Introduction
Neutrophils are essential components of the natural immune system and form the first line of defence against bacterial and fungal infections. They are terminally differentiated cells and have half-lives in the circulation of only 8–20 hr. Their nuclei are invaginated to form a number of defined lobes with thin connecting filaments.1 The DNA is condensed within these nuclear lobes and is randomly distributed into the separate lobes2,3 and probably cannot be transcribed. These cells also have little endoplasmic reticulum (ER), but can produce some protein from existing mRNA.4 They have few mitochondria and the cells generate ATP glycolytically. The long-term survival of these cells is thus insecure, and ultimately limited by the extent of their glycogen stores. One route towards death would thus occur as ATP levels diminished and ion pumps, especially plasma membrane Ca2+-ATPase, became progressively ineffective until cell death proceeds by necrosis. However, it may result in the release of hydrolases and proteases either by necrotic lysis or by Ca2+-driven exocytosis. Moreover, extracellular superoxide generation may also be triggered by Ca2+ influx.5–8 Thus, potentially disastrous extensive damage to the tissues may occur. It thus seems unlikely that the elimination of the millions of neutrophils that die physiologically would be by the necrotic route. Furthermore, neutrophil necrosis is unlikely to be the route for neutrophil clearance in inflammation. Instead, at the resolution of inflammation the neutrophil activity is reduced by an increase in the rate of neutrophil apoptosis. While our understanding of this physiological process of apoptosis as a cell biological phenomenon has increased over the last decade, the unusual role of apoptosis for neutrophils and its consequences are only now being understood.
For example, in many cells, apoptosis is characterized by nuclear condensation and invagination, a morphological event that occurs in neutrophils as they mature. In some respects then the mature neutrophil has already embarked on the route towards apoptosis. This is also evident as neutrophils undergo apoptosis spontaneously, without the need for external stimuli. In fact, external stimuli may modify the rate of this constitutive apoptosis, either by accelerating or delaying cell death, but cannot prevent it.
The goals of neutrophil apoptosis
As neutrophils have the potential to inflict harm, whether oxidative or proteolytic, it is important to the host that their activity is tightly regulated. This is especially the case in inflammation, when large numbers of neutrophils may accumulate within a single organ. In theory, the potential for neutrophil aggression could be limited by: (i) switching down the signalling or response capabilities of the neutrophils, (ii) inducing neutrophil death, or (iii) a combination of the two. The apoptotic process in neutrophils aims to reduce the number of viable and activated cells without releasing the potentially harmful enzymes of the neutrophil. Signalling shutdown may thus be an early step in achieving this goal, with morphological changes of apoptosis being necessary for end stage clearance. Apoptotic neutrophils, as in other cells, ultimately fragment to form ‘apoptotic bodies’, which can be internalized by other cells. Each apoptotic body forms by a mechanism that at no time causes the plasma membrane to be disrupted. Ultimately, these ‘apoptotic bodies’ may be phagocytosed by macrophages, perhaps recognizing them by Phosphatidyl serine (PS) expression9,10 or by lack of a CD31 ‘release/don’t eat me' signal.11 However, signalling shutdown may occur before this and be more important in limiting neutrophil activity. Apoptotic neutrophils are non-functional, being unable to move by chemotaxis, generate a respiratory burst or degranulate7,12 and there is a down-regulation of a number of immunoglobulin superfamily members10 and cell surface receptors12 on neutrophils during apoptosis. Hence, it has been suggested that the loss of the functional capacity of neutrophils undergoing apoptosis may be the result of a decrease in the number of surface receptors, preventing them from transducing signals. Indeed, part of the process of signalling shut-down may involve a decrease in the number of adhesion molecules or other receptors.13,14 However, receptor numbers usually vastly exceed that required for efficient signalling. For example, only 0.1% of formylated peptide receptors need be occupied for a maximal signal transduction.15 It is now emerging that this functional inability of neutrophils undergoing apoptosis may instead be the result of shutdown of a key component of the Ca2+ signalling pathway which controls their activation mechanism.
