Abstract
T-cell stimulation in the absence of a second, costimulatory signal can lead to anergy or deletion. There is growing evidence that peripheral tolerance to an exogenous antigen might be caused by the lack of costimulatory molecules on antigen-presenting cells (APCs). In the present study, we examined whether tolerance against orally administered antigen could be reversed by maturation of APCs via CD40 signalling. Monoclonal antibody (mAb) to CD40 efficiently induced costimulatory molecules on APCs. Treatment with anti-CD40 mAb potentiated the division of ovalbumin-specific T cells in response to oral ovalbumin in secondary lymphoid organs. However, such treatment did not prolong the presentation of oral ovalbumin on APCs. Surprisingly, treatment of anti-CD40 mAb at the time of oral administration of ovalbumin did not reverse the induction of tolerance to ovalbumin in either the high- or low-dose regimens. Furthermore, the induction of oral tolerance in our model is not the result of negative signalling by cytotoxic T-lymphocyte antigen-4. These results indicate that tolerance for oral antigen could be established regardless of APC maturation by a CD40-specific mAb, suggesting that there could be a unique mechanism to regulate immunity versus tolerance to encountered antigen in the gut-associated lymphoid tissue.
Introduction
Onset of T-cell immunity against an antigen requires the delivery of two signals. The first signal involves the specific engagement of the T-cell receptor by peptides presented by major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs). The second signal provides costimulation and involves ligation of another receptor on the T-cell surface in an antigen non-specific manner. Delivery of signal one without signal two does not fully activate the T cell but instead directs it to a non-responsive state known as anergy.1,2
Peripheral tolerance to sequestered self-antigen has been explained in this context. Non-professional APCs do not bear costimulatory molecules, such as B-7s, under normal conditions and thus cannot deliver signal two.1 Furthermore, it is widely accepted that peripheral tolerance to an exogenous antigen might be caused by the lack of costimulatory molecules on APCs.3–5
Providing costimulatory molecules on APCs would reverse the T-cell anergy. In addition, it has been reported that activation of APCs by CD40 ligation delayed the clonal deletion of antigen-specific T-cell and enhanced T-cell clonal expansion in response to super-antigen.6 Thus it is a reasonable assumption that providing signal two would ablate the induction of peripheral tolerance to an exogenous antigen and lead to immunity against the antigen.3–5,7–12 Signalling via CD40 has been used as an efficient tool to activate APCs in vivo. Indeed, accumulating evidence has shown that activation of APC via CD40 reverses tolerance induction and leads to immunity to tumour antigen or injected soluble antigen.9–12 Garza et al. reported that glycoprotein (gp) peptide treatment with CD40 ligation led to autoimmunity instead of tolerance in the RIP-gp/P14 mouse model.5 Furthermore, CD40 signalling can replace the assistance of CD4 in inducing cytotoxic T-lymphocyte responses in mouse models.10,12 Extensive approaches are underway to utilize this property in tumour immunotherapy.
A large population of immune cells resides in mucosa-associated lymphoid tissue (MALT) such as gut, nasal tissue and trachea. Since these areas are the site of pathogen entry and have unique physiological and anatomical properties, MALT has been thought to be of importance for both vaccine developments against pathogens and for tolerance induction to those proteins that cause autoimmune or allergic disorders.13,14 This is especially the case for oral administration of antigen, which is one of the oldest approaches for inducing immune tolerance. Many studies have shown that oral administration of antigen blocked the development of autoimmune or allergic disorders.15–18 Despite its importance, the mechanism of immunity and tolerance through the MALT is poorly understood. In particular, the role of APCs in establishing tolerance to encountered antigen is not defined although APCs are the platform of such immune regulation.
In this report, it was examined whether the maturation status of APCs would affect the induction of oral tolerance. To this aim, studies were designed to stimulate APCs by CD40 signals and then test whether tolerance to oral antigen would be ablated in vivo. The involvement of CTLA-4 was also examined in this model of oral tolerance. The results indicate that tolerance to oral antigen could be established regardless of APC maturation by CD40 ligation.
Materials and methods
Mice
Female BALB/c mice were purchased from Charles River (Biogenomics, Seoul, South Korea) and used at the age of 6–10 weeks. Four to six mice per group were used in all experiments. Breeding pairs of DO11.10 mice were purchased from JAX (the Jackson Laboratory Bar Harbour, ME) and were bred in our animal centre. Offspring were identified as transgenic by polymerase chain reaction of genomic DNA or by clonotypic monoclonal antibody (mAb; KJ) staining of peripheral blood lymphocytes by flow cytometer. Mice were housed at Seoul National University until use and were kept in specific-pathogen-free conditions during the entire period.
