Abstract
In mice arthritis model induced by anti-type II collagen (CII) antibodies and lipopolysaccharide (LPS), most of cells that infiltrated into the joint space were neutrophils. To investigate the role of neutrophils in the pathogenesis of arthritis, we depleted the neutrophils in vivo by injection of the antibody against Gr-1 expressed mainly on neutrophils. The neutrophil depletion completely inhibited the arthritis development. Furthermore, neutrophil depletion in mice that had already developed arthritis ameliorated the disease. These results showed that neutrophils are indispensable not only for the development, but also for the maintenance of arthritis. Next, we tried to develop arthritis in C5-deficient mice to investigate the involvement of C5a, one of chemotactic factors for neutrophils. C5-deficient mice showed significant reduction in arthritis development in comparison with wild type mice. Injection of pertussis toxin (Ptx) into the mice, which inhibits the signals from the inhibitory G-protein coupled-receptors including the C5a receptor, suppressed the development of arthritis. Furthermore, Ptx also ameliorated the arthritis when injected into mice that had already developed the disease. These results suggest the important role of chemotactic factors involving C5a and inhibitory G-protein (Gi)-coupled receptors not only in the development, but also in the maintenance of arthritis.
Keywords: neutrophils, arthritis (including rheumatoid arthritis), animal models/studies, mice/rats
Introduction
Rheumatoid arthritis (RA) is a progressive, destructive systemic autoimmune disease characterized by chronic synovial joint inflammation, including synovial hyperplasia, infiltration of inflammatory cells, fibrin deposition, and erosion of cartilage and bone.1 The underlying mechanisms of RA pathogenesis remain unknown because it is difficult to study in human cases, especially regarding the initiation of RA. To override these difficulties, experimental models of RA established in small animals are frequently used. It has been reported that a combined injection of mixtures of monoclonal anti-type II collagen antibody (anti-CII mAb) and lipopolysaccharide (LPS) can induce arthritis in mice.2 In this arthritis model, we have identified proinflammatory cytokines, especially tumour necrosis factor-α and interleukin-1β3 and Fcγ receptors4 as indispensable molecules in the development of arthritis. It is believed that not only anti-CII monoclonal antibodies (mAb), but also inflammatory cytokines, are involved in the pathogenesis of human RA.5–11
Hence, the pathogenesis of this arthritis model might partially share that of human RA. There is, however, little information about how inflammatory cells participate in the pathogenesis of this arthritis model and their relative contribution. It has been reported that various kinds of cells are involved in the development of arthritis in many animal models. In particular, neutrophils are thought to be important. Neutrophils are indispensable in the development of a streptococcal cell wall-induced arthritis model in rats,12 a spontaneous arthritis model in T-cell receptor transgenic K/BxN mice,13 and anti-CII mAb and LPS-induced arthritis.14
In a previous study using histological analysis3 we observed that massive numbers of neutrophils were infiltrated into the joint. In the present study, we examined the contribution of neutrophils to the pathogenesis of anti-CII mAb and LPS-induced arthritis model in mice, and showed that neutrophils are essential, not only in the development, but also in the maintenance of arthritis. Furthermore, the involvement of G-protein coupled-receptors (GPCRs) and C5 in the mechanism of arthritis development was speculated upon.
Materials and methods
Animals
Male BALB/cAnNCrj (BALB/c) mice were purchased from Charles River (Tokyo, Japan). Male C5-deficient mice (oSnJ) and the corresponding control mice (nSnJ) were from The Jackson Laboratory (Bar Harbor, ME).15 All mice were purchased at the age of 5–6 weeks, housed at Sankyo Laboratories (Tokyo, Japan), and given a standard rodent chow diet and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Sankyo Co., Ltd. (Tokyo, Japan).
