Abstract
Pollen grains contain reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidases and in contact with mucosal surfaces generate superoxide anion (). In the presence of iron, may be converted to more reactive oxygen radicals, such as to H2O2 and/or •OH, which may augment antigen-induced airway inflammation. The aim of the study was to examine the impact of lactoferrin (LF), an iron-binding protein, on ragweed (Ambrosia artemisiifolia) pollen extract (RWE)-induced cellular oxidative stress levels in cultured bronchial epithelial cells and accumulation of inflammatory and mucin-producing cells in airways in a mouse model of allergic airway inflammation. Results show that LF lowered RWE-induced increase in cellular reactive oxygen species (ROS) levels in bronchial epithelial cells. Most importantly, LF significantly decreased accumulation of eosinophils into airways and subepithelium of intranasally challenged, sensitized mice. LF also prevented development of mucin-producing cells. Amb a 1, the major allergenic ragweed pollen antigen lacking NAD(P)H oxidase activity, induced low-grade airway inflammation. When administered along with glucose oxidase (G-ox), a superoxide-generating enzyme, Amb a 1 induced robust airway inflammation, which was significantly lowered by LF. Surprisingly, LF decreased also inflammation caused by Amb a 1 alone. Iron-saturated hololactoferrin had only a marginal effect on RWE-induced cellular ROS levels and RWE- or Amb a 1 plus G-ox-induced inflammation. We postulate that free iron in the airways chemically reduces to more reactive species which augment antigen-induced inflammation in a mouse model of asthma. Our results suggest the utility of LF in human allergic inflammatory disorders.
Keywords: lactoferrin, allergy, airway inflammation, oxidative stress
Introduction
The prevalence of allergic lung inflammation has significantly increased over the past decades. This phenomenon is related to changes in the environment acting on susceptible individuals, both in the induction and progression of disease.1 Epidemiological studies identified multiple interacting risk factors, including inhaled pollutants (e.g. tobacco smoke, particulate matter, oxides of nitrogen, ozone), and repeated respiratory virus exposures, that generate reactive oxygen species (ROS).1 Oxidative stress occurs not only as a result of environmental exposure, but also because of inflammation, as both airway and intravascular inflammatory cells generate ROS.2,3
The physiological balance between ROS production and the rate of their elimination naturally protects against oxidative cell injury. When the detoxication system is defective or overwhelmed by ROS the cells become oxidatively stressed, often leading to additional ROS production, alterations in cellular signalling and development of pathological processes.4 Under normal physiological conditions, this dynamic interplay between pro- and antioxidant substances depends on the availability of transition metals. In particular, traces of iron can be detrimental to physiological processes under oxidative stress conditions. Superoxide anion () and hydrogen peroxide (H2O2), given their long half-lives, are moderately cytotoxic; however, in the presence of iron catalyst they are converted to the highly cytotoxic hydroxyl radical (•OH) via the metal-catalysed Fenton reaction.5 Lactoferrin (LF), an iron-binding glycoprotein present in serum and many human exocrine secretions serves as immunomodulator and a natural antioxidant.6–8 A number of physiological effects have been attributed to LF, including a wide array of antimicrobial actions, both direct9 and indirect,10 anti-inflammatory effects,11 as well as immunomodulatory properties.12,13
We have previously shown that pollen grains and their extracts generate because of reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase activity, which was essential for robust airway inflammation in a mouse model.14,15 Although and H2O2 are considered as signalling molecules,16 their conversion to •OH by free iron is important in induction and augmentation of inflammatory processes. Pro-oxidant iron is present in airway lining fluids, as well as in resident cells of airways of human and animals and it is known to have implication for oxidative stress in the lungs.17–20 Ragweed pollen extract (RWE) induced a significant increase in lipid peroxidation products, 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) in cells and airways of experimental animals.15 Therefore, we propose that LF decreases levels of highly reactive species (e.g. H2O2•OH, 4-HNE) because of its iron-binding capacity and thereby reduces RWE-induced airway inflammation. The results of our studies show that LF, but not iron-saturated lactoferrin (LFFe), significantly lowered pollen extract-induced accumulation of inflammatory cells and formation of mucin-producing cells in airways.