Flow cytometry for costimulatory molecules on dendritic cells (DC) and B cells
Anti-CD40 mAb (FGK 45.5) was purified from hybridoma culture supernatant (Ultradoma, Biowhittaker, MD) using a protein G column. Mice were treated with 200 μg of anti-CD40 mAb, or with rat immunoglobulin G (IgG) as a control, and secondary lymphoid organs were isolated at various times thereafter. DCs were isolated from the mesenteric lymph nodes (MLN) or spleen using anti-CD11c microbeads and a magnetic antibody cell-sorting (MACS) column (Miltenyi Biotec, Bergisch Gladbach, Germany). To stain B cells, single-cell suspensions of spleen, MLNs, Peyer's patches (PP), or inguinal lymph nodes (ILN) were stained in phosphate-buffered saline (PBS) containing 1% fetal bovine serum, with phycoerythrin-conjugated rat anti-mouse B220 (PharMingen, San Diego, CA). Additional staining with biotin-conjugated rat anti-mouse CD40, CD80, CD86 and I-A/E (PharMingen) and streptavidin-conjugated phycoerythrin (Pierce, Rockford, IL) was performed. Cells were analysed on a PAS III flow cytometer (Partec, Munster, Germany) using flow max software or a FACSort using cellquest (Becton Dickinson, Mountain View, CA) software.
Tolerance induction and CD40 mAb treatment
Kinetic study of CD40 ligation on oral tolerance
Groups of mice were fed 20 mg of ovalbumin (OVA; Grade V; Sigma, St Louis, MO) and received intravenous (i.v.) injections of 200 μg anti-CD40 mAb either 24 hr before feeding or at 0, 2, 6, or 24 hr after feeding. Positive control mice were fed PBS and negative control mice were fed 20 mg of OVA and received rat IgG. After 2 weeks, these mice were primed and boosted with 50 μg OVA emulsified in complete or incomplete Freund's adjuvant, respectively, at 2-week intervals. Ten days later, sera and spleens were obtained from mice and tested.
Dosage study
Groups of mice were fed 0.2, 2, or 20 mg of OVA or PBS alone and received 200 µg anti-CD40 mAb or rat IgG i.v., simultaneously. These mice were primed, boosted and tested using the same basic protocol described above.
CTL antigen-4 blocking study
The anti-CTL antigen-4 (CTLA-4) mAb 4F10 was purified from hybridoma culture supernatant using a protein G column. Groups of mice were fed 20 mg of OVA or PBS and received 200 μg anti-CTLA-4 mAb or hamster IgG at the time of feeding and 1, 2, 3 and 5 days after feeding. One group of mice also received 200 μg anti-CD40 mAb at the time of feeding.
Antigen uptake study
For uptake studies, OVA was conjugated to fluorescein isothiocyanate (FITC; Sigma). Mice received 200 μg anti-CD40 mAb or rat IgG at −24 hr or 0 hr and were injected i.v. with 3 mg/mouse of OVA-FITC. Non-fluorescent native OVA was injected into control mice to provide the DC background for FITC labelling.19 After 1 hr, spleens were removed from the mice and DCs were isolated by using anti-CD11c microbeads and a MACS column. Analysis of DC showing uptake of fluorescent antigens was performed by flow cytometer.
Enzyme-linked imunosorbent assay (ELISA) for OVA-specific IgG
OVA-specific IgG in serum was measured as described previously.20 IgG concentration in tested serum was determined from standard curves constructed using immunoaffinity-purified anti-OVA IgG.
Proliferation assay
Single cell suspensions from the spleen were plated at 5 × 105 spleen cells per well in 96-well, round-bottomed microtitre plates, and cultured for 4 days with 5, 50, or 500 μg/ml OVA or alone in 200 μl of medium. After 96 hr of incubation, including a final 22-hr pulse with [3H]thymidine (1 μCi/well), cells were harvested and label incorporation was measured.
Cytokine ELISA
Interleukin-5 (IL-5), IL-4, or interferon-γ (IFN-γ) was quantified from splenocyte culture supernatant. Cells were plated, at 8 × 106 cells per well, with 1 ml aliquots. Cells were cultured for 72 hr with 40 μg/ml OVA or alone. Culture supernatants were tested for the presence of IL-5, IL-4, or IFN-γ by sandwich ELISA. Purified rat anti-mouse IL-5 mAb (clone TRFK5), biotinylated rat anti mouse IL-5 mAb (clone TRFK4), and recombinant mouse IL-5 (PharMingen) were used for IL-5 sandwich ELISA. For IL-4 sandwich ELISA, purified rat anti-mouse IL-4 mAb (clone 11B11), biotinylated rat anti-mouse IL-4 mAb (clone BVD6-24G2), and recombinant mouse IL-4 (PharMingen) were used and for IFN-γ sandwich ELISA purified rat anti-mouse IFN-γ mAb (clone R4-6A2), biotinylated rat anti-mouse IFN-γ mAb (clone XMG1.2), and recombinant mouse IFN-γ were used.
CFSE labelling and adoptive transfer study
OVA-specific CD4+ T cells were isolated from DO11 mice using a negative selection CD4 T-cell comlumn (R&D System, Minneapolis, MN) following the manufacturer's instructions. Briefly, red-blood-cell-depleted single cell splenocyte was prepared and stained with the antibody mixture and loaded onto the column. Eluted cells were harvested (85% pure) and 5- (and 6-) carboxyfluorescein diacetate, succinimidyl ester (CFSE) labelling was performed following the manufacturer's instructions. Briefly, purified T cells were resuspended in PBS containing 0.1% bovine serum albumin (Sigma) at 107 cells/ml. For fluorescence labelling, 2 μl of a CFSE (Molecular Probes, Eugene, OR) stock solution (5 mm in dimethyl sulphoxide) was incubated with 107 cells for 10 min at 37°.