Reagents
Arthritogenic anti-CII mAb mixture that contains four monoclonal immunoglobulin Gs (IgGs; F10, A2, D8, and D1) in equal amounts, and LPS (serotype 0111:B4) were purchased from Immuno-Biological Laboratories (Gunma, Japan). Rat anti-mouse Gr-1 mAb (clone RB6-8C5) and isotype mAb (rat IgG2b) were purchased from BD Pharmingen (San Diego, CA). RB6-8C5 mAb was dialysed against sterile saline at 4°, centrifuged to remove precipitates and stored at 4° before use. Pertussis toxin (Ptx) was from CALBIOCHEM (Darmstadt, Germany).
Induction of arthritis in mice
Arthritis was induced by the method of Terato and colleagues2 using an arthritogenic anti-CII mAb mixture. Briefly, the mice were intravenously injected with 4 mg/ml of anti-CII mAb (2 mg/0·5 ml/body) into the tail vein (day 0), and 3 days later LPS (50 µg/0·2 ml/body) were intravenously injected. In the experiments with nSnJ and oSnJ mice, anti-CII mAb (4 mg/1 ml/body) were intravenously injected and LPS (50 µg/0·2 ml/body) was intraperitoneally injected.
Clinical assessment of arthritis
The mice were carefully observed daily for swelling of the hind paws as a sign of arthritis. The severity of the arthritis was graded on a 0–3 scale as follows: 0, normal; 1, swelling of one digit; 2, two digits or more; 3, swelling of the entire paw. The statistical significance was determined by a non-parametric Dunnett's test based on the arthritis score using SAS System for Windows.
Histopathological assessment of arthritis
For the histopathology, arthritis was induced in the mice by injecting anti-CII mAb and LPS, as described above. Three mice were killed each day, and the hind legs were removed by cutting them between the knee and ankle and were fixed in phosphate-buffered saline containing 10% formaldehyde, decalcified in 10% ethylenediaminetetra-acetic acid, and embedded in paraffin. Sections of the hind paws were made by horizontally slicing the footpad, and stained with haematoxylin and eosin. Evaluation of the cellularity (neutrophils, lymphocytes and macrophages) infiltrated into the synovial membranes was carried out and graded as follows: –, normal; +, slight change; ++, mild change; +++, severe change. The scoring was performed as a blind test.
Depletion of neutrophils in vivo
The mice were intravenously injected with RB6-8C5 mAb (150 µg/0·4 ml/body). RB6-8C5 mAb selectively binds Gr-1 expressed on neutrophils.16,17 After the injection of RB6-8C5 mAb, the peripheral blood was collected in order to count the concentration of neutrophils with an automatic haemocytometer (Technicon H1E; Bayer AG).
Treatment with Ptx
The mice were intravenously injected with Ptx (0·5 µg/0·2 ml/body (25 g)) 1 hr before the LPS-injection on the 7th day after the anti-CII mAb injection (day 7). At this Ptx dose, no obvious weight loss or abnormal behaviour was observed.
Results
Prominent neutrophil infiltration within the synovial membranes in the arthritis-induced mice
The mice were injected with anti-CII mAb on day 0 and with LPS on day 3. The onset of arthritis was observed on day 4. Disease severity increased on days 4–5, and then reached maximum on days 5–7 (Fig. 1a). Histological sections of the tarsal joints revealed marked inflammatory properties, such as synovial hyperplasia (Fig. 1d, asterisk), fibrin deposition (Fig. 1d, arrowheads), and prominent neutrophil infiltration within the synovial membranes (Fig. 1e, arrows) in the arthritis-induced mice on day 7. These inflammatory properties were not observed in the normal mice (Fig. 1b), or in mice that were injected with anti-CII mAb, but not LPS (Fig. 1c) on day 3. Furthermore, examination of the histological sections showed that the infiltrated cells were neutrophils and macrophages, but not lymphocytes, until day 7 (Table 1). Neutrophils and macrophages were not observed in the joints of the normal mice, but they infiltrated slightly on day 3. The number of neutrophils increased on days 3–5 and reached maximum on day 6, and then decreased on day 7. In contrast, the infiltration of macrophages was slight on days 3–7.
Figure 1.