Materials and methods
Reagents
Endotoxin-free RWE was purchased from Greer Laboratories (Lenoir, NC). Glucose oxidase (G-ox), Amb a 1, endotoxin-free human milk LF and LFFe, desferoxamine (DFO), NAD(P)H, catalase, H2O2, superoxide dismutase (SOD), butylated hydroxytoluene were purchased from Sigma-Aldrich (St. Louis, MO).
Cell cultures
A549 bronchial epithelial cells were purchased from American Type Culture Collection – ATCC (Rockville, MD). The A549 cells were cultured in F-12 Kaighn's-modified medium. Primary normal human bronchial epithelial (NHBE) cells obtained from Cambrex Bio Science (Walkersville, MD) were cultured in BEGM® BulletKit® medium supplied by the manufacturer. The culture media were supplemented with 10% heat-inactivated fetal bovine serum (FBS), l-glutamine (2 mm), penicillin (100 U/ml), and streptomycin (100 µg/ml).
Animals
BALB/c mice were purchased from Harlan Sprague-Dawley (San Diego, CA). All animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals and approved by UTMB Animal Care and Use Committee (#9708038-05).
Sensitization and challenge of animals
Eight-week-old female mice were sensitized with RWE as previously described.15,21 Briefly, mice were sensitized with two intraperitoneal administrations of endotoxin-free RWE, 150 µg/100 µl/injection, combined in a 3 : 1 ratio with alum adjuvant (Pierce Laboratories, Rockford, IL) on days 0 and 4. On day 11, parallel groups of mice (n = 6–8) were challenged intranasally with RWE (100 µg), iron-free LF (100 µg), LFFe (100 µg), RWE (100 µg) + LF (100 µg), or RWE (100 µg) + LFFe (100 µg). Some properties of LF are similar to DFO. For example, DFO binds iron 1 : 1 stoichiometrically, has impact on cell cycle, DNA synthesis, regulates gene expression and possess antiproliferative as well as anti-inflammatory effects and has been utilized in clinical practice.6,11,22 Therefore, parallel groups of mice were challenged intranasally with RWE (100 µg), RWE (100 µg) plus DFO (100 µg) or DFO (100 µg) alone. Mice were also challenged with Amb a 1 alone (25 µg), G-ox (50 µU) alone or G-ox + Amb a 1. Control groups of mice were challenged with equivalent volumes of PBS.
Evaluation of allergic inflammation
To evaluate inflammation, animals from all experimental groups were killed on day 14 with ketamine (135 mg/kg body wt) and xylazine (15 mg/kg body wt), and the lungs were lavaged with two 0·8-ml aliquots of ice-cold PBS. The cells were collected by centrifugation (1000 g, for 10 min at 4°) and resuspended in 1 ml of PBS, and total cell counts were determined. Differential cell counts were performed on cytocentrifuge preparations stained with haematoxylin and eosin. After bronchoalveolar lavage (BAL), the lungs were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned to 5 µm. Lung sections were stained with haematoxylin and eosin.21 Perivascular and peribronchial inflammation and cell composition in the BAL were evaluated by a pathologist blinded to treatment groups to obtain data for each lung. To objectively quantify cellular (eosinophilic) infiltrations of lung sections morphometric analyses were done using NIKON Eclipse TE 200 UV microscope operated via Metamorph™ software (Version 5.09r, Universal Imaging, Downingtown, PA). Images were obtained from four different levels per lung (three animals per group) and reassembled using the montage stage stitching algorithm of the Metamorph™ software.15,23 The integrated morphometric analysis function was used to transform total pixel area of the field light intensity from the sections into µm2 units after calibration.15,24 Mucin production in the epithelial cells was assessed by periodic acid–Schiff (PAS)-staining of formalin-fixed, paraffin-embedded lung sections.15 The stained sections were analysed as above and representative fields were photographed with a Photometrix CoolSNAP Fx camera mounted on a NIKON Eclipse TE 200 UV microscope.15
Measurements of reactive oxygen species
A549 cells were grown to 70% confluence and loaded with 50 µm 2′-7′-dihydro-dichlorofluorescein diacetate (H2DCF-DA) at 37° for 15 min. After removing excess probe, cells in phenol red-free medium were treated with RWE (125 µg/ml) + NAD(P)H (100 µm) with or without LF (100 µg/ml) and LFFe (100 µg/ml). In parallel experiments cells were treated with Amb a 1 (12·5 µg/ml) with or without LF (100 µg/ml) and LFFe (100 µg/ml). The changes in fluorescence intensity were assessed in an FLx800 microplate reader (Bio-Tek Instruments, Winooski, VT) at 488 nm excitation and 530 nm emission.14,15
To determine levels of H2O2 Amplex® Red (10-acetyl-3,7-dihydroxyphenoxazine; Molecular Probes, Eugene, OR) assay was used. Amplex® Red reacts with H2O2 in the presence of horseradish peroxidase (HRP) to generate a stable product, resorufin.25 Briefly, 100 µl test fluid (phenol red-free culture medium; BAL fluid) was mixed with reaction buffer and incubated at room temperature (25°) for 30 min with 0·25 U/ml Amplex® Red and 1 U/ml of HRP (determined in preliminary studies). The change in fluorescence (with absorption and emission wavelengths of 563 and 587 nm, respectively) was measured using a SpectraMax M2 microplate reader (Molecular Devices Corporation, Sunnyvale, CA). Reactions were carried out ± exogenously added SOD. The addition of catalase (400 U/ml) decreased H2O2 levels by ∼90%. As a positive control, increasing dilutions (0–400 pm) of H2O2 were used.