CFSE-labelled DO11 T cells were transferred i.v. into naive BALB/c mice (1 × 107/mouse). The next day, recipient mice were fed 20 mg OVA or PBS with anti-CD40 mAb or rat IgG. Three days later, the secondary lymphoid organs were removed and analysed by flow cytometer for their division.21
Statistics
Results are expressed as the means ± SE. Statistical analyses were performed upon comparisons made between the treated groups and the positive controls using Student's t-test. Each experiment was repeated at least twice.
Results
Signal through CD40 agonistic mAb induces up-regulation of costimulatory molecules on APCs
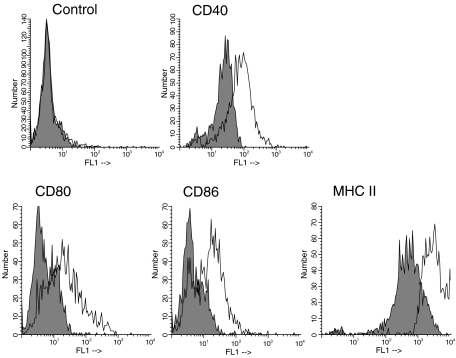
Initial studies sought to examine the effects of CD40 ligation on APC populations. Triggering with anti-CD40 mAb caused the up-regulation of CD40, B7-1, B7-2 and MHC class II on DCs in the MLN, (Fig. 1a) as well as the spleen within 24 hr. MHC class II was up-regulated as early as 12 hr in anti-CD40-treated mice. B cells also up-regulated such molecules in secondary lymphoid organs including ILN and PP (data not shown). Up-regulation of such molecules on DCs and B cells persisted for at least 72 hr after mAb treatment. The ability of anti-CD40 mAb to induce the activation of APCs described above was comparable over a range of 100–500 μg mAb per mouse and 200 μg was used during the following experiments.
Figure 1.
Anti-CD40 mAb induced costimulatory molecules. Mice received 200 μg of anti-CD40 mAb (open areas) or rat IgG (filled areas). Twenty-four hours later, mesenteric lymph nodes were removed and CD11+ DCs were isolated and analysed for the expression of CD40, CD80, CD86 and MHCII(I-A/E) by flow cytometer.
Overall, these data indicated that agonistic CD40 mAb could efficiently induce costimulatory molecules on APCs.
CD40 ligation enhanced the proliferation of OVA-specific CD4+ T cells in response to oral OVA in vivo
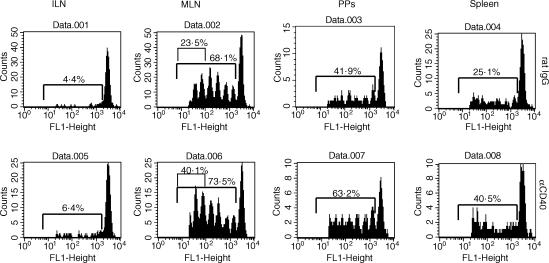
It has been reported that antigen-specific T cells proliferate primarily in PPs and MLNs in response to oral antigen.22,23 It seems reasonable to assume that providing costimulatory molecules on APCs would enhance such activation and/or proliferation of antigen-specific T cells. Thus, it was next asked whether the ligation of CD40 could affect the proliferation of T cells in vivo. CFSE-labelled DO11 T cells were transferred into naive mice. The next day, recipient mice were fed OVA and received an i.v. injection of anti-CD40 mAb simultaneously. Three days later, secondary lymphoid organs were removed and analysed by flow cytometer.
As previously reported by others, OVA-specific CD4+ T cells proliferated primarily in the PPs and MLNs after oral administration of OVA. In mice that received OVA plus anti-CD40 mAb, the proliferation of DO11 T cells was enhanced compared with that of OVA plus rat IgG-treated mice in most lymphoid tissues (Fig. 2). In PPs, a higher proportion of DO11 T cells proliferated in anti-CD40 mAb-treated mice. In MLNs, the proportion of DO11 T cells dividing was similar for both groups, though there seemed to be a slight enhancement in the rate of division in anti-CD40 mAb-treated mice. In summary, CD40 ligation weakly enhanced the division of DO11 T cells in the secondary lymphoid organs in response to oral OVA.
Figure 2.
Effect of anti-CD40 mAb on OVA-specific CD4 T-cell division in response to oral OVA. CFSE-labelled DO11 T cells (1 × 107) were transferred into syngenic BALB/c mice. Next day, the recipient mice were given 20 mg of oral OVA and received i.v. injections of anti-CD40 mAb or rat IgG simultaneously. Three days later, the indicated lymphoid organs were removed and analysed by flow cytometer.
