Abstract
Interleukin (IL)-7 and IL-15 are cytokines implicated in homeostatic control of the peripheral CD8 T-cell pool. We compared the effects of IL-7 and IL-15 on survival and proliferation of purified human CD8+ T-cell subsets. Low concentrations of either cytokine reduced the spontaneous apoptosis of all subsets, and enhancement of survival corresponded to the extent of Bcl-2 up-regulation. Surprisingly, although minimal proliferation of naïve CD8+ T cells was observed during the first week of culture with cytokines, a marked expansion of these cells occurred at later time points, particularly in response to IL-15. This occurred largely without phenotypic change or acquisition of effector function, indicating a dissociation of differentiation from proliferation. Notably, progression of naïve CD8+ T cells through several cell divisions resulted in up-regulation of telomerase and the maintenance of telomere length. These data show that IL-7 and IL-15 induce cell proliferation and rescue from apoptosis in a concentration, time and subset-dependent manner, and have implications for the homeostatic expansion of the naïve CD8+ T-cell pool.
Keywords: CD8 T cell, cytokine, telomere
Introduction
The T-cell pool comprises cells differing in phenotype and history of encounter with antigen. In humans, differential expression of CD45 isoforms was originally used to distinguish naïve T cells from previously primed cells, naïve cells selectively expressing CD45RA rather than CD45RO.1 More recently expression of the chemokine receptor CCR7 has been used to further subdivide T cells into CD45RO+ CCR7+‘central’ memory cells (T-CM) and CCR7–‘effector’ memory cells (T-EM), while CD45RA+ CD8+ T cells can be divided into CCR7+ naïve and CCR7– primed (RA-primed) subsets.2
Over the lifetime of an individual, the absolute number of cells in the recirculating T-cell pool remains relatively constant.3 Overall stability, however, is maintained in a highly dynamic manner, involving the production and death of billions of cells. Moreover, within the T-cell pool, the naïve and primed cell populations are regulated in different ways so that, in general, naïve T cells persist largely as non-dividing cells after export from the thymus, while primed T-cell populations undergo frequent cell division balanced by cell death.4,5 The relatively quiescent nature of naïve T cells may limit their ability to persist into old age, when thymic output declines. Consistent with this idea, the proportion of naïve CD8+ T cells is typically lower in the elderly6 while other populations, particularly RA-primed cells, increase.7,8 However, in mice, naïve T cells proliferate extensively after adoptive transfer into lymphopenic hosts, indicating that under certain conditions they possess the capacity for ‘homeostatic’ expansion.9,10
Cytokines are one factor influencing the kinetic behaviour of naïve and memory CD8+ T cells, both interleukin (IL)-7 and IL-15 have been implicated in CD8+ T-cell homeostasis. The importance of IL-7 in promoting survival of naïve CD8+ T cells was shown in adoptive transfer models, in which IL-7+/+ cells disappeared rapidly after transfer into IL-7–/– mice, as did IL-7R-deficient cells injected into normal hosts.11,12 IL-7 is required for homeostatic proliferation of naïve T cells in lymphopenic mice;11 conversely, IL-15 stimulates proliferation of memory-phenotype (CD44hi) but not naïve-phenotype (CD44lo) CD8+ T cells in vivo and in vitro,13 both the number and proliferation rate of CD8 memory cells being reduced in IL-15–/– and IL-15Rα–/– mice.14 However, the correlation between IL-7 and naïve cells, and IL-15 and memory cells is not absolute; high levels of IL-7 can substitute for IL-15 in driving memory CD8+ T-cell proliferation14 and IL-15 can enhance the survival of both naïve and memory-phenotype CD8+ T cells.15
In humans, the relative contribution of these cytokines to survival and proliferation of naïve and memory CD8+ T cells is less well established. IL-7-dependent expansion of CD45RA+ neonatal CD8+T cells has been reported,16 whilst IL-15 stimulates proliferation of CD45RO+ CD8+ T cells17 and induces re-expression of CD45RA on activated Epstein–Barr virus-specific CD8+ T cells.18 At present, though, the ability of IL-15 to cause proliferation of naïve cells is not clear.17,19,20
Ultimately, persistence of cell populations is determined by the balance between cell division and death. The factors controlling the survival and proliferation of different T-cell subsets are likely to be distinct. We have investigated how IL-7 and IL-15 regulate the homeostasis of the four major human CD8+ T-cell subpopulations. Both cytokines can induce proliferation and rescue cells from apoptosis, but do so in a concentration- and cell subset-dependent manner. Most strikingly, prolonged exposure to IL-15 stimulates a late expansion of naïve CD8+ cells, largely without alteration of the naïve cell phenotype but accompanied by up-regulation of telomerase activity. The implications of these findings for T-cell homeostasis are discussed.
Materials and methods
Cell sorting
Peripheral blood mononuclear cells (PBMC) were isolated from 50 ml of heparinized fresh human blood from volunteers or buffy coats (NE London Blood Transfusion Centre, Colindale, London, UK) by Ficoll-Paque (Pharmacia, St Albans, UK) density gradient centrifugation and CD8+ T cells isolated using CD8-magnetic-activated cell sorting (MACS) beads (Miltenyi Biotec, Bisley, UK) according to the manufacturer's instructions. These cells (1 × 107/ml in phosphate-buffered saline (PBS) + 0·2% bovine serum albumin (BSA) were stained with CCR7 + goat F(ab′)2 anti-mouse immunoglobulin M (IgM)–R-phycoerythrin (RPE); Southern Biotechnology Associates, Birmingham, AL), CD45RO–antigen-presenting cell [Allophycocyanin (APC); Caltag Medsystems, Towcester, UK] together with CD56–fluoroscein isothiocyanate (FITC; Serotec, Oxford, UK), to remove residual natural killer (NK)+ cells. CD45RO+ CCR7+, CD45RO+ CCR7–, CD45RO– CCR7+ and CD45RO– CCR7– subsets were sorted using a Moflo cytometer (Cytomation, Fort Collins, CO), purities >95%. CD45RO (rather than CD45RA) monoclonal antibody (mAb) was used to ensure the naïve fraction did not contain CD45RO+ CD45RA+ double positive cells or CD45RO+dim cells. For convenience CD45RO– cells are referred to as CD45RA+. Ethical approval was granted by the Human Subjects Committee, Edward Jenner Institute for Vaccine Research.