Time course of arthritis development. The mice were intravenously injected with anti-CII mAb (2 mg/0·5 ml/body) on day 0, and with LPS (50 µg/0·2 ml/body) on day 3. The severity of the arthritis was judged using the sum arthritis scores of both fore and hind paws, as described in Materials and methods. The sum scores (maximum score: 12) were expressed as the mean ± SEM of the five mice in each group (a). Histological sections of the tarsal joints stained with haematoxylin and eosin in the mice are shown (b–e). The untreated mice (b: original magnification ×100), the mice on day 3 before the LPS-injection (c: ×100), and the mice on day 7 that had developed arthritis (d: ×100, e: ×500). In sections (d) and (e), infiltrated neutrophils (arrows), proliferation of the lining cells of the synovial membrane (asterisk), and fibrin deposition (arrowheads) are indicated.
Table 1.
Cellularity in the synovial membranes of the normal mice or mice induced with arthritis by the injection of both anti-CII mAb and LPS
| Untreated | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Neutrophil | – | – | – | – | – | – | – | – | – | – | – | + | + | + | – | ++ | ++ | +++ | + | ++ | +++ | + | – | + |
| Lymphocyte | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Macrophage | – | – | – | – | – | – | – | – | – | – | + | – | + | + | + | + | + | + | + | + | + | + | + | + |
Arthritis was induced using the same procedure as Fig. 1. Three mice were killed each day and histological sections were made. The cellularity was graded as follows: –, normal; +, slight change; ++, mild change; +++, severe change.
Neutrophil depletion suppresses the development of arthritis
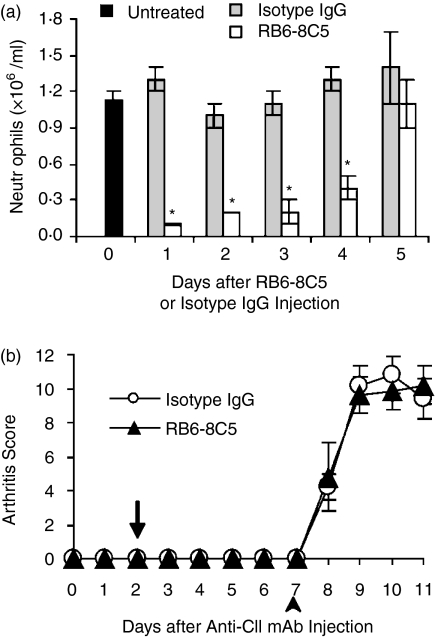
To investigate the role of neutrophils in the development of arthritis, we intravenously injected a mAb against Gr-1 (RB6-8C5 mAb) into the mice to deplete the peripheral neutrophils. The mice were intravenously injected with anti-CII mAb on day 0, with RB6-8C5 mAb or isotype mAb (IgG2b) on day 2, and with LPS on day 3. Treatment of RB6-8C5 mAb completely suppressed the development of arthritis, whereas isotype mAb did not (Fig. 2a). On day 7, histological sections of the tarsal joints of the mice revealed that RB6-8C5 mAb treatment prevented joint inflammation (Fig. 2c), while isotype mAb treatment did not show any effect (Fig. 2b). These results clearly showed that neutrophils are indispensable in the development of arthritis. Peripheral blood was taken daily from the mice injected with RB6-8C5 mAb or with isotype mAb, and the concentration of neutrophils was counted. The RB6-8C5 mAb-injected mice showed significant reduction of neutrophils on the next day after the RB6-8C5 mAb injection (approximately 96% reduction relative to the day before injection). The concentration of neutrophils gradually increased and on the 5th day reached almost the same concentration as that before injection (Fig. 3a). In contrast, there was no significant change in the isotype mAb-injected mice. To investigate the relationship between the concentration of peripheral blood neutrophils and the development of arthritis, we examined the development of arthritis in the mice that were injected with RB6-8C5 mAb and LPS, once their level had recovered after the depletion of neutrophils by the injection with RB6-8C5 mAb. The mice were injected with anti-CII mAb on day 0, with RB6-8C5 mAb or isotype mAb on day 2, and with LPS on day 7, once the level of the peripheral neutrophils was considered to have recovered. As a result, The RB6-8C5 mAb-injected mice developed arthritis commensurate to the isotype mAb-injected mice (Fig. 3b). These results showed that neutrophils are important in the development of arthritis.