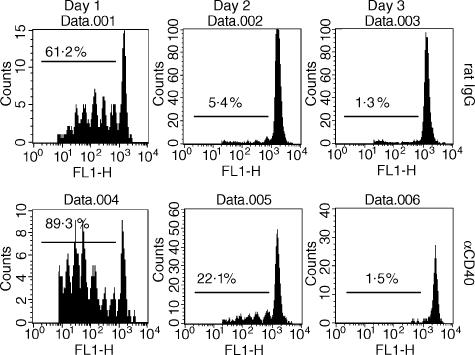
Next we examined whether CD40 ligation affected the persistence of antigen presentation. Mice were fed OVA and received antibody simultaneously. Then CFSE-labelled DO11 T cells were transferred at 1, 2, or 3 days thereafter, respectively. Three days after transfer, MLNs were removed. Figure 3 reveals strong proliferation of DO11 T cells 1 day after antigen administration, a weak response after 2 days and nothing after 3 days in both rat IgG-treated and anti-CD40 mAb-treated groups. As expected, CD40 ligation enhanced the proliferation of DO11 T cells (Fig. 3, day 1 and day 2), but it did not prolong the presentation of oral OVA in MLNs. Thus, oral OVA persisted for approximately 2 days in MLN and anti-CD40 mAb treatment did not affect this.
Figure 3.
Effect of anti-CD40 mAb on the persistence of oral OVA in APCs in mesenteric lymph nodes. Mice were given 20 mg of oral OVA and received the indicated antibody simultaneously. After 1, 2, or 3 days, respectively, 1 × 107 CFSE-labelled DO11 T cells were transferred into the OVA-fed mice. Three days later, mesenteric lymph nodes were removed and analysed by flow cytometer.
Administration of CD40 mAb before, but not after, OVA feeding blocked the induction of oral tolerance
Since mAb against CD40 efficiently induced costimulatory molecules on APCs, it was investigated next whether such maturation of APCs can ablate the induction of peripheral tolerance by oral antigen. Although proliferation of OVA-specific T cells was not greatly enhanced, it was still possible that APCs stimulated by CD40 ligation could reverse tolerance induction and lead to immunity against oral OVA.
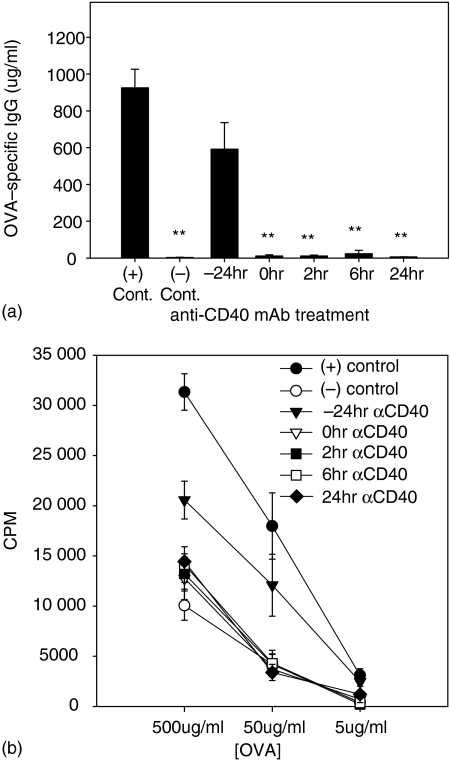
Groups of mice were fed 20 mg of OVA and received mAb at 24 hr before or at 0, 2, 6 or 24 hr after feeding and then were primed and boosted at 2-week intervals with OVA in complete and incomplete Freund's adjuvant, respectively. As expected, OVA-specific tolerance was induced in both the humoral response and splenocyte proliferation in OVA-fed rat IgG-treated mice (indicates–control in Fig. 4a,b). Treatment with anti-CD40 mAb 24 hr before oral OVA caused remarkable blocking of OVA-specific tolerance induction. However, the level of OVA-specific proliferation was still less than that in the PBS-fed group when the concentration of OVA was high (P < 0.05) and the level of OVA-specific antibody in the primary response revealed that the immune response against OVA was not primed by this regimen (data not shown). Surprisingly, anti-CD40 mAb at the time of OVA feeding could not abrogate tolerance induction by oral OVA. As shown in Fig. 4, the levels of OVA-specific IgG and OVA-specific proliferation of splenocytes were similar to those of OVA-fed rat IgG-treated mice. CD40 ligation after oral administration of OVA also failed to reverse the induction of oral tolerance. The observed suppression in OVA-fed mice was OVA-specific because immune response to an irrelevant antigen was not affected in OVA-fed mice (data not shown).
Figure 4.
Anti-CD40 mAb treatment at the inductive phase of oral tolerance. Groups of BALB/c mice were fed 20 mg of OVA and received mAb 24 hr before or 0, 2, 6, or 24 hr after feeding. After 2 weeks, these mice were primed and boosted at 2-week intervals. Ten days later, sera and splenocytes were obtained. (a) Concentration of OVA-specific IgG in serum was assessed by ELISA. (b) Proliferation of splenocytes were measured using the standard [3H]thymidine incorporation method. (+) control indicates the PBS-fed and rat IgG-injected group, (−) control indicates the OVA-fed and rat IgG-injected group. **P < 0.01 in comparison with positive control.