Measurement of 4-HNE and MDA
Mice were RWE-challenged and BAL fluids were collected after 30 min. BAL fluids were clarified by centrifugation (1000 g for 10 min at 4°) and aliquots were stored at −80°. Butylated hydroxytoluene (0·5 mm) was added to prevent further lipid oxidation. 4-HNE and MDA levels were determined using an LPO-586 assay kit (OXIS Inc., Portland, OR). The LPO-586 method is designed to assay either MDA alone (in hydrochloric acid) or MDA in combination with 4-HNE (in methanesulphonic acid). In these experiments, we assayed 4-HNE + MDA levels based on the manufacturer protocols, applying slight modifications as we previously described.15
Statistical analysis
Data collected from in vitro and in vivo experiments were analyzed by anova, followed by Bonferroni posthoc analyses for least significant difference. Differences were considered significant at P < 0·05.
Results
Effect of lactoferrin on RWE-mediated increase in intracellular ROS level in cultured cells
We have recently demonstrated that NAD(P)H oxidases intrinsic to pollen grains generate , which serves the basis of a rapid increase in oxidative stress levels in cultured bronchial epithelial (A549) and lining cells of airway and conjunctival epithelium.14,15 In this study, first we examined the effect of LF on RWE-mediated changes in intracellular ROS levels. A549 cells were grown in iron-containing medium and LF or LFFe was added for 30 min followed by loading cells with H2DCF-DA. Cells were then exposed to 125 µg/ml RWE plus 100 µm NAD(P)H,14,15 and changes in DCF fluorescence were determined. LF (100 µg/ml), but not LFFe (100 µg/ml), significantly (P < 0·001) decreased formation of fluorescent DCF (Fig. 1a). Optimal concentration of LF was determined in preliminary studies (data not shown). In control experiments G-ox (50 µU/ml), which primarily produces ,26 induced a comparable increase in ROS levels. When A549 cells were RWE-treated in iron-free medium (IFM), ROS levels were significantly lower (∼35% less) compared to the levels in cultures RWE-treated in iron-containing medium (Fig. 1a). Importantly, LF further decreased RWE-induced ROS levels in cells placed in IFM. Treatment of cells with Amb a 1, the most abundant allergen in RWE possessing no NAD(P)H oxidase activity,15 did not alter intracellular levels of ROS and LF or LFFe had no effect. DFO decreased the RWE-induced increase in cellular ROS levels by approximately 40% (Fig. 1a). is dismutated to H2O2 by SOD and by the iron-catalysed Haber–Weiss reaction.5 Accordingly, LF (and DFO) also significantly (P = 0·01) inhibited H2O2 accumulation (Fig. 1b). To further support validity of these finding, selected experiments were carried out using NHBE cells. As summarized in Fig. 1(c), LF but not LFFe significantly decreased RWE-induced ROS levels in NHBE cells.
Figure 1.
