polyclonal antibody, digested OVA fragments could not be detected in the serum. These results suggest that the macromolecules, 45 000 MW OVA antigens, were absorbed via the small intestines, transferred to the portal vein and circulated in the bloodstream in normal mice, in which oral tolerance was induced.
Figure 2.
Level of OVA antigens in plasma is dependent on the dose of orally administered OVA. BALB/c mice were orally administered with various doses of OVA. Portal and peripheral blood was collected from mice at 30 min after oral administration. Levels of OVA antigens in plasma were assessed by ELISA. The data are expressed as the mean + SE of three mice.
Figure 3.
Immunoblot of OVA antigens in the serum after the oral administration of OVA. C57BL/6 mice were orally administered with 250 mg of OVA or PBS. Blood was collected from the heart 30 min after the oral administration (po) of OVA or PBS. OVA antigens in the serum were detected by immunoprecipitation and immunoblotting. Immunoprecipitation was performed using a rabbit anti-OVA polyclonal antibody on serum from mice or OVA mixed with serum from untreated mice. Immunoprecipitated (IP) samples or 2·5 ng of OVA were loaded. Proteins were transferred to a PVDF membrane and detected using the anti-OVA polyclonal antibody. (a) 2·5 ng of OVA, (b) IP sample of OVA mixed with untreated mouse serum, (c) IP sample of the serum after oral administration of PBS, (d) IP sample of the serum after the oral administration of OVA, (e) molecular markers.
OVA antigens become undetected in the blood with an increase in mucosal and systemic OVA-specific immunoglobulin
Next, we attempted to analyse OVA antigens in blood from mice induced with OVA-specific immune responses by the oral administration of OVA plus CT adjuvant. Mice were orally administered with 1 or 100 mg of OVA or the same dose of OVA plus CT every week. Peripheral blood samples from mice were collected 30 min after oral administration every week and levels of OVA antigens and OVA-specific IgG in the blood were assessed by ELISA. The production of OVA-specific faecal IgA, OVA-specific T-cell proliferation, and secretion of cytokines were also analysed in mice.
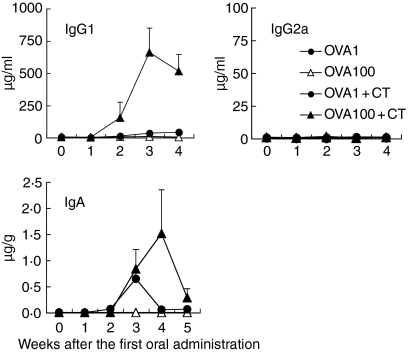
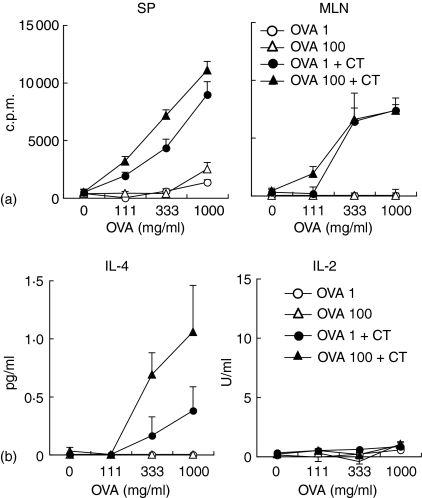
The production of OVA-specific plasma IgG1 and faecal IgA was enhanced after the oral administration of 1 or 100 mg of OVA plus CT; however, the oral administration of OVA without CT did not induce OVA-specific systemic and mucosal antibody production (Fig. 4). OVA-specific serum IgG2a, T helper (Th)1-type antibody, could not be detected by oral administration with and without CT during the experimental period. As shown in Fig. 5, OVA-specific T-cell proliferation was also observed in spleen T cells and MLN T cells from mice orally administered with OVA plus CT (Fig. 5a). IL-4 secretion was observed in culture supernatants from proliferated spleen T cells; however, IL-2 was not detected (Fig. 5b). These results demonstrate that the oral administration of OVA plus CT induces mucosal IgA and Th2-type systemic immune responses.