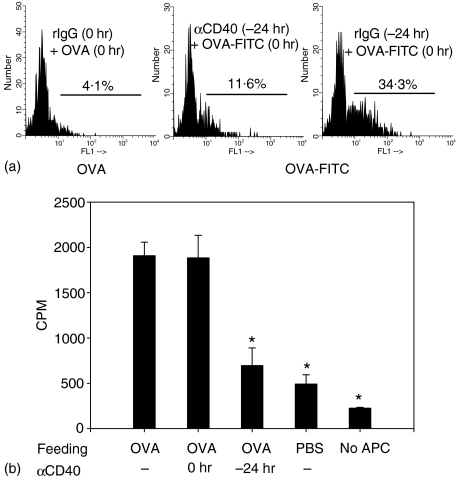
Ligation of CD40 before antigen administration blocked the induction of tolerance by oral antigen. One possible explanation for this could be that the stimulation of APCs via CD40 signals hampers the uptake of antigen. To test this possibility, mice were injected with anti-CD40 mAb or rat IgG as a control. Twenty-four hours later, these mice were injected with OVA-FITC or OVA alone. DCs were isolated from the spleen and the uptake of OVA-FITC was determined by flow cytometer. Indeed, uptake of OVA-FITC was greatly reduced in DCs isolated from anti-CD40 mAb-pretreated mice compared with rat IgG-treated mice (Fig. 5a). Consistent with this result, proliferation of DO11 T cells in response to oral OVA was reduced when cells from mesenteric lymph nodes of anti-CD40 mAb pretreated mice were used as stimulator (Fig. 5b).
Figure 5.
Preactivation of APCs with anti-CD40 mAb reduces the uptake of antigen by DCs. (a) Mice received 200 μg anti-CD40 mAb or rat IgG at −24 hr or 0 hr and were injected i.v. with 3 mg/mouse of OVA-FITC. Non-fluorescent native OVA was injected into control mice to provide the DC background for FITC labelling (left). After 1 hr spleens were removed, DCs were isolated and uptake of fluorescent OVA was performed by flow cytometer. (b) Mice received 200 μg anti-CD40 mAb rat IgG at −24 hr or 0 hr and were fed 20 mg OVA at 0 hr. Eight hours later, total lymph node cells were harvested and treated with mitomycin C. Then these cells (1 × 106 cells/well) were cultured in the presence of purified DO11 T cells (5 × 104 cells/well). After 96 hr, including 18-hr [3H]thymidine incorporation, cells were harvested and tested. *P < 0.01 versus OVA-fed anti-CD40 mAb non-treated group.
Collectively, stimulation of APCs by CD40 ligation at the time of oral administration of antigen did not reverse the induction of tolerance to that antigen.
CD40 triggering failed to prime immune response to oral OVA
Since CD40 ligation enhanced the response of DO11 T cells to oral OVA, we next examined whether ligation of CD40 primes the immune response to oral OVA. Mice received anti-CD40 mAb at the time of oral administration. The proliferation of splenocytes in the presence of OVA was examined without further immunization. As shown in Table 1, CD40 ligation did not prime the immune response to oral OVA. Since OVA-specific CD4 T cells initially proliferated (Fig. 2) and they did not prime immunity to oral OVA (Table 1), it was concluded that OVA-specific T cells became anergic after early activation by oral OVA and that CD40 triggering did not reverse this anergy.
Table 1.
Anti-CD40 mAb does not prime the immune response to oral OVA*
| Proliferation (counts/minute) | ||
|---|---|---|
| Antibody | PBS | OVA |
| Rat IgG | 5871 ± 1081 | 5122 ± 1078 |
| Anti-CD40 | 3919 ± 916 | 3787 ± 667 |
Mice were fed 20 mg OVA or PBS and received the indicated antibody simultaneously. Two weeks later, splenocytes were prepared and cultured in the presence of 50 μg/ml of OVA for 96 hr, including 18-hr [3H]thymidine incorporation.
Agonistic CD40 mAb did not reverse the induction of oral tolerance regardless of antigen dose
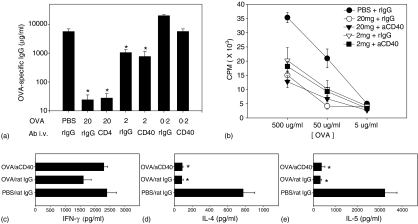
Antigen dose has been reported to influence the mechanism of oral tolerance, with high doses causing clonal deletion and anergy and low doses inducing active suppression.15 Thus our next study was designed to examine the effect of CD40 ligation on oral tolerance induced by different doses of OVA. Again, 20 mg OVA induced hyporesponsiveness both in humoral and cellular immune responses (Fig. 6) while 2 mg oral OVA led to moderate suppression (Fig. 6a,b). However, activation of APCs by CD40 ligation did not block the induction of tolerance by 2 mg of oral OVA. Data from the mice given both oral PBS and anti-CD40 mAb were similar to those from PBS-fed rat IgG-treated mice, indicating that there was no lingering effect of the anti-CD40 mAb on the following immunization (data not shown). The culture supernatant of splenocytes was tested for cytokines such as IFN-γ, IL-4 and IL-5 by ELISA. Production of IL-4 and IL-5 was also suppressed in mice that received 20 mg (Fig. 6d,e) or 2 mg (data not shown) of oral OVA regardless of treatment with anti-CD40 mAb. Suppression of T helper type 2 cytokines coincided with the suppression of humoral and cellular proliferation. Production of IFN-γ showed a marginal suppression in OVA-fed mice and this was reversed to positive control levels by anti-CD40 mAb (Fig. 6c).
