Abstract
Immune serum has a protective role against Salmonella infections in mice, domestic animals and humans. In this study, the effect of antibody on the interaction between murine macrophages and S. enterica serovar Typhimurium was examined. Detailed analysis at the single-cell level demonstrated that opsonization of the bacteria with immune serum enhanced bacterial uptake and altered bacterial distribution within individual phagocytic cells. Using gene-targeted mice deficient in individual Fc gamma receptors it was shown that immune serum enhanced bacterial internalization by macrophages via the high-affinity immunoglobulin G (IgG) receptor, Fc gamma receptor I. Exposure of murine macrophages to S. enterica serovar Typhimurium opsonized with immune serum resulted in increased production of superoxide, leading to enhanced antibacterial functions of the infected cells. However, opsonization of bacteria with immune serum did not increase either nitric oxide production in response to S. enterica serovar Typhimurium or fusion of phagosomes with lysosomes.
Keywords: antibody, Fc receptors, infection, macrophages, Salmonella
Introduction
Salmonella enterica infections are a serious medical and veterinary problem worldwide. S. enterica serovar Typhi infections in humans (typhoid fever) are still endemic in many developing countries, with a global burden of about 22 million cases and at least 200 000 deaths.1 Other Salmonella serotypes (e.g. S. enterica serovars Typhimurium, Enteritidis and Cholerasuis), which can spread from animals to humans via contaminated food, are currently a severe health hazard worldwide and cause substantial financial losses in the food industry.2
S. enterica infects animals and humans via the oral route, reaches the distal ileum and the caecum, and invades epithelial and M cells in the gut using the type III secretion system (TTSS) encoded by genes within the Salmonella pathogenicity island 1 (SPI-1).3 From the gut, the bacteria distribute to the spleen, liver and bone marrow, where they are seen mainly within phagocytic cells.
S. enterica mainly reside and replicate within phagocytes and therefore the interactions between the bacteria and macrophages are crucial to the outcome of the infection.4,5 Macrophages can kill or limit the replication of intracellular S. enterica by lysosomal enzymes, production of reactive oxygen intermediates (ROI), reactive nitrogen intermediates (RNI) and antimicrobial peptides.6,7 The solute carrier family 11a member 1 (Slc11a1) gene, which encodes a divalent metal ion pump at the membrane of the late endosome, is also involved in restraining S. enterica growth within macrophages.8 Activation of macrophages by cytokines, such as tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), increases the ability of these cells to control the intracellular growth of S. enterica,9,10 whereas other cytokines, such as interleukin-10 and interleukin-4, suppress the antimicrobial functions of mononuclear cells.11,12
The ability to grow within individual macrophages and evade killing by these cells are prerequisites for S. enterica virulence.13 Intracellular survival is likely to depend on a fine balance between the expression and targeting of antibacterial components to the Salmonella-containing vacuole (SCV) and the function of bacterial genes that allow Salmonella to evade intracellular killing. For example, genes within S. enterica pathogenicity island 2 (SPI-2) encode a TTSS that translocates effector proteins into the host cell, thus counteracting the localization of ROI and RNI to the phagolysosome.14,15Sod genes contribute to the scavenging of potentially harmful ROI.16 The phoP/phoQ regulon is involved in resistance to defensins and inhibits the fusion of SCV with lysosomes and endosomes.17,18
Despite the fact that S. enterica mainly reside inside phagocytes, the bacteria are transiently present in the extracellular compartment during infection. After oral infection, S. enterica transit from the gut to the spleen, liver and bone marrow via the lymph and blood. Bacteraemia during systemic salmonellosis has been described in animals and humans.19–21 Bacterial growth in the tissues (i.e. a net increase in bacterial numbers) is paralleled by increases in the number of infected phagocytes owing to the release of the bacteria from infected cells into the extracellular compartment from where they enter non-infected cells.4,22 In the extracellular compartment, S. enterica is targeted by specific antibodies that mediate the uptake of the bacteria by phagocytes via the interaction with Fc or complement receptors. The crucial role of antibody in host resistance to S. enterica is supported by the observation that the expression of high levels of host resistance against these bacteria requires the presence of immune serum (IS) (anti-S. enterica immunoglobulin) in addition to T-cell-mediated immunity.23 Antibodies specific for the lipopolysaccharide O-antigen, porins and flagellar epitopes and surface polysaccharides (Vi antigen) have been implicated in serum-mediated protection.24 Furthermore, vaccines consisting of whole S. enterica or purified bacterial polysaccharide preparations (Vi vaccine), which elicit good anti-S. enterica immunogloblulin responses, but fail to trigger protective T-cell responses, confer a degree of protection against typhoid fever in humans.25–27
Coupling antibody responses to phagocytic cells through the Fc region of antibodies is a fundamental objective of effective vaccine-induced humoral responses. In other infection models, mainly based on extracellular bacteria (e.g. Streptococcus), it has been shown that opsonization with IS can increase phagocytosis.28 Triggering the Fc receptor (FcR) can increase antibacterial functions of macrophages, including the production of ROI29 and cytokine production by these cells,30–32 leading to enhanced bactericidal activity of the macrophage.33
Fc receptors provide the link between antibody and cells. Mice express three receptors for immunoglobulin G (IgG): FcγRI; FcγRII; and FcγRIII. Two of these are activating receptors (FcγRI and FcγRIII) and the other is an inhibitory receptor (FcγRII). A potential fourth Fcγ receptor has also been recently reported.34 FcγRII and FcγRIII are low-affinity receptors for IgG and widely expressed on haemopoietic cells. FcγRI is a high-affinity receptor for monomeric IgG2a, which is expressed on macrophages, monocytes and dendritic cells, and its expression is up-regulated by IFN-γ.35 The activating receptors signal via two membrane-bound gamma chains containing immunoreceptor tyrosine-based activation motifs that are phosphorylated upon cross-linking of the receptor.36 FcγRII signals through an immunoreceptor tyrosine-based inhibitory motif, resulting in the inhibition of many of the functions activated by FcγRI and FcγRIII.36
Despite the crucial role of humoral immunity in host resistance to S. enterica in animals and humans, it is still unclear how antibodies exert their protective effects. Little is known regarding how IS affects the interaction of S. enterica with macrophages. In the present study, we have investigated how the humoral response, elicited by a protective live attenuated vaccine, modulates the interaction of S. enterica with murine bone marrow-derived macrophages (BMDM). We investigated how IS affects bacterial internalization and the dynamics of distribution at a single-cell level. We studied how opsonization modulates the expression of macrophage antibacterial mechanisms and how this can affect bacterial survival within macrophages. We also analysed the relative importance of individual Fcγ receptors in the interaction of IS-opsonized S. enterica with macrophages.
Materials and methods
All reagents and media were obtained from Sigma-Aldrich (Poole, UK), unless stated otherwise.
