Abstract
We analysed the regulation of human leucocyte antigen (HLA)-E, -F and -G genes, focusing on the SXY module, a promoter region that controls major histocompatibility complex (MHC) class II expression and participates in the expression of classical HLA class I molecules. It comprises the X1, X2 and Y boxes, bound by RFX, X2-BP/ATF/CREB and NFY factors, respectively. The complex recruits the master control factor CIITA. The SXY module is conserved in HLA-E and HLA-F gene promoters, whereas in the HLA-G promoter, the only conserved boxes are S and X1. Chromatin immunoprecipitation assays, performed on HLA-G positive and negative cell lines, demonstrated the in situ binding of RFX5 and CIITA to HLA-E and HLA-F, but not to HLA-G, promoters. In B cells from bare lymphocyte syndrome patients lacking RFX5 or CIITA, we observed lower steady-state levels of HLA-E and HLA-F transcripts but did not find any significant decrease in the cell-surface expression of HLA-E/classical HLA class I. In RFX5-deficient fibroblasts, the cell-surface expression of HLA molecules was decreased. RFX5 and CIITA are thus not involved in HLA-G expression and their importance for the surface expression of HLA-E/classical HLA class I molecules may vary depending on the cell type.
Introduction
The molecular mechanisms that regulate the expression of non-classical human leucocyte antigen (HLA) class I genes remain largely unknown. Using in situ analysis, we investigated the regulation of expression of HLA-E, -F and -G molecules by focusing on shared regulatory elements in the proximal promoter of HLA class I and class II genes.
Non-classical HLA class I molecules exhibit low polymorphism and specialized functions with regard to the regulation of immune responses. HLA-E functions as an inhibitor of natural killer (NK) cells through interaction with the CD94/NKG2A receptors, whereas HLA-G inhibits both T- and NK cell-mediated cytolysis through interaction with the ILT2, ILT4 and KIR2DL4/p49 receptors.1,2 HLA-G can also inhibit the rolling adhesion of activated human NK cells3 and may act as a shield against inflammatory aggression.4 Much less is known about HLA-F function. However, HLA-F tetramers bind ILT2 and ILT4 inhibitory receptors, suggesting that HLA-F may bind peptides and reach the cell surface.5 Therefore, HLA-F might also modulate immune effector-cell functions.
Like that of polymorphic classical HLA-A, -B and -C class I genes, transcription of the HLA-E gene occurs in most adult and fetal tissues6,7 and may be enhanced by interferon-γ (IFN-γ).8 However, the expression of HLA-E protein is primarily dependent on the expression levels of other class I HLA molecules, because their leader peptides are presented by, and stabilize, the HLA-E molecule.9 HLA-G leader peptides, in particular, provide the best HLA-E stabilization.10 In contrast, the expression of HLA-F and HLA-G proteins is very restricted in non-pathological conditions. HLA-F is predominantly intracellular, and confined to B cells and tissues such as adult tonsil, thymus and fetal liver.11HLA-G is transcribed at a basal level, without protein expression, in several cell types, and is highly transcribed in those expressing cell-surface HLA-G. The latter include invasive trophoblasts,12 amnion epithelial cells,13,14 thymic epithelial cells15 and fetal endothelial cells in chorionic villi.16 HLA-G is also activated, in vivo, in some tumours,17–20 under inflammatory conditions21–23 and in vitro, following exposure to interleukin (IL)-1024,25 and IFNs.26–29
The expression of non-classical HLA class I molecules is, in part, regulated at the transcriptional level.30 Constitutive and inducible expression of HLA class I genes are mainly driven by two modules in the proximal promoters (Fig. 1): the enhancer A/ISRE is the upstream module that mediates transactivation by the NF-κB/rel family of transcription factors and IFNs; the SXY region constitutes the downstream module, which interacts with the same factors as the SXY sequences of HLA class II genes.31 Most of the SXY binding factors have been identified in studies on defects causing deficiencies in HLA class II expression in type II bare lymphocyte syndrome (BLS).32,33 The SXY module comprises the X1, X2 and Y boxes. The X box is composed of the X1 box, which binds RFX factors (RFX5, -AP, -ANK/B)34–37 and of the X2 box, the binding site for X2-BP/ATF/CREB factors.38,39 The Y box is a CCAAT-binding site that is a docking element for NF-Y factors.40 The master control factor, CIITA, is expressed constitutively in antigen-presenting cells (APCs) and is inducible by IFN-γ.41–43 CIITA acts through the enhanceosome complex44 and has been shown to transactivate HLA class I genes.45,46
Figure 1.
Proximal promoter structure of human leucocyte antigen (HLA) class II and class I genes: cis regulatory elements and binding factors within the −200 bp from the transcription start site. The left of the figure represents regulatory sequences (boxes) and the corresponding DNA-binding trans-acting factors within the upstream module of HLA class I promoter. Interferon regulatory factor1 (IRF1) binds to the interferon-responsive element (ISRE). The enhancer A element within classical HLA class I promoters contains two κB sites that bind nuclear factor (NF)-κB/Rel family factors. The right of the figure corresponds to the SXY module composed of S, X1, X2 and Y sequences. RFX (RFX5, RFXAP, RFXB), X2-BP and NF-Y transcription factors co-operatively bind the SXY module of the HLA-DR gene promoter. CIITA is recruited to the enhanceosome and is physically associated with classical HLA class I promoters, where the SXY module is conserved. S and X1 are the only conserved boxes in the HLA-G promoter.
Analysis of HLA-E, -F and -G gene regulatory sequences shows that the HLA-F promoter is the only one to exhibit extensive similarities with those of classical HLA class I genes.30,47 The HLA-E gene promoter differs from the HLA class I gene promoter in the κB and ISRE sites48,49 and in the Y Box, but conserves S, X1 and X2 boxes. S and X1 boxes are the only conserved sequences in the HLA-G gene promoter.47 As expected from sequence analysis, there is now in vitro evidence that HLA-E and HLA-F are induced by CIITA, whereas HLA-G is not.30,46 The putative role of the conserved RFX-binding sequence, X1, in HLA-G was previously investigated in vitro by electrophoretic mobility shift assays (EMSAs) using oligonucleotide probes. This showed the specific binding of RFX and Sp1 factors to the X1 box of HLA-G,50 but direct physical interaction has not yet been evidenced.
In the present study, we analysed the binding of the RFX5 factor to the HLA-E, F and -G gene promoters in situ, and under conditions allowing enhanceosome formation and CIITA recruitment in vitro. In addition, we evaluated the importance of RFX5 and CIITA for non-classical and classical HLA class I gene expression in B-EBV cells and fibroblasts from BLS patients. Our studies provide the first evidence that RFX5 and CIITA are recruited to the HLA-F and HLA-E gene promoters in situ. Our results also argue in favour of a physiological role for RFX5 and CIITA in the enhancement of HLA-E and -F transcription. In contrast, the atypical status of HLA-G gene regulation was reinforced. Finally, we show that the cell-surface expression levels of both non-classical and classical HLA class I molecules may remain unaffected by RFX5 or CIITA deficiency, this varying according to the cell type investigated.
Materials and methods
Cell culture
The JEG-3 and JAR choriocarcinoma cell lines were maintained in Dulbecco's modified Eagle's minimal essential medium (DMEM) containing glutamax-I, and in RPMI-1640 containing glutamax-I, respectively (Life Technologies, Cergy Pontoise, France), supplemented with glucose to 4500 mg/l and 10% heat-inactivated fetal calf serum (FCS) (Sigma Co., Saint-Quentin-Fallavier, France). The following cell lines, Raji (Burkitt's B lymphoma),51 LCL 721.221 [gamma-ray mutated Epstein–Barr virus (EBV)-transformed lymphoblastoid B cell; kindly provided by C. Munz, Germany],52 RJ 2.2.5 (Raji-derived CIITA deficient), RJ 6.4 (RJ 2.2.5 transfected with CIITA),41 SJO (B-EBV cell line obtained from a type III RFX5-deficient BLS patient) and SJO TR RFX5 (SJO transfected with RFX5),34 were maintained in RPMI-1640 containing glutamax-I (Life Technologies) and 10% heat-inactivated FCS. Normal fibroblasts (F11) and RFX5-deficient fibroblasts (F68) were obtained via Dr P. Paul (France). F11 and F68 were grown out from skin biopsies of a healthy donor and a BLS patient defective in RFX5 factor, respectively. Fibroblasts were cultured in Iscove's modified Dulbecco's medium (IMDM) (Life Technologies) in the presence of 10% (vol/vol) heat-inactivated FCS. All cultures were supplemented with 20 µg/ml gentamicin and 0.25 µg/ml fungizone (Life Technologies).