Figure 4.
Production of OVA-specific systemic IgG and faecal IgA by oral administration of OVA plus CT. C57BL/6 mice were orally administered with 1 or 100 mg of OVA, or the same dose of OVA plus CT once weekly for 5 weeks. Peripheral blood and faeces were collected from the mice every week. OVA-specific plasma IgG1 and IgG2a and faecal IgA were assessed by ELISA. The data are expressed as the mean anti-OVA immunoglobulin in plasma (µg/ml) or faeces (µg/g) + SE of four mice.
Figure 5.
OVA-specific T cell proliferation and secretion of Th2-type cytokine by oral administration of OVA plus CT. C57BL/6 mice were orally administered with 1 mg or 100 mg of OVA, or the same dose of OVA plus CT once weekly for 3 weeks. Spleens and MLNs were removed 2 weeks after the third administration and T cells were isolated. T cells from the spleen or MLN were cultured with 0, 111, 333 or 1000 mg/ml of OVA in the presence of irradiated spleen cells from naive C57BL/6 mice for 4 days. OVA-specific T cell proliferation was assessed as described in Materials and methods (a). Splenic T cell culture supernatants were collected on day 3. IL-4-dependent cell line, CT-4S, or IL-2-dependent cell line, CTLL-2, were cultured in the presence of the supernatant for 3 or 2 days, respectively. Cytokine levels were assessed as described in Materials and methods (b). The results are expressed as the level of orally administered mice minus the level of normal mice. The results are shown as the mean + SE in triplicate. Data are representative of two separate experiments.
Although OVA antigens in the blood were sufficiently detected at 1 week after the oral administration of 100 mg of OVA plus CT, they were remarkably reduced at 2 weeks and subsequently remained reduced (Fig. 6), while OVA-specific systemic IgG1 and mucosal IgA were produced (Fig. 4). On the other hand, in mice orally administered with 100 mg of OVA, OVA antigens were detected every week. When 1 mg of OVA or the same dose of OVA plus CT was orally administered, OVA antigens were undetectable in the blood (Fig. 6).
Figure 6.
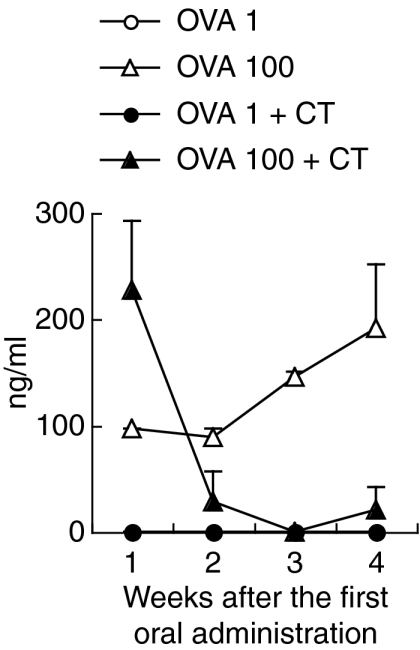
OVA antigens become undetectable after oral administration of OVA plus CT. C57BL/6 mice were orally administered with 1 or 100 mg of OVA or the same dose of OVA plus CT once weekly for 4 weeks. Peripheral blood was collected from the mice 30 min after oral administration every week. OVA antigens in plasma were assessed by ELISA. The data are expressed as the mean + SE of three mice.
These results show that in immunized mice, mucosal anti-OVA IgA may bind OVA antigens in the gastrointestinal tract and block OVA from entering into the mucosal tissues. Furthermore, OVA antigens may be caught by anti-OVA IgG and immune complexes might be undetected in ELISA.
Oral administration of intact OVA suppresses OVA-specific immune responses whereas intraportal or intravenous injection cannot induce immunological suppression
The results indicate that OVA antigens appear in the blood from mice in which oral tolerance is induced, although they are markedly reduced in mice in which OVA-specific immune responses are induced. Therefore, we attempted to induce immunological tolerance against OVA by the intravenous injection of OVA. Mice were orally administered or injected into the portal or peripheral vein with 2·5 mg or 25 mg of OVA, and then i.p. immunized with OVA plus alum. OVA-specific systemic immunoglobulin production and DTH response were assessed in the mice.