Cell culture
Cells (5 × 104) were cultured at 37° in round-bottomed 96-well plates in medium (RPMI-1640 + 10% fetal calf serum (FCS)) with or without recombinant human IL-15 or IL-7 (R & D Systems, Abingdon, UK) at final concentrations of 0·4–50 ng/ml for 24 hr to 87 days (without change of medium). In some experiments cells were cultured with CD3/CD28 T-cell expander Dynabeads (Dynal, Wirral, UK; 8 × 104 beads/well) for 6 hr.
Flow cytometric analysis
Cells were stained with FITC-conjugated mAbs (CD11a, CD27, CD28, CD38, CD62L, CD95, CD45RA, CD45RO (BD Biosciences Pharmingen, Oxford, UK) CD45RB (DAKO, Ely, UK), CD69 (Caltag), or with CCR7 mAb (BD Pharmingen) + goat F(ab′)2 anti-mouse IgM–RPE. Alternatively cells were stained with CD45RO–APC (Caltag), CD8–biotin and streptavidin. Relevant isotype controls were included. 10 000–50 000 cells were collected on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analysed using WinMDI software.
For intracellular staining cells were fixed and permeabilized at room temperature for 40 min with Permeafix (Ortho Diagnostics, High Wycombe, UK), washed, then stained with FITC-conjugated perforin, granzyme A, granzyme B or Bcl-2 mAbs.
For intracellular staining for cytokines, phorbol 12-myristate 13-actetate (PMA; 5 ng/ml, Sigma, Poole, UK), ionomycin (500 ng/ml) and GolgiPlug (according to manufacturer's instructions, BD Biosciences) were added to cultures for the last 5 hr. Cells were fixed, permeabilized and intracellular staining carried as described using FITC-conjugated mAbs to interferon-γ (IFN-γ) or tumour necrosis factor-a (TNF-α). Cells were stained with annexin V–FITC according to the manufacturer's instructions (BD Pharmingen).
Control of CD95 expression by cytokines
CD45RA+ CCR7+ cells were cultured with IL-15 or IL-7 (50 ng/ml) as described above and stained for CD95 expression at regular intervals. At day 16, when CD95 up-regulation had occurred on all cells, half the cells were extensively washed to remove residual cytokine and replaced in culture in medium without cytokine. After 9 days CD95 expression was rechecked.
Telomere length measurement by Southern blot analysis
Genomic DNA, from sorted CD45RA+ CCR7+ cells cultured with IL-7 or IL-15 (50 ng/ml), was digested with RsaI and HinfI, loaded onto a 0·5% agarose gel (1 µg/well) and separated by electrophoresis. Gels were denatured, neutralized, dried and probed overnight at 37° with 32P end-labelled oligonucleotide (CCCTAA) to detect telomeric sequences as previously described.21 After extensive washing with 0·1 × sodium chloride-sodium citrate buffer (SSC) telomeres were visualized by autoradiography. Terminal restriction fragment (TRF) length was calculated following densitometry using Bio-Rad GS700 system running Molecular Analyst software.
Cell labelling
MACs-purified or Moflo-sorted subsets of CD8+ T cells were labelled with 2·5 µm 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) (Renovar, Madison, WI) for 8 min at 37°, washed three times with RPMI-1640 + 10% FCS at room temperature then incubated at 37° for 20 min. After washing once, cells were cultured with/without cytokines at various concentrations.
Proliferation assays
2·5 × 104 cells/well were cultured, in triplicate, in 96-well plates for 3–21 days with IL-15 or IL-7. Proliferation was determined by measuring incorporation of [3H]-thymidine into cells pulsed for the last 6 hr with 1 µCi/well.
Measurement of telomerase activity
A modified version of the telomeric repeat amplification protocol (TRAP: TRAPeze Telomerase Detection Kit, Intergen Company, Oxford, UK)22 was used to measure telomerase activity in cell pellets from samples of purified CD8+ subset T cells which were snap frozen either post sorting or after in vitro culture with IL-15 or IL-7 (50 ng/ml). Polymerase chain reaction (PCR) was performed on 5000 cells per reaction. The negative control contained the PCR mix without cell extract, the positive control contained an extract of a telomerase-positive tumour line. TSR8 denotes the internal quantitative control (oligo containing the same sequence as the TS primer plus eight telomeric repeats).
Results
Characteristics and response of CD8+ subsets to high and low doses of IL-7 and IL-15
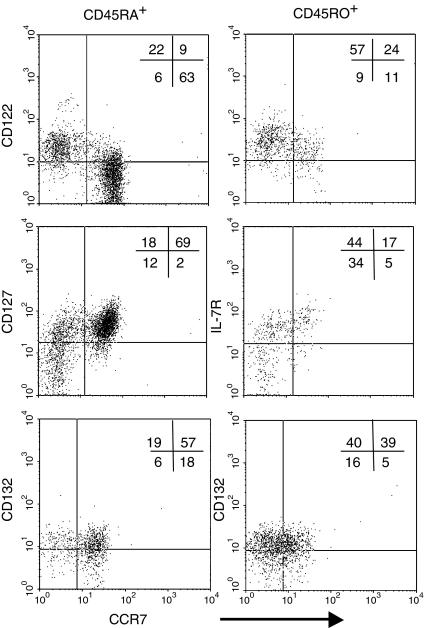
Cell surface markers indicate the state of differentiation of cells. CD45RA+ CCR7+ T cells are predominantly CD11alow and CD95low, characteristics of unprimed cells,6,23 while the three other CD8+ T-cell subsets expressed high levels of CD11a and were CD95+ (not shown). All subsets expressed moderate levels of CD132, the common interleukin receptor γ-chain. CD122, the β-chain of the IL-2 and IL-15 receptor, was most strongly expressed on CCR7– subsets while CD127, the IL-7 receptor α-chain, was most highly expressed on CD45RA+ CCR7+ naïve cells (Fig. 1).24 Although IL-15Rα expression was not analysed, this chain may not be required for T-cell responses to IL-15.24,25 These data suggest that CD45RA+ CCR7+ cells should be unresponsive to IL-15 but might respond to IL-7.