RNA extraction, reverse transcription–polymerase chain reaction (RT–PCR) and Southern blot
Total RNAs were extracted using RNA-WIZ® (Ambion, Austin, TX), according to the manufacturer's instructions. After treatment with DNAse I, RNA purity was confirmed by electrophoresis in a 1.5% agarose denaturing gel. RT–PCR was performed according to procedures validated in the 13th International Histocompatibility Workshop.53 Briefly, cDNAs were prepared from total RNA using oligo(dT)12−18 primers in the presence of MMLV-RT (Life Technologies). HLA-E, -F, -G and classical HLA class I cDNAs were then simultaneously amplified using pan-class I primers (Pan class I forward: 5′-TCCCACTCCATGAGGTATTTC; Pan class I reverse: 5′-TCCAGAAGGCACCACAG).13β-actin was used as an internal semiquantitative control (β-actin forward: 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG; β-actin reverse: 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC). After amplification, PCR products were separated on a 1.8% agarose gel, denatured for 20 min by 0.4 m NaOH, and then vacuum-transferred onto Hybond N+ membrane (Amersham-Pharmacia Biotech, Orsay, France). Successive hybridizations of the membrane were performed with the following [γ-32P]ATP-labelled probes: β-actin (5′-ATCATGTTTGAGACCTTCAACACCCCAGCC), HLA-ER (5′-ATCATTTGACTTTTGCTCGGA), HLA-FR (5′-GGCGTACCCTGTGGTCCACTC), HLA-GR (5′-GGTCTGCAGGTTCATTCTGTC)13,53 and HLA-A (5′-GGAGGACCAGACCCAGGACACG), at 60°. HLA-E, -F and -G radiolabelled probes were chosen to have the same specific activities. Dehybrizations were performed in boiling 0.5% SDS. Hybridized membranes were exposed to a molecular imager (Bio-Rad, Ivry-sur-Seine, France) for quantification. The values obtained for HLA-E, -F and -G and HLA-A signals were normalized to the β-actin signal.
Flow cytometry analysis
The following monoclonal antibodies (mAbs) were used: SV 99-85 (anti-HLA-ABC mAb; kindly provided by Professor S. Ferrone, Roswell Park Cancer Institute, Buffalo, NY, USA); MEM-E/06 (anti-HLA-E/HLA class I mAb; kindly provided by Dr V. Horejsi, Academy of Sciences of the Czech Republic, Videnska, Czech Republic);54 MEM-G/09 (anti-HLA-G mAb, which recognizes HLA-G molecules associated with β2 microglobulin: HLA-G1 at the cell surface and soluble HLA-G5 isoforms; Exbio, Praha, Czech Republic);54 goat anti-mouse phycoerythrin (PE) (Immunotech, Marseille, France); and anti-HLA-DR PE (Immunotech). Cells were analysed using a flow cytometer Epics XL (Beckman Coulter France SA, Roissy, France).
Immunoprecipitation and immunoblotting
Immunoprecipitation of cell-surface HLA molecules was carried out on 3 × 106 cells incubated for 2 hr at 4° with MEM-E0654 or W6/32 (anti-HLA class I heavy chain associated with β2-m, Sigma) antibodies, in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA). After centrifugation for 2 min at 320 g, cells were lysed by incubation on ice for 1 hr in 1 ml of Lysis Buffer containing 0.5% CHAPS (Sigma), 50 mm Tris–HCl pH 7.4 (Invitrogen) and Complete® (Roche, Meylan, France). The lysate was cleared by centrifugation (20 000 g, 30 min, 4°) and incubated for 2 hr at 4° with protein A–sepharose (Sigma). The pellet was collected by brief centrifugation (20 000 g) at 4° and then washed twice in buffer T1 (0.1% CHAPS, 20 mm Tris–HCl, pH 7.5, 150 mm NaCl), three times (5 min each wash with end-over-end agitation) in buffer T2 (10 mm Tris–HCl, pH 8.0, 300 mm NaCl, 0.1% SDS, 0.05% CHAPS) and twice in buffer T4 (0.1% CHAPS, 20 mm Tris–HCl, pH.4). For standard immunoprecipitations, 6 × 106 cells were lysed on ice in 1 ml of Lysis Buffer, as described above, for 1 hr, and the lysate was cleared by centrifugation (20 000 g, 30 min, 4°). The lysate was incubated with MEM-E06 antibody54 for 2 hr at 4° with end-over-end agitation. Protein A–sepharose was added for 2 hr under the same experimental conditions. Washes were performed as described above. Proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) (12% gel) and transferred to Nylon membrane (Hybond C-extra; Amersham), according to the supplier's instructions.
Membranes were first saturated in PBS containing 0.2% Tween-20 (Sigma) and 5% non-fat dried milk, for 30 min at room temperature with gentle agitation, and then incubated in PBS containing 0.2% Tween-20 and 2.5% non-fat dried milk for 1 hr at room temperature with MEM-E/02 antibody (specific anti-HLA-E mAb, kindly provided by Dr V. Horejsi),54 used at a 1: 10 000 dilution. Membranes were washed three times (10 min each wash) at room temperature, in PBS containing 0.2% Tween-20, with agitation. Incubation with goat anti-mouse immunoglobulin G (IgG) horseradish peroxidase (HRP) conjugate (Sigma), diluted to 1: 10 000, in PBS containing 0.2% Tween-20 and 2.5% non-fat dried milk for 30 min at room temperature with gentle agitation was followed by three washes, as described above. Bound antibody conjugate was revealed by chemoluminescence, performed with ECL+ (Amersham), and membranes were exposed to autoradiographic film (Biomax®; Kodak, New York, NY).
Protein extracts and promoter pull-down assay
Protein extracts and promoter pull-down assays were performed as previously described.44 Briefly, HLA-E, HLA-F and HLA-G promoter templates were isolated and biotinylated at the 5′ end of the upper strand by PCR using the following primer set: HLAE pdF biot (Biot-5′-GTGCAGAGATACCGAAACCT)/HLAE pdR (5′-GAGAACTTCTTGAGTCCGGA) for the HLA-E promoter sequence; HLAF pdF biot (Biot-5′-TGGAAGGCTCAGTATTGAGA)/HLAF pdR (5′-TGCGTGGGACTTTAGAACCT) for the HLA-F promoter sequence; and HLA-G pdF biot (Biot-5′-CCCGCGTTGGGGATTCTCTC)/HLA-G pdR (5′-ATGAGTCCGGGTGGGTGAGC) for the HLA-G promoter sequence.