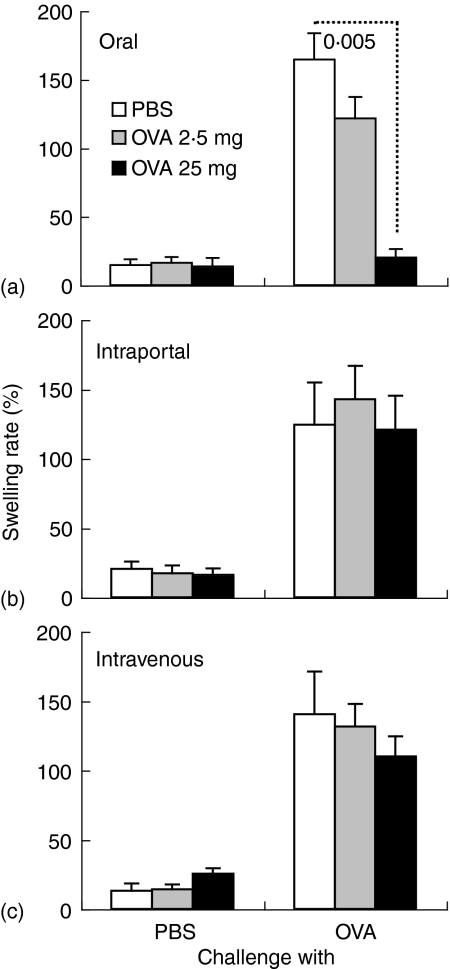
The oral administration of a high dose of intact OVA significantly suppressed DTH response against OVA (P < 0·005), compared with that in the oral treatment of PBS (Fig. 7a). The DTH response, however, was not significantly suppressed by the intraportal or intravenous injection of intact OVA (Fig. 7b, c). When control PBS was injected into ears, DTH response was not induced (Fig. 7).
Figure 7.
OVA-specific DTH response is suppressed by oral administration of intact OVA, but not by intraportal or intravenous injection of intact OVA. BDF1 mice were orally administered (a) or injected into the portal (b) or peripheral vein (c) with 2·5 mg or 25 mg of OVA or PBS, and then i.p. immunized with OVA plus alum twice. DTH response was assessed 2 weeks after the second immunization of OVA plus alum. Ear thickness was measured 24 h after challenge of OVA or PBS into the ear. The data are expressed as the mean + SE of five to six mice. The value represents statistical significance (P <) compared with control mice treated with PBS in the same way.
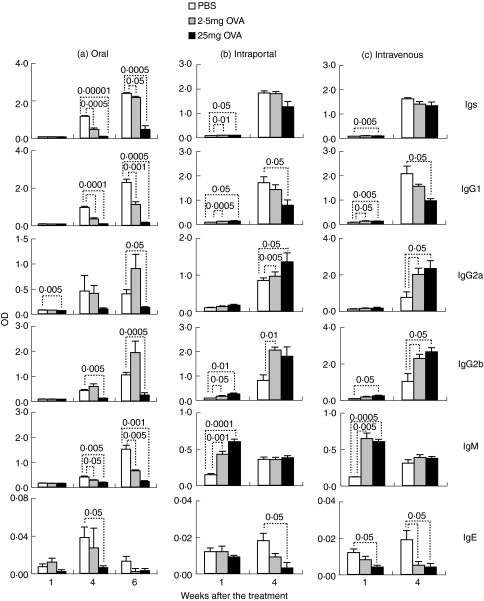
Oral administration of OVA also significantly suppressed the production of anti-OVA immunoglobulins (Fig. 8a), whereas the production was significantly enhanced rather than suppressed at 1 week after the intraportal or intravenous injection of OVA (Fig. 8b, c). All immunoglobulin subclasses, IgG1, IgG2a, IgG2b, IgM and IgE, were significantly suppressed by the oral administration of OVA (Fig. 8a). On the other hand, in mice injected with OVA intraportally or intravenously, anti-OVA IgG1 at 1 week, IgG2a, IgG2b and IgM were significantly enhanced, although IgG1 at 4 weeks and IgE were suppressed (Fig. 8b, c).
