Abstract
There is considerable interest in the possible use of cAMP-elevating agents in the treatment of autoimmune diseases such as rheumatoid arthritis. The objective of this study was to evaluate the impact of different cAMP-elevating agents on the T-cell response to type II collagen within the context of collagen-induced arthritis, a murine model of rheumatoid arthritis. Spleen cells or lymph node cells from type-II-collagen-immunized DBA/1 mice were cultured in the presence of type II collagen plus one of five different cAMP-elevating agents: rolipram, forskolin, prostaglandin E2, 8-bromo-cAMP, or cholera toxin. Levels of interferon-γ (IFN-γ), interleukin-4 (IL-4) and IL-5 were measured in culture supernatants by enzyme-linked immunosorbent assay. All of the cAMP-elevating agents tested were found to profoundly suppress IFN-γ production in a dose-dependent manner. IL-4 and IL-5 production was slightly up-regulated at low concentrations of the cAMP-elevating agents and was modestly suppressed at the highest concentrations of cAMP-elevating agents. Experiments were then carried out to determine whether T cells were directly affected by cAMP-elevating agents or whether the immunomodulatory effects were mediated via antigen-presenting cells. Pulsing T cells alone for a brief period with cholera toxin produced an almost identical effect to pulsing antigen-presenting cells alone, i.e. down-regulation of proliferation, down-regulation of IFN-γ production with little effect on IL-5 production. It was concluded that cAMP-elevating agents suppressed T helper type 1 responses to type II collagen to a greater extent than T helper type 2 responses. The cAMP-elevating agents could directly influence the activity of T cells but, in addition, influenced the ability of antigen-presenting cells to support T helper type 1 responses.
Introduction
As long ago as 1974 it was hypothesized that the second messenger, cAMP, plays an important physiological role in the control of immune and inflammatory responses. Since then, a number of studies have highlighted the therapeutic potential of cAMP-elevating agents for the treatment of diseases like rheumatoid arthritis, in which there is dysregulated activation of both immune and inflammatory pathways. Increased intracellular levels of cAMP can be achieved pharmacologically by increasing the level of activity of adenylate cyclase, which generates cAMP, or by inhibiting the level of activity of phosphodiesterase (PDE), which degrades cAMP. Both of these approaches have been used experimentally in the treatment of arthritis. For example, the PDE4 inhibitor, rolipram, was tested and shown to be effective in reducing the severity of collagen-induced arthritis (CIA),1,2 adjuvant arthritis3,4 and streptococcal cell wall-induced arthritis.5 Similarly, at least three agonists of Gs-protein-coupled receptors that elevate intracellular cAMP levels via activation of adenylate cyclase have been shown to be effective in established CIA, including the β2-adrenergic receptor agonist, salbutamol,6 and the neuropeptides, vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP).7–9
In addition to the ability of cAMP-elevating agents to ameliorate arthritis and suppress tumour necrosis factor-α production, it has also been reported that the suppression of CIA by rolipram, salbutamol, VIP and PACAP was accompanied by marked down-regulation of the T helper type 1 (Th1) response to type II collagen.1,2,7–9 There are also a number of reports suggesting that the proliferation of Th1 cells is selectively down-regulated by cAMP-elevating agents.10–12 However, contradictory reports have been published on the effect of cAMP-elevating agents on Th2 cells, with different studies suggesting that Th2 cell activity may be down-regulated,13–16 unaffected,10–12 or even up-regulated17–21 by increases in cAMP. However, many of these studies were based on the use of T-cell lines and clones and it is not clear whether these cells that are propagated in vitro respond to elevations in cAMP in the same way as primary T cells. Secondly, in many cases mitogens, such as phorbol 12-myristate 13-acetate and phytohaemagglutinin, have been used to activate the T cells and it would appear that T cells activated by mitogens respond differently to fluctuations in cAMP levels than those stimulated via the T-cell receptor.22
In this paper we have carried out a more detailed analysis of the impact of cAMP-elevating agents on Th1 and Th2 cytokine expression within the context of a polyclonal T-cell response to type II collagen. In addition, we address the important question of whether cAMP-elevating agents modulate the activity of lymphocytes directly, or whether their effects are mediated via changes in antigen-presenting cell (APC) function. Our findings confirm that elevations in cAMP have a profound immunomodulatory effect on type II collagen-stimulated T cells. In addition we show that cAMP-elevating agents affect the activity of both T cells and APC in a co-ordinated fashion.