Figure 2.
Suppression of arthritis development by neutrophil depletion. The mice were intravenously injected with anti-CII mAb on day 0, RB6-8C5 mAb (▴) or isotype mAb (○) on day 2 (arrow), and with LPS on day 3 (arrowhead) (a). The severity of the arthritis was judged using the sum arthritis scores of both fore and hind paws, as described in Materials and methods. The sum scores (maximum score: 12) were expressed as the mean ± SEM of the five mice in each group. On day 7, the hind paws of the mice injected with isotype mAb (b) or RB6-8C5 mAb (c) were removed and stained with haematoxylin and eosin. The original magnification of both photographs was ×50.
Figure 3.
Correlation between the concentration of neutrophils in the peripheral blood and arthritis development. The mice were intravenously injected with RB6-8C5 mAb (150 µg/0·4 ml/body) or isotype mAb on day 0. Peripheral blood was collected from the untreated mice (black bar) on day 0 before mAb injection, and was thereafter collected everyday from day 1 to day 5 from the RB6-8C5 mAb-injected (open bar) or the isotype mAb-treated mice (gray bar), and the concentration of neutrophils in the blood was counted (a). The five mice in each group were killed at each time point to obtain the blood. The concentrations of peripheral blood neutrophils in the RB6-8C5 mAb-injected mice were significantly different from those of the isotype mAb-injected mice (*P < 0·001). The mice were injected with anti-CII mAb on day 0, RB6-8C5 mAb (▴) or isotype mAb (○) on day 2 (arrow), and with LPS on day 7 (arrowhead) (b). The severity of the arthritis was judged using the sum of arthritis scores of both fore and hind paws, as described in Materials and methods. The sum scores (maximum score: 12) were expressed as the mean ± SEM of the five mice in each group.
Suppressive effects of RB6-8C5 mAb on maintaining inflammation of arthritis
To determine whether neutrophil depletion exerts suppressive effects on the maintenance of arthritis, RB6-8C5 mAb was injected into the mice that had developed arthritis on day 7. Neutrophil depletion significantly reduced the severity starting one day after the RB6-8C5 mAb injection, with the severity decreasing to almost normal level on day 11. In contrast, isotype mAb-injected mice maintained the arthritis until day 11 (Fig. 4a). The suppressive effects of RB6-8C5 mAb were also examined in the histological sections. There was evident inflammation (Fig. 4b), such as synovial hyperplasia and neutrophil infiltration into the synovial membrane (Fig. 4d, arrows), in the isotype mAb-injected mice on day 11. In contrast, such marked inflammation was suppressed in the RB6-8C5 mAb-injected mice, although mild hyperplasia was observed (Fig. 4c).
Figure 4.