Figure 1.
Expression of CD132 (common γ-chain), CD122 (IL-2/15R β-chain) and CD127 (IL-7R α-chain) on subsets of CD8+ T cells. PBMC were stained as described in materials and methods. One representative experiment of three is shown.
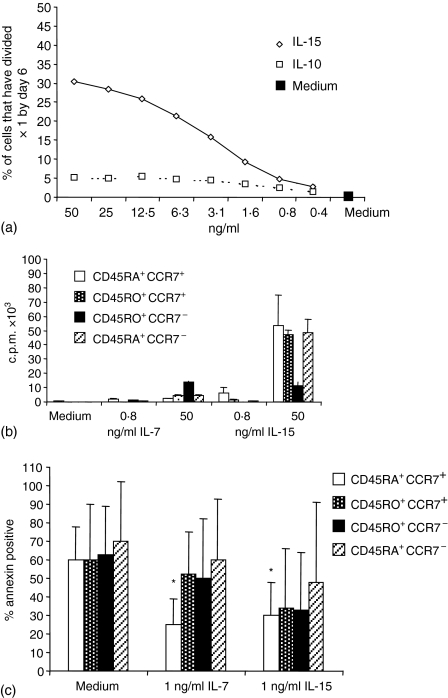
Preliminary experiments showed that IL-15 but not IL-7 induced substantial cell division of total CD8+ T cells in a dose-dependent manner (Fig. 2a). With IL-15 (50 ng/ml) 30% of CD8+ cells had divided at least once by day 6 while little cell division occurred with any dose of IL-7.
Figure 2.
Induction of proliferation and prevention of apoptosis by IL-7 and IL-15. (a) Purified CFSE-labelled CD8+ T cells were incubated with graded doses of recombinant human IL-7 or IL-15 for 6 days and the percentage of cells that had undergone division measured (one representative experiment of three). (b) Purified CD8+ T-cell subsets were stimulated with high or low doses of IL-7 or IL-15 and proliferation measured by [3H]-thymidine incorporation on day 6. (c) Apoptosis of purified subsets in the presence or absence of IL-7 or IL-15 was measured by annexin V–FITC staining on day 6 (mean ± SE of data from six individuals). *Indicates P ≤ 0·05 compared to the same cells cultured in medium alone.
Proliferation of the phenotypically defined subpopulations was assessed by culturing purified subsets with high or low concentrations of cytokines and pulsing with [3H]-thymidine (Fig. 2b). IL-7 did not induce proliferation, except for a minor response by the CD45RO+ CCR7– subset when a high dose (50 ng/ml) was used. In contrast, IL-15 (50 ng/ml) induced proliferation in all subsets although only a low response of CD45RO+ CCR7– cells was observed and the response of CD45RA+ CCR7+ cells was variable at 7 days. At a low dose (0·8 ng/ml), neither cytokine induced proliferation.
The ability of both cytokines to prevent apoptosis of purified subsets was examined by annexin V staining. Following 6 days of culture in medium alone extensive apoptosis had occurred within all subsets (Fig. 2c), although the degree of apoptosis varied between individuals. Both the CCR7– subpopulations were more susceptible to spontaneous apoptosis within the first 24 hr (data not shown). Interestingly, both cytokines induced substantial rescue from apoptosis in CD45RA+ CCR7+ cells (Fig. 2c), even though the β-chain of the IL-15 receptor (CD122) is not highly expressed in this subset (Fig. 1). There was a trend for better survival in the presence of IL-15 for all primed subsets although this did not reach statistical significance and although IL-7 slightly reduced the percentage of apoptotic cells in the primed subsets, this cytokine was a less potent survival factor for these cells than for CD45RA+ CCR7+ cells.
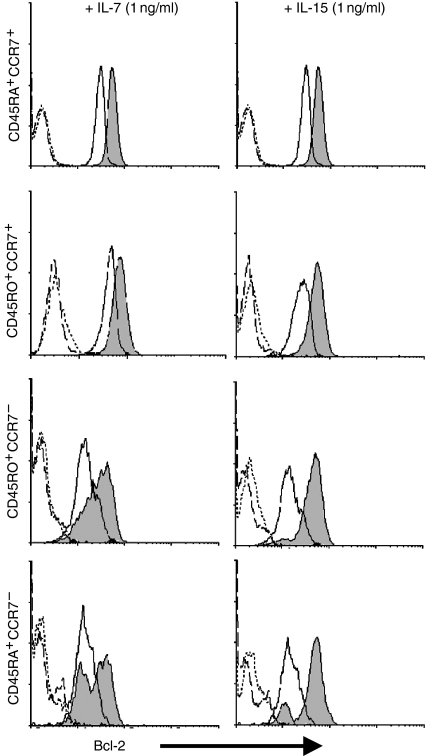
Effects of IL-15 and IL-7 on Bcl-2 levels
Since Bcl-2 has important pro-survival effects for CD8+ T cells and can be induced by γc-family cytokines16,26 we examined the role of this molecule in the anti-apoptotic effects of IL-7 and IL-15. Ex vivo expression of Bcl-2 varied between subsets, being higher on CCR7+ than CCR7– cells, which could account for the higher susceptibility of the latter to spontaneous apoptosis (see above). Consistent with their anti-apoptotic effects (Fig. 2c) both cytokines induced up-regulation of Bcl-2 in all subsets (Fig. 3) although both quantitative and qualitative differences were observed. Uniform up-regulation of Bcl-2 occurred in both CCR7+ subsets after culture with either cytokine. After culture with IL-15, a small proportion of CD45RA+ CCR7– cells did not appear to respond, in keeping with the reduced efficiency with which IL-15 inhibited apoptosis in this subpopulation. After IL-7 treatment, both the CCR7– subsets exhibited bimodal expression of Bcl-2, a sizeable proportion of cells showing no up-regulation. This could reflect the presence of IL-7Rα– cells in these subsets (data not shown). Taken together, these results show a close association between the ability of IL-15 and IL-7 to up-regulate Bcl-2 and their capacity to protect CD8+ T cells from apoptosis, providing a plausible mechanism for their pro-survival effects. For cells with a history of previous activation and replication, IL-15 appears to be a more effective survival factor than IL-7, a phenomenon that is partly related to their expression of cytokine receptors.