Figure 8.
Production of anti-OVA immunoglobulins is suppressed by the oral administration of intact OVA, but not by intraportal or intravenous injection of intact OVA. BDF1 mice were orally administered (a) or injected into the portal (b) or peripheral vein (c) with 2·5 mg or 25 mg of OVA or PBS. The mice were i.p. immunized with OVA plus alum 1 and 3 weeks after the first oral administration or injection of intact OVA. Orally treated mice were additionally i.p. immunized with OVA 5 weeks after the first oral administration. Peripheral blood was collected at various weeks. Anti-OVA immunoglobulins in plasma were assessed by ELISA. Data are expressed as the mean of OD (415 nm) + SE of five to six mice. Each value represents statistical significance (P <), compared with control mice treated with PBS in the same way.
The data clearly show that the injection of intact OVA into the portal or peripheral vein cannot induce immunological tolerance but rather enhances part of immune responses against OVA.
The injection of intact OVA into the lower intestinal tract is less effective in terms of OVA-specific tolerance induction
We therefore investigated whether the modification of intact OVA by gastrointestinal digestion was essential to induce oral tolerance. Mice were orally administered or injected into the guts, stomach, duodenum, ileum, or colon, with 25 mg of intact OVA. OVA-treated and untreated mice were i.p. immunized with OVA plus alum twice, and then OVA-specific plasma immunoglobulin and DTH response were assessed.
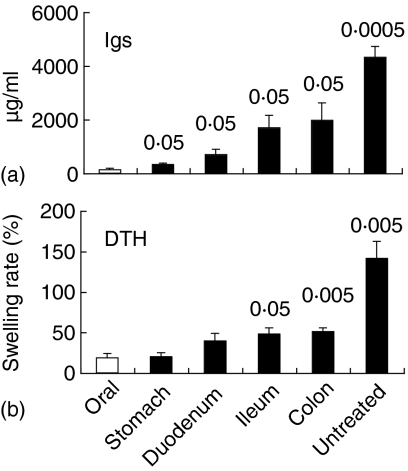
OVA-specific immunoglobulin production was remarkably suppressed by the oral administration of intact OVA, whereas immunoglobulin was sufficiently produced in untreated mice (Fig. 9a). The injection of intact OVA into the stomach, duodenum, ileum, or colon significantly enhanced OVA-specific immunoglobulin production (P < 0·05), compared with the oral administration of intact OVA (Fig. 9a). DTH response against OVA was also significantly enhanced in mice injected with intact OVA into the ileum or colon (P < 0·05 or 0·005, respectively), compared with the oral administration of OVA (Fig. 9b). Levels of OVA-specific immunoglobulins and DTH responses were higher in mice injected with intact OVA into the lower intestinal tract.
Figure 9.
Less effective induction of immunological tolerance by injection of intact OVA into lower intestinal tract. BDF1 mice were orally administrated or injected into the guts with 25 mg of OVA. OVA-treated and untreated mice were i.p. immunized with OVA plus alum 1 and 3 weeks after OVA treatment. Peripheral blood was collected 1 week after the second immunization of OVA plus alum. Plasma anti-OVA immunoglobulin was assessed by ELISA (a). DTH response against OVA was assessed 6 weeks after the second immunization (b). Data are expressed as the mean + SE of 3–9 mice. Each value represents statistical significance (P <), compared with mice orally administered with OVA.
The results demonstrate that the appropriate digestion of intact OVA in the gastrointestinal tract is crucial for the induction of oral tolerance against OVA.
Discussion
In this study, we showed that after the oral administration of OVA, OVA antigens were absorbed via the small intestines, transferred into the liver via the portal vein, and circulated in the bloodstream. This suggests that digestive enzymes do not completely digest OVA to amino acids and small peptides that do not have antigenicity, and OVA antigens can cross the intestinal surface.