The obtained fragments (≈ 200 bp) extended from the TC/ATAAA box and contained the ISRE/enhancer A and the SXY modules. Competitor DNAs were obtained by PCR using non-biotinylated primers matching the same sequence. Promoter templates were coupled to streptavidin-coated magnetic beads (Promega, Charbonnières, France) and incubated with protein extracts in the presence or absence of a 20-fold excess of competitor fragment. Protein–DNA complexes were selected magnetically and analysed by Western blotting using anti-RFX5 (sc 10667; Santa-Cruz Biotechnology, Santa Cruz, CA), donkey anti-goat secondary antibody (sc 2020; Santa-Cruz Biotechnology) and ECL plus (Amersham-Pharmacia Biotech).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitations (ChIP) were performed as described previously,39,44 with several modifications. Cells were treated for 10 min at 37° with 1% formaldehyde. After one wash with PBS, cells were lysed in buffer [5 mm PIPES, 85 mm KCl, 0.5% Nonidet P-40 (NP-40)] supplemented with Complete™ protease inhibitors (Boehringer Mannheim, Paris, France), by incubation for 5 min on ice. Nuclei were pelleted and lysed in buffer (50 mm Tris–HCl, pH 8.1, 10 mm EDTA, 1% SDS) supplemented with Complete™ protease inhibitors (Boehringer Mannheim). Cross-linked chromatin was sheared into 200-bp fragments by sonication, cleared by centrifugation at 15 000 g for 10 min and stored at −80°. Chromatin supernatants were diluted (1: 10) in chIP buffer (1.2 mm EDTA, 16.7 mm Tris–HCl, pH 8.1, 167 mm NaCl, 1.1% Triton-X-100, 0.01% SDS) supplemented with 20 µg/ml sonicated salmon-sperm DNA, 20 µg/ml yeast tRNA and 1 mg/ml BSA. After preclearing the supernatants by incubation (on a rotator at 4° for 30 min) with protein A–sepharose beads (Sigma), the supernatants were incubated for 2 hr with 5 µl of rabbit anti-RFX5c serum (purchased from two origins, as previously described,44 and kindly provided by Dr Jeremy Boss), 5 µl of rabbit anti-CIITA serum44 or 5 µl of normal rabbit serum (Dako, Glostrup, Denmark), in the presence of protein A–Sepharose beads. The beads were then washed three times as follows: twice with TSE 500 buffer (0.1% SDS, 1% Triton-X-100, 2 mm EDTA, 20 mm Tris–HCl, pH 8.1, 500 mm NaCl); once with LiCl buffer (100 mm Tris-HCl, pH 8.1, 500 mm LiCl, 1% NP-40, 1% deoxycholate); and twice with 10 mmol/l TRIS, HCl pH 8, 1 mmol/l EDTA (TE). Immune complexes were eluted twice with elution buffer (50 mm NaHCO3, 1% SDS) for 10 min at room temperature. Crosslinks were reversed by the addition of NaCl, to a final concentration of 1 m, followed by incubation at 65° for 6 hr. DNA was precipitated with 100% ethanol, resuspended in proteinase K buffer (10 mm Tris–HCl, pH 7.5, 5 mm EDTA, 0.25% SDS) and then digested with proteinase K (100 µg/ml, Sigma) for 2 hr at 45°. After extraction with phenol–chloroform (24/2: vol/vol) and chloroform, DNA was precipitated by 100% ethanol in the presence of 5 µg of yeast tRNA and 0.5 m NaCl, washed with 75% ethanol, and then resuspended in 30 µl of 1× TE. The immunoprecipitated DNA and the input chromatin were analysed by PCR (40 cycles of: 30 seconds at 94°; 30 seconds at 72°; 30 seconds at 59°) using the promoter-specific primer pairs shown in Table 1. The specificity of chromatin immunoprecipitation was assessed by PCR using primers located in the HLA-E, -F and -G genes, outside the promoter regions (for HLA-E, HLA-E int6F/HLA-E ex8R; for HLA-F, HLA-F int7 F/HLAF utr R; for HLA-G, G.1089F/G.1252 R) (see Table 1).
Table 1.
Primers used in chromatin immunoprecipitation assays
| Target | Primer name | Primer sequence |
|---|---|---|
| HLA-E promoter | HLA-E −183 F | 5′-AGTTTCCCGTTCCTCTCGTAAC |
| HLA-E −84 R | 5′-CTCTAGAAACCCGACACCCAT | |
| HLA-E exon 8 | HLA-E int6 F | 5′-TGTTCAGAGTGTCATCACTTACCG |
| HLA-E ex8 R | 5′-TGTGCATCTCAGTCGCACAC | |
| HLA-F promoter | HLA-F −187 F | 5′-GTTTCTCTTTCTCTCCCAACCC |
| HLA-F −101 R | 5′-CACTGATTGGCTTCTCTAGAAACG | |
| HLA-F intron 7 | HLA-F int7 F | 5′-ACCTCTCACTGTGACTGATACGAAT |
| HLA-F utr R | 5′-CAAGTGCAATTCTGCTACATTGA | |
| HLA-G promoter | HLA-G −181 F | 5′-TGGGGATTCTCTCCTCCTTCCTCCT |
| HLA-G +4R | 5′-AGAGGGTTCGGGGCGCCATGACCA | |
| HLA-G 3′-UT | G.1089 F | 5′-CCCTTTGTGACTTCAAGAAC |
| G.1252 R | 5′-AAGTTATAGCTCAGTGGACC |
HLA, human leucocyte antigen.
HLA-F-specific real-time amplifications were performed using a kit for quantitative PCR (Eurogentec, Seraing, Belgium) containing the intercaling fluorescent dye, SYBR Green, as previously described,55 with the PCR primers used in the standard PCR (Table 1).
Results
Differential cell-surface expression of non-classical HLA class I genes in the JEG-3 choriocarcinoma cell line and the B-cell lines Raji and LCL 721.221
As a prerequisite to the precise investigation of mechanisms involved in the regulation of non-classical HLA class I molecule expression, we re-examined HLA cell-surface expression in standard cell lines, using specific antibodies to HLA-DR, HLA-G, HLA-E and classical HLA class I molecules (Fig. 2). The HLA-G-positive JEG-3 choriocarcinomal cell line was compared with the Raji and LCL 721.221 B-cell lines that express HLA-DR antigens and thus have a functional MHC class II enhanceosome. In agreement with previous work, flow cytometry analysis using the MEMG/09 mAb, which recognizes HLA-G molecules associated with β2 microglobulin (HLA-G1 and HLA-G5), showed no significant HLA-G expression on the surface of cells from either Raji or LCL 721.221 B-cell lines.57 The levels of classical HLA class I molecules, revealed by the anti-HLA-ABC SV 99-85 mAb, were higher on the surface of Raji cells than on JEG-3 and LCL 721.221 cells. It is of note that low levels of class I antigens on the surface of LCL 721.221 cells were also previously reported after staining with the HLA class I-specific mAb, W6/32.58,59 Despite unclear identification of HLA class I specificities, immunoprecipitates of 35S-methionine-labelled cell extracts of LCL 721.221 revealed very small amounts of the 40 000- and 41 000 molecular weight (MW) class I α-chain.58 The anti-HLA-E mAb, MEM-E/06, showed cell-surface HLA-E expression in Raji cells. In contrast, HLA-E expression was moderate for JEG-3 and absent in LCL 721.221.9 Owing to possible MEM-E/06 cross-reactivity with classical HLA class specificities, HLA-E expression in the Raji cell line was confirmed using additional methodological approaches (Fig. 3a). First, cell-surface HLA-E molecules were complexed with MEM-E/06 mAb before cell lysis and the immunocomplexes were analysed by Western blot using the highly HLA-E-specific MEM-E/02 antibody (Antibody Workshop, HLA-G conference, Paris July 7–9, 2003). The HLA-E band was observed at 43 000 MW in the Raji cell line, but was absent in the LCL 721.221 cell line. Lower HLA-E expression in the JEG-3 cell line, in comparison with the Raji cell line, was confirmed by direct immunoprecipitation experiments with MEM-E/06 mAb and specific staining with MEM-E/02 mAb (Fig. 3b). Because no anti-HLA-F mAb was available, we did not investigate the expression of this protein. However, recent data have reported that HLA-F expression is intracellular only, and that intracellular HLA-F can be detected in LCL 721.221, but not in JEG-3.11
Figure 2.
Flow cytometry analysis of human leucocyte antigen (HLA) class I and class II expression at the surface of the JEG-3 choriocarcinoma cell line, and the Raji and LCL 721.221 B-cell lines. SV 99-85, MEM-E/06, MEM-G/09 and anti-HLA-DR are monoclonal antibodies directed against HLA-ABC, HLA-E/HLA class I, HLA-G and HLA-DR molecules, respectively. HLA-G expression is confined to the trophoblast-derived cell line, JEG-3. Althought we observed a minimal fluorescence intensity increase compared with the control, transcriptional analysis in Fig. 4 indicates that this shift for HLA-G in LCL 721.221 and Raji cells is not significant. HLA-E/HLA class I molecules are expressed at a low level on the cell surface of JEG-3 (HLA-C56) cells, and only low levels are detected on the surface of LCL 721.221 cells.58,59 HLA-E/HLA class I molecules are highly expressed by the Raji cell line (see also Fig. 3) and HLA class II antigens are detected in B-cell lines only.
Figure 3.
Immunoprecipitation and immunoblotting analysis of human leucocyte antigen (HLA)-E expression on the cell surface of Raji, LCL 721.221 and JEG-3 cell lines. Western blot analysis showing the reaction pattern of the HLA-E-specific MEM-E/02 monoclonal antibody (mAb) to denatured HLA-E molecules immunoprecipited with MEM-E/06 antibody (IP). MEM-E/06 mAb was complexed with HLA molecules either (a) before lysis of Raji and LCL 721.221 cells or (b) after lysis of Raji and JEG-3 cells. *The heavy chain of MEM-E/06 mAb. HLA-E molecules were not detected in supernatant (SN) used as a negative control in (b).