LF decreases RWE-mediated increase in intracellular ROS levels in cultured cells and BAL fluid. (a) A549 cells were loaded with H2DCF-DA for 15 min and challenged with RWE plus NAD(P)H. Changes in intracellular DCF fluorescence were determined fluorimetrically. G-ox, which generates , was used as control. (b) Effect of LF on H2O2 levels excreted into the medium of RWE-treated A549 cells determined by Amplex® Red assays. (c) Changes in ROS level in normal human bronchial epithelial cells. LF decreases H2O2 (d) and 4-HNE + MDA (e) levels in the BAL of RWE-challenged mice (n = 5–8). Each data point represents the mean from three or more independent experiments ± SEM. **P < 0·01, ***P < 0·001; ****P = 0·0001.
ROS, primarily •OH radicals are known to elicit, in vivo and in vitro oxidative decomposition of lipids (in the airway lining fluid, cells membrane lipids).5 This leads to the formation of a mixture of aldehydic end-products, including MDA and 4-HNE, which we showed to be present in BAL fluid after RWE challenge of mice.15 To elucidate whether LF inhibits formation of •OH radicals (because of its binding of iron) and consequently formation of lipid peroxidation products we determined 4-HNE + MDA levels in the BAL fluid from mice challenged with RWE ± LF (or ±DFO). Analysis of BAL fluids showed that RWE challenge increased 4-HNE + MDA levels (Fig. 1e). LF significantly inhibited this increase. DFO had a similar effect. •OH is also formed from H2O2 in the presence of iron.5 Results in Fig. 1(d) show that LF (and also DFO) partially inhibited the rapid (within 30 min) increase in H2O2 levels in the BAL after RWE challenge of animals. These data strongly indicate that iron-mediated dismutation of and H2O2 into •OH may serve as the basis of lipid radical formation.
Effect of lactoferrin on RWE-induced accumulation of inflammatory cells in the airways
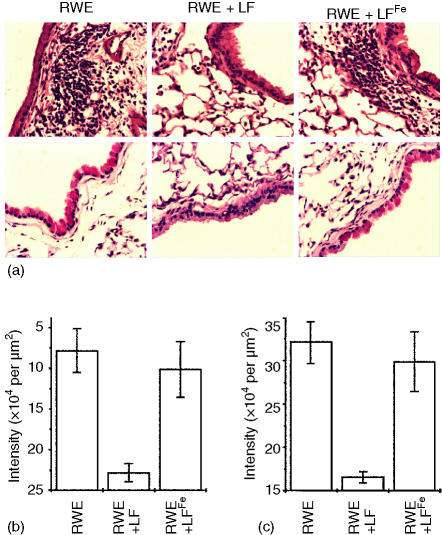
To explore the possibility that LF may decrease RWE-induced allergic airway inflammation, an experimental mouse model was used. When RWE-sensitized mice were challenged with RWE (100 µg per challenge) robust airway inflammation was observed as determined by accumulation of inflammatory cells in the BAL compartment as well as in subepithelial locations (Fig. 2a, b and Fig. 3). The BAL of mice prior to challenge contained primarily macrophages/monocytes (99 ± 0·9%) and low number of eosinophils 0·1 ± 0·05% and neutrophils (0·1 ± 0·05%). In a full-blown inflammation; however, 47 ± 6·2% cells were eosinophils, 52 ± 3·8% macrophages/monocytes and neutrophils (1 ± 0·2%). When RWE was administered together with LF (100 µg) there was a moderate accumulation of inflammatory cells in the BAL compartment (Fig. 2a, b) and in the subepithelium (Fig. 3a), the latter was quantified by morphometric analyses of sections (Fig. 3b). Likewise, LF significantly decreased the RWE-induced formation of mucin-producing cells (Fig. 3a, c). There was no statistically significant effect of LFFe (Fig. 2a, b and Fig. 3a). When DFO was added with RWE, there was only a slight decrease in inflammation (Fig. 2a, b).
Figure 2.
LF decreases RWE-induced allergic airway inflammation. Accumulation of eosinophils (a) and total inflammatory cells (b) in BAL fluids was assessed at 72 hr after RWE challenge (n = 6–8 mice per group). Results are means ± SEM. *P = 0·05; ***P = 0·001; ****P < 0·0001.
Figure 3.
Effect of LF on the RWE-induced accumulation of inflammatory cells into subepithelium and goblet cell formation. (a) Microscopic visualization of eosinophilic infiltration and goblet cell metaplasia. The mice were killed 72 hr after RWE challenge, and their lungs were processed and sections were stained with haematoxylin and eosin or PAS. Upper panels: inflammatory cell infiltration in peribronchial and perivascular regions. Lower panels: goblet cells metaplasia. Images are representative of serial sections from the lungs of seven mice in each group. (b) Morphometric quantification of peribronchial inflammatory cell infiltration. (c) Quantification of goblet cell metaplasia.
