Abstract
Hemangioblasts are thought to be one of the sources of hematopoietic progenitors, yet little is known about their localization and fate in the mouse embryo. We show here that a subset of cells co-expressing the hematopoietic marker GATA-1 and the endothelial marker VE-cadherin localize on the yolk sac blood islands at embryonic day 7.5. Clonal analysis demonstrated that GATA-1+ cells isolated from E7.0–7.5 embryos include a common precursor for hematopoietic and endothelial cells. Moreover, this precursor possesses primitive and definitive hematopoietic bipotential. By using a transgenic complementation rescue approach, GATA-1+ cell-derived progenitors were selectively restored in Runx1-deficient mice. In the rescued mice, definitive erythropoiesis was recovered but the rescued progenitors did not display multilineage hematopoiesis or intra-aortic hematopoietic clusters. These results provide evidence of the presence of GATA-1+ hemangioblastic cells in the extra-embryonic region and also their functional contribution to hematopoiesis in the embryo.
Keywords: GATA-1, hemangioblast, rescue, Runx1, VE-cadherin
Introduction
During mouse development, successive phases of hematopoiesis occur in several anatomic sites (reviewed by Speck et al, 2002). Although the origins of blood cells is still controversial, a tight developmental relationship between hematopoietic and endothelial lineages has been recognized (reviewed by Nishikawa, 2001; Choi, 2002; Dieterlen-Lievre et al, 2002). The first blood cells (primitive erythrocytes) and endothelial cells are observed within the blood islands of yolk sac at embryonic day 7.5 (E7.5) (Haar and Ackerman, 1971). This feature has led to a hypothesis that there is a transient common precursor for hematopoietic and endothelial cells, the hemangioblast (Sabin, 1920; Murray, 1932). Since it is difficult to catch a narrow time window of development in vivo and to obtain experimentally sufficient number of cells from early embryo, an in vitro model system has been developed as an alternative approach for finding hemangioblasts. The embryonic stem (ES) cell differentiation system identified a putative hemangioblast, termed the blast colony-forming cell (BL-CFC), which gives rise to primitive and definitive hematopoietic cells and endothelial cells (Kennedy et al, 1997; Choi et al, 1998). Recently, the in vivo counterpart of BL-CFC was also detected in mouse primitive streak at E7.0–7.5 (Huber et al, 2004). However, the relationship between BL-CFC and yolk sac definitive progenitors, previously reported by several groups, is unknown (Yoder et al, 1997; Nishikawa et al, 1998b; Palis et al, 1999).
Another type of close association between hematopoietic and endothelial cells has been observed in a later stage intraembryonic region. At the microscopic level, a cluster of hematopoietic cells, candidates of hematopoietic stem cells (HSCs) or progenitors, has been found in the aorta–gonad–mesonephros (AGM) region, which attach to the ventral aspect of the endothelium (Garcia-Porrero et al, 1995; Tavian et al, 1996; Yoshida et al, 1998; Bertrand et al, 2005). Using a tracing technique, the continuity between endothelial cells and hematopoietic cells has been proven in chick and murine embryos (Jaffredo et al, 1998; Sugiyama et al, 2003). Furthermore, VE-cadherin+ endothelial cells isolated from mouse embryos can generate hematopoietic cells including lymphoid lineages on OP9 stromal cells (Nishikawa et al, 1998b; Fraser et al, 2002), suggesting that a subset of definitive hematopoietic cells originate directly from hematogenic endothelial cells. Consistent with this, HSC activity was detected in the endothelium of the dorsal aorta, emphasizing that the origin of HSCs resides in endothelial cells (de Bruijn et al, 2002).
The transcription factor GATA-1 is a central regulator of erythroid gene transcription (reviewed by Ferreira et al, 2005). GATA-1 expression in the extra-embryonic mesoderm before the establishment of blood islands was demonstrated by in situ analysis (Silver and Palis, 1997), suggesting that GATA-1 gene expression begins in the very early stages of hematopoiesis. Using an in vitro ES cell differentiation system, GATA-1 was shown to be a good marker of mesodermal cells, which possess hematopoietic activity (Robertson et al, 2000; Fujimoto et al, 2001). Previously, the transcription regulatory domain that directs both the primitive and definitive erythroid cell-specific expression of GATA-1 was identified and referred to as the GATA-1 gene hematopoietic regulatory domain or G1-HRD (Onodera et al, 1997). We also confirmed that the G1-HRD is sufficient to recapitulate the expression of the GATA-1 gene in extra-embryonic mesoderm and hematopoietic mesoderm cells generated from ES cells, as well as in erythroid cells (Onodera et al, 1997; Fujimoto et al, 2001; Shimizu et al, 2001). These observations imply that it is technically feasible to express any effector gene in hematopoietic mesoderm cells using the G1-HRD.
Runx1/AML1 encodes the DNA-binding subunit of a heterodimeric transcription factor complex named polyoma enhancer binding protein 2 (PEBP2)/core-binding factor (CBF) (reviewed by Ito, 1999). Homozygous disruption of Runx1 results in embryonic lethality secondary to a complete block in fetal liver definitive hematopoiesis (Okuda et al, 1996; Wang et al, 1996; Okada et al, 1998). Previous analyses revealed that there are no hematopoietic cell clusters on endothelial cells in Runx1-deficient embryos and further, that sorted VE-cadherin+ endothelial cells from E10.5 embryos do not generate hematopoietic colonies (North et al, 1999; Yokomizo et al, 2001). These results suggested that Runx1 is required for the generation of definitive hematopoietic cells from endothelial cells.
We show here that GATA-1+ cells at E7.0–7.5 have both hematopoietic and endothelial potential. As the GATA-1-like expression of Runx1 is also detectable in the extra-embryonic mesoderm and blood islands before the formation of intra-aortic clusters (North et al, 1999; Lacaud et al, 2002), we generated transgenic mouse lines expressing Runx1 under the control of the G1-HRD and performed a complementation rescue experiment of Runx1 function. As expected, definitive hematopoiesis in the compound mutant embryos was partially rescued. However, intra-aortic clusters were absent, indicating that only GATA-1+ cell-derived progenitors were restored in Runx1-deficient mice. The rescued progenitors did not have the properties of HSC. These data demonstrate that GATA-1 expression marks hemangioblastic cells in the extra-embryonic region and that these cells display restricted hematopoietic potential in vivo.
Results
Identification of GATA-1 and VE-cadherin double-positive cells in yolk sac blood Islands
In early mouse ontogeny, GATA-1 expression is observed in the extra-embryonic mesoderm and blood islands of the yolk sac (Onodera et al, 1997; Silver and Palis, 1997). Since the function of GATA-1 at this stage has not been well characterized, we examined the expression pattern of GATA-1 using transgenic mouse lines expressing green fluorescent protein (GFP) under the control of the G1-HRD (Nishimura et al, 2000). At E7.5 (early headfold stage; Downs and Davies, 1993), GATA-1 expression is restricted to the blood islands of the yolk sac (Supplementary Figure S1). Almost all cells within the blood islands were positive for GFP (Figure 1A(a, d and h)). The GFP expression pattern overlapped with that of endogenous GATA-1 as detected with anti-GATA-1 antibody (Figure 1A(e and f)). These results indicate that the transgenic GFP reporter can effectively monitor GATA-1+ cells in the early embryonic stage.
Figure 1.