Materials and methods
Mice
Male DBA/1 mice were used at 8–12 weeks of age throughout this study.
Reagents
Rolipram was kindly supplied by Dr P. Scholz (Schering AG, Berlin, Germany). Forskolin, cholera toxin (CT) and 8-bromo-cAMP were purchased from Calbiochem (San Diego, CA). Prostaglandin E2 (PGE2) was purchased from Sigma (Gillingham, UK). Rolipram and forskolin were dissolved in dimethyl sulphoxide, 8-bromo-cAMP was dissolved in distilled water, PGE2 was dissolved in ethanol and CT was dissolved in an aqueous solution consisting of 50 mm Tris–HCl, pH 7.5, 200 mm NaCl and 1 mm ethylenediaminetetraacetic acid. None of the solvents influenced T-cell activity at the final concentrations used in this study.
Complete Freund's adjuvant (CFA) was purchased from Difco (West Molesey, UK) and alum adjuvant was purchased from Pierce and Warriner (Chester, UK). Type II collagen was purified from bovine articular cartilage, as described elsewhere.23
Immunization of mice
Immunization of DBA/1 mice with collagen in CFA leads to a profoundly Th1-skewed immune response whereas immunization with collagen in alum adjuvant leads to a more balanced Th1/Th2 cytokine profile and in this study both forms of immunization were analysed. Type II collagen was dissolved in 0.2 m NaCl/0.05 m Tris–HCl (pH 7.4) at a concentration of 1 mg/ml and emulsified in CFA (1: 1 ratio) or mixed with alum adjuvant (1: 1 ratio). Mice were immunized intradermally at two to four sites around the base of the tail. When alum was used as an adjuvant, a booster injection of type II collagen/alum adjuvant was given on day 14. Mice were killed on day 21 and spleens/lymph nodes were removed aseptically. These experiments were approved by the local Ethical Review Process Committee and by the Home Office of Great Britain.
T-cell cultures
Spleen cells or lymph node cells (LNC) from type-II-collagen-immunized mice were cultured in the presence of type II collagen (50 μg/ml) at a cell density of 5 × 106/ml in 96-well plates in RPMI-1640 containing fetal calf serum (10% v/v), 2-mercaptoethanol (20 μm), l-glutamine (1% w/v), penicillin (100 U/ml) and streptomycin (100 μg/ml).
Alternatively, CD4+ T cells were separated by positive selection from the spleens of type-II-collagen-immunized mice by magnetic-activated cell sorting (MACS) using anti-CD4-conjugated microbeads, according to the manufacturer's instructions (Miltenyi Biotec, Bisley, UK). Next, the purified T cells were stimulated with plate-bound anti-CD3ε monoclonal antibody (mAb; 5 μg/ml) and soluble anti-CD28 mAb (10 μg/ml). Both mAbs were purchased from AMS Biotechnology (Abingdon, UK).
Irrespective of the method of stimulation, cultures were assayed in triplicate after 24 hr for interleukin-4 (IL-4) and after 72 hr for IL-5 and interferon-γ (IFN-γ). To determine the rate of T-cell proliferation, triplicate cultures were cultured for 72 hr with type II collagen (50 μg/ml) and pulsed for the last 16 hr with [3H]thymidine. Cells were then harvested and assessed for incorporation of radioactivity.
Separation of T-cell/APC populations
CD4+ T cells were separated by positive selection from LNC of type-II-collagen-immunized mice by MACS using anti-CD4-conjugated microbeads, according to the manufacturer's instructions (Miltenyi Biotec). Purity of the T-cell-enriched fraction was greater than 92% as assessed by fluorescence-activated cell sorter analysis. The remaining T-cell-depleted population was subsequently gamma-irradiated (source: 137Cs; 270 Ci) for a predetermined period of time to block proliferation of any remaining lymphocytes.