The oxidatively inactive Amb a 1 (25 µg) induced low-grade airway inflammation (Fig. 2a, b). Surprisingly, LF decreased Amb a 1-induced inflammation substantially. LFFe had no effect. When Amb a 1 was administered together with the ROS-generating G-ox, eosinophil accumulation in BAL was significantly increased (Fig. 2a, b). G-ox itself did not cause inflammatory cell accumulation in airways (Fig. 2a, b). When G-ox plus Amb a 1 and LF were administered together, the number of inflammatory cells in BAL decreased significantly (Fig. 2a, b). LFFe showed no statistically significant effect.
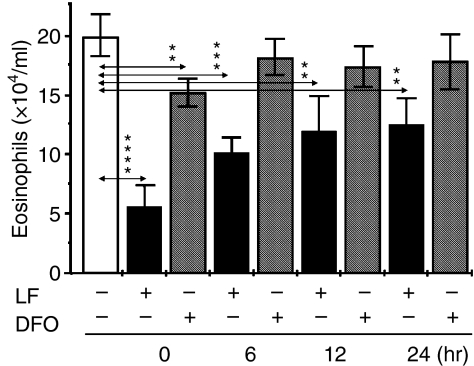
To sort out whether RWE challenge-induced immediate changes (decrease in oxidative levels) and/or some undefined inflammatory event(s) are also influenced by LF, we administered LF (or DFO) either simultaneously with RWE or 6, 12 or 24 hr following RWE-challenge. As shown in Fig. 4, LF was most effective when administered concurrently with RWE (0 hr). In addition, when LF was given 6, 12 or 24 hr after challenge also reduced inflammatory cell accumulation (Fig. 4). DFO was significantly less effective compared to LF when coadministered with RWE. Addition of DFO at later time points had no effect. These data together suggest that LF has an early effect and may regulate later events important in allergic inflammatory processes, which will be investigated in future studies.
Figure 4.
Lactoferrin is most effective when added at the time of allergen challenge. Accumulation of eosinophils in BAL fluids was assessed at 72 hr after challenge (n = 6–8 mice per group). Results are means ± SEM. **P < 0·01; ***P = 0·001; ****P = 0·0001.
Discussion
We have recently shown that ragweed pollen grains, RWE and 39 other pollen extracts generate because of their intrinsic NAD(P)H oxidase activity.14,15 RWE is a complete mixture of antigenic components of ragweed pollen grains and its addition to cultured cells or administration to sensitized animals mimics a natural exposure to pollens or subpollen particles. The redox-active RWE was shown to induce a several-fold increase in ROS levels in airway epithelium and conjunctival membranes.14,15 Protection from this oxidative insult significantly decreased allergic airway inflammation and conjunctivitis.14,15 In this study, we demonstrate that LF decreases allergic airway inflammation induced by RWE and Amb a 1. Specifically, we show that LF significantly decreases inflammatory cell accumulation in the airways induced by the redox-active RWE. Co-challenge of mice with RWE and the iron-binding DFO resulted in only a partial decrease in the level of inflammation. LF also decreased allergic inflammation induced by the non-redox Amb a 1. When mice were co-challenged with Amb a 1 and G-ox the level of inflammation increased by threefold (P = 0·0001) compared to Amb a 1 alone. Remarkably, LF decreased G-ox-mediated augmentation of Amb a 1-induced airway inflammation to level observed for Amb a 1 alone, while DFO had a less pronounced effect. Mucus hypersecretion by goblet cells is one of the major causes of airway obstruction in patients with allergic asthma.27 RWE challenge of mice caused an intense accumulation of mucin-producing cells (Fig. 3a, c). Importantly, administration of LF with RWE significantly decreased accumulation of mucin-producing goblet cells to nearly background level in the airway epithelium shown by PAS stain.