M cells that exist over PPs uptake soluble macromolecule proteins.10–12 OVA antigens may be taken up by M cells at the intestinal surface and then they may be processed and presented by immature DCs in PPs, which are shown to be inductive sites for oral tolerance where T cells secreting regulatory cytokines, IL-1021 and TGF-β,22 are induced. DCs may capture OVA antigens, present antigen epitopes to naïve T cells, and induce regulatory T cells in PPs. M cells are shown to also exist in small intestinal villi,37 suggesting that antigens may be taken up by M cells apart from PPs. Moreover, DCs may send dendrites between epithelial cells and acquire OVA antigens over epithelia. Thus, some of the OVA antigens that cross the intestinal surface are captured by DCs in these intestinal mucosal tissues.
A protein antigen is digested by digestive enzymes to amino acids and small peptides.9 They are absorbed via epithelial cells, and enter the portal vein through capillary vessels in the small intestine. After the oral administration of OVA to mice, OVA antigens are detected in peripheral blood.30,31 Furthermore, our results clearly showed that OVA antigens, 45 000 MW of proteins, enter the portal vein and then the bloodstream after the oral administration of OVA. OVA antigens taken up by M cells, or using other routes, may enter the portal vein via capillary vessels in the small intestine. Some allergens have been shown to cross the mucosal barrier by the disruption of tight junctions.38,39 Notably, it has been shown that soluble protein antigens are rapidly pinocytosed by enterocytes and co-localized with major histocompatibility complex (MHC) class II in a vesicular compartment.40 Bland et al. proposed that intestinal epithelial cells (IECs) presented antigens on their surface to local T cells41 and induced suppressor T cells.42 Recently, it has been reported that tolerosomes, which have an exosome-like structure and carry MHC class II with antigens, were released from IECs into serum.43 It has also been shown that tolerosome from mouse serum 1 hr after the oral administration of OVA-induced oral tolerance and this induction was MHC class II dependent.44 Hereafter, it is necessary to examine whether the 45 000 MW OVA antigens detected are free antigens or are included in exosomes in mouse serum.
It has been demonstrated that CD25-positive cells are crucial to immunological suppression by the transfer of serum after the oral administration of OVA.32 The liver has been shown to contribute to tolerance induction because the intraportal injection of allogeneic cells24,25 eggs of a parasite26 or insoluble protein27 induces immunological tolerance against the antigen. It is reported that liver endothelial cells endocytose OVA by a mannose receptor, CD206,45,46 and antigen presentation by cells induces T-cell tolerance against OVA.46 In this study, however, the injection of intact OVA into the portal or peripheral vein did not induce immunological tolerance but rather enhanced part of OVA-specific antibody production. As mannose receptors are also expressed on macrophages in red pulp in the spleen47 intact OVA may be captured by macrophages in the spleen after intravenous injection. In our experimental system, these antigen-presenting cells (APCs) in the liver and spleen may not induce immunological tolerance when they endocytose intact OVA.
The uptake of intact antigens untreated with digestive enzymes may lead to immunological enhancement such as allergy. Ileal injection of BSA treated with pepsin induces immunological tolerance against BSA, whereas ileal injection of intact BSA enhances anti-BSA responses.48 Correspondingly, in this study, the injection of intact OVA into the ileum or colon significantly enhanced both OVA-specific antibody production and DTH response. Induction of oral tolerance was more difficult when intact OVA was injected into the lower intestinal tract. It is reported that the impairment of gastric digestion of caviar extracts significantly enhanced caviar-specific IgG1, IgG2a, and IgE levels in mice.49 In addition, cod proteins treated with pepsin show reduced IgE-binding capability and reduced histamine release from human basophils.50 In the previous study, we demonstrated that oral tolerance against sheep red blood cells (SRBCs) was induced in young mice but rather SRBC-specific antibody response was enhanced in aged mice by the oral administration of SRBC.51 Digestive and absorptive capacity is decreased in elderly people.52 Reduced digestive capacity in aged mice might result in the failure of oral tolerance induction. In this report, it was shown that the absolute gastrointestinal ingestion of OVA via the upper gastrointestinal tract is crucial for oral tolerance induction. As macromolecular OVA antigens but not digested antigen fragments are detected in tolerant-mice serum, not only digestion but also some modification of macromolecular OVA in the gastrointestinal tract may be essential for oral tolerance induction.