Differential transcription levels of the non-classical HLA class I gene in the JEG-3 choriocarcinoma cell line and B-cell lines Raji and LCL 721.221
Transcription levels of HLA-E, -F and -G were evaluated, according to the HLA workshop procedure,53 by semiquantitative RT–PCR using validated pan-class I primers and β-actin as an internal control. As described above, in the Materials and methods, the PCR products thus obtained were hybridized (according to the workshop procedure)53 with HLA-E, HLA-F and HLA-G oligonucleotide probes exhibiting the same specific activity, and the β-actin oligonucleotide probe, successively (Fig. 4). The specificity of hybridization was assessed, using the same procedure, on the choriocarcinomal cell line, JAR (Fig. 4). The JAR cell line was used as a control because, in this trophoblast-derived cell line, the non-classical MHC genes are repressed by DNA methylation, with the exception of the HLA-E gene.60 The JAR hybridization pattern is therefore restricted to HLA-E transcripts.
Figure 4.
Steady-state levels of human leucocyte antigen (HLA)-E, -F and -G transcripts in JEG-3, JAR, Raji, LCL 721.221 and B-BLS cell lines with RFX5 or CIITA deficiencies. (a) Representative results of a Southern blot of reverse transcription–polymerase chain reaction (RT–PCR) products from JEG-3, LCL 721.221, JAR, Raji, RJ 2.2.5 (CIITA–), RJ 6.4 (CIITA+), SJO (RFX5-) and SJO TR (RFX5+) cell lines, obtained by amplification with pan-class primers and hybridized with HLA-E (ER) HLA-F (FR) or HLA-G (GR) 32P-labelled oligonucleotide probes. β-actin was used as an internal semiquantitative control. HLA-G expression is repressed in B-cell lines. In the JAR cell line, both HLA-G and HLA-F are repressed by promoter methylation and validate the specifity of probes. (b) Comparative analysis of HLA-E, HLA-F and HLA-G transcription levels in the eight cell lines. Signals were quantified using a molecular imager and normalized using the β-actin signal (two separate experiments). The value 1 was arbitrarily affected to the amounts to the amounts of HLA-E, HLA-F and HLA-G transcripts in JEG-3. The HLA-E and HLA-F transcript levels are reduced in BLS cell lines SJO and RJ 2.2.5 in comparison with the same cells transfected with RFX5 (SJO TR RFX5) and CIITA (RJ 6.4), respectively.
In addition to the absence of HLA-G gene expression in lymphoid cell lines, the steady-state levels of HLA-F transcripts were higher than in JEG-3 (Fig. 4). Comparison of the amounts of HLA-E transcripts with those of the JAR cell line indicated that the cell types investigated expressed significant levels of HLA-E transcripts, with higher levels found in LCL 721.221 than in JEG-3. Altogether, these results agree with previous reports9,11,13,53 and confirm the tissue restriction of the HLA-G gene transcription. Furthermore, they argue in favour of shared regulatory mechanisms for HLA-E and HLA-F gene expression. Some of these may act via the SXY module, as gene and protein expressions are favoured for HLA-E and HLA-F in cells expressing HLA class II molecules.
RFX5 represents part of the assembled HLA-E and -F enhanceosomes in vitro
We previously reported the in vitro ability of RFX factors to bind the conserved X1 box (common to non-classical HLA class I genes) using a probe limited to the X1X2 promoter region.50 These experimental conditions did not allow the formation of multiprotein complexes. This prompted us to use a promoter pull-down assay, with 5′ biotinylated HLA-E, HLA-F and HLA-G promoter templates that encompassed enhancerA/ISRE and SXY modules. These promoters were bound to streptavidin-coated magnetic beads and were incubated with protein extracts from JEG-3 and LCL 721.221 cell lines, with or without an excess of non-biotinylated DNA competitor. DNA–protein complexes were then magnetically isolated, and the bound proteins analysed by immunoblotting with antibody against RFX5 factor, the RFX subunit that contains a DNA-binding domain.
This procedure demonstrated that, despite the presence of RFX5 factor in JEG-3 nuclear extracts, its binding was restricted to the HLA-E promoter (Fig. 5). When the incubations were performed with nuclear extracts from the LCL 721.221 cell line, binding was observed with both HLA-E and HLA-F promoters, indicating that RFX5 may play a role in the activation of transcription of these genes in B cells (Fig. 5). The HLA-G promoter never recruited RFX5 factor. Thus, RFX5 does not participate in the HLA-G transcription activation observed in JEG-3.
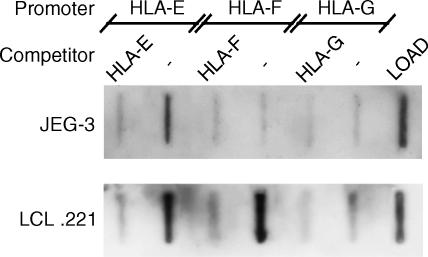
Figure 5.
RFX5 is a component of human leucocyte antigen (HLA)-E and HLA-F enhanceosomes and is not part of the HLA-G gene transcriptional machinery. Promoter pull-down assays were performed with HLA-E, HLA-F and HLA-G promoter fragments containing the C/ATAAA box, the ISRE/enhancer A and the SXY module regions. Competitors were added at 20-fold excess. The term ‘LOAD’ refers to a protein extract from JEG-3 and LCL 721.221 cell lines, used as a positive control for the presence of RFX5 factor in these cells. Immunoblotting using anti-RFX5 showed that the HLA-G promoter does not associate with RFX5 and that the binding of RFX5 to the HLA-F promoter is cell specific. RFX5 bound to the HLA-E promoter in both cell lines.
In B-cell lines, RFX5 and CIITA are recruited to HLA-E and HLA-F promoters in situ
To determine whether the in vitro analyses were a true reflection of the situation in vivo, we performed chromatin immunoprecipitation assays that targeted the RFX5 factor. Chromatin was prepared from choriocarcinoma JEG-3 cells, and from Raji and LCL 721.221 B cells that had previously been fixed with formaldehyde. After shearing the DNA, immunoprecipitations were performed with a polyclonal anti-RFX5 and non-specific antisera. DNA fragments from proteinase A sepharase selected complexes were purified and analysed by PCR for the presence of DNA corresponding to HLA-E, HLA-F or HLA-G promoters. Specificity of the assay was confirmed by amplifying gene regions located outside the promoters. As expected from promoter pull-down assays, RFX5 was physically associated with the HLA-E promoter in all cell types, while it was recruited to the HLA-F promoter only in B-cell lines (Fig. 6a). The lack of RFX5 binding to the HLA-G gene promoter observed in vitro was also confirmed in situ.
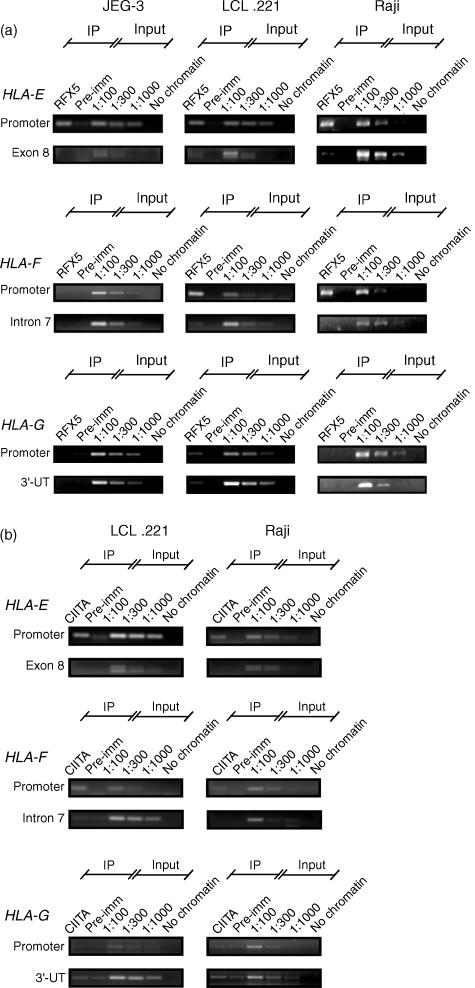
Figure 6.