Measurement of cytokines
To measure the secreted cytokines (IFN-γ, IL-4, or IL-5), 96-well enzyme-linked immunosorbent assay (ELISA) plates were coated with the appropriate capture antibody (purchased from AMS Biotechnology), blocked with bovine serum albumin (2% w/v), and then incubated with culture supernatants. After washing, bound cytokines were detected using biotinylated detect antibodies (AMS Biotechnology), followed by streptavidin biotinylated horseradish peroxidase complex (Amersham, Little Chalfont, UK). A colour reaction was developed using 3,3′,5,5′-tetramethylbenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD) and the reaction was stopped with H2SO4 (4.5 mol/l). Optical densities were measured at 450 nm using an ELX808 ELISA plate reader (Bio-tek Instruments, Winooski, VT). A standard curve was generated using known concentrations of recombinant IFN-γ, IL-4, or IL-5 (AMS Biotechnology).
Statistical analysis
Cytokine data were analysed by one-way analysis of variance, followed by the Dunnet multiple comparison test where appropriate.
Results
Conventional CIA involves immunization of genetically susceptible (e.g. DBA/1) mice with type II collagen in CFA. However, using this protocol a highly polarized Th1 response is generated and the production of Th2 cytokines is generally very low (close to the limit of detection) and subject to a high degree of variability, depending on the stage of disease, age of mice, etc. For this reason, a different immunization schedule was used for some of these studies by injecting mice with type II collagen in alum adjuvant. This generated a more balanced and reproducible Th1/Th2 response. IFN-γ was measured as the prototypic Th1 cytokine whereas IL-5 (which generally correlates strongly with IL-4 expression) was used as the marker of the Th2 response. It should be noted that IL-4 is notoriously difficult to measure in culture supernatants, probably because of uptake by cells expressing IL-4 receptors.
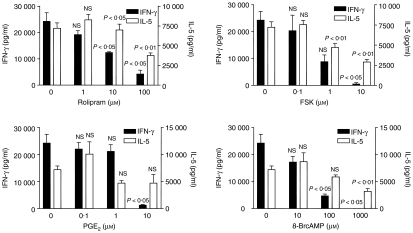
In the first experiment, mice were immunised intradermally with collagen/alum, then given an intraperitoneal booster injection 7 days later. This booster injection was given because alum is a relatively weak adjuvant compared to CFA. Spleens were harvested 3–4 weeks after primary immunization, pooled and pushed through a sieve to make a single-cell suspension. Spleen cells were then cultured for 72 hr in the presence of type II collagen (50 μg/ml) plus one of four cAMP-elevating agents; rolipram, forskolin, PGE2, or 8-bromo-cAMP (a cell permeant cAMP analogue) and the production of IFN-γ and IL-5 was measured by ELISA (Fig. 1). The doses used of each of the agents were established from preliminary studies and were designed to cover the full range of the dose–response curve. All four cAMP-elevating agents significantly suppressed IFN-γ production (maximum reduction 80–100%; P < 0.01) whilst only modestly inhibiting IL-5 production (maximum reduction 35–60%; P < 0.05). Furthermore, the modest reductions in IL-5 production were observed only at the highest concentrations of cAMP-elevating agents, which would be difficult to achieve in vivo. Thus, the first conclusion to be drawn from this study was that activation of the cAMP pathway in the spleen cell cultures had the effect of reducing the Th1 response to a greater extent than the Th2 response.
Figure 1.
Effect of cAMP-elevating agents on IFN-γ and IL-5 production. Spleen cells from DBA/1 mice immunized with type II collagen in alum adjuvant were cultured in the presence of type II collagen (50 μg/ml) plus one of the four cAMP-elevating agents. The agents were added 30 min prior to addition of collagen and cytokines were measured by ELISA after 72 hr. Data shown are mean ± SE and the experiment is a representative of three comparable experiments, giving consistent results. P-values refer to differences between groups treated with cAMP-elevating agents and untreated controls.