Animals
Wild-type (WT) female C57BL/6 mice were obtained from Harlan Olac Ltd (Blackthorn, Bichester, UK). Slc11a1[formerly natural resistance-associated macrophage protein 1 (Nramp1)] encodes a late endosomal/lysosomal divalent cation transporter, which regulates iron homeostasis in macrophages.37,38Cybb encodes the gp91 subunit of cytochrome b-245, an essential component of phagocyte NADPH oxidase. Nos2A encodes inducible nitric oxide synthase (iNOS).8FcγR1, FcγR2 and FcγR3 encode FcγRI, FcγRII and FcγRIII, respectively. Slc11a1–/– Cybb–/–, Slc11a1+/+ Nos2A–/– and Slc11a1+/+ Nos2A+/+ mice8 were bred from animals provided by Prof. J. M. Blackwell (University of Cambridge, UK). FcγRI–/– and FcγRIII–/– mice were bred from animals provided by Dr J. S. Verbeek (University of Leiden, the Netherlands).39,40Slc11a1–/– FcγR2–/– mice were provided by Dr K. G. Smith (University of Cambridge, UK).41 All the aforementioned mice were on a C57BL/6 background. Slc11a1–/– FcγR1–/– FcγR2–/– FcγR3–/– mice, lacking simultaneously FcγRI, -II and -III, as well as WT control mice, were on a 129Ola/C57Bl6 background (n = 5). Strain-, age- and gender-matched controls were used in all experiments.
Bacterial strains
S. enterica serovar Typhimurium SL1344 is a mouse virulent strain.42S. enterica serovar Typhimurium SL1344–GFP constitutively expresses the green fluorescent protein (GFP) from Aequoria victoria from a single-copy gfp gene integrated into the chromosome.43 This strain was obtained from Dr I. Hautefort, Institute of Food Research, Norwich, UK. S. enterica serovar Typhimurium 12023 and S. enterica serovar Typhimurium 12023 phoP–/– were obtained from Prof. D. Holden (Imperial College, London, UK). These strains contained plasmid pFPV25.1, carrying gfpmut3a under the control of a constitutive promoter for green fluorescence visualization.18 Bacteria were grown at 37° as stationary overnight cultures in Luria–Bertani (LB) broth.
To opsonize bacteria, cultures were spun and resuspended in an equal volume of phosphate-buffered saline (PBS) containing a 1 : 250 dilution of IS obtained from C57BL/6 mice previously immunized intravenously with 106 colony-forming units (CFU) of the attenuated aroA–S. enterica serovar Typhimurium SL3261 vaccine strain. The profile of anti-Salmonella immunoglobulins in the IS was similar to that previously reported.27,44 The serum contained detectable levels of IgG2a, IgG2b, IgG1 and IgG3 subclasses, as well as immunoglobulin M (IgM) and immunoglobulin A (IgA), as measured by enzyme-linked immunosorbent assay (ELISA) (data not shown). Normal mouse serum (NMS), from non-immunized C57BL/6 mice, was used as a control. IS and NMS were heat treated for 45 min at 55°. The IS was used at the lowest dilution (1 : 250) that would not induce agglutination of the bacterial cells. Opsonization was performed by shaking (180 r.p.m., 37°, 30 min). Pilot experiments indicated that the uptake of bacteria exposed to a 1 : 250 dilution of NMS was similar to that of non-opsonized bacteria (no serum added). Bacteria were then washed once and resuspended in PBS. Effective opsonization of the bacterial suspension was confirmed by immunofluorescence after staining the bacteria with goat anti-mouse IgG Alexa 568-conjugated (BD Biosciences, Cowley, UK), diluted 1 : 100 in 10% normal goat serum (NGS) and examination of individual bacterial cells with a Leica DM6000B fluorescence microscope using the Leica FW4000 software (Leica, Milton Keynes, UK).
Preparation of BMDM
Femurs were flushed with Hanks' balanced salt solution and the cells were grown at 37° in a 5% CO2 atmosphere on bacterial Petri dishes in RPMI-1640 supplemented with 10% fetal calf serum, 2 mm l-glutamine, 0·05 mm 2-mercaptomethanol, 1 mm sodium pyruvate, 4% horse serum and 15% medium from L929 cells (BMDM medium). After 7 days of culture, cells were firmly adherent, uniformly CD11b+ and CD11c–, and capable of internalizing latex beads, as determined by flow cytometry and fluorescence microscopy. Expression of individual FcR on cells from WT and gene-targeted mice was routinely monitored by flow cytometry (data not shown).
Visualization of intra- and extracellular bacteria by fluorescence microscopy
BMDM were seeded on 22 × 22-mm glass coverslips (Fisher Scientific, Loughborough, UK), at 3 × 105 cells per coverslip, 12 hr before use, and cultured as described above. BMDM were infected at a bacteria : cell ratio of 10 : 1 with IS- or NMS-opsonized S. enterica serovar Typhimurium SL1344–GFP, resuspended in RPMI-1640, and incubated at 37°, 5% CO2 for 15 or 45 min. Cells were fixed on the coverslips with 4% paraformaldehyde for 15 min before being washed twice in PBS containing 0·05% Tween 20 (PBS–Tween). Coverslips were then incubated in diluent buffer [PBS containing 10% NGS (DakoCytomation, Ely, UK)], and in some cases 0·02% saponin, for 30 min at 37° and washed twice in PBS–Tween. BMDM were immunostained with rabbit anti-Salmonella O4 antigen serum (Murex Biotech Ltd, Dartford, UK) and 1 µg/ml rat anti-mouse CD18 (BD Biosciences) for 1 hr at 37°. BMDM were then washed three times, after which 5 µg/ml Pacific Blue-conjugated goat anti-rabbit IgG and 5 µg/ml Alexa Fluor 568-conjugated goat anti-rat IgG were added (Invitrogen-Molecular Probes, Paisley, UK) for 30 min at 37°. The cells were then washed five times in PBS–Tween and the specimens were mounted with Vectashield (Vector Laboratories, Peterborough, UK) and observed using a Leica DM6000B fluorescence microscope.
Survival of S. enterica within BMDM, evaluated by gentamicin protection assays
BMDM were seeded in 24-well plates (Fisher Scientific, Loughborough, UK), 12 hr prior to use, at a concentration of 5 × 105 cells per well and cultured as described above. Cells were infected at a bacteria : cell ratio of 10 : 1 with IS- or NMS-opsonized S. enterica serovar Typhimurium SL1344 and incubated for 15 or 45 min at 37° in a 5% CO2 atmosphere. Cells were washed three times in RPMI-1640 to remove extracellular bacteria. RPMI-1640 containing 100 µg/ml gentamicin was added to each well for 1 hr at 37° and then was removed and replaced with medium containing 10 µg/ml gentamicin. This was taken as time point 0. At this time and at specific time-points thereafter, BMDM were lysed using 1 ml of 0·1% Triton X-100 per well and viable bacteria in the lysates were counted in pour plates of LB agar.
Measurement of ROI and RNI
BMDM were cultured overnight in 96-well clear flat-bottom white plates (Fisher Scientific), at 5 × 105 cells per well, in BMDM medium containing 50 U/ml recombinant IFN-γ. For the measurement of ROI, medium was replaced with fresh RPMI containing 25 µm lucigenin and stimulant [0·2 µm phorbol 12-myristate 13-acetate (PMA) or opsonized bacteria (bacteria : cell ratio of 10 : 1)]. Chemiluminesence was then measured using a luminometer (Lumistar Galaxy; BMG Labtech, Eylesbury, UK).