Human leucocyte antigen (HLA)-E and HLA-F promoters bind RFX5 and may recruit CIITA in situ. Chromatin immunoprecipitations performed with (a) anti-RFX5 on JEG-3, LCL 721.221 and Raji cells or (b) anti-CIITA on LCL 721.221 and Raji cells (CIITA is repressed in JEG-3). Pre-imm, preimmune serum for control of reaction specificity; Input, dilutions of input chromatin used as polymerase chain reaction (PCR) controls. A sample without chromatin was included to check for PCR contaminations. Blot labels: Promoter, PCR reactions performed with primer pairs specific for the SXY box of HLA-E, HLA-F or HLA-G promoters; exon 8, intron 7 and 3′-UT, primer pairs designed to visualize non-specific chromatin immunoprecipitations. These primers amplify a DNA fragment that encompasses part of the exon 8 of HLA-E, part of the intron 7 of HLA-F, and part of the 3′ untranslated region of HLA-G, respectively. Note that the RFX5-immunoprecipitated chromatin from LCL 721.221 is amplified using the HLA-G primer targeting SXY and 3′-UT regions and thus should be considered as non-specific. RFX5 binding to the HLA-F promoter is restricted to B-cell lines, whereas its binding to the HLA-E promoter is observed in all cells. CIITA is constitutively expressed in B-cell lines and is recruited to both HLA-E and HLA-F enhanceosomes. (c) Chromatin immunoprecipitations performed with anti-RFX5 (RFX) and anti-CIITA (CIITA) on the CIITA-positive Raji cell line and CIITA-negative RJ 2.2.5 cell line (two experiments). Real-time PCR was performed to target HLA-F promoter and Intron 7 as a negative control. PI, preimmune serum. Note that the binding of RFX5 to the HLA-F promoter is reduced when CIITA is not expressed.
In parallel, we performed chromatin immunoprecipitation assays to analyse the binding of CIITA to the non-classical HLA class I promoters in B-cell lines (Fig. 6b). Both HLA-E and HLA-F promoters were immunoprecipitated with anti-CIITA, suggesting that CIITA is constitutively recruited to these promoters in situ.
Because of the absence of RFX5 binding to the HLA-F promoter in CIITA-negative JEG-3, we further investigated whether, in the absence of CIITA, formation of the MHC enhanceosome occurs at the HLA-F promoter in situ. Both RFX5 and CIITA binding to the HLA-F promoter were investigated by chromatin immunoprecipitation assays (two independent experiments) of the CIITA-deficient cell line, RJ 2.2.5, in comparison with the CIITA-positive Raji cell line. Real-time PCR, carried out to target either the HLA-F gene promoter or the HLA-F intron 7, indicated that the recruitment of RFX5 is favoured by the presence of CIITA (Fig. 6c).
RFX-5 deficiency induces the down-regulation of HLA class I expression in a cell-type-specific manner
To better evaluate the impact of RFX5 deficiency on the expression of HLA-E, HLA-F and classical HLA class I genes, we compared the SJO B-cell line from RFX5-deficient BLS patients (no MHC class II expression; Fig. 7a) with the SJO B-cell line that is stably transfected with RFX5 (recovery of MHC class II expression; Fig. 7a). In the absence of RFX5, cell-surface expression levels of HLA-E/HLA class I, as revealed by MEM-E/06 mAb, did not vary significantly (Fig. 7a). Similarly, classical HLA class I expression, as revealed by SV 99-85 mAb, was not affected, suggesting that RFX5 binding is not crucial for high levels of HLA class I cell-surface expression (Fig. 7a). These results were reproduced using the CIITA-deficient cell line, RJ 2.2.5, and the RJ 6.4 cell line, which is stably transfected with CIITA. In this case, no significant changes in the cell-surface expression of HLA-E/classical HLA class I molecules were observed, despite a clear increase in the number of MHC class II molecules in CIITA-transfected cells. However, RT–PCR analysis revealed a two- to threefold increase in the steady-state levels of HLA-E, and a two- to eightfold enhancement of HLA-F transcription levels in the presence of RFX5 and CIITA, respectively (Fig. 4). This confirmed the active participation of these factors in the transcription regulation of HLA-E and HLA-F genes.
Figure 7.
The impact of RFX5 and CIITA deficiencies on the cell-surface expression of human leucocyte antigen HLA-E/classical HLA class I depends on the cell type. (a) Flow cytometry analysis of B cells from bare lymphocyte syndrome (BLS) patients: CIITA-deficient RJ 2.2.5 versus CIITA-positive RJ 6.4 cells and RFX5-deficient SJO cells versus SJO cells transfected with RFX5, were analysed for HLA-ABC (SV 99-85 monoclonal antibody), HLA-E/HLA class I (MEM-E/06), HLA-G (MEM-G/09) and HLA-DR (HLA-DR) cell-surface expression. The cell-surface expression of HLA-E/HLA-ABC are not affected by the presence or absence of RFX5 and CIITA. (b) Flow cytometry analysis of fibroblasts from BLS patients: RFX5-deficient fibroblasts F68 (RFX−) versus normal fibroblasts F11 (RFX+) were analysed for the cell-surface expression of HLA-E/HLA-ABC and HLA-G. A lack of RFX5 factor generates a decrease of HLA-E/HLA-ABC protein expression. (c) Immunoprecipitation and Western blot analysis of HLA-E expression at the cell surface of normal fibroblasts. MEM-E/06 monoclonal antibody was complexed with HLA molecules before fibroblast cell lysis and revealed with MEM-E/02 antibody. *The heavy chain of MEM-E/06 monoclonal antibody.
To investigate whether the HLA class I activation pathway was dependent on cell type, we analysed a possible modulation of HLA cell-surface expression in normal fibroblasts (F11) versus fibroblasts from RFX-5-deficient BLS (F68). In contrast to B-BLS cell lines, a clear decrease in the level of expression of HLA molecules was observed, both with SV 99-85 and MEM-E/06 antibodies (Fig. 7b), which may detect HLA-E molecules because they were demonstrated, by immunoprecipitation and immunoblotting, to be present on the cell surface of normal cells (Fig. 7c). The decrease of both non-classical and HLA class I expression was also confirmed at the transcriptional level (Fig. 8). Notably, it is apparent that the down-regulation of classical HLA class I transcriptional activity (HLA-A) in RFX-deficient cell lines is more important in fibroblast cells than in the B-EBV cell line.
Figure 8.
Human leucocyte antigen (HLA)-A and HLA-E transcription in RFX-deficient B-EBV and fibroblast cell lines. Semiquantification of reverse transcription–polymerase chain reaction (RT–PCR) products obtained by amplification with pan-class I primers and hybridized with HLA-A, HLA-E (ER) or HLA-F (FR) 32P-labelled oligonucleotide probes. β-actin was used as an internal semiquantitative control. Results are relative to β-actin and the value 1 was arbitrarily affected to the amounts of HLA-A, HLA-E and HLA-F transcripts in undefective cells (SJO TR RFX, F RFX+) to evaluate the effect of RFX deficiency in B-EBV (SJO) and fibroblast cell lines (F RFX−).
Discussion
The present study demonstrates, for the first time, that in situ, RFX-5 and CIITA factors are recruited to the promoter of the non-classical HLA class I genes HLA-E and HLA-F, both of which contain an SXY module, but not to the promoter of HLA-G, which has only intact S and X1 boxes. This finding may be related to the findings of previous reports, using transient transfection of luciferase reporter constructs containing HLA-E, HLA-F or HLA-G promoters, which showed that only HLA-E and HLA-F genes were transactivated in vitro by CIITA in B- and tera-2 cell lines.30,46 Thus, in the light of recently published work demonstrating that the conserved SXY module of classical HLA class I genes interacts with the same proteins as the SXY module of HLA class II genes, our results support the hypothesis that common mechanisms, which act via the SXY module, may be involved in the expression of both HLA class I and class II proteins.