Figure 6.
Effect of anti-CD40 mAb treatment on induction of oral tolerance with various doses of OVA. Groups of mice were fed 20, 2, or 0.2 mg OVA and received antibody simultaneously. After 2 weeks, these mice were primed and boosted at 2-week intervals. Ten days later, sera and splenocytes were obtained. (a) Concentration of OVA-specific IgG in serum was assessed by ELISA. (b) Proliferation of splenocytes was measured using standard [3H]thymidine incorporation method. (c)–(e) Spleen cells were cultured for 72 hr with 40 μg/ml OVA or alone. Culture supernatants were tested for the presence of IFN-γ (c), IL-4 (d), or IL-5 (e) by sandwich ELISA. *P < 0.01 versus PBS-fed rat IgG-treated group.
Taken together, these data indicate that CD40 ligation does not block the induction of hyporesponsiveness by either high- or low-dose feeding regimens.
Induction of oral tolerance in anti-CD40 mAb treated mice is not the result of negative signalling by CTLA-4
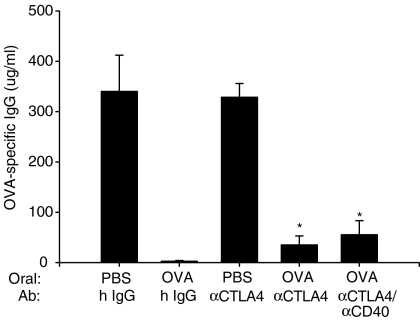
As CD28 and CTLA-4 deliver opposing signals, B-7s up-regulated by anti-CD40 mAb may deliver both positive and negative signals to the T cells. It was therefore investigated whether blockade of CTLA-4 signal would leave the up-regulated B-7s on APCs free to interact with CD28 and result in productive immunity in response to oral OVA. Mice were injected with anti-CTLA-4 mAb at the time of oral OVA administration and 1, 2, 3, 5 days after oral OVA administration. As shown in Fig. 7, coadministration of anti-CTLA-4 mAb with oral OVA resulted in a profound suppression of the OVA-specific humoral response, although there was a weak reversal of tolerance suggesting that CTLA-4 did not play a major role in our model of oral tolerance. In mice that received both anti-CD40 mAb and anti-CTLA-4 mAb at the time of oral OVA administration, production of OVA-specific IgG was also significantly suppressed (Fig. 7). These results demonstrated that oral tolerance in anti-CD40 mAb-treated mice was not the result of the ligation of CTLA-4 and B-7s being up-regulated by anti-CD40 mAb.
Figure 7.
CD40 ligation plus CTLA-4 blocking did not break oral tolerance. Mice were fed 20 mg OVA or PBS and received 200 μg anti-CTLA-4 mAb or hamster IgG at 0, 1, 2, 3 and 5 days after feeding. One group of mice additionally received 200 μg anti-CD40 mAb at the time of feeding. *P < 0.01 versus the PBS-fed hamster IgG-treated group.
Discussion
APCs are the platform of immune response. Especially, DCs are sentinels of the immune system. They sense ‘danger signals’ such as invasive pathogens and tissue damage and initiate immunity to remove such danger.24,25 It seems evident that DCs also regulate tolerance as well as immunity, although it is controversial whether there is a specialized DC for tolerance.26–28 It is well documented that antigen-specific T cells are anergized or deleted by oral antigen.15 However, although uptake, processing and presentation of antigen by APCs and their interaction with specific T helper cells are the initial event of tolerance induction, they are none of them well defined. Dissecting the role of APCs in the induction and maintenance of tolerance will provide insights into the mechanism and provide considerations for clinical trial.
It is well documented that T-cell stimulation in the absence of a second signal can lead to tolerance and that peripheral tolerance to an exogenous antigen might be caused by the lack of costimulatory molecules on APCs.3–5,12 CD40 ligation is generally used to stimulate APCs to prime immunity.6–9 Indeed, stimulation of APCs with CD40 agonistic mAb enhanced the division of OVA-specific T cells against oral OVA in secondary lymphoid organs in the present study. However, in several oral tolerance models, we found that the stimulation of APCs with CD40 agonistic antibody at the time of oral administration of OVA reversed the induction of tolerance to OVA in neither the high-dose nor the low-dose feeding regimen.