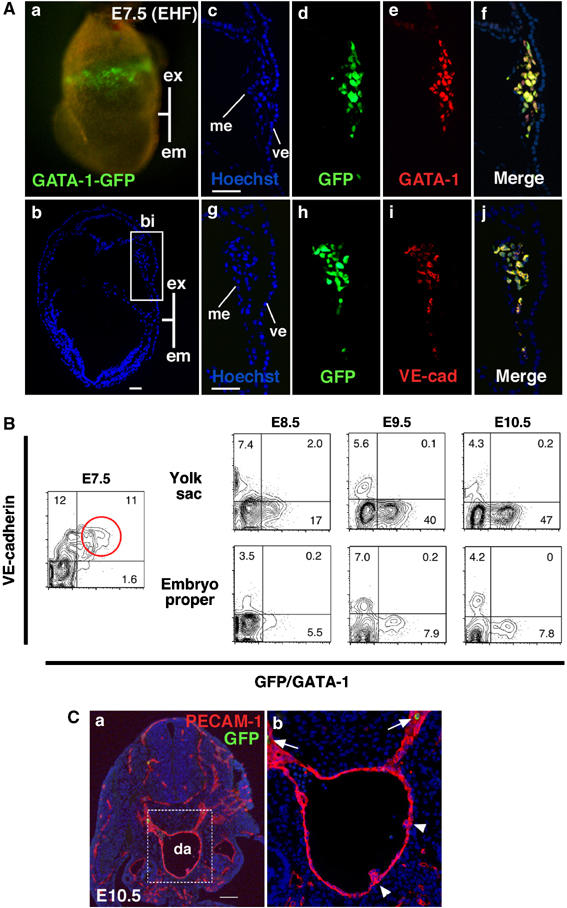
GATA-1+ cells in blood islands coexpress VE-cadherin. (A) Immunohistochemical analysis of G1-HRD-GFP transgenic embryo at E7.5 (early headfold stage, EHF). (a) Fluorescence microscopic analysis shows GFP+ cells are present in the blood islands. (b) Section of (a). The blue color represents staining with Hoechst 33342. (c–j) Serial sections of the blood island depicted in the boxed region in (b). Hoechst dye staining is shown in (c) and (g). Staining with anti-GFP (d and h), anti-GATA-1 (e) and anti-VE-cadherin (i) antibodies are also shown. Merged images of GFP and GATA-1 (f) and GFP and VE-cadherin (j). (B) FACS analysis of G1-HRD-GFP transgenic embryos. Note that most GFP+ cells are positive for VE-cadherin+ (highlighted by a red circle) at E7.5. (C) Immunohistochemical analysis of G1-HRD-GFP transgenic embryo at E10.5. Transverse section of AGM region, stained with anti-GFP (green) and anti-PECAM-1 (red) antibodies. The dorsal aspect is upward. (b) This is higher magnification of the area within (a) indicated by the box. GFP+ cells (white arrows) are observed in circulating cells, not in endothelial cells or hematopoietic cell clusters (white arrowheads). Abbreviations: ex, extra-embryonic region; em, embryonic region; bi, blood island; ve, visceral endoderm; me, mesothelium; da, dorsal aorta. Scale bars: (A) 50 μm; (C) 100 μm.
To our surprise, these GATA-1+ cells co-expressed VE-cadherin (Figure 1A(i)), a known endothelial cell marker in midgestation (Nishikawa et al, 1998b). The expression profiles of GFP and VE-cadherin merged nicely (Figure 1A(j)). This observation was further confirmed by fluorescence activated cell sorter (FACS) analysis (Figure 1B). In E7.5 embryos, almost all GFP+ cells were VE-cadherin+ (Figure 1B, highlighted by a red circle). By contrast, the expression of GATA-1 and VE-cadherin became mutually exclusive after E8.5 both in the yolk sac and embryo proper (Figure 1B, right panels). At E10.5, GATA-1 expression in the AGM region was predominantly observed in erythroid cells and not in PECAM-1+ endothelial cells (Figure 1C), in agreement with our previous analysis (Onodera et al, 1997).
PECAM-1 was also expressed in blood island GFP+ cells at E7.5 (Supplementary Figure S2; Ema et al, 2006). Although the morphology of the blood island GFP+ cells at E7.5 did not coincide with that of a typical endothelial cell (not shown), these results demonstrate that hematopoietic and endothelial markers are co-expressed in blood island cells and suggest that these cells might be early precursors before or during commitment to the hematopoietic or endothelial lineage.
Definitive hematopoietic potential of GATA-1+ cells
It was previously demonstrated that VE-cadherin+ cells derived from embryos or ES cells have definitive hematopoietic potential (Nishikawa et al, 1998a, 1998b). To further characterize the blood island GATA-1+ cells, they were sorted from E7.5 G1-HRD-GFP transgenic mouse embryos. Approximately 5% of these cells were GFP+ and these cells were efficiently recovered (Figure 2A). GFP+ cells expressed the transcription factors GATA-1, GATA-2, Runx1 and SCL, all of which are known to be important for hematopoiesis (Figure 2B). To test the functional potential of the recovered GFP+ fraction, cells were cultured on OP9 stromal cells (Figure 2C(a and b)). GFP+ cells were capable of generating enucleated erythroid cells and mature myeloid cells (Figure 2C(c)). Also CD45 and c-Kit hematopoietic progenitor cells, as well as erythroid (Ter119) and myeloid (Mac-1 and Gr-1) cells, were recovered from these cultures (Figure 2C(d)). In contrast, GFP− cells from the E7.5 embryos (early or late headfold stage) did not produce any hematopoietic cell colonies, even when plated at 5000 cells per well (Figure 2C(b) and D), indicating that the definitive hematopoietic potential was enriched in the GATA-1+ cell fraction in E7.5 blood islands. Whereas, colony forming potential was still found in the GFP+ fraction at E8.25, the potential shifted to the GFP− fraction after E8.5 (Figure 2D). The period in which the GFP+ fraction contained progenitor cells with definitive hematopoietic potential correlated with the presence of VE-cadherin-expressing cells (Figure 1B).
Figure 2.
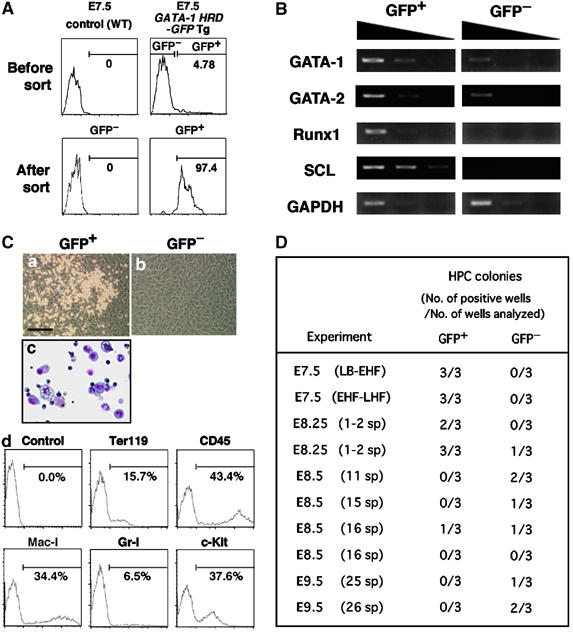
Definitive hematopoietic potential resides in GATA-1+ cells at E7.5. (A) FACS profile of the cells derived from E7.5 G1-HRD-GFP transgenic embryos. GFP+ and GFP− cells were sorted (upper panel, right) and re-analyzed (lower panels). (B) RT–PCR analysis of the expression of hematopoietic transcription factor mRNAs in GFP+ and GFP− cells sorted from E7.5 embryos. (C) Definitive hematopoietic potential of GFP+ cells derived from E7.5 G1-HRD-GFP transgenic embryo. Both GFP+ and GFP− cells were cultured on an OP9 stromal cell layer in the presence of SCF, IL-3, G-CSF and Epo. A phase-contrast microscopic photograph was taken after 4 days of culture (a and b). After 9 days of culture, the cells formed in the culture of GFP+ cells were harvested and analyzed for morphology (c) and the expression of surface markers (d). Erythrocytes, granulocytes and monocytes/macrophages were identified. Scale bar: 200 μm. (D) Definitive hematopoietic potential of GFP+ and GFP− cells sorted from embryos of different stages. The frequency of hematopoietic colony development was examined with the OP9 stromal cell culture. 500 cells were cultured per well under the same conditions as described in (C). The generation of hematopoietic colonies was judged by their morphology after 7 days of culture. Stages determined by morphological appearance are presented in parentheses. LB–EHF represents a mixture of LB and EHF stage embryos. Abbreviations: LB, late bud; EHF, early headfold; LHF, late headfold; sp, somite pairs.