The detection of OVA antigens has been shown in mouse serum 1 hr after the oral administration of OVA, and serum transfer induced significant suppression of OVA-specific immune responses.31 Recently, it was shown that tolerosomes including MHC class II are produced by IEC at 1 hr after the oral administration of OVA, and tolerosomes induce oral tolerance.44 Also in our results, OVA antigens were remarkably detected at 30 min and 1 hr after the oral administration of OVA.
In this study, it was clearly demonstrated that the absolute gastrointestinal ingestion of OVA via the upper gastrointestinal tract is crucial to the establishment of oral tolerance. Although macromolecular OVA antigens are detected after the oral administration of OVA in tolerant-mouse serum, the injection of intact OVA cannot induce tolerance. Therefore, some modification of macromolecular OVA in the gastrointestinal tract and ingestion may be essential for oral tolerance induction.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Sciences (JSPS). The authors are grateful to Dr Eiji Shinya, Ms Atsuko Owaki and Dr Megumi Takahashi for advice on the immunoprecipitation and immunoblotting.
Abbreviations
- ABTS
2,2′-azino-bis diammonium salt
- APC
antigen-presenting cell
- BSA
bovine serum albumin
- CT
cholera toxin
- CTM
complete T-cell medium
- DC
dendritic cell
- DTH
delayed-type hypersensitivity
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- IL
interleukin
- i.p.
intraperitoneally
- IP
immunoprecipitated
- M cell
microfold cell
- MHC
major histocompatibility complex
- IEC
intestinal epithelial cell
- MLN
mesenteric lymph node
- OVA
ovalbumin
- PBS
phosphate-buffered saline
- PP
Peyer's patch
- SE
standard error
- SRBC
sheep red blood cell
- TGF
transforming growth factor
- Th
T helper
- TMB
tetramethyl benzidine
References
- 1.Mowat AM, Strobel S, Drummond HE, Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982;45:105–13. [PMC free article] [PubMed] [Google Scholar]
- 2.Lider O, Santos LM, Lee CS, Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J Immunol. 1989;142:748–52. [PubMed] [Google Scholar]
- 3.Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. doi: 10.1016/j.jaci.2004.11.008. quiz 3. [DOI] [PubMed] [Google Scholar]
- 4.Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000;106(1 Part 1):27–36. doi: 10.1067/mai.2000.106929. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006;117(2 Suppl.):S470–5. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Khakoo A, Lack G. Preventing food allergy. Curr Allergy Asthma Rep. 2004;4:36–42. doi: 10.1007/s11882-004-0041-1. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL, Friedman A, Miller A, et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 8.Ma S, Jevnikar AM. Autoantigens produced in plants for oral tolerance therapy of autoimmune diseases. Adv Exp Med Biol. 1999;464:179–94. doi: 10.1007/978-1-4615-4729-7_14. [DOI] [PubMed] [Google Scholar]
- 9.Erickson RH, Kim YS. Digestion and absorption of dietary protein. Annu Rev Med. 1990;41:133–9. doi: 10.1146/annurev.me.41.020190.001025. [DOI] [PubMed] [Google Scholar]
- 10.Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. 1973;136:455–77. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- 11.von Rosen L, Podjaski B, Bettmann I, Otto HF. Observations on the ultrastructure and function of the so-called ‘microfold’ or ‘membraneous’ cells (M cells) by means of peroxidase as a tracer. Virchows Arch a Pathol Anat Histol. 1981;390:289–312. doi: 10.1007/BF00496560. [DOI] [PubMed] [Google Scholar]
- 12.Neutra MR, Phillips TL, Mayer EL, Fishkind DJ. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res. 1987;247:537–46. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- 13.Wolf JL, Kauffman RS, Finberg R, Dambrauskas R, Fields BN, Trier JS. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983;85:291–300. [PubMed] [Google Scholar]