The accumulation of nitrite, a stable metabolite of the reaction of nitric oxide (NO) with oxygen, within the supernatant of infected BMDM was measured by the Griess reaction. An equal volume of Griess reagent (0·05%N-1-naphthylethylenediamide hydrochloride, 0·5% sulphanilamide in 2·5% phosphoric acid) was added to BMDM supernatants and the absorbance was measured at 550 nm. The nitrite concentration was determined from a standard curve prepared using sodium nitrite (2–200 µm), 2 µm being the lower limit of detection.
Colocalization of S. enterica serovar Typhimurium with Texas Red ovalbumin and cathepsin D
Colocalization of S. enterica serovar Typhimurium with Texas Red ovalbumin (TROv; Invitrogen Molecular Probes) and cathepsin D was performed as a modification of the method described by Garvis et al.18 Briefly, BMDM were cultured overnight on glass coverslips (Fisher Scientific) at 2·5 × 105 cells per coverslip.
For experiments investigating the colocalization of TROv with bacteria, cells were pulsed with 50 µg/ml TROv for 30 min and then incubated for 1 hr to allow colocalization of TROv to the lysosomes. The pulsed BMDM were exposed with IS- or NMS-opsonized bacteria (bacteria : cell ratio of 10 : 1) for 45 min. Cells were then washed three times with RPMI-1640 and incubated for 1 hr with medium containing 100 µg/ml gentamicin. Following the incubation, medium was removed and replaced with BMDM medium containing 10 µg/ml gentamicin and the cells were incubated for a further 24 hr. BMDM were then fixed in 4% paraformaldehyde for 15 min and washed three times in PBS containing 1% Tween 20.
For experiments investigating the colocalization of cathepsin D with bacteria, BMDM were infected, as described above, and permeabilized for 30 min at 37° with PBS containing 1% saponin and 10% NGS. Cells were immunostained with 5 µg/ml goat anti-mouse cathepsin D polyclonal antibody (R & D systems, Abingdon, UK) for 1 hr at 37°, washed three times and incubated with 5 µg/ml Alexa fluor 568 donkey anti-goat IgG for 1 hr at 37°.
BMDM were then washed five times and the specimens were mounted with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Peterborough, UK) and observed using a Leica TCS-NT-UV confocal laser-scanning microscope.
Statistical analysis
Statistical analyses of the numbers of infected cells and the distribution of bacteria within infected cells were undertaken. Statistical significance was set at P = 0·05.
Differences in the proportion of infected cells were compared statistically between cell types using the Mantel–Haenszel test. Any effect of the presence of IS and of different cell types on the likelihood of cells being infected and the number of bacteria within the cells were determined by regression analysis. Most of the data sets were not described well by Poisson or negative binomial distributions, owing to a relative excess of uninfected cells, and so the effects of the different treatment types were explored using zero-inflated Poisson and zero-inflated negative binomial (ZINB) models.45 Such generalized linear models allow for the high proportions of zeros by splitting the model into two parts, including a logistic portion (which assesses the probability of any cell not being infected), and a Poisson or negative binomial portion (which models the number of bacteria in infected cells). Saturated models were fitted and variables were then removed if they were not significant (P > 0·05). Most-parsimonious models were selected using Akaike information criteria. Initially, data from each experiment were analysed separately and, where results appeared to be qualitatively and quantitatively similar, they were combined and re-analysed, treating each experiment as a fixed effect. Analyses were performed in sas/stat, version 8·02 (SAS Institute, Cary, NC), using proc nlmixed, with models being fit using maximum likelihood.
For all other experiments, statistical analysis was carried out using a Student's t-test, assuming equal variance. Error bars in figures indicate standard deviations, unless otherwise stated.
Results
Opsonization of S. enterica serovar Typhimurium with IS results in increased numbers of viable bacteria within BMDM
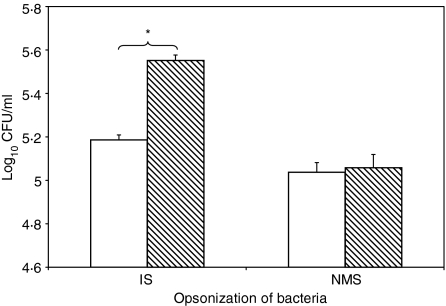
We investigated whether opsonization with IS affects the number of viable bacteria that are recovered from BMDM at various time-points after in vitro infection. BMDM were exposed to S. enterica serovar Typhimurium SL1344 that had previously been incubated with IS or NMS. After an incubation period of 45 min, BMDM were washed and incubated with a bactericidal concentration (100 µg/ml) of gentamicin for 1 hr to kill extracellular bacteria. At the end of the gentamicin treatment, BMDM were lysed in 1 ml of 0·1% Triton X-100 and viable intracellular bacteria were counted. Significantly higher numbers of viable bacteria were recovered from BMDM exposed to IS-opsonized bacteria (5·1 ± 0·05 log CFU/ml) compared with BMDM exposed to NMS-opsonized bacteria (4·5 ± 0·04 log CFU/ml; P = 0·013). Thus, opsonization with IS increases the number of viable S. enterica serovar Typhimurium within BMDM.
Opsonization with IS increases the proportion of infected macrophages and alters the distribution of the bacteria in individual BMDM
Having established that opsonization with IS increases the total number of viable intracellular S. enterica serovar Typhimurium in a BMDM culture, we then proceeded to analyse how IS affects the interaction between bacteria and BMDM at the single-cell level. We evaluated the numbers of individual macrophages containing S. enterica serovar Typhimurium and/or the numerical distribution of the bacteria within individual cells.
BMDM were incubated for 45 min with S. enterica serovar Typhimurium SL1344–GFP that had previously been exposed to NMS or IS. At the end of the incubation period, the cells were washed, fixed and extracellular bacteria were stained with a rabbit anti-Salmonella O4 serum followed by a goat anti-rabbit Alexa Fluor 350-conjugated antibody (which would stain only extracellular bacteria). The number of infected cells and the number of intracellular S. enterica serovar Typhimurium–GFP (GFP positive, Alexa Fluor 350 negative) per infected BMDM were then counted.
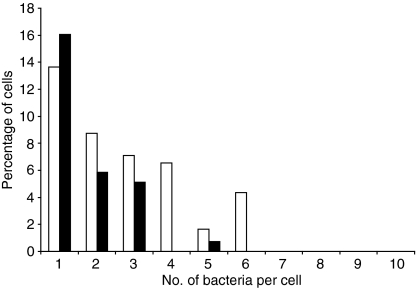
As only a small percentage of BMDM were infected, the distribution of the number of bacteria per cell was skewed towards zero. Therefore, we chose a zero-inflated model to analyse the results. Data from three experiments were amalgamated. Analysis using ZINB models, with experiment included as a fixed logistic effect, was the most parsimonious. The data analysis indicated that opsonization with IS was associated with significantly increased numbers of bacteria within infected cells (P < 0·01) (Fig. 1). Opsonization with IS was also associated with a significantly increased proportion of infected cells. These conclusions were consistent with the results from separate analyses of data from three similar individual experiments, although significance levels were reduced when individual data sets were considered separately.