Figure 3.
Induction of Bcl-2 by IL-7 and IL-15. Purified subsets of CD8+ T cells were cultured for 4 days with non-proliferative doses (1 ng/ml) of IL-15 or IL-7 and up-regulation of Bcl-2 measured by flow cytometry using a live cell gate. Solid fill, Bcl-2 on day 4; solid line, Bcl-2 on day 0; dotted line, isotype control for day 0 and; dashed line, isotype control for day 4.
IL-15 induces a late expansion of CD45RA+ CCR7+ CD8+ T cells
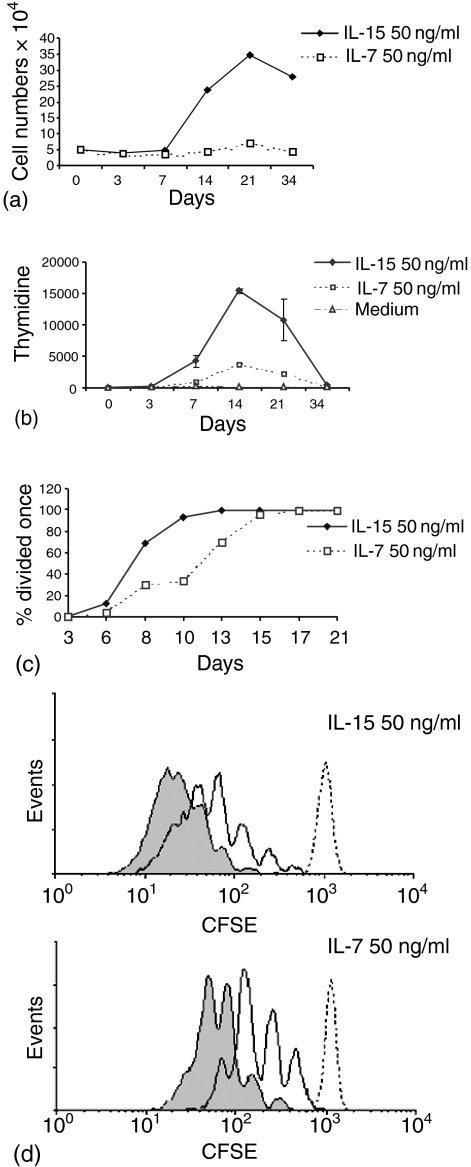
Although in most experiments little cell division was detected among CD45RA+ CCR7+ cells cultured for 6 days in IL-15, an unexpected increase in cell numbers was observed at later time points. For example, after 21 days of culture with IL-15 (50 ng/ml), there was a sevenfold increase in cell numbers (5–10-fold increase in four of four experiments) (Fig. 4a). Subsequently the number of cells gradually decreased, although viable cells were maintained as long as 87 days without further addition of cytokine or medium (data not shown). In contrast, with IL-7, only a small expansion (2×) was observed at day 21 (Fig. 3a), although, in one experiment (out of four) a larger rise, similar to that observed with IL-15, occurred at day 31 (data not shown). These data suggest that IL-15- or IL-7-induced proliferation of CD45RA+ CCR7+ cells can occur, but only after prolonged exposure to these cytokines.
Figure 4.
Delayed proliferation of CD45RA+ CCR7+ CD8+ T cells in response to IL-7 or IL-15. (a, b) Purified cells cultured with 50 ng/ml of IL-7 or IL-15 for 34 days (representative data shown for one experiment of four). Spontaneous apoptosis of cells in medium alone was ≥90% by day 7 (data not shown). (a) Viable cell numbers were determined at different time points by sampling bulk cultures repeatedly (trypan blue exclusion). (b) Proliferation was measured by [3H]-thymidine incorporation at the indicated time points. (c–e) CFSE-labelled cells were cultured as indicated and cell division measured by flow cytometry. (d, e) dotted line day 3, solid line day 13, solid fill day 21.
Cell proliferation in these long-term cultures was measured directly using thymidine incorporation (Fig. 4b). Substantial proliferation was detected in IL-15-containing cultures at day 14. In a separate experiment, CFSE-labelled CD45RA+ CCR7+ cells were cultured with IL-15 or IL-7. Figure 4(c) shows the number of cells that had divided at least once at different time points. With IL-15, there was a burst of cell division beginning around day 8 (as seen with thymidine, Fig. 4b) and by day 13 all cells had divided once, the response to IL-7 occurring more slowly. Differences between the responses to IL-15 and IL-7 were more evident when CFSE labelling was compared at days 13 and 21 (Fig. 4d, e). At day 13, 88% of CD45RA+ CCR7+ cells cultured with IL-15 had undergone more than three divisions compared to 37% of IL-7-treated cells. By day 21, most IL-15-stimulated cells had undergone five or more divisions (98%) whereas 14% of IL-7-cultured cells had undergone five divisions, 85% having divided three times.
These long-term effects of the cytokines were surprising, given that IL-15 and IL-7 were only added to the cultures on day 0, however, at least immunoreactive IL-15 could be demonstrated in supernatants by enzyme immunoassay up to 87 days after the initiation of culture (data not shown).
Phenotypic characteristics of cytokine-expanded CD45RA+ CCR7+ CD8+ T cells
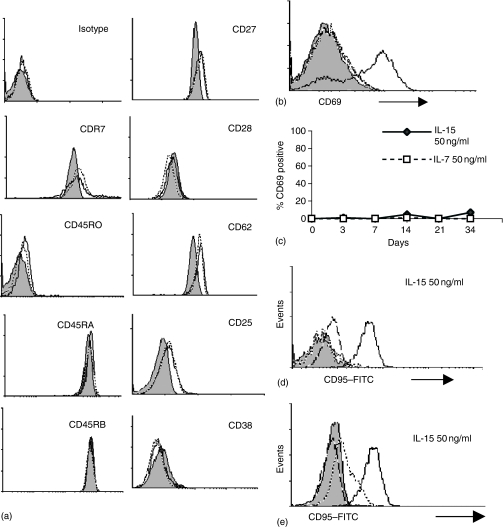
To further characterize the effects of prolonged cytokine exposure on CD45RA+ CCR7+ cells, we analysed their phenotype over time. The cells remained largely unchanged (Fig. 5a). They did not up-regulate CD45RO or CD38, although there was a slight increase in CD25 and CD11a (data not shown). Retention of the CD8+ CD45RO– CCR7+ phenotype excluded the possibility that the observed proliferation was caused by outgrowth of a contaminating population, a conclusion supported by the absence of changes in Vβ family usage among cultured cells (Table 1).