The DO11 T-cell adoptive transfer study showed that orally administered antigen was presented on APCs in mesenteric lymph nodes for 2 days regardless of the activation state of the APCs. To our knowledge, this is the first study to show the duration of antigen presentation after oral administration of antigen. Since up-regulated B7-1 and B7-2 molecules induced by CD40 ligation could bind to CTLA-4 on T cells and thus override the positive signal delivered through CD28, we examined CTLA-4 involvement using anti-CTLA-4 blocking mAb. Our result demonstrated that oral tolerance could be induced in CD40-treated mice in the absence of a CTLA-4 signal. This observation is not consistent with a previous report, which described that CTLA-4 was required for the induction of high dose of oral tolerance.29 Such discordance is probably the result of the difference in the frequency of antigen feeding.
In most models of peripheral tolerance, immunity to antigen has been shown to occur by CD40 ligation, which coincides with the ‘two-signal theory’.10–12 In this study, however, it was demonstrated that tolerance was efficiently established in mice upon the oral administration of OVA regardless of APCs activation with anti-CD40 mAb (providing costimulatory molecules on APCs). There are several possible reasons for the discordance with the ‘two-signal theory’ in our oral tolerance model. First, there might be a unique subset of APC that specialized for inducing and maintaining tolerance.26,27 In this case, this subset of APC (tolerogenic DCs) may overcome other CD40-activating APC (immunogenic DCs) and lead to tolerance to oral antigen. Recently, Wakkach et al. reported that a subpopulation of DCs specifically induce tolerance in vivo through the differentiation of Tr1 cells.30 Furthermore, a recent study reported that there are several inhibitory receptors on DCs rendering these cells tolerogenic to CD4 T cells.31 Characterization of the DC subtype responsible for tolerance in mucosal tissue would be interesting. Second, T cells may use an active mechanism for tolerance induction to harmless antigens rather than the passive mechanism provided by APCs in some unique environments, such as mucosal tissues. Accumulating reports have described cytokines such as transforming growth factor-β and IL-10 from T cells as critical in peripheral tolerance.32,33 In addition, T cells also express negative regulators such as CTLA-4 and PD-1 in tolerogenic circumstances.34–36 Such negative regulatory molecules might have a role in inducing oral tolerance. In our study, however, CTLA-4 blockade did not affect the induction of oral tolerance. Third, maturation of APCs by the CD40 signal may not be sufficient to stimulate antigen-specific CD4 T cells to differentiate into effector cells in our model of oral tolerance. Several recent studies demonstrated that mature DCs induce CD4 T-cell tolerance (reviewed in ref. 37). Menges et al. described how injection of mature DCs induces antigen-specific protection from autoimmunity in mice.38 Akbari et al. showed that DCs responsible for the induction of intranasal tolerance are phenotypically mature.39 Furthermore, a recent study demonstrated that maturation of DCs is required for cross-tolerance.40 Thus it is possible to assume that the DCs of anti-CD40 mAb-treated mice were mature phenotypically but were not activated functionally. Additional signal might be required to overcome tolerance, such as inflammatory cytokines as a third signal.41,42
Our results are in agreement with the recent report of Sun and Houten43 who showed that a single high dose of oral antigen induced T-cell tolerance regardless of CD40 ligation using an adoptive transfer study. In that study, mice were given anti-CD40 mAb twice at the time of adoptive transfer (day −1) and at the time of oral administration of antigen (day 0). Based on our results, treatment of anti-CD40 mAb prior to oral antigen could affect the uptake of the oral antigen by DC. Our study is novel in that we dissected the effects of CD40 ligation in terms of ‘antigen-uptake’ and ‘antigen-presentation’ and ruled out the possible involvement of CTLA-4 in vivo. Furthermore, our study demonstrates that coadministratioin of CD40 agonistic antibody and antigen fails to reverse the induction of oral tolerance regardless of ‘dose’. Our findings suggest that there could be another mechanism in the immune system to regulate immunity versus tolerance to encountered antigen especially in gut-associated lymphoid tissue.
Acknowledgments
We thank Dr William R. Heath (WEHI, Australia) for kindly providing the anti-CD40-mAb-secreting hybridoma (FGK45.5) and for discussion and review of this manuscript. This work was supported by grants, HMP-00-CH-06-0006, from the Ministry of Health & Welfare of Korea.