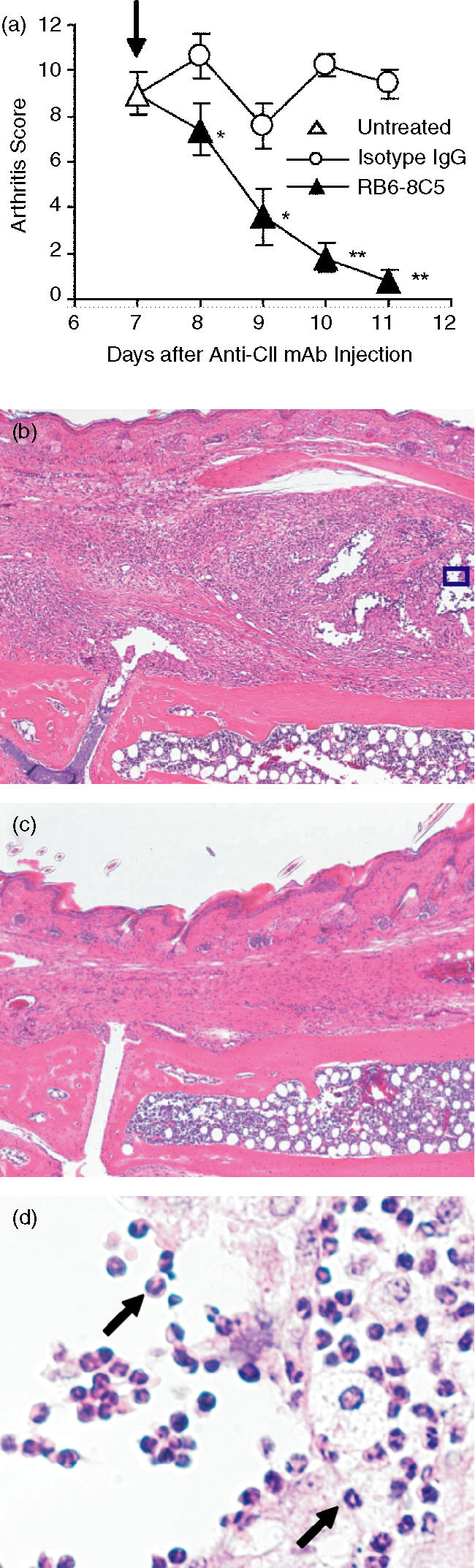
Amelioration of the maintaining of arthritis by neutrophil depletion. The mice were injected with anti-CII mAb on day 0 and with LPS on day 3. On day 7, arthritis-developed mice were picked up (uninjected: ▵), and were injected with RB6-8C5 mAb (▴) and isotype mAb (○) (arrow) (a). The severity of arthritis was judged using the sum arthritis scores of both fore and hind paws as described in Materials and methods. The sum scores (maximum score: 12) were expressed as the mean ± SEM of the five mice. The sum scores of the RB6-8C5 mAb-injected mice are significantly different from those of the isotype mAb-injected mice (*P < 0·05; **P < 0·01). Histological sections of the tarsal joints of the mice stained with haematoxylin and eosin are shown (b–d). The isotype mAb-injected mice on day 11 (original magnification, b: ×100, d: ×500) and the RB6-8C5 mAb-injected mice on day 11 (c: ×100). (d) A photograph of the inset in (b) at higher magnification, arrows indicate infiltrated neutrophils.
Involvement of C5 in the development of arthritis
To evaluate the involvement of the complement system in the development of this arthritis model, we used C5-deficient mice (oSnJ) and the corresponding control mice (nSnJ). The development of arthritis was significantly suppressed in the oSnJ mice compared to the nSnJ mice (Fig. 5a). In the histological sections, synovial hyperplasia (Fig. 5b) and infiltration of neutrophils (Fig. 5d, arrows) were observed in the nSnJ mice. In the oSnJ mice, synovial hyperplasia was mild and little infiltration of neutrophils was observed (Fig. 5c). These results indicate that C5 participates in the development of arthritis.
Figure 5.

Partial inhibition of arthritis development in C5-deficient mice. The C5-deficient oSnJ mice (▵) and the corresponding wild type nSnJ mice (○) were intravenously injected with anti-CII mAb (4 mg/1 ml/body) on day 0, and with LPS (50 µg/0·2 ml/body) on day 3 (a). The severity of arthritis was judged using the sum arthritis scores of both fore and hind paws as described in Materials and methods. The sum scores (maximum score: 12) were expressed as the mean ± SEM of the four (nSnJ) or five mice (oSnJ) mice. The sum scores of oSnJ mice are significantly different from those of nSnJ mice (*P < 0·05). Histological sections of the tarsal joints stained with haematoxylin and eosin in mice are shown (b–d). The nSnJ mice on day 14 (original magnification, b: ×100, d: ×500), and the oSnJ mice on day 14 (c: ×100). (d) A photograph of the inset in (b) at higher magnification, arrows indicate infiltrated neutrophils.