Figure 1.
Distribution of immune serum (IS)- or normal mouse serum (NMS)-opsonized Salmonella enterica serovar Typhimurium within bone marrow-derived macrophages (BMDM) infected for 45 min. Cells were infected with IS- (white bars) or NMS- (black bars) opsonized S. enterica serovar Typhimurium SL1344–green fluorescent protein (GFP) conjugate for 45 min, fixed and examined by fluorescence microscopy. Numbers of uninfected and infected cells, as well as the number of bacteria within each cell, were counted. For each experiment, at least 200 cells were counted. The graph illustrates one of three individual experiments that yielded similar results.
Opsonization of S. enterica serovar Typhimurium with IS enhances the production of ROI
Having established that IS increases the internalization of S. enterica serovar Typhimurium by BMDM, we then proceeded to investigate whether opsonization with IS would enhance BMDM functions known to be associated with increased antibacterial activity of phagocytic cells.6
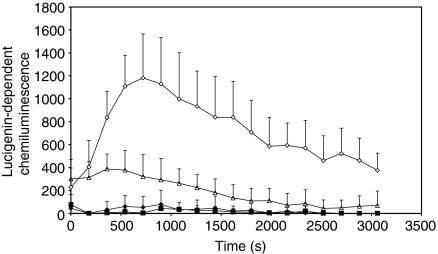
BMDM were exposed to S. enterica serovar Typhimurium SL1344 previously opsonized with NMS or IS. The production of 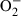 was assessed at time-points thereafter by lucigenin chemiluminescence (Fig. 2). Exposure to NMS-opsonized S. enterica serovar Typhimurium did not result in the production of detectable amounts of
was assessed at time-points thereafter by lucigenin chemiluminescence (Fig. 2). Exposure to NMS-opsonized S. enterica serovar Typhimurium did not result in the production of detectable amounts of 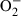 by BMDM, whereas exposure to IS-opsonized S. enterica serovar Typhimurium resulted in a significantly increased production of
by BMDM, whereas exposure to IS-opsonized S. enterica serovar Typhimurium resulted in a significantly increased production of 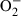 that peaked around 500 s after exposure to the bacteria and then gradually declined. As expected, the addition of PMA to BMDM induced high levels of
that peaked around 500 s after exposure to the bacteria and then gradually declined. As expected, the addition of PMA to BMDM induced high levels of 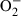 , whilst control cells treated with medium alone did not produce detectable amounts of
, whilst control cells treated with medium alone did not produce detectable amounts of 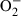 . Thus, opsonization of S. enterica serovar Typhimurium with IS enhances the production of ROI in BMDM.
. Thus, opsonization of S. enterica serovar Typhimurium with IS enhances the production of ROI in BMDM.
Figure 2.
Production of superoxide by bone marrow-derived macrophages (BMDM) in response to phorbol 12-myristate 13-acetate (PMA) (white diamonds), immune serum (IS)-opsonized bacteria (white triangles), normal mouse serum (NMS)-opsonized bacteria (black diamonds), or medium alone (black squares), as assessed by the reduction of lucigenin. Lucigenin and stimulant were added to interferon-γ (IFN-γ)-activated macrophages and the reduction of lucigenin was measured immediately and at 150-s intervals thereafter. The graph illustrates one of three individual experiments that yielded similar results. Data are expressed as the mean of five individual wells. The error bars represent the standard deviation.
Opsonization of S. enterica serovar Typhimurium with IS enhances the antibacterial activity of BMDM via the production of ROI
ROI are crucial mediators of the antibacterial activity of phagocytes, and increased levels of ROI were produced by BMDM upon exposure to IS-opsonized S. enterica serovar Typhimurium. We therefore wished to assess whether opsonization of S. enterica serovar Typhimurium with IS would enhance the antibacterial activity of BMDM via the production of ROI.
BMDM from C57BL/6 WT mice and from Cybb–/– mice were exposed to IS-opsonized S. enterica serovar Typhimurium SL1344–GFP or NMS-opsonized S. enterica serovar Typhimurium SL1344–GFP for 15 min. The numbers of intracellular S. enterica serovar Typhimurium were counted by microscopy.
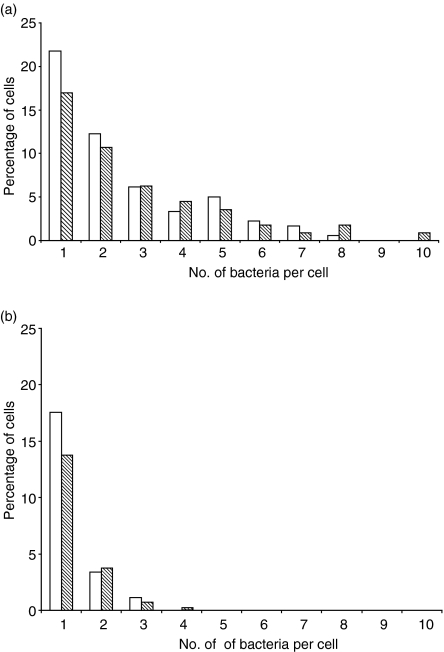
The number of NMS-opsonized intracellular bacteria were similar in the BMDM cultures from WT and Cybb–/– mice (Fig. 3). Opsonization of S. enterica serovar Typhimurium with IS resulted in a similar increase in the numbers of intracellular bacteria in BMDM cultures from WT and Cybb–/– mice (Fig. 3). Statistical analysis of data from all experiments demonstrated that, at 15 min, the counts of intracellular bacteria were significantly increased by the addition of IS (P < 0·001), but that differences between WT and Cybb–/– cells were not significant, suggesting that BMDM from Cybb–/– mice are as efficient as cells from WT mice at internalizing NMS- and IS-opsonized S. enterica serovar Typhimurium.
Figure 3.
Distribution of immune serum (IS)- and normal mouse serum (NMS)-opsonized bacteria within wild-type (WT) bone marrow-derived macrophages (BMDM) or Cybb–/– BMDM. WT (white bars) or Cybb–/– (shaded bars) BMDM were infected with (a) IS- or (b) NMS-opsonized Salmonella enterica serovar Typhimurium SL1344–green fluorescent protein (GFP) conjugate for 15 min, after which the cells were fixed and examined by fluorescence microscopy. Numbers of uninfected and infected cells were counted, and the number of bacteria within infected cells was also determined. For each experiment, at least 200 cells were counted. Each graph illustrates one of three individual experiments that yielded similar results.
We then proceeded to study whether IS enhances bacterial killing by BMDM and whether this is mediated by the production of ROI. BMDM cultures from WT and Cybb–/– mice were exposed to IS-opsonized or NMS-opsonized S. enterica serovar Typhimurium SL1344 for 15 min, washed and then incubated with medium containing 100 µg/ml of gentamicin for 1 hr. Then, the cells were lysed and viable intracellular bacteria were counted. The addition of 100 µg/ml gentamicin after the short 15-min infection period ensures that there is little or no further phagocytosis of live bacteria, and the assay therefore reveals the survival rate of the bacteria phagocytosed by BMDM in the initial 15 min.