The CIITA recruitment has been shown to require multiple protein–protein interactions with SXY module binding factors.44 Thus, the binding that we observed of CIITA to HLA-E and HLA-F is strongly indicative of the docking of the whole RFX-X2-BP/ATF-NFY multiprotein complex to the promoters of these genes. CIITA may contribute to high HLA-E and HLA-F transcription levels in B-cell lines and to the down-regulation of their transcription in RFX- or CIITA-deficient BLS B-cell lines. Concerning HLA-G, it was proposed that differences in the X2 and Y boxes of the gene promoter do not allow higher-order complex formation, thus preventing CIITA recruitment.30
Our work demonstrates that, despite the presence of an intact HLA-G X1 box with binding capacity in vitro,50 this box does not bind RFX5 in situ. The mechanism responsible for the lack of RFX5 binding to the HLA-G promoter may not be linked to the methylation of the X box, as promoter pull-down assays performed with PCR-amplified promoter templates yielded the same results as chromatin immunoprecipitation experiments. Chromatin structure and/or competition between RFX5 and other X-box binding factors are attractive hypotheses to investigate. Indeed, it has been previously shown that glucocorticoids may negatively regulate the expression of the MHC class II gene, IAβ, by interfering with the docking of the X-box binding protein.61
Tight control of the transcription of non-classical HLA class I genes is observed in the JEG-3 cell line. Indeed, we show here that the steady-state levels of HLA-E and HLA-F transcripts are lower in this cell line than in B-cell lines. As demonstrated for MHC class II antigens, this may be related, in part, to the absence of CIITA in trophoblasts,62 owing to hypermethylation of the MHC2TA gene promoter IV.63,64 The transcriptional down-regulation of HLA-E and HLA-F, observed in CIITA-deficient BLS B cells, argues in favour of this hypothesis. Furthermore, it was proposed that additional pathways may be critical to the control of transcription of classical HLA class I genes in JEG-3. In particular, it has been suggested that the reduced expression of RFX5 in this cell line may prevent the assembly of the multiprotein complex to the XY promoter.65 In agreement with this hypothesis, we observed no binding of RFX5 to the HLA-F promoter. However, we did observe RFX5 binding to the HLA-E promoter in JEG-3, both in vitro and in situ. This supports the notion that mechanisms other than RFX5 expression levels are involved. For example, one hypothesis may be promoter occupancy by a cell-specific competitor factor, which may constitute an HLA-F repressosome.66 Because the X2 box of HLA-F differs from those of the other non-classical HLA class I genes, another possible explanation is that CIITA may exert a stabilization effect on the RFX–X2-BP complex, as described for MHC class II genes.44 This hypothesis is supported by the chromatin immunoprecipitation assays performed with CIITA-deficient EBV-transformed B cells (RJ 2.2.5), indicating that RFX5 recruitment to the HLA-F promoter in situ is reduced in the absence of CIITA (Fig. 6c). Interestingly, despite the HLA-F promoter being very similar to that of classical HLA class I molecules, HLA-F protein expression is not ubiquitous and was shown to be mostly confined to B cells constitutively expressing CIITA.11 The above observation, and the in situ demonstration of an association of CIITA with the HLA-F promoter in the B-cell lines LCL 721.221 and Raji, suggests a close relationship between HLA-F protein expression and CIITA. It is therefore tempting to postulate that CIITA might be a master regulator of HLA-F expression as it is of MHC class II molecules.
Unlike that of HLA-E and -F, HLA-G activation does not involve the RFX5 factor. Moreover, HLA-G transcription activity is absent in B cells expressing RFX factors, confirming that the HLA-G gene-induction pathways are radically different67 from those of HLA-E and -F. The possible involvement of a locus control region (LCR) has been previously put forth68,69 and was shown to bind factors of the CREB/ATF family.70 The importance of epigenetic mechanisms, such as methylation and chromatin structure, is also emerging60,71 and may explain that CREB/ATF factors do not account for the tissue-specific expression of HLA-G.70
Moreover, although HLA-E is expressed at the transcriptional level in basically all tissues, we did not find any correlation between the level of HLA-E gene transcription and the modulation of HLA-E cell-surface protein expression. However, low levels of HLA-E transcripts in comparison with JAR (Fig. 4) might indicate that MEM-E/06 staining is mostly caused by other HLA class I genes in RJ 6.4 and SJO TR RFX5 cells, a hypothesis that cannot be excluded. Nonetheless, this is clear in Raji and LCL 721.221 cell lines, both of which exhibit HLA-E transcripts, with HLA-E protein expression only detected at the cell surface of Raji (Figs 3 and 4). Therefore, post-transcriptional regulatory mechanisms, such as the HLA-E cell-surface stabilization by HLA class I leader peptides,9 might play a more important role than the enhancement of transcription levels. In agreement with this interpretation, we showed that in B-BLS cells, the lack of RFX5 (SJO cells) and of CIITA (RJ 2.2.5 cells) led to a decrease in the steady-state levels of HLA-E mRNA, but not significant changes of HLA-E/HLA class I cell-surface expression. Whether this decrease affected HLA-F protein expression remains to be determined.
HLA-A, -B and -C protein expression was affected by neither RFX5 nor CIITA deficiencies in BLS B-cell lines. In agreement with our data, a recent publication cited unpublished results showing that CIITA-deficient EBV-transformed B cells did not show any change in MHC class I expression.46 The authors hypothesized a compensation for CIITA deficiency by the EBV-related high expression levels of NF-κB- and IFN regulatory factors. The situation is different when RFX5-deficient fibroblasts are used, as, in these cells, we found a lack of both HLA-E and HLA class I protein expression, which is also observed at the transcriptional level. The fact that HLA-A transcriptional activity is strongly down-regulated in RFX-deficient fibroblast cells in comparison with B-EBV cells reinforces the hypothesis of the existence of a compensatory mechanism in B-EBV cells. Because HLA-E and -F promoters exhibit divergences in enhancer A and/or ISRE sites, one suspects that they might be less dependent upon EBV-related factors. This could account for the smaller variation in HLA-E and HLA-F transcription between fibroblast and B-EBV cell lines with RFX deficiencies (Fig. 8). Regardless of the putative compensatory mechanism that occurs in B-EBV cell lines, these data indicate that RFX5 and CIITA factors are not so crucial for HLA class I protein expression. These data also show that the degree of involvement of RFX5 and CIITA in the regulation of HLA-E and HLA class I protein expression depends on the cell type. Thus, in the absence of HLA-G, HLA-E cell-surface expression is clearly dependent on the expression of other HLA class I proteins.
In conclusion, RFX5 and CIITA participate in the regulation of HLA-E and HLA-F gene transcription. RFX5 was previously identified as an important component of the HLA class I enhanceosome. Our work suggests that, for this reason, the absence of RFX5 may induce a deficit in HLA class I protein in some cell types. This raises the issue of pregnancies being successful when the fetus present a bare lymphocyte syndrome owing to RFX5 deficiency. Indeed, in the absence of compensatory mechanisms, MHC class I expression, including HLA-E and HLA-C molecules, would be very reduced or absent. We show that HLA-G gene regulation is not dependent on this factor, which thus may be expressed in trophoblasts from these patients and may permit HLA-E expression. HLA-G then appears to be a fundamental molecule for the protection of the fetus against maternal immunity.
Acknowledgments
We are grateful to Dr Jean Villard for SJO and SJO-TR RFX5 B cells and to Dr Pascale Paul for F11 and F68 fibroblasts. We thank Professor Soldano Ferrone, Dr Vàclav Horejsi, Professor Jeremy Boss and Dr Guy Beresford for antibodies. We also thank Madelaine Zufferey and Celine Marcou for excellent technical assistance. We very much appreciate the helpful advice of, and discussions with, Dr Nathalie Rouas-Freiss, Dr Joël le Maoult and Catherine Menier. This work was supported by the Commissariat à l'Energie Atomique. Work carried out in the laboratory of Dr W. Reith was supported by the Swiss National Science Foundation, the Gabriella Giorgi-Cavaglieri Foundation, the Ernst and Lucie Schmidheiny Foundation, the Swiss Multiple Sclerosis Foundation, and NovImmune SA. Ph Rousseau is a recipient of a grant from the Ministère de L'Education Nationale de la Recherche et de la Technologie.