Figure 5.
Phenotype of purified CD45RA+ CCR7+ CD8+ T cells cultured for a prolonged period in IL-7 or IL-15. (a) Flow cytometric analysis of cell surface markers on purified cells cultured for 15 days with 50 ng/ml of IL-7 or IL-15. Filled histogram represents expression on day 0, dotted line cells cultured with IL-7, and solid line cells cultured in the presence of IL-15. (b) CD69 expression measured after 6 hr culture with CD3/CD28 expander beads/cytokines, filled histogram represents CD69 expression on day 0, dotted line and dashed line represent culture with IL-15 and IL-7, respectively, solid line represents culture with CD3/CD28 expander beads. (c) CD69 expression during 34 days culture with 50 ng/ml of IL-7 or IL-15. (d) CD95 expression was measured on cells at day 0 (dotted line) or cultured for 16 days (solid line) with 50 ng/ml IL-15. Solid fill represents isotype day 0, dashed line isotype day 16. (e) CD95 expression measured after culture with IL-15 for 25 days (solid line), or after 16 days culture with cytokines followed by washing and culture in medium alone for 9 days (dotted line). Isotype control for day 25 represented by dashed line, isotype for washed and recultured cells represented by solid fill histogram.
Table 1.
Vβ expression on purified CD8+ CD45RA+ CCR7+ T cells before or after culture with either IL-7 or IL-15 at 50 ng/ml for 15 days
| Day 0 | Day 15 + IL-15 | Day 15 + IL-7 | |
|---|---|---|---|
| Vβ 3.1 | 3·1 | 2·7 | 3·7 |
| Vβ 5.1 | 7 | 7 | 6·9 |
| Vβ 5.3(5a) | 4·2 | 3·8 | 4·3 |
| Vβ 5.1 (5b) | 1·3 | 1·2 | 1·1 |
| Vβ 6.7 | 1·4 | 2·9 | 3 |
| Vβ 8 | 1·7 | 1·4 | 1·3 |
| Vβ 12 | 8·8 | 8·1 | 5·8 |
| Vβ 13.1 | 6·6 | 6·1 | 6 |
| Vβ 2 | 2·4 | 2·2 | 1·4 |
| Vβ 12.1 | 6·6 | 8·7 | 7·3 |
CD45RA+ CCR7+ cells cultured for 6 hr with CD3/CD28 beads up-regulated CD69 (Fig. 5b). Surprisingly, however, when cells were cultured with IL-15 or IL-7 almost no CD69 up-regulation occurred at any time point (Fig. 5c). In contrast, the other CD8+ T-cell subsets up-regulated CD69 after culture with IL-15 (data not shown).
Although most activation markers were absent on CD45RA+ CCR7+ cells cultured with cytokines, one exception was CD95 (Fig. 5d), a surface marker that is absent from naïve CD8+ T cells.6 By day 16 most cells cultured with IL-7 or IL-15 (50 ng/ml) expressed CD95, although up-regulation of CD95L (FasL) did not occur (data not shown).
To determine whether CD95 expression on cytokine-stimulated CD45RA+ CCR7+ cells represented a permanent change or a transient response to stimulation, cells were incubated for 16 days in IL-15 or IL-7, then either left with cytokine or washed extensively and cultured for a further 9 days in medium alone. As shown in Fig. 5(e), down-regulation of CD95 was observed following removal of cytokines indicating that high level expression of CD95 by CD45RA+ CCR7+ cells appears to require continuous stimulation with cytokines.
IL-15 and IL-7 do not induce effector activity in CD45RA+ CCR7+ CD8+ T cells
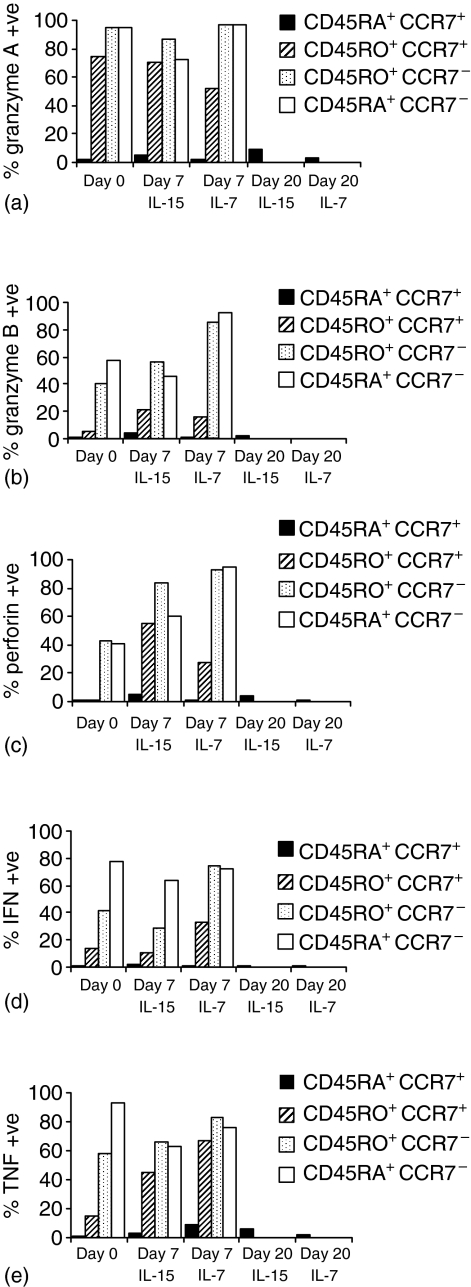
Effector function was investigated by measuring the effects of cytokines on expression of three key components of the cytotoxic T lymphocyte killing machinery: granzyme A, granzyme B and perforin.24 CD45RA+ CCR7+ cells remained negative for granzyme A, granzyme B and perforin after culture with either cytokine for 7–20 days. In contrast, in the other subsets, there was constitutive expression of granzyme A, and by day 7 up-regulation of granzyme B and perforin had occurred in the primed subsets (by day 20 extensive cell death had occurred in these subsets making further analysis impossible) (Fig. 6a–c). As an additional assay for effector activity in CD45RA+ CCR7+ cells, we examined the effects of IL-15 and IL-7 on expression of IFN-γ and TNF-α (Fig. 6d, e). Constitutive expression of IFN-γ and TNF-α was detected in a proportion of all primed subsets but not in CD45RA+ CCR7+ cells. Whilst IL-7 and IL-15 were able to augment cytokine production in some primed subsets, importantly, no significant induction of cytokine production by CD45RA+ CCR7+ cells was observed. Therefore, these results indicate that CD45RA+ CCR7+ cells do not acquire effector activity in response to stimulation with IL-15 or IL-7 alone.