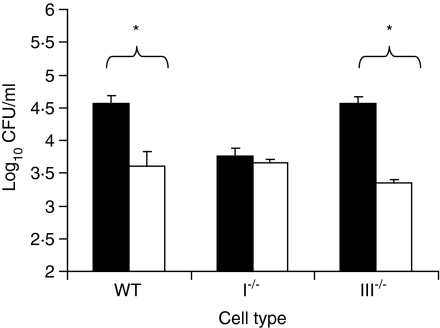
The number of viable intracellular bacteria in BMDM from WT and Cybb–/– mice were similar when the cells were infected with NMS-opsonized bacteria (Fig. 4). However, higher numbers of viable intracellular S. enterica serovar Typhimurium were present in the cells from Cybb–/– mice infected with IS-opsonized S. enterica serovar Typhimurium compared with similarly infected BMDM from WT mice.
Figure 4.
Survival of immune serum (IS)- or normal mouse serum (NMS)-opsonized bacteria within Cybb–/– and wild-type (WT) BMDM. WT (unshaded) or Cybb–/– (shaded) bone marrow-derived macrophages (BMDM) were infected with IS- or NMS-opsonized Salmonella enterica serovar Typhimurium SL1344 for 15 min. Bacteria were then removed and the remaining extracellular bacteria were killed by incubation with 100 µg/ml gentamicin for 1 hr. Cells were lysed and the numbers of viable bacteria were counted. The graph illustrates one of three individual experiments that yielded similar results. Data are expressed as the mean of three individual wells. The error bars represent the standard deviation. *P < 0·05, as determined by the Student's t-test.
Taken together, these results indicate that opsonization with IS results in a similar increase in the numbers of intracellular bacteria in BMDM from WT and Cybb–/– mice (Fig. 3) and enhances the antibacterial activity of BMDM via the production of ROIs (Fig. 4).
Opsonization of Salmonella with IS does not enhance the production of RNIs
RNIs are important in the control of intracellular S. enterica within phagocytes.6 We therefore assessed whether opsonization with IS affects the ability of BMDM to produce RNIs in response to infection with S. enterica serovar Typhimurium.
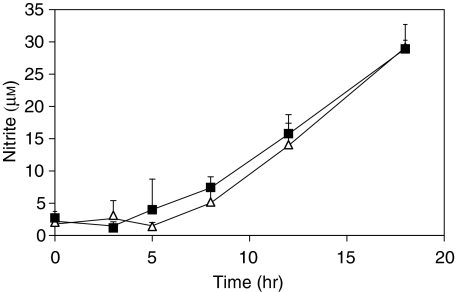
BMDM from Nos2A+/+ mice were exposed to S. enterica serovar Typhimurium SL1344 that had previously been incubated with IS or NMS. After 45 min, BMDM were washed and incubated with 100 µg/ml of gentamicin for 1 hr. Nitrite levels were monitored in the supernatants at 0, 3, 5, 8 and 18 hr after infection. Nitrites remained below detectable levels until 8 hr after infection and then steadily increased until 18 hr, indicating continued production of NO (Fig. 5). Nitrite levels in the supernatants were similar in the cultures infected with IS-opsonized S. enterica serovar Typhimurium and NMS-opsonized S. enterica serovar Typhimurium. BMDM from mice lacking iNOS (Nos2A–/–) were used as additional negative controls in the experiments described above and no nitrites were detected in the supernatants of BMDM from these mutant mice (data not shown).
Figure 5.
Production of reactive nitrogen intermediates (RNI) by bone marrow-derived macrophages (BMDM) exposed to immune serum (IS)- and normal mouse serum (NMS)-opsonized bacteria. Cells were exposed to IS- (triangles) or NMS- (squares) opsonized bacteria for 45 min. Bacteria were then removed and the remaining extracellular bacteria killed by incubation with 100 µg/ml gentamicin for 1 hr. Medium was then removed at this time-point (0 hr) and at specific time-points thereafter (3, 5, 8, 12 and 18 hr). Nitrite levels within the medium were then assessed by the Griess reaction. The graph illustrates one of three individual experiments that yielded similar results. Data are expressed as the mean of three individual wells. The error bars represent standard deviations.
Opsonization with IS resulted in a similar increase in the intracellular viable numbers of S. enterica serovar Typhimurium in BMDM from Nos2A+/+ and Nos2A–/– mice (data not shown). To assess the effect of the lack of iNOS on the intracellular numbers of IS- and NMS-opsonized bacteria, BMDM cultures from Nos2A+/+ and Nos2A–/– mice were exposed to S. enterica serovar Typhimurium for 45 min, washed and then incubated with medium containing 100 µg/ml of gentamicin for 1 hr. At this time-point and at time-points thereafter (2, 8 and 18 hr), the cells were lysed and viable intracellular bacteria were counted. The number of viable intracellular bacteria was similar in BMDM from Nos2A+/+ and Nos2A–/– mice when the cells were infected with NMS-opsonized bacteria. A similar increase in intracellular bacterial numbers was seen in BMDM from Nos2A+/+ and Nos2A–/– mice when these were infected with IS-opsonized bacteria (data not shown).
Therefore, opsonization of S. enterica serovar Typhimurium with IS does not enhance the production of RNI from BMDM and does not affect the antibacterial activity of these cells via RNI.
Opsonization of S. enterica serovar Typhimurium with IS does not increase the rate of phagosome-lysosome fusion within BMDM
S. enterica serovar Typhimurium are able to avoid the fusion of the phagosome when they reside within lysosomes, resulting in an enhanced ability to survive within macrophages.18 We wished to investigate whether opsonization with IS could overcome the ability of S. enterica serovar Typhimurium to avoid phagosome–lysosome fusion. TROv, which is colocalized to lysosomes within the macrophages, and cathepsin D, a cell-surface marker that is colocalized to the phagosome membrane following lysosome fusion,18 were used to assess the percentage of Salmonella-containing phagosomes that fuse with lysosomes.
BMDM were pulsed with TROv for 30 min and then infected with IS- or NMS-opsonized S. enterica serovar Typhimurium 12023 or a phoP mutant, both strains expressing GFP. The phoP– mutant was used as a control, as it has previously been shown that this mutant does not avoid phagosome–lysosome fusion with the same efficiency as WT bacteria.18 Cells were fixed and examined at specific time-points after infection, by using laser-scanning confocal microscopy.
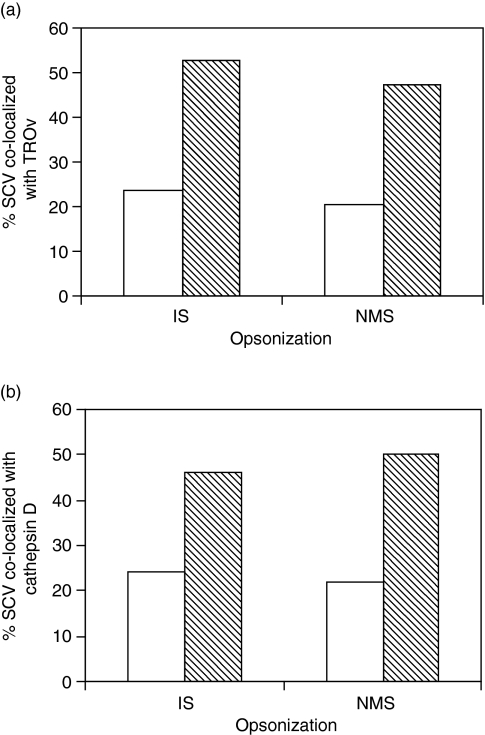
Figure 6(a) shows a higher level of colocalization of the phoP– mutant with TROv compared with WT S. enterica, indicating, as expected, that this mutant is unable to avoid phagosome–lysosome fusion. The opsonization of WT S. enterica serovar Typhimurium 12023, or of the phoP– mutant, with IS did not affect the percentage of phagosome–lysosome fusion in the BMDM (Fig. 6a).