Clonal analysis of GATA-1+ cells sorted from E7.0–7.5 embryos
The presence of cells co-expressing the hematopoietic marker GATA-1 and the endothelial marker VE-cadherin suggested that GATA-1+ cells might include the common precursor, the hemangioblast. To test this possibility, we performed a single cell deposition assay. GFP+ cells isolated from G1-HRD-GFP transgenic mouse embryos at E7.5 (early headfold and late headfold stages) were deposited into individual wells of a 96-well plate. After 1 day of culture, colonies of round cells appeared in almost 30% of wells. These cells were positive for βH1 globin, and morphologically identical to primitive erythrocytes (Supplementary Figure S3), indicating that the majority of GATA-1+ cells at E7.5 are committed primitive erythroid progenitors. After 2 days of culture, another type of colony appeared in 1.04% (6/576) of wells. These colonies expanded rapidly and generated definitive hematopoietic cells including erythrocytes, granulocytes and monocytes/macrophages (Figure 3A). Immunostaining with anti-PECAM-1 antibody was used to identify endothelial cell colonies in day 7 cultures. Among 576 cells, one cell (0.17%, three experiments) gave rise to both hematopoietic and endothelial cells. This shows that a portion of GATA-1+ cells at E7.5 has hemangioblastic activity, although at a very low frequency. Thus, we did an examination at an earlier stage, E7.0. Similar to the E7.5 embryo, GFP+ cells at E7.0 were observed in the extra-embryonic region and co-expressed endothelial markers, VE-cadherin and PECAM-1 (Figure 3B(a); Ema et al, 2006). These GFP+ cells were isolated from mid-streak to no bud stage embryos and cultured with OP9 stromal cells. After 2 days of culture, we found densely packed cell clusters in some wells (Figure 3B(b)). These clusters expanded and generated round cells around themselves (Figure 3B(c and d)). After 4 days, cell clusters were harvested and divided into two fractions. One fraction was seeded on an OP9 stromal layer and the other was subjected to methylcellulose culture to test for primitive erythroid progenitors. The culture remaining after harvest was also tested for endothelial cell colony forming potential. Among 720 cells, 5 cells (0.69%, five experiments) displayed bipotential definitive hematopoietic and endothelial potential (Figure 3B(e, f, and g)). Moreover, clusters could generate primitive erythroid colonies, which were comparable to those from E7.5 embryos (Figure 3B(h–k)) and were positive for βH1 globin (Figure 3B(l)). No hemangioblastic activity was observed in the GFP− fraction, even when up to 6600 cells were investigated. These results clearly demonstrate that a single GATA-1+ cell can differentiate into primitive and definitive hematopoietic and endothelial cells.
Figure 3.
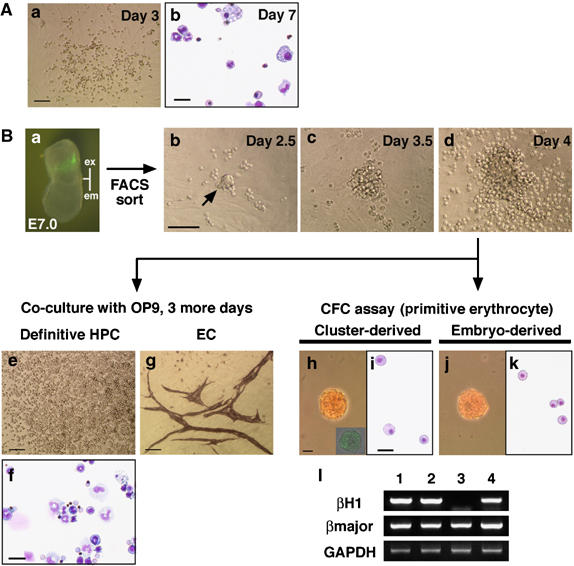
Single GATA-1+ cells can differentiate into primitive and definitive hematopoietic cells and endothelial cells. GFP+ cells isolated from G1-HRD-GFP transgenic mouse were deposited into individual wells of a 96-well plate and cultured with OP9 stromal cells in the presence of SCF, IL-3, G-CSF, Epo, VEGF and Ang-1. (A) Analysis of GFP+ cells sorted from E7.5 (early to late headfold stage) embryos. A phase-contrast microscopic photograph was taken after 3 days of culture (a). After 7 days of culture, the cells formed in the culture of GFP+ cells were harvested and analyzed for morphology (b). Erythrocytes, granulocytes and monocytes/macrophages were identified. (B) Analysis of GFP+ cells sorted from E7.0 (mid-streak to no bud stage) embryos. (a) Representative picture of E7.0 embryo (no bud/early bud stage). Abbreviations: ex, extra-embryonic region; em, embryonic region. A densely packed cluster was detected at day 2 of culturing (b, arrow) and grown to a larger size (c), with the generation of round cells around it (d). Note that the shape of the colony is different from that of a typical colony formed in E7.5 culture (A(a)). After 4 days of culture, the progeny of the cluster was replated onto fresh OP9 stromal cells (e) and in methylcellulose (h). The culture remaining after harvesting was also investigated for the detection of endothelial cell colonies (g). (e and f) Erythrocytes, granulocytes and monocytes/macrophages were identified in the replated dish after an additional 3 days of culture. (g) Endothelial cell colonies formed on OP9 stromal cells were visualized by immunostaining with anti-PECAM-1 antibody. (h–l) Generation of primitive erythroid colonies from clusters. Morphology of primitive erythroid colony generated from replated clusters (h and i) was similar to those from E7.5 embryo (j and k). Inset in (h) shows a GFP-expressing colony detected by fluorescence microscopy. (l) RT–PCR analysis of globin gene expression in the colonies. Both βH1 and βmajor globins were detected in the colonies derived from the clusters (lane 4). Peripheral blood from E10.5 embryo, primitive erythroid colonies from E7.5 embryo (primitive erythrocytes, lanes 1 and 2, respectively) and BFU-E from E10.5 yolk sac (definitive erythrocytes, lane 3) were used as controls. Scale bars: (Aa, Bb, Be, and Bg) 100 μm; (Ab, Bf, Bh, and Bi) 25 μm.
GATA-1+ cell-specific expression of Runx1 in a Runx1−/− background
The data so far suggest that a portion of definitive hematopoietic progenitors may be generated from GATA-1+ hemangioblastic cells in the yolk sac. To visualize the emergence of these progenitors, immunohistochemical staining for CD45 was performed (Nishikawa et al, 1998b; Takakura et al, 2000). CD45+ cells localized to the blood island region that was positively marked with the G1-HRD-LacZ transgene (Supplementary Figure S4), supporting the notion that the blood islands of the yolk sac contain precursors of definitive hematopoiesis.
To elucidate the fate of definitive hematopoietic progenitors derived from GATA-1+ hemangioblastic cells, we attempted to generate a mouse composed only of this population of definitive progenitors. We postulated that in a Runx1 null background where definitive hematopoiesis does not commence, the G1-HRD directed expression of Runx1 would rescue definitive hematopoiesis. To this end, we generated G1-HRD-Runx1 transgenic mice (Figure 4A; Supplementary Figure S5) and crossed them with Runx1 mutant mice.
Figure 4.
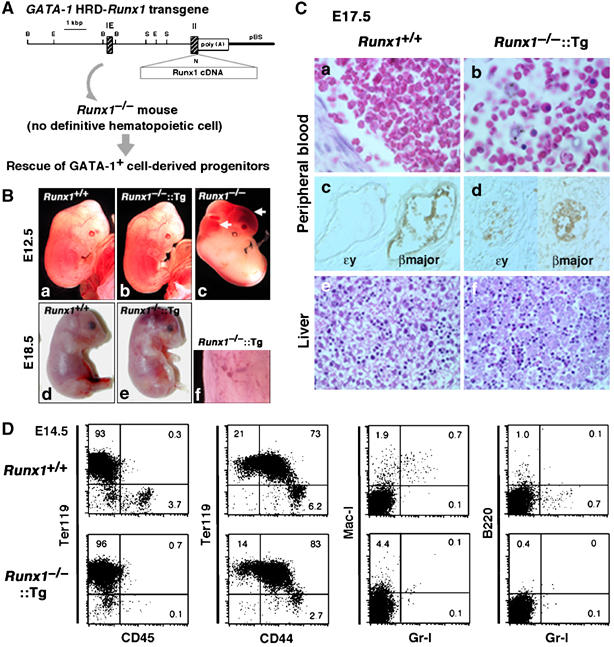
Rescue of GATA-1+ cell-derived progenitors in Runx1−/− embryos. (A) Strategy for the rescue of GATA-1+ cell-derived progenitors in Runx1−/− embryos. The G1-HRD-Runx1 transgene contains the 3.9-kb sequence 5′ of the IE exon, the IE exon itself, the first intron, and a part of the second exon of the mouse GATA-1 gene in front of Runx1 cDNA. The initiation Met codon in the second exon was replaced by a unique NotI site (shown as N) for subsequent cloning. Restriction enzyme sites are B, BamHI; E, EcoRI; N, NotI; S, SacI. (B) Macroscopic appearance of G1-HRD-Runx1 transgene-rescued embryos. Embryos with each genotype at E12.5 (a–c) and E18.5 (d and e) are shown. A higher magnification picture of the skin of transgene-rescued embryo (f) reveals micro-hemorrhages in the skin. (C) Histological analysis of wild type and Runx1−/−∷Runx1-Tg+ embryos at E17.5. Hematoxylin and eosin staining of peripheral blood from wild type (a) and Runx1−/−∷Runx1-Tg+ (b) embryos are shown. Note that numerous enucleated erythrocytes are present in the Runx1−/−∷Runx1-Tg+ embryos. Panels (c) and (d) show sections stained with anti-βmajor globin antibody or anti-ɛy globin antibody. Hematoxylin and eosin-stained livers of wild type (e) and Runx1−/−∷Runx1-Tg+ (f) embryos. (D) FACS analysis of E14.5 fetal liver cells from wild type and Runx1−/−∷Runx1-Tg+ embryos.