Figure 6.
Analysis of effector function in purified subsets of CD8+ T cells stimulated with 50 ng/ml of IL-7 or IL-15. Expression of granzyme A (a), granzyme B (b), or perforin (c) measured by intracellular staining of purified subsets on day 0 or after culture with IL-15 or IL-7 for 7, 15 or 20 days (CD45RA+ CCR7+ cells only) as indicated. (d, e) Expression of IFN-γ and TNF-α, on purified subsets cultured for 7, 15 or 20 days (CD45RA+ CCR7+ cells only) with cytokines, incubated for the last 5 hr with PMA + ionomycin + GolgiPlug. Cytokines were detected by intracellular staining. All results are representative of three independent experiments.
Culture of CD45RA+ CCR7+ CD8+ T cells with IL-15 or IL-7 promotes telomerase induction
Because telomere erosion occurs with cell division27 cytokine-induced proliferation of CD45RA+ CCR7+ cells might be expected to cause a reduction in telomere length over time. However, telomere shortening can be counteracted by induction of the enzyme telomerase, which can occur following stimulation of T cells through the T-cell receptor28 or when memory CD8+ T cells are cultured with IL-15.29
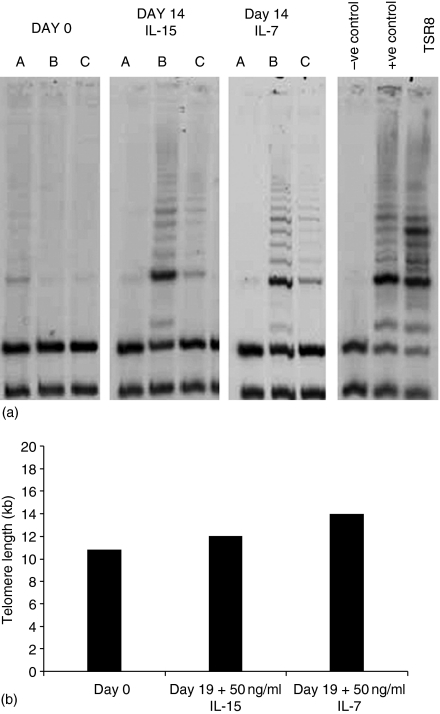
To ascertain whether IL-7 or IL-15 induce telomerase in the CD45RA+ CCR7+ subset, purified cells were cultured with concentrations of cytokines that supported proliferation, telomerase activity was measured using the TRAP assay. In seven of eight individuals endogenous telomerase activity in freshly isolated CD45RA+ CCR7+ cells was minimal. Figure 7(a) shows the results of one experiment with cells from three separate individuals after 14 days culture in either cytokine, induction of telomerase activity was observed. The effects of both cytokines were dose dependent, higher levels being detected with 50 ng/ml than with 3 ng/ml (data not shown). The effect of telomerase up-regulation was assessed by comparing telomere length in the same population of CD45RA+ CCR7+ cells on day 0 and after 19 days of culture with cytokines (Fig. 7b). Strikingly, telomeres were, if anything, slightly longer in the cytokine-treated cells, implying cytokine-mediated up-regulation of telomerase activity plays an important role in preventing telomere erosion and maintaining replicative potential of CD45RA+ CCR7+ cells.
Figure 7.
Induction of telomerase activity and maintenance of telomere length by CD45RA+ CCR7+ CD8+ T cells cultured with 50 ng/ml of IL-15 or IL-7. (a) Purified cells (from three individuals A, B, C) analysed on day 0 or after 14 days of culture with cytokines. Telomerase activity in cell lysates was measured by Southern blot analysis. TSR8 denotes the internal quantitative control. (b) Telomere length analysis of purified cells on day 0 or after 19 days of culture with 50 ng/ml of IL-7 or IL-15. 1 µg of genomic DNA was digested with HinfI and RsaI and probed with 32P-labelled (CCCTAA) to detect repeat sequences.
Discussion
We have investigated the potential for IL-15 and IL-7 to regulate the homeostasis of human CD8+ T subpopulations by examining the effects of these cytokines on the survival and proliferation of purified subpopulations representing naïve, T-CM, T-EM and RA-primed cells in vitro. We found that these cytokines were capable both of promoting cell survival and stimulating cell division. The anti-apoptotic effects of the cytokines were apparent at lower concentrations than those required to induce proliferation, in accordance with the reported effects of IL-15 on mouse CD8+ T cells.15 These dose-dependent effects could provide an effective ‘homeostat’ for regulating T-cell numbers. Under steady-state conditions, the size of the T-cell pool would be limited by the number of cells that could successfully compete for access to cytokines in amounts sufficient to sustain cell viability. If cell numbers drop, the effective concentration of cytokines would increase so that some cells now receive proliferation-inducing signals, allowing for expansion and return to steady-state cell numbers. Alterations in the production of these cytokines would be analogous to changing the setting on the ‘homeostat’.