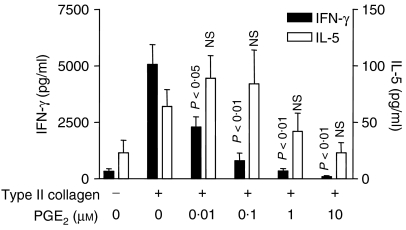
A second observation of significance to emerge from this study was that there was a small but consistent trend towards increased IL-5 production, at low concentrations of all the cAMP-elevating agents, although this did not reach the level of statistical significance. The possibility was considered that this trend towards increased IL-5 production was related to the fact that the spleen cells were taken from mice immunized with type II collagen in alum (a strong Th2 adjuvant). Hence, a second experiment was carried out in which LNC from arthritic mice immunized with type II collagen in CFA were cultured in the presence of collagen and increasing concentrations of PGE2. As in the previous experiment, a trend towards increased IL-5 production was observed at low PGE2 concentrations (0.1–0.1 μm) which was not statistically significant, whilst IFN-γ production was significantly inhibited (P < 0.01; Fig. 2).
Figure 2.
Induction of IL-5 expression by low doses of PGE2. DBA/1 mice were immunized with type II collagen in CFA and draining (inguinal) lymph nodes were removed after 21 days. LNC were cultured in the presence or absence of type II collagen and PGE2 was added at the doses shown 30 min prior to addition of type II collagen. IFN-γ and IL-5 were measured by ELISA after 72 hr. Data are shown as mean ± SE and are representative of three similar experiments. P-values refer to differences between PGE2-treated groups and untreated controls.
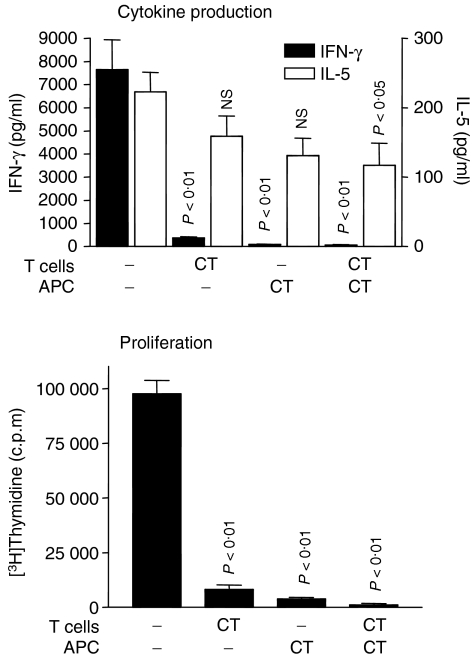
It is well known that the way in which antigen is presented to T cells strongly influences the outcome of the ensuing immune response. Hence, having confirmed that activation of the cAMP pathway in spleen cell cultures is capable of profoundly altering the Th1/Th2 profile, the next step was to identify the principle target of the cAMP-mediated effects: the T cell itself, or the APC. To address this question, LNC from mice immunized with type II collagen in CFA were separated into T-cell-enriched and T-cell-depleted populations by positive selection of CD4+ cells using the MACS system. The T-cell-depleted population was then gamma-irradiated to block proliferation of any contaminating lymphocytes and this population was named the APC fraction. The purity of the T-cell fraction was >92% whereas the T cells were undetectable in the APC fraction after irradiation. The purity of the cell populations was also assessed on a functional basis. Thus, neither the T-cell fraction alone nor the APC fraction alone responded to antigenic stimulation whereas when the two fractions were recombined a robust T-cell response was observed following stimulation with type II collagen (data not shown). Furthermore, we have previously shown that positive selection of T cells using the MACS system does not cause any obvious changes in cytokine production (data not shown).
Next, the T-cell fraction and the APC fraction were separately treated with CT (an irreversible activator of adenylate cyclase) or vehicle control for 1 hr at 37°. CT was used for this study because it is known to produce a sustained rise in cAMP that persists after its removal from the culture supernatant. Next, the cells were washed and recombined to give four groups: (1) control T cells plus control APC, (2) CT-treated T cells plus control APC, (3) control T cells plus CT-treated APC, and (4) CT-treated T cells plus CT-treated APC. These groups were cultured for 72 hr in the presence of type II collagen, levels of IFN-γ and IL-5 were measured, as before, and cell proliferation was assessed by incorporation of [3H]thymidine. The results of this study clearly showed that treatment of either T cells or APC with CT had a remarkably similar effect, i.e. marked down-regulation of IFN-γ production (∼95%) and T-cell proliferation (∼90%) with only a limited reduction (∼40%) in IL-5 production (Fig. 3).