Unregulated and regulated neutrophil apoptosis
Neutrophils undergo constitutive apoptosis both in vivo and experimentally. However, the rate of apoptosis can also be accelerated or delayed experimentally. This suggests that although the neutrophil apoptotic programme is running, its rate can be regulated.16,17 For example, in acute inflammation, the neutrophil numbers within tissues may be extremely high both because of targeted influx from the circulation and also by slowing of the constitutive apoptotic pathway as reported in sepsis or systemic inflammatory response syndrome (SIRS).18,19 This decreased apoptosis is thought to promote an unbalanced tissue load of neutrophils with resultant uncontrolled release of toxic metabolites exacerbating tissue injury in acute respiratory distress syndrome, SIRS, acute pancreatic inflammation and burn injury.18,20,21 Although delayed neutrophil apoptosis correlates with the severity of clinical sepsis19,22 and multiple organ dysfunction syndrome23 the mechanism for the delay is unclear. However, experimentally bacterial products and cytokines released during sepsis can delay neutrophil apoptosis24 and glucocorticoids, which are commonly given in these conditions, also inhibit apoptosis of human neutrophils.25
Perhaps the clearest trigger for accelerated apoptosis is via the fas (APO-1, CD95) receptor. Fas ligand17,25 or cross-linking fas, on the neutrophil surface leads to a significant increase in apoptotic rate.26–29 Activation of the fas receptor results in the formation of the death-inducing signalling complex (DISC) that contains CD95, the CD95-associated death domain containing molecule, FADD and procaspase-8. As a result of association with this complex, procaspase-8 is cleaved, generating caspase-8. Caspase-8 is then released and activates a cascade of protein-cleaving caspases. This cascade has been seen in a number of cell-types. In some cell types, caspase-8 cleaves Bid, a pro-apoptotic member of the Bcl-2 protein family; which in turn leads to activation of the apoptogenic function of mitochondria through the release of cytochrome c, resulting in the cleavage of other caspases downstream of the mitochondria.30 It has been unclear so far whether this route is important in neutrophil apoptosis, but in the remainder of this review, we will highlight the recent evidence that points to a molecular explanation for Ca2+ signalling shutdown in fas-triggered apoptotic neutrophils and which points to an involvement of mitochondria.
Ca2+ Influx is important for neutrophil activity
In neutrophils, as in most other cell types, Ca2+ signalling is important for a number of cellular activities, including the generation of oxidants and the release of proteases. Ca2+ signalling in neutrophils is initiated by the release of Ca2+ from storage sites. However, this event is coupled to the opening of Ca2+ influx channels on the plasma membrane.31,32 While the initial Ca2+ release event is a necessary prelude, it is the influx of Ca2+ into the cytosol that is responsible for many of the neutrophil responses.33 The influx of Ca2+ through channels in the plasma membrane raises the concentration of Ca2+ just under the plasma membrane to significantly higher levels than in the bulk cytosol34 and is sufficient to activate degranulation35 and calpain activation.36 In neutrophils, as in other cell types, the route to Ca2+ influx can follow a conventional route, namely activation of phospholipase C β (or possibly γ), generation of inositol-1,4,5-trisphosphate (IP3), which diffuses through the cytosol to the ‘vestigial endoplasmic reticulum’ (vER) near the neutrophil nucleus37 releasing stored Ca2+. Following this, store-operated Ca2+ channels (SOCs) in the plasma membrane are also opened and Ca2+ influx results. Although this process of ‘capacitative Ca2+entry’ or ‘store-operated Ca2+ entry’ (for a review, see 38) is common to a number of non-excitable cells39 the mechanism by which it occurs has yet to be fully resolved and may involve different channels in different cell types.38,40 There may be two mechanisms, one involving conformational coupling of IP3 receptors (IP3R)41 with a Ca2+ channel located in the plasma membrane42 and another involving a diffusible second messenger released in response from an internal organelle which causes Ca2+ channel opening. Although the molecular identity is unknown, the latter factor, termed Ca2+ influx factor (CIF) may be a small membrane permeable phosphorylated molecule (MW < 1000)43,44 and CIF activity can be isolated from Ca2+ store depleted neutrophils.45 The characteristics of this store operated Ca2+ influx are perhaps best documented in the related myeloid cell type, the basophil, where the Ca2+ influx response generates a characteristic Ca2+-release activated current, ICRAC.46,47
Ca2+ Influx shutdown during apoptosis
In T cells, Ca2+ influx through SOCs is also important and is required for cell activation, cytokine synthesis, and proliferation. Lepple-Wienhues et al. (1999) have shown that fas-stimulation of T cells blocks Ca2+ influx triggered by Ca2+ store release. They further showed that the block of Ca2+ influx was lacking in fas-defective lymphocytes.48 In neutrophils, we have shown that there is a similar uncoupling of Ca2+ influx from the Ca2+ store release Ayub et al. unpublished observation (2003). As fas-treated neutrophils progress towards apoptosis, Ca2+ influx in response to formylated peptide becomes increasingly diminished although the Ca2+ release component remains the same. This phenomenon cannot therefore be explained by loss of receptor function, but must result from an uncoupling of part of the stimulus-response linkage. The inability of SOCs to open was an early event occurring before detectable externalization of PS or gross morphological changes. It was unlikely to be the result of proteases mediated damage to the Ca2+ channel protein, as this shutdown was insensitive to caspase inhibitors. This may mean that unlike the irreversible step of cell-death, the Ca2+ influx shutdown may be reversible. However, it is clear that uncoupling Ca2+ influx from transduction by activating stimuli would have a profound effect on the responsiveness of neutrophils and render them unable to mount potentially pathogenic actions such as extracellular oxidant and protease liberation which depend on high subplasma membrane Ca2+ and are triggered by Ca2+ influx.