The two cytokines did not promote cell survival equally among all subsets. IL-7 strongly inhibited the apoptosis of CD45RA+ CCR7+ cells, but was less effective for the other subsets. These differential effects might be partially related to IL-7R expression, because T-EM and RA-primed populations include some cells that are IL-7Rαlow/–. However, variation in IL-7R expression could not account for all observed differences in responsiveness, because T-CM cells are uniformly IL-7Rαhigh but were only weakly rescued from cell death by IL-7. IL-15 was relatively efficient in preventing apoptosis within all subsets, although rescue was less effective for the RA-primed subset (this subset also showed the poorest response to IL-7). For both cytokines, enhancement of CD8+ T-cell survival correlated with the extent to which they induced up-regulation of the anti-apoptotic molecule Bcl-2. A role for Bcl-2 in IL-7-mediated protection from cell death would fit with a previous report showing that enforced expression of Bcl-2 in IL-7Rα–/– mice could rescue T-cell lymphopoiesis.30 However, in addition, signals through the IL-7R also lead to inactivation of the proapoptotic molecules Bax26 and Bad31 promotion of glucose metabolism and maintenance of cell viability through a phosphatidylinositol 3-kinase/Akt-dependent, Bcl-2-independent pathway.32 Therefore, it is likely that multiple mechanisms are involved in cytokine-mediated rescue of CD8+ T cells from apoptotic death.
IL-15 and IL-7 differed substantially in their ability to stimulate CD8+ T-cell proliferation. In short-term cultures, high concentrations of IL-7 induced low-level proliferation only of the naïve subset, whilst high doses of IL-15 caused marked proliferation of T-CM, T-EM and RA-primed cells, but a minor response by naïve cells. Taken together with the selective effects of IL-7 on cell survival, these data are consistent with the view that IL-7 is primarily involved in regulating the homeostasis of naïve rather than antigen-primed CD8+ T cells. Moreover, although IL-15 is able to inhibit apoptosis of cells in all CD8+ subsets, it is clear that the overall response of naïve CD8+ T cells to IL-15 is very different from that of other subpopulations.
Analysis of long-term cultures of cytokine-treated cells revealed a striking late expansion of naïve CD8+ T cells in response to IL-15. This was associated with a surge in the number of dividing cells, beginning at around 1 week of culture and peaking at approximately day 14; a slower response, more variable in magnitude, was observed for cells treated with IL-7. The delayed, cytokine-induced, proliferation of naïve cells was distinct from the response of primed cells to IL-15 in several respects. In particular, no induction of effector molecules was observed in cytokine-treated naïve cells. In addition, despite actively progressing through cell cycle, they did not express typical markers of activation such as CD69. In fact, the expanded population essentially exhibited a naïve phenotype, with the exception of the up-regulation of CD95 and a transient and minor increase in CD11a (data not shown). Expression of CD95 was dependent on the continued presence of the cytokines.
Our results showing that naïve CD8+ T cells proliferate without differentiating in response to IL-15 or IL-7 are in line with previous observations that IL-7 can expand and preserve a naïve repertoire when added to total CD45RA+ cord blood cells.16 Others have shown that culture of CD45RA+ CD8+ cord blood cells in IL-15 leads to expansion of CD56+ NK T cells that arise from CD56-precursors.33 However, no experiments were carried out on CD56-depleted adult naïve cells, as in the present study. Our CD56-depleted adult naïve CD8+ cells do not acquire CD56 when cultured in IL-7 or IL-15, supporting the view that adult and cord blood T cells differ in their response to cytokines. At first sight our data contrast with those of Alves et al.20 showing that naïve CD8+ T cells in short-term (7 days) IL-15-containing cultures underwent conspicuous phenotypic and functional changes, including down-regulation of CD45RA, CD28, CCR7 and CD62L and up-regulation of IFN-γ, TNF-α, perforin, granzyme B and cytolytic activity. Closer examination indicates several differences between these experiments and our own. First, many of the published experiments used cord blood lymphocytes and where adult naïve CD8+ T cells were used, the cells were separated using CD45RA rather than CD45RO and CD27 rather than CCR7 mAbs. The CD45RA+ cells may include double positive CD45RA+ CD45RO+ cells, while separation with CD45RO excludes these double positives. Furthermore, because CD27 is a costimulatory molecule and CD27 mAbs activate naïve T cells treated with a variety of stimuli34 it is possible that signals through CD27 could synergize with IL-15 in stimulating effector function. In contrast to these results, but in accord with our own, others have found that CD45RA+ CCR7+ cells do not lose CCR7 or gain perforin expression when treated with a combination of IL-7 and IL-15.19 However, these authors cultured the cells for only 7 days so that they did not observe the late proliferation to IL-15 and IL-7 reported here19.
One phenotypic change that occurred in cytokine-stimulated naïve CD8+ T cells was the up-regulation of CD95. Expression of CD95 could indicate that these cells are susceptible to apoptosis.35 However, expression of CD95 is not strictly correlated with sensitivity to apoptosis, and under some circumstances signals mediated through CD95 appear to promote T-cell proliferation.36
The delayed response of naïve CD8 T cells to both IL-7 and IL-15 is surprising and we have not yet investigated the underlying biochemical events. We speculate that at least two factors may contribute to this prolonged delay. First, we have shown that CD4+ CD45RA+ CCR7+ T cells are extremely quiescent dividing on average only once a year.37 If CD8+ CD45RA+ CCR7+ cells are similarly quiescent it might be expected that they would be difficult to stimulate. Second, T-cell activation by antigen requires multiple signals delivered through antigen-specific and costimulatory receptors. Cytokine-mediated stimulation initiates signalling via a single receptor complex; this may mean that it is relatively inefficient in highly quiescent naïve cells.
Although exposure to cytokines frequently results in rapid up-regulation of cytokine receptors, continuous exposure can also lead to down-regulation.38,39 In preliminary experiments we have stained cells for CD122, CD127 and CD132 after exposure of naïve CD8+ T cells to IL-7 or IL-15. We have not seen obvious up-regulation of the receptor chains but this may be complicated by interference of the bound cytokines with staining by the monoclonal antibodies. Resolution of these issues will require studies of the expression of IL-7 and IL-15 receptor chain genes in naïve cells during prolonged culture in the cytokines.