Our cell culture studies show that LF indeed possesses an antioxidant activity, which is nearly sufficient to protect cells from RWE-mediated oxidative stress. When cells were transferred into iron-free medium RWE-induced ROS levels were lower suggesting a significant role of iron in conversion of into highly reactive species. Most importantly LF was more effective in decreasing cellular ROS levels than in absence of iron in the culture medium or addition of the iron-binding DFO to the cells. Similar results were observed when cells were LF-treated and exposed to G-ox, a ROS-producing enzyme.26 Because in iron-free medium LF further decreased RWE-induced cellular ROS levels it suggests that iron-binding is only one of the actions of LF. LFFe showed only marginal effects due to the fact that it cannot bind iron that remain available to participate as a catalyst for the generation of the •OH.7 In our previous studies, we showed that 4-HNE and MDA levels significantly increased in airway lining fluid after RWE challenge.15 Indeed, partial reduction of molecular oxygen by pollen NAD(P)H oxidases yields , which in the presence of iron is converted to •OH (the Fenton and Haber–Weiss reactions). has a relatively long half life, a limited reactivity with some proteins, but not with lipids or DNA.28 On the other hand •OH is an extremely active oxidant, which is known to participate in lipid peroxidation and causes protein oxidation and DNA damage in cells. Our data show that LF significantly lowers RWE-induced increase in 4-HNE + MDA level, thus appears to inhibit conversion of to highly reactive species.
In parallel experiments, we also show that LF is most effective in decreasing inflammation when added together with RWE. Interestingly, when added at later time points after RWE challenge its effect is still significant. This phenomenon indicates that in addition to binding of iron, LF may have other actions for decreasing allergic immune responses. Among others, one of the key attributes of LF is that it can decrease the expression of pro-inflammatory mediators, including tumour necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and IL-8, major regulators of airway inflammation.11,29–31 Topical administration of LF inhibits the mobilization and migration of epidermal Langerhans cells and of TNF-α and IL-1β production.32,33 As a cationic molecule, LF interacts with metabolically important molecules.34 In fact, it has been shown that by virtue of binding heparin, LF can inactivate serine proteases released from activated mast cells.35 Another study reported that immunoglobulin E-dependent histamine release from skin mast cells was inhibited by up to 50% following incubation with LF.36 LF given to mice intraperitoneally, either prophylactically (18 hr before lipopolysaccharide (LPS) challenge) or therapeutically (1 hr post LPS challenge) is capable of substantially decreasing the levels of nitric oxide and TNF-α.37 LF is also capable of stimulating cell-mediated immunity in mice, including augmentation of the delayed type hypersensitivity response to specific antigens.38,39 In a sheep model, LF abolished both late-phase bronchoconstriction and airway hyperresponsiveness.35
Research into free iron metabolism in the lower respiratory tract shows that 80% of iron is present in the airway lining cells, especially in macrophages, and 20% in the epithelial lining fluid of the lung.40,41 LF is also present in airway lining fluid, however, its concentration is approximately 10 times lower than that of other iron chelators e.g. transferritin.42,43 Because LF supplementation along with allergen challenge significantly decreases RWE-induced oxidative stress levels (e.g. 4-HNE and MDA) in the BAL and RWE-induced airway inflammation it suggests that LF, along with its other properties, may have a particular role in iron metabolism in airways different from other iron-binding proteins. It appears that the free iron exacerbates RWEs' NAD(P)H oxidase generated oxidative stress. Redox active pollens or subpollen particles, and exogenous oxidant substances (ozone, cigarette smoke, NO2), oxidant particulate matters (found in diesel, cigarette smoke) exacerbate antigen-induced airway inflammations.2 Therefore, our data suggest that LF can ameliorate impact of other oxidants on antigen-induced inflammation. Finally, while the precise mechanism of LF action remains to be elucidated our data along with LF's ability to inhibit pro-inflammatory cytokine production and immunoglobulin E-dependent activation of human mast cells hold a promise for the therapeutic utility of LF in human allergic inflammatory disorders.
Acknowledgments
Research was supported by National Institute of Environmental Health and Sciences Center Grant (ES06676), and grants from National Institute of Health (RO1-HL07163-01; Sur, Boldogh) and National Institute Allergic and Infectious Diseases (PO1-AI062885-01; Boldogh, Brasier). We thank Dr Garg (UTMB, Department of Microbiology) and Dr Jeffrey K. Actor (Department of Pathology, UT Medical School, Houston) for their scientific and editorial advice.