Figure 6.
Colocalization of immune serum (IS)- or normal mouse serum (NMS)-opsonized bacteria within Salmonella enterica-containing vesicles (SCV) with lysosomes. Bone marrow-derived macrophages (BMDM) were infected with opsonized S. enterica serovar Typhimurium 12023–green fluorescent protein (GFP) conjugate (white bars), or with S. enterica serovar Typhimurium 12023-GFP phoP– (shaded bars), for 45 min before the extracellular bacteria were killed by incubation with 100 µg/ml gentamicin for 1 hr. Cells were then incubated for a further 24 hr with 10 µg/ml gentamicin, then fixed and examined by confocal microscopy for colocalization of bacteria with (a) Texas Red-ovalbumin (TROv) or (b) cathepsin D. Each graph illustrates one of two individual experiments that yielded similar results. For each experiment, at least 100 cells were counted.
In parallel experiments, BMDM were infected as described above and immunostained for cathepsin D. Figure 6(b) shows that the percentage of phoP– mutant bacteria colocalized with Cathepsin D was higher than that of WT S. enterica serovar Typhimurium. No difference in the level of colocalization was detected in cells infected with IS- and NMS-opsonized bacteria. Thus, opsonization with IS does not affect the fusion of S. enterica serovar Typhimurium-containing phagosomes with lysosomes within BMDM.
FcγRI mediates the increase in intracellular bacterial numbers seen after exposure of BMDM to IS-opsonized S. enterica serovar Typhimurium
To define the requirement for individual Fcγ receptors in the increased intracellular bacterial numbers seen in BMDM exposed to IS-opsonized S. enterica serovar Typhimurium–GFP, we obtained BMDM from mice lacking individual Fcγ receptors (FcγRI–/–, FcγRII–/– or FcγRIII–/– mice). BMDM from WT mice and from mice lacking all three Fcγ receptors (FcγRI–/–, FcγRII–/– and FcγRIII–/–) were used as controls. We measured the number of infected BMDM and intracellular bacterial numbers in individual phagocytes by fluorescence microscopy. We also evaluated the number of intracellular viable bacteria by gentamicin protection assays.
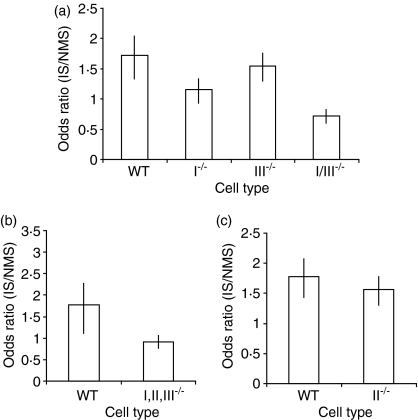
BMDM cultures from WT, FcγRI–/–, FcγRII–/– and FcγRIII–/– mice, as well as from FcγRI–/– FcγRII–/– FcγRIII–/– triple-mutant mice, were exposed to IS- or NMS-opsonized bacteria for 45 min. Cells were then washed, fixed and extracellular bacteria stained, as previously described. The number of cells infected, and the number of bacteria within these cells, was enumerated by fluorescence microscopy. Differences in the proportion of infected cells were analysed statistically using the Mantel–Haenszel test. The uptake by non-opsonized bacteria (no serum at all) was similar in WT and FcγR phagocytes. Opsonization of bacteria with IS significantly increased the proportion of infected WT and FcγRIII–/– BMDM, but not the proportion of infected FcγRI–/– BMDM (Fig. 7a). The absence of all three Fcγ receptors resulted in no increase in the proportion of BMDM containing intracellular bacteria (Fig. 7b). BMDM from FcγRII–/– mice showed an increase in the number of intracellular bacteria similar to that seen in BMDM from WT mice (Fig. 7c). Thus, FcγRI appears to play the dominant role in the increase in intracellular bacterial numbers seen after exposure of BMDM to IS-opsonized S. enterica serovar Typhimurium.
Figure 7.
Ratio between the proportion of normal mouse serum (NMS)- and immune serum (IS)-opsonized Salmonella enterica serovar Typhimurium in mice lacking individual Fcγ receptors (I, II and/or III). Bone marrow-derived macrophages (BMDM) were exposed to NMS- or IS-opsonized S. enterica serovar Typhimurium SL1344–green fluorescent protein (GFP) conjugate for 45 min, then fixed and examined by fluorescence microscopy. The numbers of uninfected and infected cells were counted. The figure shows the ratio between the proportion of infected cells following exposure to IS-opsonized bacteria and the proportion of infected cells following exposure to NMS-opsonized bacteria. Each panel shows the data from two individual experiments. Data were analysed using the Mantel–Haenszel test. The bars indicate 95% confidence intervals. For each experiment, at least 200 cells were counted.
To assess the effect of the lack of individual Fcγ receptors on the number of viable intracellular S. enterica serovar Typhimurium, BMDM cultures from mice lacking individual Fcγ receptors (FcγRI–/–, FcγRII–/– or FcγRIII–/– mice) were exposed to IS- and NMS-opsonized bacteria for 45 min. Cells from WT mice and from mice lacking all three Fcγ receptors (FcγRI–/–, FcγRII–/– and FcγRIII–/–) were used as controls. The cells were then washed and incubated, for 1 hr, with medium containing 100 µg/ml of gentamicin. At this (0) and at subsequent (4, 8 and 18 hr) time-points, the cells were lysed and viable intracellular bacteria counted.
Higher numbers of viable bacteria were recovered from WT and FcγRIII–/– BMDM exposed to IS-opsonized bacteria compared with BMDM exposed to NMS-opsonized bacteria (Fig. 8). Conversely, similar numbers of viable bacteria were recovered from FcγRI–/– BMDM exposed to IS-opsonized bacteria compared with NMS-opsonized bacteria, indicating that FcγRI–/– is required for the IS-induced increase in the numbers of viable bacteria inside BMDM (Fig. 8). Parallel experiments in BMDM from FcγRII–/– showed results similar to those obtained in WT or FcγRIII–/–, with an increase in the number of intracellular viable bacteria following opsonization with IS (data not shown). As expected, BMDM from triple-mutant mice (FcγRI–/– FcγRII–/– FcγRIII–/–) showed no increase in the numbers of viable intracellular bacteria following opsonization with IS (data not shown).
Figure 8.