Abbreviations
- GALT
gut-associated lymphoid tissue
- ILN
inguinal lymph nodes
- MALT
mucosa-associated lymphoid tissue
- MLN
mesenteric lymph nodes
- PP
Peyer's patch
- Tr
regulatory T cells
References
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 3.Legge KL, Gregg RK, Maldonado-Lopez R, Li L, Caprio JC, Moser M, Zaghouani H. On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J Exp Med. 2002;196:217–27. doi: 10.1084/jem.20011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garza KM, Chan SM, Suri R, Nguyen LT, Odermatt B, Choenberger SSP, Ohashi PS. Role of antigen presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191:2021–7. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maxwell JR, Campbell JD, Kim CH, Vella AT. CD40 activation boosts T cell immunity in vivo by enhancing T cell clonal expansion and delaying peripheral T cell deletion. J Immunol. 1999;162:2024–34. [PubMed] [Google Scholar]
- 7.Grohmann U, Fallarino F, Silla S, et al. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2001;166:277–83. doi: 10.4049/jimmunol.166.1.277. [DOI] [PubMed] [Google Scholar]
- 8.Ichikawa HT, Williams LP, Segal BM. Activation of APCs through CD40 or Toll-like receptor 9 overcomes tolerance and precipitates autoimmune disease. J Immunol. 2002;169:2781–7. doi: 10.4049/jimmunol.169.5.2781. [DOI] [PubMed] [Google Scholar]
- 9.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–7. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 10.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–53. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 11.Lefrancois L, Altman JD, Williams K, Olson S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000;164:725–32. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- 12.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 13.Hiroi T, Goto H, Someya K, Yanagita M, Honda M, Yamanaka N, Kiyono HHIV. Mucosal Vaccine. Nasal immunization with rBCG-V3J1 induces a long term V3J1 peptide-specific neutralizing immunity in Th1- and Th2-deficient conditions. J Immunol. 2001;167:5862–7. doi: 10.4049/jimmunol.167.10.5862. [DOI] [PubMed] [Google Scholar]
- 14.Iijima H, Takahashi I, Kiyono H. Mucosal immune network in the gut for the control of infectious diseases. Rev Med Virol. 2001;11:117–33. doi: 10.1002/rmv.307. [DOI] [PubMed] [Google Scholar]
- 15.Weiner HL, Friedman A, Miller A, et al. Oral tolerance. immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 16.Chung Y, Choi J, Chang Y-S, Cho S-H, Kang C-Y. Preventive and therapeutic effects of oral tolerance in a murine model of asthma. Immunobiology. 2002;206:408–23. doi: 10.1078/0171-2985-00190. [DOI] [PubMed] [Google Scholar]
- 17.Weiner HL, Mackin GA, Matsui M, Khoury EJ, Dawson DM, Hafler DA. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321–4. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 18.Trentham DE, Dyneisius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Swell KL, Hafler DA, Weiner HL. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–30. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 19.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8– dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–30. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 20.Chung Y, Chang SY, Kang CY. Kinetic analysis of oral tolerance: memory lymphocytes are refractory to oral tolerance. J Immunol. 1999;163:3692–8. [PubMed] [Google Scholar]
- 21.Mintern J, Li M, Davey GM, Blanas E, Kurts C, Carbone FR, Heath WR. The use of carboxyfluorescein diacetate succinimidyl ester to determine the site, duration and cell type responsible for antigen presentation in vivo. Immunol Cell Biol. 1999;77:539–43. doi: 10.1046/j.1440-1711.1999.00868.x. [DOI] [PubMed] [Google Scholar]
- 22.Blanas E, Davey GM, Carbone FR, Heath WR. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J Immunol. 2000;164:2890–6. doi: 10.4049/jimmunol.164.6.2890. [DOI] [PubMed] [Google Scholar]
- 23.Lee HO, Cooper CJ, Choi JH, Alnadjim Z, Barrett TA. The state of CD4+ T cell activation is a major factor for determining the kinetics and location of T cell responses to oral antigen. J Immunol. 2002;168:3833–8. doi: 10.4049/jimmunol.168.8.3833. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 25.Watts C. Immunology Inside the gearbox of the dendritic cell. Nature. 1997;388:724–5. doi: 10.1038/41900. [DOI] [PubMed] [Google Scholar]
- 26.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabrielle TB, Behrens GMN, Smith CM, et al. The CD8+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldonado-López R, De Smedt T, Michel P, et al. CD8α+ and CD8α– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samoilova EB, Horton JL, Zhang H, Khoury SJ, Weiner HL, Chen Y. CTLA-4 is required for the induction of high dose oral tolerance. Int Immunol. 1998;10:491–8. doi: 10.1093/intimm/10.4.491. [DOI] [PubMed] [Google Scholar]
- 30.Wakkach A, Fournier N, Brun V, Breittmayer J-P, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 31.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by TS cells. the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 32.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–67. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 33.Moore KW, Malefyt R, deWaal, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–65. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 34.Perez VL, Parijs LVan, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 35.Carreno BM, Collins M. The B7 family of ligands and its receptors. new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002 1997;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald RJ, Latchman YE, Sharpe AH. Negative co-receptors on lymphocytes. Curr Opin Immunol. 2002;14:391–6. doi: 10.1016/s0952-7915(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 37.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 38.Menges M, Rößner S, Voigtländer C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor-α induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2001;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nature Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 40.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–7. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 41.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–51. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeths MJ, Kedl RM, Mescher MF. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J Immunol. 1999;163:102–10. [PubMed] [Google Scholar]
- 43.Sun J, Houten NV. CD40 stimulation in vivo does not inhibit CD4 T cell tolerance to soluble antigens. Immunol Letter. 2002;84:125–32. doi: 10.1016/s0165-2478(02)00153-0. [DOI] [PubMed] [Google Scholar]