Abbreviations
- 4-HNE
4-hydroxynoneal
- BAL
bronchoalveolar lavage
- DCF
dichlorofluorescein
- DFO
desferoxamine
- G-ox
glucose oxidase
- H2DCF-DA
2′-7′-dihydro-dichlorofluorescein diacetate
- HRP
horseradish peroxidase
- IFM
iron-free medium
- IL
interleukin
- LF
lactoferrin
- LFFe
iron-saturated lactoferrin
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- NAD(P)H
reduced nicotinamide adenine dinucleotide phosphate
- NHBE
normal human bronchial epithelial cells
superoxide anion
- PAS
periodic acid Schiff
- ROS
reactive oxygen species
- RWE
ragweed pollen extract
- SOD
superoxide dismutase
- TNF
tumour necrosis factor
References
- 1.Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402(6760 Suppl.):B2–4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 2.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002;110:349–56. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- 3.Dworski R. Oxidant stress in asthma. Thorax. 2000;55(Suppl. 2):S51–3. doi: 10.1136/thorax.55.suppl_2.S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp C, Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970;245:4641–6. [PubMed] [Google Scholar]
- 6.Beutler E, Hoffbrand AV, Cook JD. Iron deficiency and overload. Hematology (Am Soc Hematol Educ Program) 2003;1:40–61. doi: 10.1182/asheducation-2003.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg ED. The therapeutic potential of lactoferrin. Expert Opin Invest Drugs. 2003;12:841–51. doi: 10.1517/13543784.12.5.841. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–6. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 10.Zagulski T, Lipinski P, Zagulska A, Broniek S, Jarzabek Z. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br J Exp Pathol. 1989;70:697–704. [PMC free article] [PubMed] [Google Scholar]
- 11.Britigan BE, Serody JS, Cohen MS. The role of lactoferrin as an anti-inflammatory molecule. Adv Exp Med Biol. 1994;357:143–56. doi: 10.1007/978-1-4615-2548-6_14. [DOI] [PubMed] [Google Scholar]
- 12.Brock J. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 1995;16:417–9. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 13.Brock JH. The physiology of lactoferrin. Biochem Cell Biol. 2002;80:1–6. doi: 10.1139/o01-212. [DOI] [PubMed] [Google Scholar]
- 14.Bacsi A, Dharajiya N, Choudhury BK, Sur S, Boldogh I. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J Allergy Clin Immunol. 2005;116:836–43. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boldogh I, Bacsi A, Choudhury BK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:1–13. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–67. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Gutteridge JM, Mumby S, Quinlan GJ, Chung KF, Evans TW. Pro-oxidant iron is present in human pulmonary epithelial lining fluid: implications for oxidative stress in the lung. Biochem Biophys Res Commun. 1996;220:1024–7. doi: 10.1006/bbrc.1996.0518. [DOI] [PubMed] [Google Scholar]
- 18.Mateos F, Brock JH, Perez-Arellano JL. Iron metabolism in the lower respiratory tract. Thorax. 1998;53:594–600. doi: 10.1136/thx.53.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park YY, Hybertson BM, Wright RM, Repine JE. Serum ferritin increases in hemorrhaged rats that develop acute lung injury: effect of an iron-deficient diet. Inflammation. 2003;27:257–63. doi: 10.1023/a:1025044732423. [DOI] [PubMed] [Google Scholar]
- 20.Zhao G, Ayene IS, Fisher AB. Role of iron in ischemia-reperfusion oxidative injury of rat lungs. Am J Respir Cell Mol Biol. 1997;16:293–9. doi: 10.1165/ajrcmb.16.3.9070614. [DOI] [PubMed] [Google Scholar]
- 21.Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, Sur S. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164:2701–10. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 22.Dayani PN, Bishop MC, Black K, Zeltzer PM. Desferoxamine (DFO)-mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J Neurooncol. 2004;67:367–77. doi: 10.1023/b:neon.0000024238.21349.37. [DOI] [PubMed] [Google Scholar]
- 23.Loo BW, Jr, Meyer-Ilse W, Rothman SS. Automatic image acquisition, calibration and montage assembly for biological X-ray microscopy. J Microsc. 2000;197:185–201. doi: 10.1046/j.1365-2818.2000.00644.x. [DOI] [PubMed] [Google Scholar]