There are several suggestions for the mechanism by which fas ligation uncouples Ca2+ channel opening. The effect of fas activation on Ca2+ influx was prevented in T cells lacking in acidic sphingomyelinase, and was restored by transfection with the enzyme.48 This shed light both on the mechanism of capacitiative Ca2+ entry and on how the signalling shutdown is achieved.49 Fas may not be the only member of the superfamily of tumour-necrosis-factor (TNF) receptors to inhibit Ca2+ channels in the plasma membrane through activation of acid sphingomyelinase. TNF-receptor ligands TNF-α and neural growth factor have also been reported to attenuate store-operated Ca2+ entry in thyroid and mast cells, respectively.50,51 However, this is not the only plausible mechanism. The production of IP3 that is triggered by ligation of CD3 in lymphocytes is also inhibited by fas-ligation.52 Also, it has been suggested that there may be an effect on membrane potential, a negative membrane potential providing the electrical component for Ca2+ entry. It has been shown that activation of fas or the addition of ceramide inhibits N-type K+ channels through activation of a protein kinase, p56lck.53 Inhibition of K+ channels would cause membrane depolarization and consequently reduce the electrochemical gradient and inhibit Ca2+ influx. However, valinomycin, a K+ ionophore, which would restore K+ flux across the cell, failed to prevent the block on Ca2+ entry induced by fas ligation on T cells.48 It should be noted that fas ligation itself has been reported to increase cytosolic free Ca2+ in some cell types54,55 and that in Syrian hamster embryo cells reduced capacitative calcium entry was reported to result in increased apoptosis.56,57
The link between mitochondria in apoptosis and control of ca2+ influx
There would therefore seem to be no clear explanation at present for the mechanism of Ca2+ influx shutdown during apoptosis. However, the work from Hoth and Parekh's laboratories have provided an exciting possible explanation, which links the well-established roles of mitochondria in apoptosis with a new role in signalling Ca2+ influx.
Hoth's group have showed that in T-lymphoid cells58 and Parekh's group in the myeloid cell line, basophilic leukaemia59,60 that ‘energized’ mitochondria play an important role in the opening of the SOCs. As mitochondria are known to take-up Ca2+ from the cytosol and are often placed close to the endoplasmic reticulum where Ca2+ release is occurring, it was thought that they assisted in emptying storage sites of their Ca2+ by acting as a Ca2+‘sink’ or lowering local cytosolic Ca2+ below a level that may activate the SOCs. However, these effects alone are unable to account for the role of mitochondria in Ca2+ influx. Although, SOC opening induced experimentally by thapsigargin can occur in the absence energized mitochondria, Parekh's group have shown in basophils, that mitochondrial depolarization effectively uncouples Ca2+ influx from Ca2+ store release. These authors ruled out effects of changes in intracellular ATP, oxidants, cytosolic acidification, nitric oxide or the permeability transition pore and their data61 suggests a new role for mitochondria as the generator of the signal for Ca2+ influx. One possibility (see Fig. 1) is that released Ca2+ is taken up by nearby mitochondria and that the elevated intramitochondrial Ca2+ generates the signal for Ca2+ influx (e.g. CIF). Because the uptake of Ca2+ into the mitochondria is dependent on its energized state (i.e. membrane potential), one way in which Ca2+ influx could be blocked would be for the energized state of mitochondria to be altered. As pointed out earlier, in many cell types there is a clear involvement of mitochondrial metabolism in apoptosis, culminating in the release of cytochrome c. Although neutrophils have few mitochondria, many of the molecule family members of this mitochondrial pathway have been identified in neutrophils.62 These include Bcl-2 family proteins Al, Mcl-1, Bcl-XL, and Bad. Furthermore, although mitochondria do not normally play an important role in ATP production in neutrophils, these cells being fully active at low oxygen levels are essentially anaerobic, yet they often have membrane potentials.63 The few mitochondria that exist in neutrophils therefore have the required property for Ca2+ uptake and provide them with an important role in interorganelle signalling. Furthermore, recently it has been shown that an early event in the progress towards neutrophil apoptosis, which precedes externalization of PS, is loss of mitochondrial membrane potential63 this would provide the link to Ca2+ signalling shutdown (Fig. 1). As Edwards' group have shown that depolarization of the mitochondrial membrane in itself does not alter the rate of apoptosis63 it is unlikely that mitochondrial depolarization drives apoptosis. It would make teleological sense for Ca2+ shutdown in neutrophils to precede and perhaps be independent of total commitment to apoptosis, as neutrophil inactivation may be the main ‘purpose’ of neutrophil apoptosis in inflammation.