References
- 1.Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr Opin Immunol. 1999;11:100–8. doi: 10.1016/s0952-7915(99)80018-1. [DOI] [PubMed] [Google Scholar]
- 2.Carosella ED, Paul P, Moreau P, Rouas-Freiss N. HLA-G and HLA-E: fundamental and pathophysiological aspects. Immunol Today. 2000;21:532–4. [PubMed] [Google Scholar]
- 3.Forte P, Pazmany L, Matter-Reissmann UB, Stussi G, Schneider MK, Seebach JD. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167:6002–8. doi: 10.4049/jimmunol.167.10.6002. [DOI] [PubMed] [Google Scholar]
- 4.Carosella ED, Moreau P, Aractingi S, Rouas-Freiss N. HLA-G: a shield against inflammatory aggression. Trends Immunol. 2001;22:553–5. doi: 10.1016/s1471-4906(01)02007-5. [DOI] [PubMed] [Google Scholar]
- 5.Lepin EJ, Bastin JM, Allan DS, et al. Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur J Immunol. 2000;30:3552–61. doi: 10.1002/1521-4141(200012)30:12<3552::AID-IMMU3552>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Wei XH, Orr HT. Differential expression of HLA-E, HLA-F, and HLA-G transcripts in human tissue. Hum Immunol. 1990;29:131–42. doi: 10.1016/0198-8859(90)90076-2. [DOI] [PubMed] [Google Scholar]
- 7.Ulbrecht M, Honka T, Person S, Johnson JP, Weiss EH. The HLA-E gene encodes two differentially regulated transcripts and a cell surface protein. J Immunol. 1992;149:2945–53. [PubMed] [Google Scholar]
- 8.Gustafson KS, Ginder GD. Interferon-gamma induction of the human leukocyte antigen-E gene is mediated through binding of a complex containing STAT1alpha to a distinct interferon-gamma-responsive element. J Biol Chem. 1996;271:20035–46. doi: 10.1074/jbc.271.33.20035. [DOI] [PubMed] [Google Scholar]
- 9.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. 1998;160:4951–60. [PubMed] [Google Scholar]
- 10.Llano M, Lee N, Navarro F, Garcia P, Albar JP, Geraghty DE, Lopez-Botet M. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur J Immunol. 1998;28:2854–63. doi: 10.1002/(SICI)1521-4141(199809)28:09<2854::AID-IMMU2854>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Wainwright SD, Biro PA, Holmes CH. HLA-F is a predominantly empty, intracellular, TAP-associated MHC class Ib protein with a restricted expression pattern. J Immunol. 2000;164:319–28. doi: 10.4049/jimmunol.164.1.319. [DOI] [PubMed] [Google Scholar]
- 12.McMaster MT, Librach CL, Zhou Y, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154:3771–8. [PubMed] [Google Scholar]
- 13.Houlihan JM, Biro PA, Harper HM, Jenkinson HJ, Holmes CH. The human amnion is a site of MHC class Ib expression: evidence for the expression of HLA-E and HLA-G. J Immunol. 1995;154:5665–74. [PubMed] [Google Scholar]
- 14.Hammer A, Hutter H, Blaschitz A, Mahnert W, Hartmann M, Uchanska-Ziegler B, Ziegler A, Dohr G. Amnion epithelial cells, in contrast to trophoblast cells, express all classical HLA class I molecules together with HLA-G. Am J Reprod Immunol. 1997;37:161–71. doi: 10.1111/j.1600-0897.1997.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 15.Crisa L, McMaster MT, Ishii JK, Fisher SJ, Salomon DR. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J Exp Med. 1997;186:289–98. doi: 10.1084/jem.186.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaschitz A, Lenfant F, Mallet V, Hartmann M, Bensussan A, Geraghty DE, Le Bouteiller P, Dohr G. Endothelial cells in chorionic fetal vessels of first trimester placenta express HLA-G. Eur J Immunol. 1997;27:3380–8. doi: 10.1002/eji.1830271237. [DOI] [PubMed] [Google Scholar]
- 17.Paul P, Cabestre FA, Le Gal FA, et al. Heterogeneity of HLA-G gene transcription and protein expression in malignant melanoma biopsies. Cancer Res. 1999;59:1954–60. [PubMed] [Google Scholar]
- 18.Urosevic M, Kurrer MO, Kamarashev J, et al. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am J Pathol. 2001;159:817–24. doi: 10.1016/S0002-9440(10)61756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim EC, Guerra N, Lacombe MJ, Angevin E, Chouaib S, Carosella ED, Caignard A, Paul P. Tumor-specific up-regulation of the nonclassical class I HLA-G antigen expression in renal carcinoma. Cancer Res. 2001;61:6838–45. [PubMed] [Google Scholar]
- 20.Urosevic M, Willers J, Mueller B, Kempf W, Burg G, Dummer R. HLA-G protein up-regulation in primary cutaneous lymphomas is associated with interleukin-10 expression in large cell T-cell lymphomas and indolent B-cell lymphomas. Blood. 2002;99:609–17. doi: 10.1182/blood.v99.2.609. [DOI] [PubMed] [Google Scholar]
- 21.Wagner SN, Rebmann V, Willers CP, Grosse-Wilde H, Goos M. Expression analysis of classic and non-classic HLA molecules before interferon alfa-2b treatment of melanoma. Lancet. 2000;356:220–1. doi: 10.1016/S0140-6736(00)02486-7. [DOI] [PubMed] [Google Scholar]
- 22.Aractingi S, Briand N, Le Danff C, Viguier M, Bachelez H, Michel L, Dubertret L, Carosella ED. HLA-G and NK receptor are expressed in psoriatic skin: a possible pathway for regulating infiltrating T cells? Am J Pathol. 2001;159:71–7. doi: 10.1016/S0002-9440(10)61675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khosrotehrani K, Le Danff C, Reynaud-Mendel B, Dubertret L, Carosella ED, Aractingi S. HLA-G expression in atopic dermatitis. J Invest Dermatol. 2001;117:750–2. doi: 10.1046/j.0022-202x.2001.01487.x. [DOI] [PubMed] [Google Scholar]
- 24.Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, Carosella ED, Paul P. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11:803–11. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- 25.Spencer JV, Lockridge KM, Barry PA, Lin G, Tsang M, Penfold ME, Schall TJ. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol. 2002;76:1285–92. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Chu W, Geraghty DE, Hunt JS. Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J Immunol. 1996;156:4224–31. [PubMed] [Google Scholar]
- 27.Chu W, Yang Y, Geraghty DE, Hunt JS. Interferons enhance HLA-G mRNA and protein in transfected mouse fibroblasts. J Reprod Immunol. 1999;42:1–15. doi: 10.1016/s0165-0378(98)00077-1. [DOI] [PubMed] [Google Scholar]
- 28.Chu W, Fant ME, Geraghty DE, Hunt JS. Soluble HLA-G in human placentas: synthesis in trophoblasts and interferon-gamma-activated macrophages but not placental fibroblasts. Hum Immunol. 1998;59:435–42. doi: 10.1016/s0198-8859(98)00045-7. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre S, Berrih-Aknin S, Adrian F, et al. A specific interferon (IFN)-stimulated response element of the distal HLA-G promoter binds IFN-regulatory factor 1 and mediates enhancement of this nonclassical class I gene by IFN-beta. J Biol Chem. 2001;276:6133–9. doi: 10.1074/jbc.M008496200. [DOI] [PubMed] [Google Scholar]
- 30.Gobin SJ, van den Elsen PJ. Transcriptional regulation of the MHC class Ib genes HLA-E, HLA-F, and HLA-G. Hum Immunol. 2000;61:1102–7. doi: 10.1016/s0198-8859(00)00198-1. [DOI] [PubMed] [Google Scholar]
- 31.van den Elsen PJ, Gobin SJ, van Eggermond MC, Peijnenburg A. Regulation of MHC class I and II gene transcription: differences and similarities. Immunogenetics. 1998;48:208–21. doi: 10.1007/s002510050425. [DOI] [PubMed] [Google Scholar]
- 32.Waldburger JM, Masternak K, Muhlethaler-Mottet A, Villard J, Peretti M, Landmann S, Reith W. Lessons from the bare lymphocyte syndrome: molecular mechanisms regulating MHC class II expression. Immunol Rev. 2000;178:148–65. doi: 10.1034/j.1600-065x.2000.17813.x. [DOI] [PubMed] [Google Scholar]
- 33.Masternak K, Muhlethaler-Mottet A, Villard J, Peretti M, Reith W. Molecular genetics of the Bare lymphocyte syndrome. Rev Immunogenet. 2000;2:267–82. [PubMed] [Google Scholar]
- 34.Steimle V, Durand B, Barras E, Zufferey M, Hadam MR, Mach B, Reith W. A novel DNA-binding regulatory factor is mutated in primary MHC class II deficiency (bare lymphocyte syndrome) Genes Dev. 1995;9:1021–32. doi: 10.1101/gad.9.9.1021. [DOI] [PubMed] [Google Scholar]
- 35.Durand B, Sperisen P, Emery P, Barras E, Zufferey M, Mach B, Reith W. RFXAP, a novel subunit of the RFX DNA binding complex is mutated in MHC class II deficiency. EMBO J. 1997;16:1045–55. doi: 10.1093/emboj/16.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masternak K, Barras E, Zufferey M, et al. A gene encoding a novel RFX-associated transactivator is mutated in the majority of MHC class II deficiency patients. Nat Genet. 1998;20:273–7. doi: 10.1038/3081. [DOI] [PubMed] [Google Scholar]