Involvement of inhibitory G-protein-coupled receptor in the development and maintenance of arthritis
It has been reported that the receptors of neutrophil chemotactic factors are coupled to the inhibitory G-protein (Gi).18,19 Ptx binds Gi and blocks the signal transduction from the Gi-coupled-receptors.20 To investigate the effects of Gi-protein-coupled receptors on the development of arthritis, mice were injected with anti-CII mAb on day 0 and with Ptx 1 hr before the LPS-injection on day 3. The Ptx-injected mice showed complete suppression of the development of arthritis, while the mice that were injected with saline instead of Ptx developed arthritis (Fig. 6a). Furthermore, once Ptx was injected into the mice that had developed arthritis on day 7, the severity of the mice was ameliorated significantly on day 9. The severity then further decreased on day 11, while it was maintained in the saline-injected mice until day 11 (Fig. 6b).
Figure 6.
Suppression of arthritis development and amelioration of established arthritis by Ptx injection. The mice were intravenously injected with anti-CII mAb on day 0, and with LPS on day 3. One hr before the LPS-injection, the mice were intravenously injected with Ptx (0·5 µg/0·2 ml/body) (▴) or saline (○) (arrow) (a). The mice were injected with anti-CII mAb on day 0, and with LPS on day 3. On day 7, arthritis-developed mice whose sum arthritis scores were 12 were selected (untreated: ▵), and were injected with Ptx (▴) or saline (○) (arrow) (b). The severity of the arthritis was judged using the sum arthritic scores of both fore and hind paws, as described in Materials and methods. The sum of scores (maximum score: 12) were expressed as the mean ± SEM of the five mice in each group. The sum scores of the Ptx-injected mice were significantly different from those of the saline-injected mice (*P < 0·05; **P < 0·01).
Discussion
In the present study, we demonstrated the essential roles of neutrophils in anti-CII mAb and LPS-induced arthritis. From the histological analysis (Fig. 1 and Table 1), it was clear that the major population of infiltrated cells in the joint space of arthritic mice are neutrophils, and we showed that these neutrophils are necessary for the development of arthritis using neutrophil-depleting RB6-8C5 mAb (Fig. 2). The degree of neutrophil infiltration was prominent on days 5–6 (Table 1), and decreased on day 7 when the arthritis was already established (the arthritis score maintained on days 5–7; Fig. 1a). However, neutrophils are considered to be important in the maintenance of arthritis because RB6-8C5 mAb injection ameliorated the established arthritis (Fig. 4). These results were similar to the previously reported in the K/BxN mouse arthritis model.13 Macrophages were also observed to infiltrate into the joints, but there were fewer macrophages than neutrophils (Table 1). Although we did not examine the involvement of macrophages in the development of arthritis, macrophages might play some role in the pathogenesis of this arthritis model. As for T cells and B cells, they are considered to be unnecessary in the development of anti-CII mAb and LPS-induced arthritis, because severe combined immunodeficient mice also normally develop arthritis3 and the infiltration of T cells or B cells in the joints of arthritis-developed mice is very low compared with that of neutrophils (Table 1).