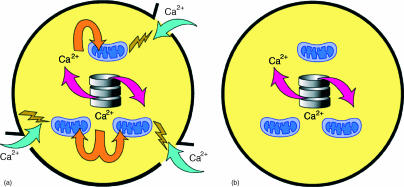
Figure 1.
(a) Signalling competent neutrophil: showing (i) release of Ca2+ from vER; (ii) uptake by energized mitochondria; and (iii) signalling Ca2+ influx. (b) Neutrophil approaching apoptosis with non-energized mitochondria; showing (i) release of Ca2+ from vER, (ii) no uptake by non-energized mitochondria, and (iii) lack of signalling Ca2+ influx.
Consequences of ca2+ influx shutdown
The apoptotic shutdown of the signalling pathway to Ca2+ influx in neutrophils may have important implications for their behaviour in the large number of inflammatory conditions encountered in clinical practice. It may be speculated that a failure of the shut-down mechanism could give rise to inappropriately prolonged activity, which may result in inflammatory tissue damage. The Ca2+ signalling shutdown mechanism may be of more importance for neutrophils than for other cell types as these cells are probably unique in their potential pathogenic effects. It may therefore also be possible to utilize this physiological pathway to trigger this ‘off signal’ pharmacologically. If it could be hijacked and used to prematurely inactive neutrophil sensitivity to stimuli, this may have therapeutic benefit. Clearly, it is important to understand the mechanism of Ca2+ influx shutdown during apoptosis in neutrophils before this knowledge may be beneficially exploited in future. The recent discoveries outlined here may be the first steps towards this understanding.
References
- 1.Bessis M, de Boisfleury-Chevance A. Facts and speculation about necrotaxis (chemotaxis toward a dying cell) Blood Cells. 1984;10:5–22. [PubMed] [Google Scholar]
- 2.Campbell MS, Lovell MA, Gorbsky GJ. Stability of nuclear segments in human neutrophils and evidence against a role for microfilaments or microtubules in their genesis during differentiation of HL60 myelocytes. J Leukoc Biol. 1995;58:659–66. doi: 10.1002/jlb.58.6.659. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez JA, Wangh LJ. New insights into the mechanisms of nuclear segmentation in human neutrophils. J Cell Biochem. 1999;73:1–10. doi: 10.1002/(sici)1097-4644(19990401)73:1<1::aid-jcb1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Simon HU. Regulation of eosinophil and neutrophil apoptosis – similarities and differences. Immunol Rev. 2001;179:156–62. doi: 10.1034/j.1600-065x.2001.790115.x. [DOI] [PubMed] [Google Scholar]
- 5.Afford S, Randhawa S. Apoptosis. Mol Pathol. 2000;53:55–63. doi: 10.1136/mp.53.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papathanassoglou ED, Moynihan JA, Ackerman MH. Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? A review and a theoretical framework. Crit Care Med. 2000;28:537–49. doi: 10.1097/00003246-200002000-00042. [DOI] [PubMed] [Google Scholar]
- 7.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–80. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 8.Simchowitz L, Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Laboratory Clin Med. 1979;93:583–93. [PubMed] [Google Scholar]
- 9.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 10.Homburg CH, de Haas M, dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D. Human neutrophils lose their surface Fc gamma RIII and acquire annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–40. [PubMed] [Google Scholar]
- 11.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–3. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 12.Whyte MK, Meagher LC, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–34. [PubMed] [Google Scholar]
- 13.Dransfield I, Stocks SC, Haslett C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood. 1995;85:3264–73. [PubMed] [Google Scholar]
- 14.Jones J, Morgan BP. Apoptosis is associated with reduced expression of complement regulatory molecules, adhesion molecules and other receptors on polymorphonuclear leucocytes: functional relevance and role in inflammation. Immunology. 1995;86:651–60. [PMC free article] [PubMed] [Google Scholar]
- 15.Sklar LA, Finney DA, Oades ZG, Jesaitis AJ, Painter RG, Cochrane CG. The dynamics of ligand–receptor interactions. Real-time analyses of association, dissociation, and internalization of an N-formyl peptide and its receptors on the human neutrophil. J Biol Chem. 1984;259:5661–9. [PubMed] [Google Scholar]