Figure 3.
Treatment of T cells or APC with CT has the same effect on IFN-γ and IL-5 production and proliferation. LNC from mice immunized with type II collagen in CFA were separated into CD4+ T-cell and APC fractions by positive selection of CD4+ T cells, followed by gamma-irradiation of the APC fraction. The T-cell and APC populations were treated separately with CT for 1 hr at 37°, washed and then recombined, as shown. The recombined cells were cultured in the presence of type II collagen (50 μg/ml) for 72 hr for measurement of IFN-γ and IL-5 production. To measure proliferation, [3H]thymidine was added for the last 16 hr. Data are shown as mean ± SE and the experiment is representative of three experiments, all of which gave the same result. P-values refer to differences between CT-treated groups and untreated controls.
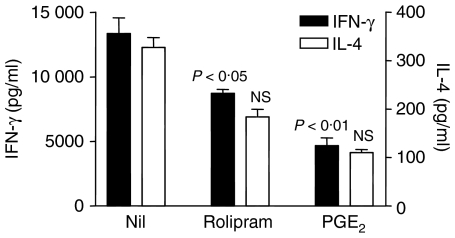
One potential criticism of the previous experiment is that CT, whilst a potent cAMP-elevating agent, may have effects on T cells and/or APC that are unrelated to cAMP. Hence, another experiment was carried out in which we were able to confirm, using rolipram and PGE2, that T cells are directly affected by elevations in cAMP. Here, CD4+ T cells were purified from the spleens of naive mice, then stimulated by a combination of plate-bound anti-CD3ε mAb and soluble anti-CD28 mAb in the presence of increasing concentrations of either rolipram or PGE2 in the absence of APC. IFN-γ and IL-4 (which were found to be detectable in anti-CD3ε/anti-CD28-stimulated T-cell cultures) were measured by ELISA. This experiment confirmed that both rolipram and PGE2 were capable of modulating the activity of purified T cells in the absence of APC (Fig. 4). It should also be noted, however, that the inhibitory effects of both rolipram and PGE2 were less pronounced when T cells were stimulated with anti-CD3ε/anti-CD28 than when spleen cells or LNC were stimulated with type II collagen in the presence of APC. This may be attributed to the fact that T cells stimulated with mitogens are more refractory than TCR-stimulated T cells to incoming cAMP signals.22
Figure 4.
Modulation of Th1/Th2 cytokine production by cAMP in the absence of APC. CD4+ T cells were purified, using the MACS system, from mouse spleens and cultured on anti-CD3ε-coated plates in the presence of soluble anti-CD28 alone or with rolipram (100 μm) or PGE2 (10 μm). Data are shown as mean ± SE. P-values refer to differences between groups treated with rolipram or PGE2 and untreated controls.