- 24.Klimaschewski L, Nindl W, Pimpl M, Waltinger P, Pfaller K. Biolistic transfection and morphological analysis of cultured sympathetic neurons. J Neurosci Methods. 2002;113:63–71. doi: 10.1016/s0165-0270(01)00473-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–8. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson R, Pick FM, Bray RC. EPR studies on reduction of oxygen to superoxide by some biochemical systems. Biochim Biophys Acta. 1969;192:145–8. doi: 10.1016/0304-4165(69)90022-1. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein RA, Paul WE, Metcalfe DD, Busse WW, Reece ER. NIH conference. Asthma. Ann Intern Med. 1994;121:698–708. doi: 10.7326/0003-4819-121-9-199411010-00011. [DOI] [PubMed] [Google Scholar]
- 28.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–97. [PubMed] [Google Scholar]
- 29.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–6. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 30.Legrand D, Elass E, Pierce A, Mazurier J. Lactoferrin and host defence. an overview of its immuno-modulating and anti-inflammatory properties. Biometals. 2004;17:225–9. doi: 10.1023/b:biom.0000027696.48707.42. [DOI] [PubMed] [Google Scholar]
- 31.Zimecki M, Wlaszczyk A, Zagulski T, Kubler A. Lactoferrin lowers serum interleukin 6 and tumor necrosis factor alpha levels in mice subjected to surgery. Arch Immunol Ther Exp (Warsz) 1998;46:97–104. [PubMed] [Google Scholar]
- 32.Griffiths CE, Cumberbatch M, Tucker SC, Dearman RJ, Andrew S, Headon DR, Kimber I. Exogenous topical lactoferrin inhibits allergen-induced Langerhans cell migration and cutaneous inflammation in humans. Br J Dermatol. 2001;144:715–25. doi: 10.1046/j.1365-2133.2001.04125.x. [DOI] [PubMed] [Google Scholar]
- 33.Kimber I, Cumberbatch M, Dearman RJ, Headon DR, Bhushan M, Griffiths CE. Lactoferrin. influences on Langerhans cells, epidermal cytokines, and cutaneous inflammation. Biochem Cell Biol. 2002;80:103–7. doi: 10.1139/o01-227. [DOI] [PubMed] [Google Scholar]
- 34.Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005;62:2549–59. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elrod KC, Moore WR, Abraham WM, Tanaka RD. Lactoferrin, a potent tryptase inhibitor, abolishes late-phase airway responses in allergic sheep. Am J Respir Crit Care Med. 1997;156(2 Part 1):375–81. doi: 10.1164/ajrccm.156.2.9607012. [DOI] [PubMed] [Google Scholar]
- 36.He S, McEuen AR, Blewett SA, Li P, Buckley MG, Leufkens P, Walls AF. The inhibition of mast cell activation by neutrophil lactoferrin. uptake by mast cells and interaction with tryptase, chymase and cathepsin G. Biochem Pharmacol. 2003;65:1007–15. doi: 10.1016/s0006-2952(02)01651-9. [DOI] [PubMed] [Google Scholar]
- 37.Kruzel ML, Harari Y, Mailman D, Actor JK, Zimecki M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin Exp Immunol. 2002;130:25–31. doi: 10.1046/j.1365-2249.2002.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002;2:475–86. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 39.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett. 2000;74:183–8. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 40.Corhay JL, Weber G, Bury T, Mariz S, Roelandts I, Radermecker MF. Iron content in human alveolar macrophages. Eur Respir J. 1992;5:804–9. [PubMed] [Google Scholar]
- 41.Wesselius LJ, Flowers CH, Skikne BS. Alveolar macrophage content of isoferritins and transferrin. Comparison of nonsmokers and smokers with and without chronic airflow obstruction. Am Rev Respir Dis. 1992;145(2 Part 1):311–6. doi: 10.1164/ajrccm/145.2_Pt_1.311. [DOI] [PubMed] [Google Scholar]
- 42.Pacht ER, Davis WB. Role of transferrin and ceruloplasmin in antioxidant activity of lung epithelial lining fluid. J Appl Physiol. 1988;64:2092–9. doi: 10.1152/jappl.1988.64.5.2092. [DOI] [PubMed] [Google Scholar]
- 43.Thompson AB, Bohling T, Heires A, Linder J, Rennard SI. Lower respiratory tract iron burden is increased in association with cigarette smoking. J Lab Clin Med. 1991;117:493–9. [PubMed] [Google Scholar]