E12.5 Runx1−/− embryos showed the expected lethality and massive hemorrhaging in the central nervous system (Figure 4B(c)). By contrast, the embryos compound for the G1-HRD-Runx1 transgene (Runx1-Tg) were viable, showed no such morphological defects (Figure 4B(b)) and we could not distinguish the compound mutant embryos from wild-type embryos (Figure 4B(a)). To identify whether Runx1−/−∷Runx1-Tg+ embryos can complete embryogenesis, we analyzed newborn pups. Of 52 newborn mice, no live Runx1-null pups were found (an expected number of pups was 6.5). Examination at late-gestational stages revealed that Runx1−/−∷Runx1-Tg+ embryos begin to die at E14.5 (Supplementary Table S1). At E18.5, Runx1−/−∷Runx1-Tg+ pups showed flushing and massive hemorrhaging (Figure 4B(e)). Histological analysis revealed general bleeding within the central nervous system, subarachnoid space, urinary bladder, subcutaneous area and peritoneal cavity of these rescued embryos (not shown). Microaneurysms were also observed in the skin (Figure 4B(f)). Vascular abnormality in Runx1−/−∷Runx1-Tg+ mice at E18.5 was more prominent than that in Runx1-null pups at E12.5 (Supplementary Figure S6). Thus, G1-HRD directed Runx1-Tg expression rescues the Runx1 deficient hemorrhaging phenotype and embryonic lethality up to E18.5.
Partial restoration of definitive erythropoiesis in Runx1−/−∷Runx1-Tg+ embryos
Peripheral blood analysis of the E17.5 embryos revealed an accumulation of enucleated erythroid cells in both wild type and Runx1−/−∷Runx1-Tg+ embryos (Figure 4C(a and b), respectively), albeit nucleated and abnormal erythroid cells were observed frequently in Runx1−/−∷Runx1-Tg+ embryos (Figure 4C(b)). Immunohistochemical studies revealed that, as is the case for wild-type embryos (Figure 4C(c)), a major part of the erythroid cells were positive for βmajor globin and negative for ɛy globin (Figure 4C(d)) in Runx1−/−∷Runx1-Tg+ embryos, indicating their derivation from definitive hematopoietic progenitors. In addition, microscopic analysis revealed that the size of erythroid cells in Runx1−/−∷Runx1-Tg+ embryos was similar to that of wild-type mice (not shown), supporting the notion that these are definitive erythroid cells. E17.5 liver sections showed an accumulation of erythroid cells in both wild type and Runx1−/−∷Runx1-Tg+ embryos (Figure 4C(e and f)). Intriguingly, megakaryocytes were not observed in the livers of Runx1−/−∷Runx1-Tg+ embryos (Figure 4C(f)), in clear contrast to the wild-type embryos (Figure 4C(e)). We also examined the E14.5 fetal liver for the presence of other hematopoietic cell lineages. FACS analysis revealed markedly lower percentages of B220+ or Gr-1+ cells in Runx1−/−∷Runx1-Tg+ embryos at E14.5 (Figure 4D) while percentages of erythroid cells (Ter119+ and Ter119+CD44+) were near wild-type values. FACS analysis performed on Runx1−/−∷Runx1-Tg+ embryos at E10.5, 11.5, 12.5 and 15.5 showed results almost identical to those obtained at E14.5 (not shown). Finally, histological analysis showed a complete loss of thymocytes in the thymus rudiment of Runx1−/−∷Runx1-Tg+ embryos at E17.5 (not shown). These results strongly argue that definitive erythroid cells were selectively rescued by the Runx1-Tg.
Absence of transition from endothelial cells to hematopoietic cells in E10.5 Runx1−/−∷Runx1-Tg+ embryos
Hematopoietic cell clusters (CD34+ and c-Kit+) associated with the endothelia of the dorsal aorta, vitelline artery and umbilical artery (Wood et al, 1997; Yoshida et al, 1998) are thought to be a visible manifestation of the transition from endothelial to definitive hematopoietic progenitor/stem cell (reviewed by Jaffredo et al, 2005). Since such clusters are completely absent in Runx1−/− embryos (Yokomizo et al, 2001), we investigated whether cluster formation is rescued in Runx1−/−∷Runx1-Tg+ mice. E10.5 embryos were stained with anti-c-Kit antibody and treated to increase tissue transparency. Intriguingly, while c-Kit+ cell clusters in these regions were clearly detectable in wild-type embryo dorsal aorta (Figure 5A(a)) the vitelline (Figure 5A(c)) and umbilical artery (Figure 5A(e)) and yolk sac (Figure 5A(g)), these cell clusters were completely absent in Runx1−/−∷Runx1-Tg+ embryos (Figure 5A(b, d, f and h)).
Figure 5.
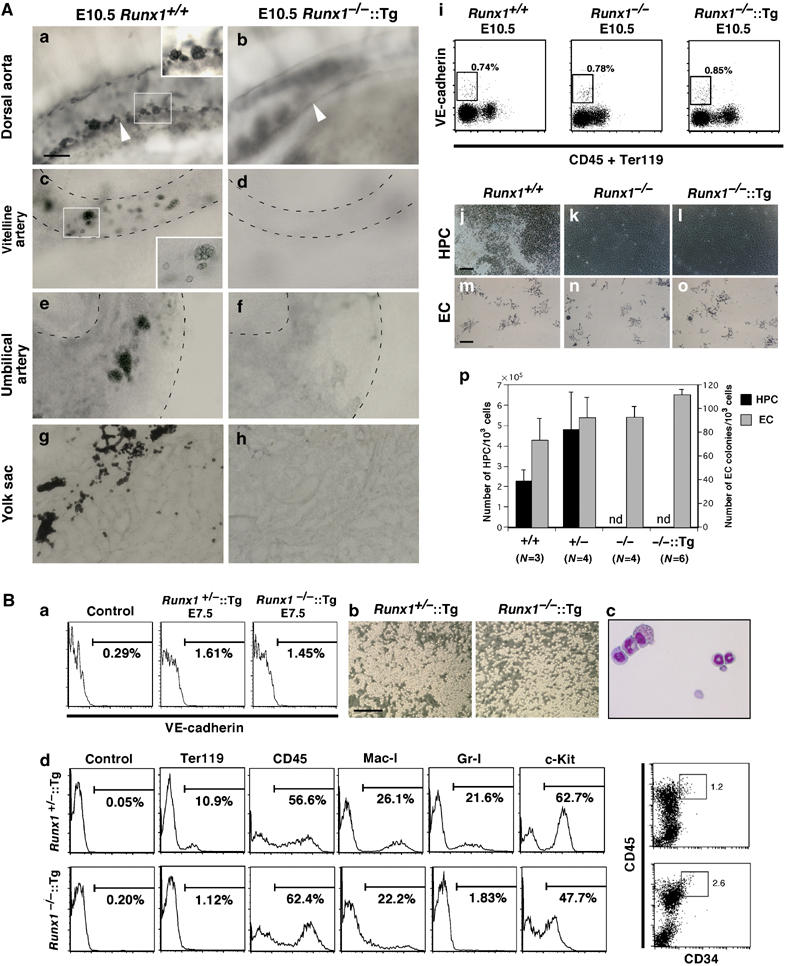
Definitive hematopoietic potential is recovered in VE-cadherin+ cells from E7.5, but not from E10.5, Runx1−/−∷Runx1-Tg+ embryos. (A) The absence of hematopoietic cell clusters and definitive hematopoietic potential of VE-cadherin+ endothelial cells in E10.5 Runx1−/−∷Runx1-Tg+embryos. (a–h) Detection of hematopoietic cell clusters in wild type and Runx1−/−∷Runx1-Tg+ embryos. Embryos at E10.5 were processed for whole-mount c-Kit staining and cleared to visualize the hematopoietic cell clusters. (a and b) Side-view of the dorsal aorta. In the wild-type embryos, many c-Kit+ cell clusters were attached to the ventral wall of the dorsal aorta (a, white arrowhead), whereas no c-Kit+ cell clusters were present in the transgene-rescued Runx1−/− embryos (b). Similar results were obtained in the vitelline artery (c and d), umbilical artery (e and f) and yolk sac (g and h; flat-mount preparation). Scale bar: 20 μm. (i–p) Endothelial cells (VE-cadherin+CD45−Ter119−) sorted from the transgene-rescued Runx1−/−embryos could not differentiate into hematopoietic cells in vitro. (i) Surface expression of VE-cadherin, CD45 and Ter119 in Runx1+/+, Runx1−/− and Runx1−/−∷Runx1-Tg+ embryos at E10.5. Cells from the caudal half of the embryo proper and yolk sac were stained with APC-anti-VE-cadherin, FITC-anti-CD45 and OrGreen-anti-Ter119 antibodies. Percentages of cells that fell within the sorting parameters (boxes) are indicated. (j–o) Development of hematopoietic and endothelial cell colonies in the culture of sorted endothelial cells. VE-cadherin+/CD45−/Ter119− endothelial cells sorted from Runx1+/+, Runx1−/− and Runx1−/−∷Runx1-Tg+ embryos as indicated in (i) were cultured on an OP9 stromal layer in the presence of