Discussion
This study has shed further light on the immunomodulatory properties of cAMP-elevating agents within the context of the T-cell response to type II collagen. Thus, all of the five different cAMP-elevating agents tested (rolipram, forskolin, PGE2, 8-bromo-cAMP and CT) caused a dose-dependent reduction in the production of the Th1 cytokine, IFN-γ, whilst having only a modest inhibitory effect at very high doses on the Th2 cytokines, IL-4 and IL-5. It is concluded that elevations in cAMP are likely to reduce Th1 cell activity to a greater extent than Th2 activity. Elevations in intracellular levels of cAMP cause activation of protein kinase A (PKA) by causing disassociation of the regulatory and catalytic subunits. PKA is thought to have negative regulatory influences on multiple components of the T-cell signalling machinery, including the nuclear accumulation of nuclear factor of activated T cells (NF-AT),24 and the activation of nuclear factor κB (NFκB) and mitogen-activated protein kinase (MAPK) pathways.25 In addition, PKA has been shown to activate Csk, a negative regulator of Lck.26 It is also reported that cAMP negatively regulates T-cell activity independently of PKA,16 although the precise mechanism(s) remain to be elucidated. There are therefore a number of possible explanations for the inhibitory effects of cAMP on T-cell activity. What is not clear, however, is why Th2 cells should be less sensitive than Th1 cells to the inhibitory effects of cAMP. We did not measure intracellular levels of cAMP following treatment with cAMP-elevating agents. However, previous studies have shown that Th1 and Th2 cells accumulate cAMP at comparable levels following stimulation with PGE2,12 therefore we cannot attribute the relative insensitivity of Th2 cells to the inhibitory effects of cAMP-elevating agents to a failure to generate or accumulate cAMP. It is becoming increasingly apparent that a group of proteins, known as A-kinase anchor proteins (AKAPs), play an important role in regulating and directing the activity of PKA in a variety of different cell types,27,28 including lymphocytes.29,30 Indeed, one purely speculative possibility that is currently under investigation is that Th1 cells and Th2 cells may differ in their expression of AKAPs, such that PKA activity is reduced in Th2 cells compared to Th1 cells, or that the substrate specificity of PKA differs between the two subsets.
Another observation to emerge from this study was that low doses of all the cAMP-elevating agents caused a small but consistent increase in IL-5 production, although this was not statistically significant. The concentration range of PGE2 that gave maximal IL-5 levels was 0.01–0.1 μm and a pertinent question is what levels of PGE2 are likely to be found in vivo? This is a difficult question to answer because concentration gradients within the vicinity of PGE2-producing cells may create large local variations. Nevertheless, with this proviso, the level of PGE2 in the plasma of normal individuals was reported to be around 0.001 μm, although this can rise to 0.03 μm in patients with cancer.31 Synovial fluid PGE2 levels of 0.001 μm were detected in patients with rheumatoid arthritis32 whereas tissue PGE2 levels of 0.1–0.4 μm were measured following trauma.33
It was confirmed in this study that cAMP-elevating agents are able to influence T-cell activity independently of APC. This was unsurprising given the known inhibitory effects of cAMP/PKA on T-cell signalling pathways.25 However, a less expected finding was that pulsing APC for 1 hr with a cAMP-elevating agent (CT) had the same, or even greater, effect on Th1/Th2 cytokine expression as pulsing T cells. The ability of APC to alter profoundly T-cell activation may be the result of one or more of a number of possible factors, including an increase in IL-10 production,34 a decrease in IL-12 production,35 a reduction in major histocompatibility complex class II expression,34 or an alteration in costimulatory molecule expression.36 Further studies will be required to elucidate the precise mechanisms involved.
A number of previous studies have examined the effects of cAMP-elevating agents on T-cell lines and clones10–12 and in this study we have demonstrated the potent immunomodulatory effects of cAMP within the context of a T-cell response to type II collagen. In addition, we have shown that this immunomodulatory effect is mediated by T cells directly and by changes in APC function, although further work will be required to elucidate the precise mechanisms involved. Understanding the molecular targets of the cAMP/PKA pathway in T cells or APC may provide important clues as to how we can regulate pathogenic T-cell activity in diseases such as rheumatoid arthritis.
Acknowledgments
We are indebted to Paul Warden and the staff of the Biological Services Unit for support with the animal work. This research was supported by the Arthritis Research Campaign.
