Abstract
Toll-like receptors (TLR) have been described as partially sharing signalling pathways but showing unique ligand specificity and tissue distribution. Here, the response of bovine macrophages (Mφ) and dendritic cells (DC), both derived from monocytes, was compared by exposing them to the TLR-specific ligands lipopolysaccharide, poly(I:C)-double-stranded RNA, and CpG-DNA, as well as inactivated Gram-negative and Gram-positive bacteria, shown to bind to TLR. The production of NO, superoxide anion, interleukin-10 (IL-10), IL-12 and tumour necrosis factor (TNF) was determined. Compared to monocytes, Mφ expressed more TLR2 and similar levels of TLR4 mRNA transcripts, as analysed by quantitative polymerase chain reaction, whereas DC expressed reduced amounts. Although both DC and Mφ recognized the TLR ligands, dramatic differences were seen in their reaction pattern to them. Both cell types responded with the production of TNF, but DC produced more IL-12, whereas Mφ produced more IL-10, regardless of the TLR agonist used. Co-stimulation with interferon-γ influenced the amount of cytokine production, but did not alter the cell type-specific response pattern. Compared to Mφ, DC produced >10 times less NO upon triggering with TLR ligands. In addition, DC produced superoxide anion to opsonized and non-opsonized zymosan, but not to phorbol 12-myristate 13-acetate, a response pattern confirmed for human Mφ and DC, respectively. Different protein kinase C isoforms and extracellular signal-regulated kinase patterns were detected in cell lysates of resting and stimulated Mφ and DC. Collectively, our results point to profound differences in pathogen-derived signal–response coupling occurring commensurate with distinct functions carried out by Mφ or DC.
Introduction
Terminal myeloid differentiation exhibits functional and phenotypic heterogeneity. A dynamic and complex pattern of gene expression influenced by cytokines and cell–cell interactions direct the differentiation along two major pathways prototypically represented by macrophages (Mφ) on the one hand and by dendritic cells (DC) on the other. Mφ and myeloid DC may be generated in vitro from monocytes exposed to different conditions1 with a common set of genes being expressed in their non-activated stage.2 Both cell types belong to the innate immune system and are specialized to mediate different functions, e.g. phagocytosis and secretion of inflammatory modulators/mediators in the case of Mφ, and antigen uptake, processing and presentation to T cells in the case of DC. Whereas, extensive studies were devoted to the question of how Mφ handle the pathogens they harbour, much less is known about DC in this regard.3,4 It is assumed that both differentiation forms of monocytoid cells recognize pathogens non-specifically by sensing pathogen-associated molecular patterns (PAMP) with pattern recognition receptors (PRR).
An important family of PRR are Toll-like receptors (TLR). Mammalian cells may express any of 10 distinct TLR thereby allowing recognition of, and reaction to a broad range of PAMP.5 TLR are constitutively as well as inducibly expressed in different tissues, and possess a unique specificity for certain ligands.6–8 Recent data suggest that the pattern of TLR expressed by different antigen-presenting cell (APC) subsets varies.9,10 Thus, levels of messenger RNA (mRNA) transcripts for TLR have been reported to change during the maturation of monocytes into Mφ or DC9,11 activating a cell-specific set of genes upon encounter with the same pathogen.2
The differences in gene activation may be the result of either engagement of a different set of PRR, or to different intracellular signalling pathways being activated in distinct differentiation stages. TLR share some common signalling pathways. Thus, TLR engagement by means of the intracellular Toll–interleukin-1 receptor (TIR) domain results in the activation of NF-κB.12,13 In addition, ligation of TLR stimulates phosphatidylinositol 3-kinase14 and protein kinase C (PKC).15,16 Furthermore, the evolutionarily conserved signalling intermediate in Toll pathway (ESCIT) bridges the common signalling pathway with the mitogen-activated protein kinase (MAPK) signalling cascade.17,18 Signalling through one of these pathways strongly activates Mφ and DC to up-regulate costimulatory molecules19 and to produce pro-inflammatory cytokines [tumour necrosis factor (TNF), interleukin-6 (IL-6) and IL-12],20–22 nitric oxide (NO)23 and recent evidence suggests an involvement of TLR2 and TLR4 signalling in the production of reactive oxygen intermediates (ROI).24,25
Few direct functional comparisons between monocyte-derived DC and Mφ exist, and none for the bovine system. In the present study, expression of TLR2 and TLR4 transcripts was compared between bovine monocytes, monocyte-derived Mφ and monocyte-derived DC. In addition, Mφ and DC were assessed with regard to effector functions induced by prototypic bacteria and by established TLR ligands. It is concluded
that members of the TLR family are differentially expressed by monocyte-derived Mφ and DC, and, of particular interest,
that TLR ligands induce distinct signals in these two forms of terminally differentiated monocytes, leading to
a different response to the same ligand.