To evaluate the role of complement C5 in the development of arthritis, we used C5-deficient mice15 and have shown that arthritis development was significantly suppressed in C5-deficient mice. As well, we observed that the infiltration of neutrophils into the joints was reduced in a histological analysis (Fig. 5). These results are consistent with the previous reports that showed the importance of C5 in other arthritis models using C5-deficient mice.21–26 We did not examine the mechanism how C5 or C5a, which are generated from C5, are involved in the development of arthritis. We previously observed that anti-CII mAb injected into mice bound onto the surface of the cartilage, where CII is rich, and that complement C3 was deposited at the same site.4 This observation suggested the possibility that C5a, a potent neutrophil chemoattractant, might be generated at the site of C3 deposition on the surface of the cartilage after LPS-injection, and that it attracts neutrophils into the joints. A previous report showed that the genetic deletion of C5a receptor completely protects mice from arthritis induced by anti-CII mAb and LPS.25 However, the data is controversial in this study, because the C5-deficient mice did not show complete resistance to arthritis, even though C5-deficient mice are considered to lack C5a.26 We speculate that this contradiction is caused by a difference in the experimental conditions, in that we used a higher dose of anti-CII mAb to induce the arthritis, in comparison with the previous reports.3,4 The partial suppression of arthritis development in C5-deficient mice (Fig. 5) suggests the involvement of neutrophil chemoattractants other than C5a in the development of arthritis. We have previously reported that arthritis-developed hind paws contain macrophage inflammatory protein-1α (MIP-1α), a chemoattractant protein for monocytes/macrophages and neutrophils, and showed that the development of arthritis was inhibited by injection of neutralizing antibodies against MIP-1α.3
It has been reported that C5a and other neutrophil chemoattractants, such as leukotriene B4, platelet-activating factor and formylmethionylleucylphenylalanine, bind to each GPCR.27–30 Ptx blocks the signal from GPCRs, and inhibits the chemotaxis of the neutrophils, both in vitro and in vivo.27,31 To address the involvement of GPCRs, we injected Ptx into mice and examined the development and maintenance of the arthritis. Administration of Ptx inhibited not only the development, but also the maintenance of the arthritis (Fig. 6). These results suggested that GPCRs might be indispensable in both the development and the maintenance of arthritis. It is possible that neutrophils expressing GPCRs may respond to some of the chemoattractants generated at the inflammatory site in the joints and may also infiltrate into the joints.
Acknowledgments
We greatly thank Dr Azusa Seki for his technical support on histological analyses. We also acknowledge Snider Philip for proofreading this manuscript.
Abbreviations
- CII
type II collagen
- Gi
inhibitory G-protein
- GPCR
G-protein-coupled receptor
- LPS
lipopolysaccharide
- Ptx
pertussis toxin
- RA
rheumatoid arthritis
References
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.Terato K, Harper DS, Griffiths MM, Hasty DL, Ye XJ, Cremer MA, Seyer JM. Collagen-induced arthritis in mice. synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmunity. 1995;22:137–47. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- 3.Kagari T, Doi H, Shimozato T. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002;169:1459–66. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]
- 4.Kagari T, Tanaka D, Doi H, Shimozato T. Essential role of Fcγ receptors in anti-type II collagen antibody-induced arthritis. J Immunol. 2003;170:4318–24. doi: 10.4049/jimmunol.170.8.4318. [DOI] [PubMed] [Google Scholar]
- 5.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 6.Koch AE, Kunkel SL, Harlow LA, et al. Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994;93:921–8. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch AE, Kunkel SL, Harlow LA, et al. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90:772–9. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton S, Duwel LE, Boivin GP, Ma Y, Hirsch R. Association of the course of collagen-induced arthritis with distinct patterns of cytokine and chemokine messenger RNA expression. Arthritis Rheum. 1999;42:1109–18. doi: 10.1002/1529-0131(199906)42:6<1109::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Marinova-Mutafchieva L, Williams RO, Mason LJ, Mauri C, Feldmann M, Maini RN. Dynamics of proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis (CIA) Clin Exp Immunol. 1997;107:507–12. doi: 10.1046/j.1365-2249.1997.2901181.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith-Oliver T, Noel LS, Stimpson SS, Yarnall DP, Connolly KM. Elevated levels of TNF in the joints of adjuvant arthritic rats. Cytokine. 1993;5:298–304. doi: 10.1016/1043-4666(93)90060-i. [DOI] [PubMed] [Google Scholar]