- 37.Nagarajan UM, Louis-Plence P, DeSandro A, Nilsen R, Bushey A, Boss JM. RFX-B is the gene responsible for the most common cause of the bare lymphocyte syndrome, an MHC class II immunodeficiency. Immunity. 1999;10:153–62. doi: 10.1016/s1074-7613(00)80016-3. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa SL, Boss JM. Two B cell factors bind the HLA-DRA X box region and recognize different subsets of HLA class II promoters. Nucleic Acids Res. 1991;19:6269–76. doi: 10.1093/nar/19.22.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno CS, Beresford GW, Louis-Plence P, Morris AC, Boss JM. CREB regulates MHC class II expression in a CIITA-dependent manner. Immunity. 1999;10:143–51. doi: 10.1016/s1074-7613(00)80015-1. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani R. The molecular biology of the CCAAT-binding factor NF-Y. Gene. 1999;239:15–27. doi: 10.1016/s0378-1119(99)00368-6. [DOI] [PubMed] [Google Scholar]
- 41.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–46. [PubMed] [Google Scholar]
- 42.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–9. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 43.Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994;180:1367–74. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masternak K, Muhlethaler-Mottet A, Villard J, Zufferey M, Steimle V, Reith W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. 2000;14:1156–66. [PMC free article] [PubMed] [Google Scholar]
- 45.Gobin SJ, Peijnenburg A, van Eggermond M, van Zutphen M, van den Berg R, van den Elsen PJ. The RFX complex is crucial for the constitutive and CIITA-mediated transactivation of MHC class I and beta2-microglobulin genes. Immunity. 1998;9:531–41. doi: 10.1016/s1074-7613(00)80636-6. [DOI] [PubMed] [Google Scholar]
- 46.Gobin SJ, van Zutphen M, Westerheide SD, Boss JM, van den Elsen PJ. The MHC-specific enhanceosome and its role in MHC class I and beta (2)-microglobulin gene transactivation. J Immunol. 2001;167:5175–84. doi: 10.4049/jimmunol.167.9.5175. [DOI] [PubMed] [Google Scholar]
- 47.van den Elsen PJ, Peijnenburg A, van Eggermond MC, Gobin SJ. Shared regulatory elements in the promoters of MHC class I and class II genes. Immunol Today. 1998;19:308–12. doi: 10.1016/s0167-5699(98)01287-0. [DOI] [PubMed] [Google Scholar]
- 48.Gobin SJ, Keijsers V, van Zutphen M, van den Elsen PJ. The role of enhancer A in the locus-specific transactivation of classical and nonclassical HLA class I genes by nuclear factor kappa B. J Immunol. 1998;161:2276–83. [PubMed] [Google Scholar]
- 49.Gobin SJ, van Zutphen M, Woltman AM, van den Elsen PJ. Transactivation of classical and nonclassical HLA class I genes through the IFN-stimulated response element. J Immunol. 1999;163:1428–34. [PubMed] [Google Scholar]
- 50.Rousseau P, Paul P, O'Brien M, Dausset J, Carosella ED, Moreau P. The X1 box of HLA-G promoter is a target site for RFX and Sp1 factors. Hum Immunol. 2000;61:1132–7. doi: 10.1016/s0198-8859(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 51.Epstein MA, Achong BG, Barr YM, Zajac B, Henle G, Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji) J Natl Cancer Inst. 1966;37:547–59. [PubMed] [Google Scholar]
- 52.Shimizu Y, Geraghty DE, Koller BH, Orr HT, DeMars R. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci USA. 1988;85:227–31. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul P, Rouas-Freiss N, Moreau P, et al. HLA-G-E-F preworkshop: tools and protocols for analysis of non-classical class I genes transcription and protein expression. Hum Immunol. 2000;61:1177–95. doi: 10.1016/s0198-8859(00)00154-3. [DOI] [PubMed] [Google Scholar]
- 54.Menier C, Saez B, Horejsi V, et al. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: new tools to analyze the expression of nonclassical HLA class I molecules. Hum Immunol. 2003;64:315–26. doi: 10.1016/s0198-8859(02)00821-2. [DOI] [PubMed] [Google Scholar]
- 55.Masternak K, Peyraud N, Krawczyk M, Barras E, Reith W. Chromatin remodeling and extragenic transcription at the MHC class II locus control region. Nat Immunol. 2003;4:132–7. doi: 10.1038/ni883. [DOI] [PubMed] [Google Scholar]
- 56.King A, Boocock C, Sharkey AM, Gardner L, Beretta A, Siccardi AG, Loke YW. Evidence for the expression of HLAA-C class I mRNA and protein by human first trimester trophoblast. J Immunol. 1996;156:2068–76. [PubMed] [Google Scholar]
- 57.Blaschitz A, Hutter H, Leitner V, Pilz S, Wintersteiger R, Dohr G, Sedlmayr P. Reaction patterns of monoclonal antibodies to HLA-G in human tissues and on cell lines: a comparative study. Hum Immunol. 2000;61:1074–85. doi: 10.1016/s0198-8859(00)00207-x. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989;142:3320–8. [PubMed] [Google Scholar]
- 59.Chumbley G, King A, Robertson K, Holmes N, Loke YW. Resistance of HLA-G and HLA-A2 transfectants to lysis by decidual NK cells. Cell Immunol. 1994;155:312–22. doi: 10.1006/cimm.1994.1125. [DOI] [PubMed] [Google Scholar]
- 60.Boucraut J, Guillaudeux T, Alizadeh M, et al. HLA-E is the only class I gene that escapes CpG methylation and is transcriptionally active in the trophoblast-derived human cell line JAR. Immunogenetics. 1993;38:117–30. doi: 10.1007/BF00190899. [DOI] [PubMed] [Google Scholar]
- 61.Celada A, McKercher S, Maki RA. Repression of major histocompatibility complex IA expression by glucocorticoids: the glucocorticoid receptor inhibits the DNA binding of the X box DNA binding protein. J Exp Med. 1993;177:691–8. doi: 10.1084/jem.177.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy SP, Tomasi TB. Absence of MHC class II antigen expression in trophoblast cells results from a lack of class II transactivator (CIITA) gene expression. Mol Reprod Dev. 1998;51:1–12. doi: 10.1002/(SICI)1098-2795(199809)51:1<1::AID-MRD1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 63.van den Elsen PJ, van der Stoep N, Vietor HE, Wilson L, van Zutphen M, Gobin SJ. Lack of CIITA expression is central to the absence of antigen presentation functions of trophoblast cells and is caused by methylation of the IFN-gamma inducible promoter (PIV) of CIITA. Hum Immunol. 2000;61:850–62. doi: 10.1016/s0198-8859(00)00159-2. [DOI] [PubMed] [Google Scholar]
- 64.Morris AC, Spangler WE, Boss JM. Methylation of class II trans-activator promoter IV: a novel mechanism of MHC class II gene control. J Immunol. 2000;164:4143–9. doi: 10.4049/jimmunol.164.8.4143. [DOI] [PubMed] [Google Scholar]
- 65.van den Elsen PJ, Gobin SJ, van der Stoep N, Datema G, Vietor HE. Transcriptional control of MHC genes in fetal trophoblast cells. J Reprod Immunol. 2001;52:129–45. doi: 10.1016/s0165-0378(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 66.Courey AJ, Jia S. Transcriptional repression: the long and the short of it. Genes Dev. 2001;15:2786–96. doi: 10.1101/gad.939601. [DOI] [PubMed] [Google Scholar]
- 67.Solier C, Mallet V, Lenfant F, Bertrand A, Huchenq A, Le Bouteiller P. HLA-G unique promoter region: functional implications. Immunogenetics. 2001;53:617–25. doi: 10.1007/s00251-001-0373-0. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt CM, Ehlenfeldt RG, Athanasiou MC, Duvick LA, Heinrichs H, David CS, Orr HT. Extraembryonic expression of the human MHC class I gene HLA-G in transgenic mice: evidence for a positive regulatory region located 1 kilobase 5′- to the start site of transcription. J Immunol. 1993;151:2633–45. [PubMed] [Google Scholar]
- 69.Moreau P, Paul P, Gourand L, Prost S, Dausset J, Carosella E, Kirszenbaum M. HLA-G gene transcriptional regulation in trophoblasts and blood cells: differential binding of nuclear factors to a regulatory element located 1.1 kb from exon 1. Hum Immunol. 1997;52:41–6. doi: 10.1016/S0198-8859(96)00242-X. [DOI] [PubMed] [Google Scholar]
- 70.Gobin SJ, Biesta P, De Steenwinkel JE, Datema G, Van Den Elsen PJ. HLA-G transactivation by cAMP-response element-binding protein (CREB): an alternative transactivation pathway to the conserved major histocompatibility complex class I regulatory routes. J Biol Chem. 2002;277:39525–31. doi: 10.1074/jbc.M112273200. [DOI] [PubMed] [Google Scholar]
- 71.Moreau P, Mouillot G, Rousseau P, Marcou C, Dausset J, Carosella ED. HLA-G gene repression is reversed by demethylation. Proc Natl Acad Sci USA. 2003;100:1191–96. doi: 10.1073/pnas.0337539100. [DOI] [PMC free article] [PubMed] [Google Scholar]