The ability of naïve CD8+ cells to expand after prolonged exposure to IL-15 or IL-7 in vitro raises the question whether this mechanism contributes to naïve T-cell homeostasis in vivo. In theory, naïve T cells could be exposed to elevated concentrations of cytokines for extended periods of time under conditions of lymphopenia. IL-7- or IL-15-stimulated cells retain their naïve phenotype and maintain telomere length through up-regulation of telomerase, although we have not examined telomere length or telomerase in cultures maintained for more than 3 weeks, so that we do not know yet whether eventually telomerase is down-regulated and telomere shortening occurs, as in T-cell cultures repeatedly re-stimulated in vitro by mitogens or alloantigens.40 However, as cytokine-mediated expansion of naïve CD8 T cells appears to cease after several cell divisions, even though cytokine is still present in the cultures, it might be an effective way of increasing the size of the naïve pool without loss of telomere length, alteration of functional properties or significant narrowing of the repertoire. Whether or not this occurs under natural conditions, these findings suggest that like IL-7, IL-15 could have potential therapeutic benefits in certain conditions of T-cell deficiency, or in bone marrow transplantation.41 These data also indicate a profound difference in the cytokine response machinery of naïve versus primed CD8+ T cells.
Acknowledgments
This work was supported by the Edward Jenner Institute for Vaccine Research (publication number 110). We would like to thank Dr C. Jones and Dr D. Baird, Department of Pathology, University of Wales College of Medicine, for performing Southern blots for telomere length analysis and Sarah Jackson for excellent technical assistance.
References
- 1.Beverley PC. Kinetics and clonality of immunological memory in humans. Semin Immunol. 2004;16:315–21. doi: 10.1016/j.smim.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 3.Hulstaert F, Hannet I, Deneys V, Munhyeshuli V, Reichert T, De Bruyere M, Strauss K. Age-related changes in human blood lymphocyte subpopulations. II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol. 1994;70:152–8. doi: 10.1006/clin.1994.1023. [DOI] [PubMed] [Google Scholar]
- 4.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–35. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macallan DC, Asquith B, Irvine AJ, et al. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–26. doi: 10.1002/eji.200323763. [DOI] [PubMed] [Google Scholar]
- 6.Fagnoni F, F0 Vescovini R, Passeri G, et al. Shortage of circulating naive CD8 (+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–8. [PubMed] [Google Scholar]
- 7.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 8.Dunne PJ, Faint JM, Gudgeon NH, et al. Epstein–Barr virus-specific CD8 (+) T cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood. 2002;100:933–40. doi: 10.1182/blood-2002-01-0160. [DOI] [PubMed] [Google Scholar]
- 9.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–7. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 10.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–64. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 14.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–22. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–26. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 16.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol. 1998;161:5909–17. [PubMed] [Google Scholar]
- 17.Kanegane H, Tosato G. Activation of naive and memory T cells by interleukin-15. Blood. 1996;88:230–5. [PubMed] [Google Scholar]
- 18.Dunne PJ, Belaramani L, Fletcher JM, et al. Quiescence and functional reprogramming of Epstein–Barr virus (EBV)-specific CD8+ T cells during persistent infection. Blood. 2005;106:558–65. doi: 10.1182/blood-2004-11-4469. [DOI] [PubMed] [Google Scholar]
- 19.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–6. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 20.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;10:2541–6. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 21.Jones CJ, Soley A, Skinner JW, et al. Dissociation of telomere dynamics from telomerase activity in human thyroid cancer cells. Exp Cell Res. 1998;240:333–9. doi: 10.1006/excr.1998.3944. [DOI] [PubMed] [Google Scholar]
- 22.Kim NW, Wu F. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP) Nucl Acids Res. 1997;25:2595–7. doi: 10.1093/nar/25.13.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okumura M, Fujii Y, Inada K, Nakahara K, Matsuda H. Both CD45RA+ and CD45RA– subpopulations of CD8+ T cells contain cells with high levels of lymphocyte function-associated antigen-1 expression, a phenotype of primed T cells. J Immunol. 1993;15:429–37. [PubMed] [Google Scholar]
- 24.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 25.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci USA. 2003;10:4724–9. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaled AR, Durum SK. Death and Baxes: mechanisms of lymphotrophic cytokines. Immunol Rev. 2003;193:48–57. doi: 10.1034/j.1600-065x.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 27.Son NH, Murray S, Yanovski J, Hodes RJ, Weng N. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with age. J Immunol. 2000;165:1191–6. doi: 10.4049/jimmunol.165.3.1191. [DOI] [PubMed] [Google Scholar]
- 28.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Zhi W, Wareski P, Weng NP. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J Immunol. 2005;174:4019–24. doi: 10.4049/jimmunol.174.7.4019. [DOI] [PubMed] [Google Scholar]
- 30.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–41. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 31.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–6. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 32.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–76. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 33.Cookson S, Reen D. IL-15 drives neonatal T cells to acquire CD56 and become activated effector cells. Blood. 2003;102:2195–7. doi: 10.1182/blood-2003-01-0232. [DOI] [PubMed] [Google Scholar]
- 34.Kobata T, Agematsu K, Kameoka J, Schlossman SF, Morimoto C. CD27 is a signal-transducing molecule involved in CD45RA+ naive T cell costimulation. J Immunol. 1994;153:5422–32. [PubMed] [Google Scholar]
- 35.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 36.Wajant H, Pfizenmaier K, Scheurich P. Non-apoptotic Fas signaling. Cytokine Growth Factor Rev. 2003;14:53–66. doi: 10.1016/s1359-6101(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 37.Macallan DC, Wallace D, Zhang Y, et al. Rapid turnover of effector-memory CD4 (+) T cells in healthy humans. J Exp Med. 2004;200:255–60. doi: 10.1084/jem.20040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR, Strom TB. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–8. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 39.Kumaki S, Armitage R, Ahdieh M, Park L, Cosman D. Interleukin-15 up-regulates interleukin-2 receptor alpha chain but down-regulates its own high-affinity binding sites on human T and B cells. Eur J Immunol. 1996;26:1235–9. doi: 10.1002/eji.1830260608. [DOI] [PubMed] [Google Scholar]
- 40.Effros R, Pawelec G. Replicative senescence of T cells: does the Hayflick limit lead to immune exhaustion? Immunol Today. 1997;18:450–4. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- 41.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]