Abbreviations
- APC
antigen-presenting cell
- CFA
complete Freund's adjuvant
- CIA
collagen-induced arthritis
- CT
cholera toxin
- LNC
lymph node cells
- MACS
magnetic-activated cell sorting
- PACAP
pituitary adenylate cyclase activating peptide
- PDE
phosphodiesterase
- VIP
vasoactive intestinal peptide
References
- 1.Nyman U, Mussener A, Larsson E, Lorentzen J, Klareskog L. Amelioration of collagen II-induced arthritis in rats by the type IV phosphodiesterase inhibitor Rolipram. Clin Exp Immunol. 1997;108:415–9. doi: 10.1046/j.1365-2249.1997.3931291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross SE, Williams RO, Mason LJ, Mauri C, Marinova-Mutafchieva L, Malfait A-M, Maini RN, Feldmann M. Suppression of TNF-α expression, inhibition of Th1 activity, and amelioration of collagen-induced arthritis by rolipram. J Immunol. 1997;159:6253–9. [PubMed] [Google Scholar]
- 3.Sekut L, Yarnall D, Stimpson SA, et al. Anti-inflammatory activity of phosphodiesterase (PDE)-IV inhibitors in acute and chronic models of inflammation. Clin Exp Immunol. 1995;100:126–32. doi: 10.1111/j.1365-2249.1995.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francischi JN, Yokoro CM, Poole S, Tafuri WL, Cunha FQ, Teixeira MM. Anti-inflammatory and analgesic effects of the phosphodiesterase 4 inhibitor rolipram in a rat model of arthritis. Eur J Pharmacol. 2000;399:243–9. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- 5.Laemont KD, Schaefer CJ, Juneau PL, Schrier DJ. Effects of the phosphodiesterase inhibitor rolipram on streptococcal cell wall-induced arthritis in rats. Int J Immunopharmacol. 1999;21:711–25. doi: 10.1016/s0192-0561(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 6.Malfait AM, Malik AS, Marinova-Mutafchieva L, Butler DM, Maini RN, Feldmann M. The beta2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J Immunol. 1999;162:6278–83. [PubMed] [Google Scholar]
- 7.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–8. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 8.Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M. Pituitary adenylate cyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol. 2001;167:3182–9. doi: 10.4049/jimmunol.167.6.3182. [DOI] [PubMed] [Google Scholar]
- 9.Williams RO. Therapeutic effect of vasoactive intestinal peptide in collagen-induced arthritis. Arthritis Rheum. 2002;46:271–3. doi: 10.1002/1529-0131(200201)46:1<271::AID-ART10039>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 10.Gajewski TF, Schell SR, Fitch FW. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990;144:4110–20. [PubMed] [Google Scholar]
- 11.Munoz E, Zubiaga AM, Merrow M, Sauter NP, Huber BT. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990;172:95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–13. [PubMed] [Google Scholar]
- 13.Crocker IC, Townley RG, Khan MM. Phosphodiesterase inhibitors suppress proliferation of peripheral blood mononuclear cells and interleukin-4 and -5 secretion by human T-helper type 2 cells. Immunopharmacology. 1996;31:223–35. doi: 10.1016/0162-3109(95)00053-4. [DOI] [PubMed] [Google Scholar]
- 14.Essayan DM, Kagey-Sobotka A, Lichtenstein LM, Huang SK. Differential regulation of human antigen-specific Th1 and Th2 lymphocyte responses by isozyme selective cyclic nucleotide phosphodiesterase inhibitors. J Pharmacol Exp Ther. 1997;282:505–12. [PubMed] [Google Scholar]
- 15.Braun CM, Huang SK, Kagey-Sobotka A, Lichtenstein LM, Essayan DM. Co-regulation of antigen-specific T lymphocyte responses by type I and type II cyclic AMP-dependent protein kinases (cAK) Biochem Pharmacol. 1998;56:871–9. doi: 10.1016/s0006-2952(98)00238-x. [DOI] [PubMed] [Google Scholar]
- 16.Staples KJ, Bergmann M, Tomita K, Houslay MD, McPhee I, Barnes PJ, Giembycz MA, Newton R. Adenosine 3′,5′-cyclic monophosphate (cAMP) -dependent inhibition of IL-5 from human T lymphocytes is not mediated by the cAMP-dependent protein kinase A. J Immunol. 2001;167:2074–80. doi: 10.4049/jimmunol.167.4.2074. [DOI] [PubMed] [Google Scholar]