Survival of immune serum (IS)-opsonized (black bars) or normal mouse serum (NMS)-opsonized (white bars) bacteria within wild-type (WT), FcγRI–/– and FcγRIII–/– bone marrow-derived macrophages (BMDM). BMDM were infected with opsonized Salmonella enterica serovar Typhimurium SL1344 for 45 min. The bacteria were then removed and the remaining extracellular bacteria killed by incubation with 100 µg/ml gentamicin for 1 hr. Cells were lysed and the number of viable bacteria were counted. The graph illustrates one of three individual experiments that yielded similar results. Data are expressed as the mean of three individual wells. The error bars represent standard deviations. *P < 0·05, determined by the Student's t-test.
Thus, the increase in the numbers of viable IS-opsonized S. enterica serovar Typhimurium within BMDM occurs mainly via FcγRI, with FcγRIII playing a minor role. Furthermore, the absence of FcγRII does not enhance the phagocytosis of IS-opsonized S. enterica serovar Typhimurium by BMDM.
Discussion
Detailed study of the role of IS in the interactions between S. enterica serovar Typhimurium and murine macrophages has revealed that the presence of opsonizing antibodies on the bacterial surface affects the dynamics of bacterial internalization and enhances bacterial killing by mononuclear cells via increased production of ROI.
Increased uptake of S. enterica serovar Typhimurium following opsonization with normal serum (complement opsonization) or IS has been reported in the past, but the dynamics of these interactions at the single-cell level are not clear.46–49 In fact, passive administration of IS, or active immunization, can accelerate bacterial uptake by macrophages from the blood (blood clearance) or from the peritoneal cavity, but the numerical distribution of the bacteria in individual phagocytes has not been clarified. Here, we report that more bacteria are found inside individual phagocytes when the micro-organisms are opsonized with Salmonella-IS. This modulation of the dynamics of bacterial distribution may have consequences for the local tissue spread of micro-organisms during secondary infections in immunized individuals. In fact, the bacterial load in individual phagocytes can affect cell viability and the time after the initial infection when the cell is lysed and releases the micro-organisms into the surrounding space (our unpublished observations). These factors would ultimately affect the pattern of spread of the bacteria in the tissues and the ability of the vaccinated host to contain the dissemination of the infection.
The production of ROI and RNI, and the fusion of lysosomes with maturing phagosomes, all contribute to the antibacterial activity of mononuclear cells. In the present report, we showed that opsonization with IS enhances ROI production within BMDM early after the bacteria–cell interaction and increases the ability of BMDM to control intracellular bacteria numbers (i.e. differences seen in WT versus Cybb–/– BMDM). The role of ROI in the enhanced antibacterial efficiency of BMDM against opsonized Salmonella is illustrated by the higher numbers of viable opsonized bacteria seen in Cybb–/– BMDM as compared to WT BMDM, despite the similar initial bacterial internalization seen in the Cybb–/– and WT BMDM. This effect of IS on the early stages of the in vitro infection is consistent with the reported bactericidal activity of ROI that is mainly confined to the early phases of the infection process.6 We found that after the initial ROI-dependent events, the relative numbers of opsonized versus non-opsonized bacteria within BMDM remain constant throughout the in vitro infection. This observation is consistent with the fact that the later stages of the interactions between S. enterica serovar Typhimurium and macrophages are influenced by the production of RNI6 and with the fact that RNI levels are not affected by opsonization of the bacteria with IS.
The relative importance of different Fcγ receptors in the interactions of bacteria and immune complexes with phagocytes is still the object of study. We have shown that the effects of IS on the interactions between S. enterica serovar Typhimurium and BMDM are mainly dependent on FcγRI. FcγRI is a high-affinity receptor for IgG2a and previous work indicates that immunization with live S. enterica serovar Typhimurium leads to production of antibody responses with a high IgG2a content, probably as a result of the simultaneous induction of high levels of IFN-γ by Salmonella-specific T helper 1-type T cells.27,44 In the absence of FcγRI, internalization of S. enterica serovar Typhimurium is abrogated and other Fcγ receptors (e.g. FcγRIII) are unable to compensate for the FcγRI deficiency. The inability of FcγRIII to mediate phagocytosis of S. enterica serovar Typhimurium by BMDM is surprising given the fact that this receptor can efficiently bind several IgG subclasses present in the serum of immunized mice27 and can mediate the effects of immune complexes in autoimmune diseases and hypersensitivity reactions.40 FcγRI has also been shown to have a prominent role in the control of other bacterial infections, being essential for the resistance to Bordetalla pertussis in the lung after an intranasal infection.39
We found that the absence of the inhibitory FcγRII does not affect either internalization or survival of S. enterica serovar Typhimurium within BMDM. Stimulation of FcγRII counterbalances the activating signals provided by FcγRI and III, often resulting in the down-regulation of cell functions and in the control of certain aspects of immune reactivity.36 Additionally, results from the Streptococcus pneumoniae model indicate that the absence of FcγRII increases the antibody-dependent phagocytosis of the bacteria by peritoneal macrophages in vitro.41 There are no simple explanations for the discrepancies seen between the Salmonella and Streptococcus in vitro infection models. It is possible that the concentration and isotype profile of the antibody in opsonizing sera was different with subtle effects on the relative engagement of individual receptors. It is also possible that other non-Fcγ receptor innate immunity receptors triggered by Salmonella and Streptococcus may differentially modulate the functions of individual Fcγ receptors upon binding of opsonized bacteria. It is also possible that the balance between activating and inhibitory receptors is different in individual macrophage populations (e.g. peritoneal versus BMDM).
In the work presented here, we have investigated the events occurring after the interaction of bacteria (coated with a subagglutinating amount of specific antibody) with BMDM cultured in the absence of additional mouse serum. In vivo, FcγRI is partially engaged by serum monomeric IgG2a and therefore opsonized particles will have to compete for access to the partially engaged receptor.50 We have found that the prevalence of FcγRI in the internalization of IS-opsonized S. enterica serovar Typhimurium by BMDM also persisted when the assays were performed in the presence of NMS or non-specific monomeric IgG2a (data not shown), indicating that FcγRI function is required also when these receptors are largely occupied by non-specific monomeric IgG2a. However, it is possible that the relative requirement and functions of different Fcγ receptors are different in cultured cells and phagocytes exposed to opsonized bacteria in vivo. This is currently under investigation.
Acknowledgments
This work was funded by a Medical Research Council grant to P. Mastroeni. H. Uppington was supported by studentship from the Biotechnology and Biological Sciences Research Council.