SCF, IL-3, G-CSF and Epo. Photographs were taken by a phase-contrast microscope after 4 days of culture (j–l). Hematopoietic cells were generated from Runx1+/+ endothelial cells (j), whereas no hematopoietic cells were detected in the culture containing Runx1−/− or Runx1−/−∷Runx1-Tg+ endothelial cells (k and l). (m–o) Immunostaining with anti-PECAM-1 antibody after 7 days of culture shows endothelial cell colonies. No significant differences were detectable in the size or morphology of the colonies between Runx1+/+ and Runx1−/−∷Runx1-Tg+ endothelial cells. (p) Numbers of hematopoietic and endothelial cell colonies in the culture of sorted endothelial cells. Scale bars: (j–l) 100 μm; (m–o) 200 μm. Abbreviations: HPC, hematopoietic cells; EC, endothelial cells; ND, not detected. (B) Definitive hematopoietic potential in VE-cadherin+ cells from E7.5 Runx1−/−∷Runx1-Tg+ embryos. (a) Surface expression of VE-cadherin in Runx1+/–∷Runx1-Tg+ and Runx1−/−∷Runx1-Tg+ embryos at E7.5 (early headfold stage). The cells that disaggregated from whole embryo were stained with APC-anti-VE-cadherin antibody. Genotyping was performed using the sorted VE-cadherin− fraction. (b) Development of hematopoietic cell colonies in the culture of sorted VE-cadherin+ cells. VE-cadherin+ cells derived from Runx1+/–∷Runx1-Tg+ and Runx1−/−∷Runx1-Tg+ embryos as indicated in (a) were cultured on an OP9 stromal layer in the presence of SCF, IL-3, G-CSF and Epo. Photographs were taken by phase-contrast microscopy after 6 days of culture. Scale bar: 200 μm. (c) Cytocentrifuge preparation of cultured cells. May–Grunwald Giemsa staining. Erythrocytes, granulocytes and macrophages were identified. (d) Hematopoietic potential of VE-cadherin+ cells from E7.5 Runx1+/–∷Runx1-Tg+ embryo. Cells were harvested and analyzed after 9 days of culture.
VE-cadherin+CD45−Ter119− cells of E10.5 embryos have been previously defined as endothelial cells and approximately 10% of these cells when cultured on an OP9 layer give rise to definitive hematopoietic cells (Nishikawa et al, 1998b). We found comparable percentages of VE-cadherin+CD45−Ter119− cells in wild type, Runx1−/− and Runx1−/−∷Runx1-Tg+ embryos (Figure 5A(i)). When these cells were sorted and cultured on an OP9 cell layer, no hematopoietic cells were generated from Runx1−/−∷Runx1-Tg+ cells or Runx1−/− embryos, as expected, whereas E10.5 endothelial cells from wild-type embryos gave rise to hematopoietic cells (Figure 5A(j–l and p)). These cultures were also evaluated for endothelial colony potential by staining with anti-PECAM-1 antibody (Figure 5A(m–o and p)). After 7 days of culture, there was no significant difference in size, morphology or number of the PECAM-1-positive endothelial colonies among Runx1+/+, Runx1−/− and Runx1−/−∷Runx1-Tg+ embryos. We therefore conclude that the transition from endothelial cells to hematopoietic cells was not rescued in E10.5 Runx1−/−∷Runx1-Tg+ embryos, a conclusion consistent with the foregoing data that G1-HRD is not active in VE-cadherin+ cells at E10.5 (Figure 1B). These results are consistent with our contention that, while the production of definitive erythroid cells is rescued, the ability to generate definitive hematopoietic cells from endothelial cells is not recovered in the E10.5 Runx1−/−∷Runx1-Tg+ embryos.
CD45+ cells are re-established in Runx1−/−∷Runx1-Tg+ embryos
The data up to this point have indicated that the definitive hematopoietic cells identified in the E17.5 embryos are generated somewhere in Runx1−/−∷Runx1-Tg+ embryos, but that the cells are not derived from endothelial cells at E10.5. If the definitive progenitors were generated from GATA-1+ cells in blood islands of the yolk sac, the emergence of CD45+ cells should be detected in the yolk sacs of rescued embryos. Indeed, we found CD45+ cells, albeit fewer than those in the control embryos, at the proximal side of the yolk sac in E9.5 Runx1−/−∷Runx1-Tg+mice (Supplementary Figure S7). This result indicates that CD45+ cells are re-established in the early stage in Runx1−/−∷Runx1-Tg+ embryos.
Rescue of definitive hematopoietic potential in E7.5 Runx1−/−∷Runx1-Tg+ embryos
We speculated that the transgene-rescued progenitors must be derived from VE-cadherin+ cells. As G1-HRD is active in these cells at E7.5 (Figure 1), VE-cadherin+ cells were sorted from E7.5 Runx1−/−∷Runx1-Tg+ embryos and cultured on OP9 cells (Figure 5B(a and b)). In accordance with our expectation, VE-cadherin+ cells from E7.5 Runx1−/−∷Runx1-Tg+ embryos formed hematopoietic colonies (Figure 5B(b)). After 9 days of culture, we found definitive erythrocytes and myeloid cells in the culture (Figure 5B(c and d)). Similar results were obtained from sorted E7.5 PECAM-1+ cells (not shown). These results unequivocally demonstrate that the G1-HRD-Runx1 transgene recovered hematogenic potential in VE-cadherin+ cells from E7.5 embryos. It should be noted that, in contrast to the fetal liver analysis (Figure 4D), the observed cell lineages were not restricted to erythrocytes.
Absence of an HSC population in Runx1−/−∷Runx1-Tg+ fetal liver
The development of hematopoietic cells derived from E7.5 Runx1−/−∷Runx1-Tg+ embryos appeared to be within the normal range in vitro, and this fact implies that the erythroid-restricted differentiation observed in the rescued livers may not be solely due to specificity of the G1-HRD promoter. Consistent with this notion, E9.5 Runx1−/−∷Runx1-Tg+ yolk sac (but not embryo proper) cells formed CFU-mix, although found at a much lower frequency than in the wild-type control (Figure 6A). In contrast, methylcellulose colony assays of liver cells of E17.5–18.5 Runx1−/−∷Runx1-Tg+ embryos were completely negative for CFU-mix. Only colony-forming unit-erythroid (CFU-E) were observed (Figure 6B). No colonies were found when E14.5 Runx1−/−∷Runx1-Tg+ liver cells were cultured on the OP9 cells (Figure 6C). Moreover, FACS analysis showed no detectable CD34, c-Kit, Sca-1 or CD45 positive cells in E14.5 Runx1−/−∷Runx1-Tg+ livers (Figure 6D).
Figure 6.