- 17.Lacour M, Arrighi JF, Muller KM, Carlberg C, Saurat JH, Hauser C. cAMP up-regulates IL-4 and IL-5 production from activated CD4+ T cells while decreasing IL-2 release and NF-AT induction. Int Immunol. 1994;6:1333–43. doi: 10.1093/intimm/6.9.1333. [DOI] [PubMed] [Google Scholar]
- 18.Siegel MD, Zhang DH, Ray P, Ray A. Activation of the interleukin-5 promoter by cAMP in murine EL-4 cells requires the GATA-3 and CLE0 elements. J Biol Chem. 1995;270:24548–55. doi: 10.1074/jbc.270.41.24548. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Zhang DH, LaPorte JM, Ray A. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J Immunol. 2000;165:5597–605. doi: 10.4049/jimmunol.165.10.5597. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O'Garra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192:105–15. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suarez A, Mozo L, Gutierrez C. Generation of CD4(+) CD45RA(+) effector T cells by stimulation in the presence of cyclic adenosine 5′-monophosphate-elevating agents. J Immunol. 2002;169:1159–67. doi: 10.4049/jimmunol.169.3.1159. [DOI] [PubMed] [Google Scholar]
- 22.Staples KJ, Bergmann M, Barnes PJ, Newton R. Stimulus-specific inhibition of IL-5 by cAMP-elevating agents and IL-10 reveals differential mechanisms of action. Biochem Biophys Res Commun. 2000;273:811–15. doi: 10.1006/bbrc.2000.3023. [DOI] [PubMed] [Google Scholar]
- 23.Miller EJ. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972;11:4903–9. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- 24.Sheridan CM, Heist EK, Beals CR, Crabtree GR, Gardner P. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J Biol Chem. 2002;25:25. doi: 10.1074/jbc.M207029200. [DOI] [PubMed] [Google Scholar]
- 25.Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Tasken K. Molecular mechanisms for protein kinase A-mediated modulation of immune function. Cell Signal. 2002;14:1–9. doi: 10.1016/s0898-6568(01)00214-5. [DOI] [PubMed] [Google Scholar]
- 26.Vang T, Torgersen KM, Sundvold V, et al. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J Exp Med. 2001;193:497–507. doi: 10.1084/jem.193.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feliciello A, Gottesman ME, Avvedimento EV. The biological functions of A-kinase anchor proteins. J Mol Biol. 2001;308:99–114. doi: 10.1006/jmbi.2001.4585. [DOI] [PubMed] [Google Scholar]
- 28.Michel JJ, Scott JD. AKAP mediated signal transduction. Annu Rev Pharmacol Toxicol. 2002;42:235–57. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- 29.Schillace RV, Andrews SF, Liberty GA, Davey MP, Carr DW. Identification and characterization of myeloid translocation gene 16b as a novel a kinase anchoring protein in T lymphocytes. J Immunol. 2002;168:1590–9. doi: 10.4049/jimmunol.168.4.1590. [DOI] [PubMed] [Google Scholar]
- 30.Williams RO. Cutting Edge: a-kinase anchor proteins are involved in maintaining resting T cells in an inactive state. J Immunol. 2002;168:5392–6. doi: 10.4049/jimmunol.168.11.5392. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe BM, Behrman HR, Parker CW. Radioimmunoassay measurement of prostaglandins E, A, and F in human plasma. J Clin Invest. 1973;52:398–405. doi: 10.1172/JCI107196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seppala E, Nissila M, Isomaki H, Wuorela H, Vapaatalo H. Effects of non-steroidal anti-inflammatory drugs and prednisolone on synovial fluid white cells, prostaglandin E2, leukotriene B4 and cyclic AMP in patients with rheumatoid arthritis. Scand J Rheumatol. 1990;19:71–5. doi: 10.3109/03009749009092624. [DOI] [PubMed] [Google Scholar]
- 33.Berenbaum MC, Cope WA, Bundick RV. Synergistic effect of cortisol and prostaglandin E2 on the PHA response. Relation to immunosuppression induced by trauma. Clin Exp Immunol. 1976;26:534–41. [PMC free article] [PubMed] [Google Scholar]
- 34.Kambayashi T, Wallin RP, Ljunggren HG. cAMP-elevating agents suppress dendritic cell function. J Leukoc Biol. 2001;70:903–10. [PubMed] [Google Scholar]
- 35.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delgado M, Leceta J, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide stimulate the induction of Th2 responses by up-regulating B7.2 expression. J Immunol. 1999;163:3629–35. [PubMed] [Google Scholar]