Abbreviations
- BMDM
bone marrow-derived macrophages
- CFU
colony-forming units
- FcR
Fc receptor
- GFP
green fluorescent protein
- IFN-γ
interferon-γ
- iNOS
inducible nitric oxide synthase
- IS
immune serum
- LB
Luria–Bertani
- NGS
normal goat serum
- NMS
normal mouse serum
- NO
nitric oxide
- PBS
phosphate-buffered saline
- PMA
phorbol 12-myristate 13-acetate
- RNI
reactive nitrogen intermediates
- ROI
reactive oxygen intermediates
- SCV
Salmonella-containing vacuole
- Slc11a1
solute carrier family 11a member 1
- SPI
Salmonella pathogenicity island
- TNF-α
tumour necrosis factor-α
- TROv
Texas Red-ovalbumin
- TTSS
type III secretion system
- WT
wild type
- ZINB
zero-inflated negative binomial
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: immune responses and vaccines. Vet J. 2001;161:132–64. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 3.Lee VT, Schneewind O. Type III secretion machines and the pathogenesis of enteric infections caused by Yersinia and Salmonella spp. Immunol Rev. 1999;168:241–55. doi: 10.1111/j.1600-065x.1999.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 4.Sheppard M, Webb C, Heath F, Mallows V, Emilianus R, Maskell D, Mastroeni P. Dynamics of bacterial growth and distribution within the liver during Salmonella infection. Cell Microbiol. 2003;5:593–600. doi: 10.1046/j.1462-5822.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 5.Richter-Dahlfors A, Buchan AMJ, Finlay BB. Murine salmonellosis studied by confocal microscopy. Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–80. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–36. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–48. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White JK, Mastroeni P, Popoff JF, Evans CA, Blackwell JM. Slc11a1-mediated resistance to Salmonella enterica serovar Typhimurium and Leishmania donovani infections does not require functional inducible nitric oxide synthase or phagocyte oxidase activity. J Leukoc Biol. 2005;77:311–20. doi: 10.1189/jlb.0904546. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez-Torres A, Fantuzzi G, Edwards CK, 3rd, Dinarello CA, Fang FC. Defective localization of the NADPH phagocyte oxidase to Salmonella- containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc Natl Acad Sci USA. 2001;98:2561–5. doi: 10.1073/pnas.041618998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastroeni P, Harrison JA, Robinson JH, Clare S, Khan S, Maskell DJ, Dougan G, Hormaeche CE. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–76. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denich K, Borlin P, O'Hanley PD, Howard M, Heath AW. Expression of the murine interleukin-4 gene in an attenuated aroA strain of Salmonella typhimurium: persistence and immune response in BALB/c mice and susceptibility to macrophage killing. Infect Immun. 1993;61:4818–27. doi: 10.1128/iai.61.11.4818-4827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai T, Hiromatsu K, Nishimura H, Kimura Y, Kobayashi N, Ishida H, Nimura Y, Yoshikai Y. Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defence mechanism against murine Salmonella infection. Immunology. 1995;85:381–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–93. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–8. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 15.Chakravortty D, Hansen-Wester I, Hensel M. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med. 2002;195:1155–66. doi: 10.1084/jem.20011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Groote MA, Ochsner UA, Shiloh MU, et al. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci USA. 1997;94:13997–4001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SI. PhoP/PhoQ. macrophage-specific modulators of Salmonella virulence? Mol Microbiol. 1991;5:2073–8. doi: 10.1111/j.1365-2958.1991.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 18.Garvis SG, Beuzon CR, Holden DW. A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella-containing vacuoles in macrophages. Cell Microbiol. 2001;3:731–44. doi: 10.1046/j.1462-5822.2001.00153.x. [DOI] [PubMed] [Google Scholar]
- 19.Parry CM, Hoa NT, Diep TS, et al. Value of a single-tube widal test in diagnosis of typhoid fever in Vietnam. J Clin Microbiol. 1999;37:2882–6. doi: 10.1128/jcm.37.9.2882-2886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, White NJ. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol. 1998;36:1683–7. doi: 10.1128/jcm.36.6.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter PB, Collins FM. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastroeni P, Sheppard M. Salmonella infections in the mouse model: host resistance factors and in vivo dynamics of bacterial spread and distribution in the tissues. Microbes Infect. 2004;6:398–405. doi: 10.1016/j.micinf.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–4. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- 25.Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis. 1989;11(Suppl. 3):S552–67. doi: 10.1093/clinids/11.supplement_3.s552. [DOI] [PubMed] [Google Scholar]
- 26.Acharya IL, Lowe CU, Thapa R, et al. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987;317:1101–4. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 27.Harrison JA, Villarreal-Ramos B, Mastroeni P, Demarco de Hormaeche R, Hormaeche CE. Correlates of protection induced by live Aro–Salmonella typhimurium vaccines in the murine typhoid model. Immunology. 1997;90:618–25. doi: 10.1046/j.1365-2567.1997.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston RB., Jr The host response to invasion by Streptococcus pneumoniae: protection and the pathogenesis to tissue damage. Rev Infect Dis. 1981;3:282–8. doi: 10.1093/clinids/3.2.282. [DOI] [PubMed] [Google Scholar]
- 29.Wright S, Silverstein S. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–23. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosser DM, Karp CL. Receptor mediated subversion of macrophage cytokine production by intracellular pathogens. Curr Opin Immunol. 1999;11:406–11. doi: 10.1016/s0952-7915(99)80068-5. [DOI] [PubMed] [Google Scholar]
- 31.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–85. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 1998;188:217–22. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellwig SM, van Oirschot HF, Hazenbos WL, van Spriel AB, Mooi FR, van De Winkel JG. Targeting to Fcγ receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J Infect Dis. 2001;183:871–9. doi: 10.1086/319266. [DOI] [PubMed] [Google Scholar]
- 34.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Guyre PM, Morganelli PM, Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983;72:393–7. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 37.Vidal SM, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–85. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 38.Blackwell JM, Goswami T, Evans CA, et al. SLC11A1 (formerly NRAMP1) and disease resistance. Cell Microbiol. 2001;3:773–84. doi: 10.1046/j.1462-5822.2001.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, et al. FcγRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 40.Hazenbos WL, Gessner JE, Hofhuis FM, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–8. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 41.Clatworthy MR, Smith KG. FcγRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004;199:717–23. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–9. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 43.Hautefort I, Proenca MJ, Hinton JC. Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl Environ Microbiol. 2003;69:7480–91. doi: 10.1128/AEM.69.12.7480-7491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinha K, Mastroeni P, Harrison J, de Hormaeche RD, Hormaeche CE. Salmonella typhimurium aroA, htrA, and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect Immun. 1997;65:1566–9. doi: 10.1128/iai.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nodtvedt A, Dohoo I, Sanchez J, Conboy G, DesCjteaux L, Keefe G, Leslie K, Campbell J. The use of negative binomial modelling in a longitudinal study of gastrointestinal parasite burdens in Canadian dairy cows. Can J Vet Res. 2002;66:249–57. [PMC free article] [PubMed] [Google Scholar]
- 46.Liang-Takasaki CJ, Saxen H, Makela PH, Leive L. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect Immun. 1983;41:563–9. doi: 10.1128/iai.41.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saxen H, Reima I, Makela PH. Alternative complement pathway activation by Salmonella O polysaccharide as a virulence determinant in the mouse. Microb Pathog. 1987;2:15–28. doi: 10.1016/0882-4010(87)90111-2. [DOI] [PubMed] [Google Scholar]
- 48.Liang-Takasaki CJ, Makela PH, Leive L. Phagocytosis of bacteria by macrophages: changing the carbohydrate of lipopolysaccharide alters interaction with complement and macrophages. J Immunol. 1982;128:1229–35. [PubMed] [Google Scholar]
- 49.Collins FM. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974;38:371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unkeless JC, Eisen HN. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975;142:1520–33. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]