- 16.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 2001;15:879–92. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 17.Renshaw SA, Timmons SJ, Eaton V, et al. Inflammatory neutrophils retain susceptibility to apoptosis mediated via the Fas death receptor. J Leukoc Biol. 2000;67:662–8. doi: 10.1002/jlb.67.5.662. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez MF, Watson RW, Parodo J, et al. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132:1263–9. doi: 10.1001/archsurg.1997.01430360009002. discussion 1269–70. [DOI] [PubMed] [Google Scholar]
- 19.Keel M, Ungethum U, Steckholzer U, et al. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–63. [PubMed] [Google Scholar]
- 20.Matute-Bello G, Liles WC, Radella F, 2nd, et al. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–77. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- 21.Ayub K, Serracino-Inglott F, Williamson RC, Mathie RT. Expression of inducible nitric oxide synthase contributes to the development of pancreatitis following pancreatic ischaemia and reperfusion. Br J Surg. 2001;88:1189–93. doi: 10.1046/j.0007-1323.2001.01841.x. [DOI] [PubMed] [Google Scholar]
- 22.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–20. [PubMed] [Google Scholar]
- 23.Nolan B, Collette H, Baker S, et al. Inhibition of neutrophil apoptosis after severe trauma is NFêα dependent. J Trauma. 2000;48:599–604. doi: 10.1097/00005373-200004000-00004. discussion 604–5. [DOI] [PubMed] [Google Scholar]
- 24.Liles WC, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–8. [PubMed] [Google Scholar]
- 25.Mincheff M, Loukinov D, Zoubak S, Hammett M, Meryman H. Fas and Fas ligand expression on human peripheral blood leukocytes. Vox Sang. 1998;74:113–21. [PubMed] [Google Scholar]
- 26.Iwai K, Miyawaki T, Takizawa T, et al. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–8. [PubMed] [Google Scholar]
- 27.Liles WC, Klebanoff SJ. Regulation of apoptosis in neutrophils – Fas track to death? J Immunol. 1995;155:3289–91. [PubMed] [Google Scholar]
- 28.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–40. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson RW, O'Neill A, Brannigen AE, et al. Regulation of Fas antibody induced neutrophil apoptosis is both caspase and mitochondrial dependent. FEBS Lett. 1999;453:67–71. doi: 10.1016/s0014-5793(99)00688-2. [DOI] [PubMed] [Google Scholar]
- 30.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies-Cox EV, Laffafian I, Hallett MB. Control of Ca2+ influx in human neutrophils by inositol 1,4,5-trisphosphate (IP3) binding: differential effects of micro-injected IP3 receptor antagonists. Biochem J. 2001;355:139–43. doi: 10.1042/0264-6021:3550139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montero M, Alvarez J, Garcia-Sancho J. Control of plasma-membrane Ca2+ entry by the intracellular Ca2+ stores. Kinetic evidence for a short-lived mediator. Biochem J. 1992;288:519–25. doi: 10.1042/bj2880519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dillon SB, Verghese MW, Snyderman R. Signal transduction in cells following binding of chemoattractants to membrane receptors. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55:65–80. doi: 10.1007/BF02896561. [DOI] [PubMed] [Google Scholar]
- 34.Davies EV, Hallett MB. High micromolar Ca2+ beneath the plasma membrane in stimulated neutrophils. Biochem Biophys Res Commun. 1998;248:679–83. doi: 10.1006/bbrc.1998.9031. [DOI] [PubMed] [Google Scholar]
- 35.Theander S, Lew DP, Nusse O. Granule-specific ATP. requirements for Ca2+-induced exocytosis in human neutrophils. Evidence for substantial ATP-independent release. J Cell Sci. 2002;115:2975–83. doi: 10.1242/jcs.115.14.2975. [DOI] [PubMed] [Google Scholar]
- 36.Dewitt S, Hallett MB. Cytosolic free Ca (2+) changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159:181–9. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettit EJ, Hallett MB. Two distinct Ca2+ storage and release sites in human neutrophils. J Leukoc Biol. 1998;63:225–32. doi: 10.1002/jlb.63.2.225. [DOI] [PubMed] [Google Scholar]