- 14.Sicinski P, Rowinski J, Warchol JB, Jarzabek Z, Gut W, Szczygiel B, Bielecki K, Koch G. Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology. 1990;98:56–8. doi: 10.1016/0016-5085(90)91290-m. [DOI] [PubMed] [Google Scholar]
- 15.Amerongen HM, Weltzin R, Farnet CM, Michetti P, Haseltine WA, Neutra MR. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4:760–5. [PubMed] [Google Scholar]
- 16.Owen RL, Pierce NF, Apple RT, Cray WC., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986;153:1108–18. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- 17.Grutzkau A, Hanski C, Hahn H, Riecken EO. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–5. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J Exp Med. 1996;183:237–47. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 21.Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR. Peyer's patches are required for oral tolerance to proteins. Proc Natl Acad Sci USA. 2001;98:3310–5. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji NM, Mizumachi K, Kurisaki J. Antigen-specific, CD4+ CD25+ regulatory T cell clones induced in Peyer's patches. Int Immunol. 2003;15:525–34. doi: 10.1093/intimm/dxg051. [DOI] [PubMed] [Google Scholar]
- 23.Spahn TW, Fontana A, Faria AM, et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur J Immunol. 2001;31:1278–87. doi: 10.1002/1521-4141(200104)31:4<1278::aid-immu1278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Nakano Y, Monden M, Valdivia LA, Gotoh M, Tono T, Mori T. Permanent acceptance of liver allografts by intraportal injection of donor spleen cells in rats. Surgery. 1992;111:668–76. [PubMed] [Google Scholar]
- 25.Yu S, Nakafusa Y, Flye MW. Portal vein administration of donor cells promotes peripheral allospecific hyporesponsiveness and graft tolerance. Surgery. 1994;116:229–34. discussion 34–5. [PubMed] [Google Scholar]
- 26.Cuison A, Tasaka K, Chuang CK, Minai M, Yoshikawa H, Nakajima Y. Schistosome eggs in the portal vein can induce tolerance. Int J Parasitol. 1995;25:993–8. doi: 10.1016/0020-7519(95)00004-l. [DOI] [PubMed] [Google Scholar]
- 27.Ogasawara H, Watari E, Shirai Y, Yokomuro K. [Inhibitory effects of portal venous injection of type II collagen in collagen-induced arthritis] Nippon Ika Daigaku Zasshi. 1997;64:220–4. doi: 10.1272/jnms1923.64.220. [DOI] [PubMed] [Google Scholar]
- 28.May CD, Remigio L, Feldman J, Bock SA, Carr RI. A study of serum antibodies to isolated milk proteins and ovalbumin in infants and children. Clin Allergy. 1977;7:583–95. doi: 10.1111/j.1365-2222.1977.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 29.Vance GH, Thornton CA, Bryant TN, Warner JA, Warner JO. Ovalbumin-specific immunoglobulin G and subclass responses through the first 5 years of life in relation to duration of egg sensitization and the development of asthma. Clin Exp Allergy. 2004;34:1542–9. doi: 10.1111/j.1365-2222.2004.02058.x. [DOI] [PubMed] [Google Scholar]
- 30.Bruce MG, Ferguson A. The influence of intestinal processing on the immunogenicity and molecular size of absorbed, circulating ovalbumin in mice. Immunology. 1986;59:295–300. [PMC free article] [PubMed] [Google Scholar]
- 31.Peng HJ, Turner MW, Strobel S. The generation of a ‘tolerogen’ after the ingestion of ovalbumin is time-dependent and unrelated to serum levels of immunoreactive antigen. Clin Exp Immunol. 1990;81:510–5. doi: 10.1111/j.1365-2249.1990.tb05365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlsson MR, Kahu H, Hanson LA, Telemo E, Dahlgren UI. Tolerance and bystander suppression, with involvement of CD25-positive cells, is induced in rats receiving serum from ovalbumin-fed donors. Immunology. 2000;100:326–33. doi: 10.1046/j.1365-2567.2000.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato H, Fujihashi K, Kato R, Yuki Y, McGhee JR. Oral tolerance revisited: prior oral tolerization abrogates cholera toxin-induced mucosal IgA responses. J Immunol. 2001;166:3114–21. doi: 10.4049/jimmunol.166.5.3114. [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi A, Eishi Y, Nakamura K. Development of the immune system in severe combined immunodeficiency mice reconstituted with transferred fetal liver cells. J Med Dent Sci. 1997;44:21–9. [PubMed] [Google Scholar]