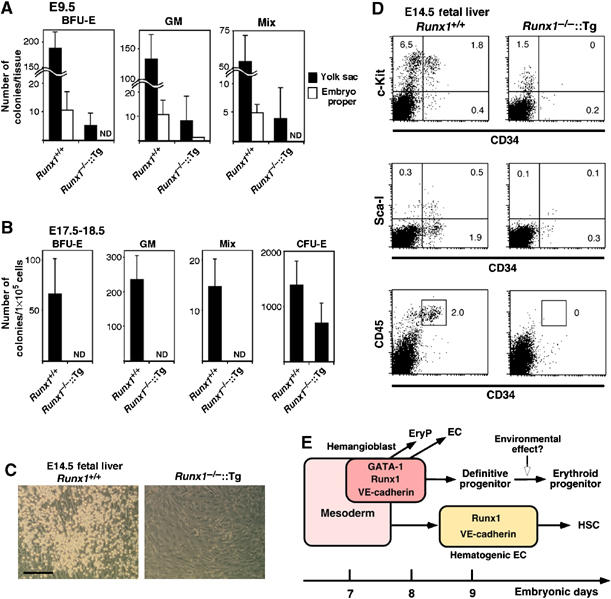
The absence of uncommitted progenitors and HSCs in Runx1−/−∷Runx1-Tg+ fetal liver. Colonies arising from E9.5 (21–28 somite stage) embryos (A) and E17.5–18.5 fetal liver cells (B) in the semisolid medium. nd means not detected. (C) Hematopoietic potential of E14.5 fetal liver progenitors on OP9 stromal cells. Phase-contrast microscopic views of the culture are shown. Adherent, round hematopoietic colonies were generated from 103 cells from Runx1+/+ fetal liver, whereas no colonies were detected in the culture of 106 cells from Runx1−/−∷Runx1-Tg+ fetal liver. Scale bar: 200 μm. (D) Expression of hematopoietic stem cell markers in E14.5 fetal liver cells from wild type or rescued embryos. (E) Schematic representation of multiple pathways for hematopoiesis in mouse embryos. Abbreviations: EC, endothelial cells; EryP, primitive erythrocytes.
These results thus indicate that erythroid-committed progenitors, but not uncommitted progenitors, reside in the livers of Runx1−/−∷Runx1-Tg+ embryos and suggest that definitive hematopoietic progenitors generated from E7.5 VE-cadherin+ cells most likely undergo a commitment to the erythroid lineage before or during fetal liver hematopoiesis.
Discussion
Expression of GATA-1 in hemangioblastic cells
The close temporal and spatial relationship between hematopoietic and endothelial development observed in the early embryo led to the hypothesis that two lineages share a common precursor, the hemangioblast (Sabin, 1920; Murray, 1932). We show here that a subset of GATA-1+ cells at E7.0–7.5 possess hematopoietic and endothelial bipotential (1/144 frequency at E7.0). One such cell type has already been identified in the mouse ES cell differentiation system and is referred to as the BL-CFC (Kennedy et al, 1997). Recently, an in vivo counterpart of the BL-CFC was also identified in E7.0–7.5 embryos (Huber et al, 2004). These embryo-derived BL-CFCs are enriched in the brachyury+Flk1+ fraction (approximately 1/400 frequency), displaying hematopoietic (including primitive erythroid) and endothelial potential. Despite similarities in their potential, GATA-1+ and brachyury+Flk1+ cells differ in their localization. While GATA-1+ cells reside in the extra-embryonic region at E7.0–7.5 (primitive streak to headfold stage), brachyury+Flk1+ cells were mainly detected in the posterior region of the primitive streak at the neural plate stage. Although there is a difference in localization, it is possible that brachyury+Flk1+ cells might be a precursor of GATA-1+ cells. Alternatively, the BL-CFC assay system may detect a more primitive precursor than in our OP9 culture system.
The molecular mechanisms that operate downstream of GATA-1 in hemangioblastic cells are unclear. Like GATA-1, GATA-2 has also been detected in the endothelial marker-positive fraction at E7.5 (our unpublished data). Thus, GATA-1 and GATA-2 may have overlapping functions at this stage. In support of this notion, GATA-1-GATA-2-double deficient mice exhibit no visible blood cells but do form normal yolk sac endothelium. Thus, the double deficient mice display a more severe phenotype than either single knockout mouse (Fujiwara et al, 2004), suggesting that GATA factors may instruct hematopoietic cell commitment in hemangioblastic cells. Indeed, we observed that the incidence of endothelial cell colony formation from the E7.5 PECAM-1+GATA-1+ fraction is lower than that from the PECAM-1+GATA-1− fraction (not shown), indicating that GATA-1 expression leads to a loss of endothelial potential.
Rescued progenitors in Runx1−/−∷Runx1-Tg+ mice
Recent studies suggest that there may be a transient definitive hematopoiesis from progenitors, probably not from stem cells, during embryonic stage (Palis et al, 1999; Cumano et al, 2001). Therefore, definitive hematopoietic cells in this study correspond to the definitive-type hematopoietic cells, which include transient definitive progenitors and differentiated hematopoietic cells, but not definitive (adult) HSCs.
We showed in this study that definitive hematopoiesis in Runx1−/− embryos can be rescued, albeit partially, by the expression of the G1-HRD-Runx1 transgene. This finding supports our contention that the GATA-1+ cell fraction in the E7.0–7.5 yolk sac contains definitive hematopoietic precursors requiring the contribution of Runx1 for their growth and differentiation. It has been reported that the expression level of Runx1 influences the fate or generation of HSCs (Cai et al, 2000; Lacaud et al, 2004). To accurately examine the expression level of the transgene, we performed quantitative real-time PCR analysis with RNA samples from E7.5 and E14.5 embryos. As shown in Supplementary Figure S5, the transgene expression level in rescued embryos is comparable to that in wild-type embryos at E7.5. At E14.5, the expression level of the transgene in rescued embryos becomes much higher than that of endogenous Runx1. The latter observation suggests that there remains a possibility that certain ectopic overexpression of Runx1 might affect hematopoietic cell differentiation in a midgestation embryonic stage.
However, based on the following observations, we surmise that the overexpression did not greatly influence the development of hematopoietic progenitors in Runx1−/−∷Runx1-Tg+ mice. First, the cell-sorting experiment showed that G1-HRD-GFP+ cells from E7.5 wild-type embryos expressed Runx1, indicating that the GATA-1 and Runx1 expression overlaps at this stage. In addition, similar to the case for Runx1-deficient mice, PEBP2β-deficient mice could be rescued by the G1-HRD-PEBP2β transgene (Yoshida et al, 2002; Supplementary Figure S8). Since PEBP2β enhances the DNA-binding activity of Runx proteins, but cannot function without Runx proteins (Adya et al, 2000), Runx1 must be expressed in the same cells as those expressing PEBP2β in order to exert its activity. Thus, the fact that both G1-HRD-Runx1 and G1-HRD-PEBP2β rescued mice showed a similar hematopoietic phenotype provides decisive evidence that the rescued progenitors recapitulate the physiological differentiation profile of yolk sac progenitors (Supplementary Figure S8).
In support of this conclusion, G1-HRD-Runx1 transgenic mice showed only a mild influence in adult hematopoiesis (Yanagida et al, 2005). Furthermore, results from the OP9 culture system revealed that VE-cadherin+ cells from Runx+/–∷Runx1-Tg+ or Runx1−/−∷Runx1-Tg+ embryos at E7.5 can generate multilineage hematopoietic cells that are similar to G1-HRD-GFP+ cells from E7.5 wild-type embryos, which also express VE-cadherin. This suggests that excessive expression of Runx1 in GATA-1+ cells does not substantially change normal hematopoietic events.
Does the microenvironment influence the fate of definitive hematopoietic progenitors?
An interesting observation is that erythroid cells are predominant in the livers of Runx1−/−∷Runx1-Tg+ embryos. A simple and straightforward explanation for the erythroid lineage-restriction is to assume the critical contribution of promoter specificity to transgene function; the G1-HRD-Runx1 transgene could not rescue the myeloid and lymphoid lineages since the G1-HRD does not drive transgene expression in these lineages (Onodera et al, 1997). However, we feel that there may be some alternative interpretations of the data, since the transgene-rescued hematopoietic progenitors differentiated into the myeloid lineage in vitro in a cell culture analysis. We surmise that the hematopoietic microenvironment in the yolk sac and embryo proper may be critical (below), but that Runx1 may be dispensable for generating the other lineages. In agreement with this notion, hematopoiesis in the PEBP2β-deficient mouse is rescued by the endothelial cell-specific expression of PEBP2β; in the rescued mouse, almost all lineages of hematopoietic cells exist, including lymphocytes, indicating that hematopoietic cells can differentiate autonomously, even in the absence of PEBP2β (Miller et al, 2002). Similarly, conditional targeting of Runx1 in adult mice did not influence maintenance of the HSC population (Ichikawa et al, 2004).