- 38.Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–72. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 39.Putney JW, Jr, Bird GS. The signal for capacitative calcium entry. Cell. 1993;75:199–201. doi: 10.1016/0092-8674(93)80061-i. [DOI] [PubMed] [Google Scholar]
- 40.Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–9. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 41.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 42.Berridge MJ. Capacitative calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–14. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 44.Parekh AB, Terlau H, Stuhmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993;364:814–8. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- 45.Davies EV, Hallett MB. A soluble cellular factor directly stimulates Ca2+ entry in neutrophils. Biochem Biophys Res Commun. 1995;206:348–54. doi: 10.1006/bbrc.1995.1048. [DOI] [PubMed] [Google Scholar]
- 46.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–6. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 47.Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994;15:77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 48.Lepple-Wienhues A, Belka C, Laun T, et al. Stimulation of CD95 (Fas) blocks T lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Natl Acad Sci USA. 1999;96:13795–800. doi: 10.1073/pnas.96.24.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hueber AO. CD95: more than just a death factor? Nat Cell Biol. 2000;2:E23–5. doi: 10.1038/35000092. [DOI] [PubMed] [Google Scholar]
- 50.Mathes C, Fleig A, Penner R. Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J Biol Chem. 1998;273:25020–30. doi: 10.1074/jbc.273.39.25020. [DOI] [PubMed] [Google Scholar]
- 51.Tornquist K, Malm AM, Pasternack M, et al. Tumor necrosis factor-alpha, sphingomyelinase, and ceramide inhibit store-operated calcium entry in thyroid FRTL-5 cells. J Biol Chem. 1999;274:9370–7. doi: 10.1074/jbc.274.14.9370. [DOI] [PubMed] [Google Scholar]
- 52.Kovacs B, Tsokos GC. Cross-linking of the Fas/APO-1 antigen suppresses the CD3-mediated signal transduction events in human T lymphocytes. J Immunol. 1995;155:5543–9. [PubMed] [Google Scholar]
- 53.Szabo I, Gulbins E, Apfel H, et al. Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J Biol Chem. 1996;271:20465–9. doi: 10.1074/jbc.271.34.20465. [DOI] [PubMed] [Google Scholar]
- 54.Oshimi Y, Miyazaki S. Fas antigen-mediated DNA fragmentation and apoptotic morphologic changes are regulated by elevated cytosolic Ca2+ level. J Immunol. 1995;154:599–609. [PubMed] [Google Scholar]
- 55.Scoltock AB, St Bortner CDJBG, Putney JW, Jr, Cidlowski JA. A selective requirement for elevated calcium in DNA degradation, but not early events in anti-Fas-induced apoptosis. J Biol Chem. 2000;275:30586–96. doi: 10.1074/jbc.M004058200. [DOI] [PubMed] [Google Scholar]
- 56.Preston GA, Barrett JC, Biermann JA, Murphy E. Effects of alterations in calcium homeostasis on apoptosis during neoplastic progression. Cancer Res. 1997;57:537–42. [PubMed] [Google Scholar]
- 57.Jayadev S, Petranka JG, Cheran SK, Biermann JA, Barrett JC, Murphy E. Reduced capacitative calcium entry correlates with vesicle accumulation and apoptosis. J Biol Chem. 1999;274:8261–8. doi: 10.1074/jbc.274.12.8261. [DOI] [PubMed] [Google Scholar]
- 58.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–48. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilabert JA, Parekh AB. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current I (CRAC) EMBO J. 2000;19:6401–7. doi: 10.1093/emboj/19.23.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilabert JA, Bakowski D, Parekh AB. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. EMBO J. 2001;20:2672–9. doi: 10.1093/emboj/20.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glitsch MD, Bakowski D, Parekh AB. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002;21:6744–54. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J Leukoc Biol. 2001;70:783–92. [PubMed] [Google Scholar]
- 63.Fossati G, Moulding DA, Spiller DG, Moots RJ, White MR, Edwards SW. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol. 2003;170:1964–72. doi: 10.4049/jimmunol.170.4.1964. [DOI] [PubMed] [Google Scholar]