- 11.Schrier DJ, Schimmer RC, Flory CM, Tung DK, Ward PA. Role of chemokines and cytokines in a reactivation model of arthritis in rats induced by injection with streptococcal cell walls. J Leukoc Biol. 1998;63:359–63. doi: 10.1002/jlb.63.3.359. [DOI] [PubMed] [Google Scholar]
- 12.Schimmer RC, Schrier DJ, Flory CM, et al. Streptococcal cell wall-induced arthritis. Requirements for neutrophils, P-selectin, intercellular adhesion molecule-1, and macrophage-inflammatory protein-2. J Immunol. 1997;159:4103–8. [PubMed] [Google Scholar]
- 13.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167:1601–8. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 14.Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice. description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003;163:1827–37. doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi YM, Colten HR. Genetic defect in secretion of complement C5 in mice. Nature. 1979;282:207–8. doi: 10.1038/282207a0. [DOI] [PubMed] [Google Scholar]
- 16.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–68. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romani L, Mencacci A, Cenci E, et al. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–56. [PubMed] [Google Scholar]
- 18.Cicchetti G, Allen PG, Glogauer M. Chemotactic signaling pathways in neutrophils: from receptor to actin assembly. Crit Rev Oral Biol Med. 2002;13:220–8. doi: 10.1177/154411130201300302. [DOI] [PubMed] [Google Scholar]
- 19.Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol. 2003;35:1619–38. doi: 10.1016/s1357-2725(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 20.Becker EL, Kanaho Y, Kermode JC. Nature and functioning of the pertussis toxin-sensitive G protein of neutrophils. Biomed Pharmacother. 1987;41:289–97. [PubMed] [Google Scholar]
- 21.Ji H, Gauguier D, Ohmura K. Genetic influences on the end-stage effector phase of arthritis. J Exp Med. 2001;194:321–30. doi: 10.1084/jem.194.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson AC, Sundler M, Kjellen P, et al. Genetic control of collagen-induced arthritis in a cross with NOD and C57BL/10 mice is dependent on gene regions encoding complement factor 5 and FcgammaRIIb and is not associated with loci controlling diabetes. Eur J Immunol. 2001;31:1847–56. doi: 10.1002/1521-4141(200106)31:6<1847::aid-immu1847>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Kristan J, Hao L, Lenkoski CS, Shen Y, Matis LA. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J Immunol. 2000;164:4340–7. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 24.Watson WC, Brown PS, Pitcock JA, Townes AS. Passive transfer studies with type II collagen antibody in B10.D2/old and new line and C57Bl/6 normal and beige (Chediak–Higashi) strains: evidence of important roles for C5 and multiple inflammatory cell types in the development of erosive arthritis. Arthritis Rheum. 1987;30:460–5. doi: 10.1002/art.1780300418. [DOI] [PubMed] [Google Scholar]
- 25.Grant EP, Picarella D, Burwell T, et al. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J Exp Med. 2002;196:1461–71. doi: 10.1084/jem.20020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawlisch H, Wills-Karp M, Karp CL, Kohl J. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Mol Immunol. 2004;41:123–31. doi: 10.1016/j.molimm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Becker EL, Kermode JC, Naccache PH, Yassin R, Marsh ML, Munoz JJ, Sha'afi RI. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985;100:1641–6. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLeish KR, Gierschik P, Schepers T, Sidiropoulos D, Jakobs KH. Evidence that activation of a common G-protein by receptors for leukotriene B4 and N-formylmethionyl-leucyl-phenylalanine in HL-60 cells occurs by different mechanisms. Biochem J. 1989;260:427–34. doi: 10.1042/bj2600427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Painter RG, Zahler-Bentz K, Dukes RE. Regulation of the affinity state of the N-formylated peptide receptor of neutrophils: role of guanine nucleotide-binding proteins and the cytoskeleton. J Cell Biol. 1987;105:2959–71. doi: 10.1083/jcb.105.6.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siciliano SJ, Rollins TE, Springer MS. Interaction between the C5a receptor and Gi in both the membrane-bound and detergent-solubilized states. J Biol Chem. 1990;265:19568–74. [PubMed] [Google Scholar]
- 31.Spangrude GJ, Sacchi F, Hill HR, Van Epps DE, Daynes RA. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J Immunol. 1985;135:4135–43. [PubMed] [Google Scholar]