In clear contrast to the predominant erythroid-lineage in the livers of Runx1-Tg-rescued embryos, the multipotential of hematopoietic cells was identified in the in vitro culture of E7.5 and E9.5 transgene-rescued embryonic cells. Our interpretation of this discrepancy is that the microenvironment of the yolk sac may have restricted the differentiation potential of hematopoietic progenitors, such that the majority of the multipotential progenitors committed to the erythroid lineage before migration to the liver. An alternative explanation is that Runx1-deficiency in the hematopoietic progenitors could not sustain the immature state of the progenitors. We assume both mechanisms might be operating, since the Runx1-Tg-rescued progenitors did not appear to be intrinsically defective in their differentiation potential. Indeed, the E7.5 hematopoietic progenitors from Runx1−/−∷Runx1-Tg+ embryos grew for at least 9 days in the culture with OP9 cells. In fact, a change in the hematopoietic potential within the yolk sac was previously observed. VE-cadherin+ cells isolated from the yolk sac and embryo proper at E9.5 displayed lymphohematopoietic potential in a cell culture system (Nishikawa et al, 1998b). CD45+ cells from the E9.5 embryo proper also showed a similar potential. In contrast to the CD45+ cells from the embryo proper, CD45+ cells from the E9.5 yolk sac could not differentiate into lymphocytes, implying that the microenvironment of the yolk sac might not effectively sustain the multipotential properties of the hematopoietic progenitor cells.
In summary, we have demonstrated that E7.0–7.5 GATA-1+ cells have primitive and definitive hematopoietic as well as endothelial potential in vitro, and give rise to definitive erythrocytes in vivo. Based on these results, we propose a model of hematopoietic development from hemangioblasts and hematogenic endothelium (Figure 6E). At least these two types of precursors are involved in definitive hematopoiesis at embryonic stage in close association with endothelial development. Detailed comparison between hemangioblasts and hematogenic endothelium would provide insights into the developmental regulation of hematopoietic and vascular systems.
Materials and methods
Histological analysis
E7.5 and E17.5 embryos were fixed with 4% paraformaldehyde or 10% buffered formalin, respectively. For histological examination, sections were stained with hematoxylin and eosin. Cryosections were prepared for immunostaining with anti-GFP antibody (Molecular Probes, Eugene, OR), anti-VE-cadherin antibody (VCAD1, a generous gift from Dr N Matsuyoshi), anti-GATA-1 antibody (N6; Ito et al, 1993), anti-ɛy globin antibody (kindly provided by Dr T Atsumi), anti-βmajor globin antibody (Research Plus, Bayonne and Denville, NJ), anti-PECAM-1 antibody (MEC13.3, PharMingen) or anti-smooth muscle actin antibody (1A4, NeoMarkers, Union City, CA). For the detection of immunofluorescence, Cy3- or Alexa-488-conjugated secondary antibodies (Jackson Immuno. Res., West Grove, PA and Molecular Probe, respectively) were used. Anti-PECAM-1, anti-c-Kit (ACK2 or 2B8) and anti-CD45 (30-F11, PharMingen) antibodies were also used for whole mount immunostaining of embryos as described previously (Yokomizo et al, 2001). Stained yolk sacs were dissected completely from the embryo proper and flat-mounted. Dehydration and treatment with BABB, a 1:2 mix of benzyl alcohol and benzyl benzoate, was carried out to increase tissue transparency. Glutaraldehyde fixation (2%) was used for examination by electron microscope.
Cell preparation and flow cytometry
Pre-somite embryos were staged according to the report (Downs and Davies, 1993). For analyzing E10.5 embryos, the caudal half was used as the embryo proper. Dispase (Gibco BRL) and Cell Dissociation Buffer or Collagenase S-1 (Nitta Gelatin Co, Osaka) was used to prepare the cells from embryos as described (Nishikawa et al, 1998b).
Procedures for surface staining and cell sorting were as described previously (Nishikawa et al, 1998b). The monoclonal antibodies used for flow cytometry were allophycocyanin (APC), phycoerythrin (PE) or fluorescein isothiocyanate (FITC) conjugated-antibody to VE-cadherin, PECAM-1, Ter119, CD45, Mac-I (M1/70), Gr-1 (RB6–8C5), CD34 (RAM34) and c-Kit (2B8).
Culture with OP9 stromal cells
For the generation of hematopoietic cells, dissociated cells were cultured in a 12-well plate for 7–9 days on an OP9 stromal cell layer in αMEM medium (Gibco BRL) containing 10% fetal bovine serum (FBS) and 5 × 10−5 M 2-mercaptoethanol (2-ME), supplemented with 100 ng/ml of stem cell factor (SCF), 200 U/ml of Interleukin-3 (IL-3), 100 ng/ml of granulocyte-colony stimulating factor (G-CSF) and 2 U/ml of erythropoietin (Epo). Hematopoietic cells were harvested and analyzed for the expression of several surface markers using flow cytometry in addition to morphological analysis with May–Grunwald Giemsa staining. To detect the endothelial cell colony formation on the OP9 cell layer, cultured cells were fixed with 4% paraformaldehyde and stained with anti-PECAM-1 antibody. The subsequent colorization step with alkaline phosphatase-conjugated anti-rat IgG (H+L) was performed as described previously (Yokomizo et al, 2001).
Single-cell culture
Single GFP+ cells were sorted using the Clone-Cyt system of FACS Vantage (Becton Dickinson, San Jose, CA) into separate wells of 96-well plates. Sorted cells were cocultured with OP9 stromal cells with 100 ng/ml of SCF, 200 U/ml of IL-3, 100 ng/ml of G-CSF, 2 U/ml of Epo, 5 ng/ml of human VEGF (R&D systems, Minneapolis, MN) and 100 ng/ml of human Angiopoietin-1 (Ang-1, R&D systems).
In vitro hematopoietic progenitor assay
Cells from the embryos were dispersed into single cell suspensions and plated in duplicate in 1.0% methylcellulose in αMEM containing 30% FBS, 50 μM 2-ME, 1% bovine serum albumin (Sigma, St Louis, MO) and Nutridoma-SP (Behringer Mannheim), supplemented with 2 U/ml human Epo, 50 ng/ml murine SCF, 50 ng/ml human thrombopoietin, 10 ng/ml murine IL-3, 10 ng/ml murine GM-CSF and 10 ng/ml murine IL-6. Cultures were maintained at 37°C under humidified conditions with 5% CO2. CFU-E was counted on day 3, while BFU-E, CFU-GM and CFU-Mix were counted on days 7–10. The colony assay for the primitive erythroid lineage was performed as described (Palis et al, 1999).
Mice, genotyping and RT–PCR analyses and statistical analysis
See Supplementary data.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Table S1
Supplementary data
Acknowledgments
We would like to thank Drs T Fujimoto, S Fraser, D Sugiyama, H Hirai, T O'Connor, N Suzuki, F Sugiyama and K Yagami for discussion and help, Ms N Kaneko and N Kajiwara for help, Dr N Matsuyoshi for the VE-cadherin antibody and Dr E Dzierzak for critical comments on this manuscript. We also thank Kirin brewery for their generous supply of cytokines. This work was supported in part by Grants-in-Aids from the Ministry of Education, Science, Sports and Technology, JST-ERATO Environmental Protection Project, Ministry of Health, Labor and Welfare, Special Coordination Funds for Promoting Science and Technology and the Program for Promotion of Basic Research Activities for Innovative Biosciences.