- 35.Wakabayashi A, Eishi Y, Nakamura K. Regulation of experimental autoimmune orchitis by the presence or absence of testicular antigens during immunological development in SCID mice reconstituted with fetal liver cells. Immunology. 1997;92:84–90. doi: 10.1046/j.1365-2567.1997.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kweon MN, Fujihashi K, VanCott JL, Higuchi K, Yamamoto M, McGhee JR, Kiyono H. Lack of orally induced systemic unresponsiveness in IFN-gamma knockout mice. J Immunol. 1998;160:1687–93. [PubMed] [Google Scholar]
- 37.Jang MH, Kweon MN, Iwatani K, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110–5. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan H, Winton HL, Soeller C, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–33. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mine Y, Zhang JW. Surfactants enhance the tight-junction permeability of food allergens in human intestinal epithelial Caco-2 cells. Int Arch Allergy Immunol. 2003;130:135–42. doi: 10.1159/000069009. [DOI] [PubMed] [Google Scholar]
- 40.Gonnella PA, Wilmore DW. Co-localization of class II antigen and exogenous antigen in the rat enterocyte. J Cell Sci. 1993;106:937–40. doi: 10.1242/jcs.106.3.937. [DOI] [PubMed] [Google Scholar]
- 41.Bland PW, Warren LG. Antigen presentation by epithelial cells of the rat small intestine. I. Kinetics, antigen specificity and blocking by anti-Ia antisera. Immunology. 1986;58:1–7. [PMC free article] [PubMed] [Google Scholar]
- 42.Bland PW, Warren LG. Antigen presentation by epithelial cells of the rat small intestine. II. Selective induction of suppressor T cells. Immunology. 1986;58:9–14. [PMC free article] [PubMed] [Google Scholar]
- 43.Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E. ‘Tolerosomes’ are produced by intestinal epithelial cells. Eur J Immunol. 2001;31:2892–900. doi: 10.1002/1521-4141(2001010)31:10<2892::aid-immu2892>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Ostman S, Taube M, Telemo E. Tolerosome-induced oral tolerance is MHC dependent. Immunology. 2005;116:464–76. doi: 10.1111/j.1365-2567.2005.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kindberg GM, Magnusson S, Berg T, Smedsrod B. Receptor-mediated endocytosis of ovalbumin by two carbohydrate-specific receptors in rat liver cells. The intracellular transport of ovalbumin to lysosomes is faster in liver endothelial cells than in parenchymal cells. Biochem J. 1990;270:197–203. doi: 10.1042/bj2700197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Limmer A, Ohl J, Kurts C, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–54. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 47.Linehan SA, Martinez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–72. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michael JG. The role of digestive enzymes in orally induced immune tolerance. Immunol Invest. 1989;18(9–10):1049–54. doi: 10.3109/08820138909030606. [DOI] [PubMed] [Google Scholar]
- 49.Untersmayr E, Scholl I, Swoboda I, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–23. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 50.Untersmayr E, Poulsen LK, Platzer MH, Pedersen MH, Boltz-Nitulescu G, Skov PS, Jensen-Jarolim E. The effects of gastric digestion on codfish allergenicity. J Allergy Clin Immunol. 2005;115:377–82. doi: 10.1016/j.jaci.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Wakabayashi A, Utsuyama M, Hosoda T, Sato K, Hirokawa K. Differential age effect of oral administration of an antigen on antibody response: an induction of tolerance in young mice but enhancement of immune response in old mice. Mech Ageing Dev. 1999;109:191–201. doi: 10.1016/s0047-6374(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 52.Masamune O. Digestive and absorptive capacity in the elderly. Rinsho Byori. 1989;37:629–33. [PubMed] [Google Scholar]