References
- Adya N, Castilla LH, Liu PP (2000) Function of CBFβ/Bro proteins. Semin Cell Dev Biol 11: 361–368 [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I, Cumano A (2005) Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci USA 102: 134–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZL, de Bruijn M, Ma XQ, Dortland B, Luteijn T, Downing JR, Dzierzak E (2000) Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity 13: 423–431 [DOI] [PubMed] [Google Scholar]
- Choi K (2002) The hemangioblast: a common progenitor of hematopoietic and endothelial cells. J Hematother Stem Cell Res 11: 91–101 [DOI] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G (1998) A common precursor for hematopoietic and endothelial cells. Development 125: 725–732 [DOI] [PubMed] [Google Scholar]
- Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I (2001) Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15: 477–485 [DOI] [PubMed] [Google Scholar]
- de Bruijn M, Ma XQ, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E (2002) Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16: 673–683 [DOI] [PubMed] [Google Scholar]
- Dieterlen-Lievre F, Pardanaud L, Bollerot K, Jaffredo T (2002) Hemangioblasts and hemopoietic stem cells during ontogeny. C R Biologies 325: 1013–1020 [DOI] [PubMed] [Google Scholar]
- Downs KM, Davies T (1993) Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 118: 1255–1266 [DOI] [PubMed] [Google Scholar]
- Ema M, Yokomizo T, Wakamatsu A, Terunuma T, Yamamoto M, Takahashi S (2006) Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin and CD34 in the mouse embryo. Blood, Aug. 22 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ferreira R, Ohneda K, Yamamoto M, Philipsen S (2005) GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol 25: 1215–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ST, Ogawa M, Yu RT, Nishikawa S, Yoder MC, Nishikawa S-I (2002) Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin (+) population. Exp Hematol 30: 1070–1078 [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Ogawa M, Minegishi N, Yoshida H, Yokomizo T, Yamamoto M, Nishikawa S-I (2001) Step-wise divergence of primitive and definitive haematopoietic and endothelial cell lineages during embryonic stem cell differentiation. Genes Cells 6: 1113–1127 [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Chang AN, Williams AM, Orkin SH (2004) Functional overlap of GATA-1 and GATA-2 in primitive hematopoietic development. Blood 103: 583–585 [DOI] [PubMed] [Google Scholar]
- Garcia-Porrero JA, Godin IE, Dieterlen-Lievre F (1995) Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol Berl 192: 425–435 [DOI] [PubMed] [Google Scholar]
- Haar JL, Ackerman GA (1971) A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat Rec 170: 199–223 [DOI] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G (2004) Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432: 625–630 [DOI] [PubMed] [Google Scholar]
- Ichikawa M, Asai T, Saito T, Yamamoto G, Seo S, Yamazaki I, Yamagata T, Mitani K, Chiba S, Ogawa S, Kurokawa M, Hirai H (2004) AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat Med 10: 299–304 [DOI] [PubMed] [Google Scholar]
- Ito E, Toki T, Ishihara H, Ohtani H, Gu L, Yokoyama M, Engel JD, Yamamoto M (1993) Erythroid transcription factor GATA-1 is abundantly transcribed in mouse testis. Nature 362: 466–468 [DOI] [PubMed] [Google Scholar]
- Ito Y (1999) Molecular basis of tissue-specific gene expression mediated by the runt domain transcription factor PEBP2/CBF. Genes Cells 4: 685–696 [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F (1998) Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125: 4575–4583 [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Nottingham W, Liddiard K, Bollerot K, Pouget C, de Bruijn M (2005) From hemangioblast to hematopoietic stem cell: an endothelial connection? Exp Hematol 33: 1029–1040 [DOI] [PubMed] [Google Scholar]
- Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G (1997) A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 386: 488–493 [DOI] [PubMed] [Google Scholar]
- Lacaud G, Gore L, Kennedy M, Kouskoff V, Kingsley P, Hogan C, Carlsson L, Speck N, Palis J, Keller G (2002) Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood 100: 458–466 [DOI] [PubMed] [Google Scholar]
- Lacaud G, Kouskoff V, Trumble A, Schwantz S, Keller G (2004) Haploinsufficiency of Runx1 results in the acceleration of mesodermal development and hemangioblast specification upon in vitro differentiation of ES cells. Blood 103: 886–889 [DOI] [PubMed] [Google Scholar]
- Miller J, Horner A, Stacy T, Lowrey C, Lian JB, Stein G, Nuckolls GH, Speck NA (2002) The core-binding factor β subunit is required for bone formation and hematopoietic maturation. Nat Genet 32: 645–649 [DOI] [PubMed] [Google Scholar]
- Murray PDF (1932) The development in vitro of the blood of early chick embryo. Proc Roy Soc London 11: 497–521 [Google Scholar]
- Nishikawa S-I (2001) A complex linkage in the developmental pathway of endothelial and hematopoietic cells. Curr Opin Cell Biol 13: 673–678 [DOI] [PubMed] [Google Scholar]
- Nishikawa S-I, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H (1998a) Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125: 1747–1757 [DOI] [PubMed] [Google Scholar]
- Nishikawa S-I, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y (1998b) In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8: 761–769 [DOI] [PubMed] [Google Scholar]
- Nishimura S, Takahashi S, Kuroha T, Suwabe N, Nagasawa T, Trainor C, Yamamoto M (2000) A GATA box in the GATA-1 gene hematopoietic enhancer is a critical element in the network of GATA factors and sites that regulate this gene. Mol Cell Biol 20: 713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin Padilla M, Speck NA (1999) Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126: 2563–2575 [DOI] [PubMed] [Google Scholar]
- Okada H, Watanabe T, Niki M, Takano H, Chiba N, Yanai N, Tani K, Hibino H, Asano S, Mucenski ML, Ito Y, Noda T, Satake M (1998) AML1(−/−) embryos do not express certain hematopoiesis-related gene transcripts including those of the PU.1 gene. Oncogene 17: 2287–2293 [DOI] [PubMed] [Google Scholar]
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR (1996) AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84: 321–330 [DOI] [PubMed] [Google Scholar]
- Onodera K, Takahashi S, Nishimura S, Ohta J, Motohashi H, Yomogida K, Hayashi N, Engel JD, Yamamoto M (1997) GATA-1 transcription is controlled by distinct regulatory mechanisms during primitive and definitive erythropoiesis. Proc Natl Acad Sci USA 94: 4487–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J, Robertson S, Kennedy M, Wall C, Keller G (1999) Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126: 5073–5084 [DOI] [PubMed] [Google Scholar]
- Robertson SM, Kennedy M, Shannon JM, Keller G (2000) A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-l. Development 127: 2447–2459 [DOI] [PubMed] [Google Scholar]
- Sabin FR (1920) Studies of the origin of blood vessels and of red corpuscles as seen in the living blastoderm of the during second day of incubation. Contrib Embryol 9: 213–262 [Google Scholar]
- Shimizu R, Takahashi S, Ohneda K, Engel JD, Yamamoto M (2001) In vivo requirements for GATA-1 functional domains during primitive and definitive erythropoiesis. EMBO J 20: 5250–5260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L, Palis J (1997) Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood 89: 1154–1164 [PubMed] [Google Scholar]
- Speck NA, Peeters M, Dzierzak E (2002) Development of the vertebrate hematopoietic system. In Mouse Development, Rossant J, Tam PPL (eds), pp 191–210. San Diego: Academic Press [Google Scholar]
- Sugiyama D, Ogawa M, Hirose I, Jaffredo T, Arai K, Tsuji K (2003) Erythropoiesis from acetyl LDL incorporating endothelial cells at the preliver stage. Blood 101: 4733–4738 [DOI] [PubMed] [Google Scholar]
- Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T (2000) A role for hematopoietic stem cells in promoting angiogenesis. Cell 102: 199–209 [DOI] [PubMed] [Google Scholar]
- Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lievre F, Peault B (1996) Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood 87: 67–72 [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin Padilla M, Sharpe AH, Speck NA (1996) Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci USA 93: 3444–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood HB, May G, Healy L, Enver T, Morriss Kay GM (1997) CD34 expression patterns during early mouse development are related to modes of blood vessel formation and reveal additional sites of hematopoiesis. Blood 90: 2300–2311 [PubMed] [Google Scholar]
- Yanagida M, Osato M, Yamashita N, Liqun H, Jacob B, Wu F, Cao X, Nakamura T, Yokomizo T, Takahashi S, Yamamoto M, Shigesada K, Ito Y (2005) Increased dose of Runx1/AML1 acts as a positive modulator of myeloid leukemogenesis in BXH2 mice. Oncogene 24: 4477–4485 [DOI] [PubMed] [Google Scholar]
- Yoder MC, Hiatt K, Mukherjee P (1997) In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 post coitus. Proc Natl Acad Sci USA 94: 6776–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Ogawa M, Osato M, Kanno T, Yoshida H, Fujimoto T, Fraser S, Nishikawa S, Okada H, Satake M, Noda T, Nishikawa S-I, Ito Y (2001) Requirement of Runx1/AML1/PEBP2αB for the generation of haematopoietic cells from endothelial cells. Genes Cells 6: 13–23 [DOI] [PubMed] [Google Scholar]
- Yoshida CA, Furuichi T, Fujita T, Fukuyama R, Kanatani N, Kobayashi S, Satake M, Takada K, Komori T (2002) Core-binding factor β interacts with Runx2 and is required for skeletal development. Nat Genet 32: 633–638 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Hirashima M, Kataoka H, Tsuchida K, Nishikawa S, Nishikawa S-I (1998) Hematopoietic tissues, as a playground of receptor tyrosine kinases of the PDGF-receptor family. Dev Comp Immunol 22: 321–332 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Table S1
Supplementary data