The differences observed are consistent with Mφ primarily responding to bacteria-derived PAMP by expressing anti-microbial defence-related effector functions whereas these functions are down-regulated in DC and possibly compensated by other host defence mechanisms. The exact signalling pathways resulting in differential effector function activation remain to be elucidated.
Materials and methods
Animals and cell culture
Peripheral blood was collected from Swiss Brown cattle. Bovine peripheral blood mononuclear cells (PBMC) isolated by an adapted Ficoll–metrizoate procedure were used either for flow cytometry, or for the generation of monocyte-derived Mφ.26 For generation of Mφ, PBMC were sealed in Teflon bags (10–20 ml, 4 × 106 PBMC/ml) as described previously26 and cultured for 6–8 days at 37° in a humidified atmosphere of 5% CO2. The medium was RPMI-1640 containing 10 mm HEPES (pH 7.4), 100 IU/ml penicillin, 100 μg/ml streptomycin, 1% v/v non-essential amino acids for minimal essential medium (MEM; Invitrogen, Basel, Switzerland), 0.4% v/v vitamin solution for MEM (Invitrogen), 2 μm glutamine (Invitrogen), 40 μg/ml folic acid, 1 mm sodium pyruvate (Invitrogen), 2.5 μm amphotericin B (Invitrogen) and 15% heat-inactivated fetal calf serum (FCS; Invitrogen). This medium will be referred to as MφM. During this time, monocytes had matured to non-activated Mφ, which optimally responded to lipopolysaccharide (LPS) and Gram-negative organisms by NO generation and TNF production.26 From the cell mixture of variable composition, Mφ were purified by selective adherence to microtitre plate wells for 3 hr. After washing, the level of T-cell contamination was estimated to be 1–2%, based on immunocytochemical analysis (unpublished observation), with a viability >98%.
For the generation of bovine monocyte-derived DC, PBMC were also derived as described above, and DC were generated from PBMC as described elsewhere27 but with minor modifications. PBMC were incubated with a monoclonal antibody to the bovine CD14 molecule28 followed by goat anti-mouse immunoglobulin G1 (IgG1)-coated super-paramagnetic particles (Miltenyi-Biotech, Bergisch Gladbach, Germany), and labelled cells were isolated from a MidiMacs column (Miltenyi-Biotech) according to the manufacturer's instructions. The purity of the cells was evaluated by flow cytometry and shown in each case to be >98%. Cell viability was >98%. Monocytes were adjusted to 1 × 106/ml in RPMI-1640 medium containing Glutamax-I (Invitrogen), 10% heat-inactivated FCS, 5 × 10−5 m 2-mercaptoethanol, penicillin (100 IU/ml), streptomycin (100 μg/ml), recombinant bovine (rbo) IL-4 (33 ng/ml human IL-4 equivalents) expressed in Trypanosoma bruzei29 and rbogranulocyte–macrophage colony-stimulating factor (GM-CSF; 50 ng/ml).30 After 5–7 days of culture, DC were harvested, washed, and resuspended in RPMI-1640 medium containing FCS (10%) and 2-mercaptoethanol. At this time, the above cells had acquired the morphology and surface phenotype of bovine monocyte-derived DC described previously.27 They had down-regulated CD14, completely lacked expression of T-cell and B-cell markers, but had a more heterogeneous expression of major histocompatibility complex class II molecules.
Human monocyte-derived DC were generated essentially as described for bovine DC. In brief, PBMC were isolated from human buffy coats, followed by monocyte isolation using the above-described magnetic bead method. Culture condition and time were as described.27
TLR ligands and bacterial stimuli
The CpG oligode-oxynucleotide (ODN) 2059 (TCGTCGTTTTGTCGTTTGTCGTT; termed boCpG) and m2059 with methylated cysteines (TQGTQGTTTTGTQGTTTGTQGTTC; termed coCpG) were used to stimulate bovine Mφ and DC, or as control, respectively (TIB MOLBIOL, Berlin, Germany). These CpG ODN have been shown to induce a species-specific activation in bovine B cells and mononuclear phagocytes31 and were used at a final concentration of 5 μg/ml. Poly(I:C) double-stranded (ds) RNA (Sigma, Buchs, Switzerland) was used at a final concentration of 100 μg/ml. LPS (Escherichia coli O55:B5 Sigma L2637) was obtained from Sigma. The bacterial strains Listeria monocytogenes (NCTC 10,527) and Salmonella dublin (NZL 24–90) were kindly provided by Dr J. Nicolet (Institute of Veterinary Bacteriology, University of Berne, Switzerland). Bacteria were heat-inactivated (60°, 120 min), washed three times in phosphate-buffered saline (PBS) and stored frozen. They were used at a final concentration of 20 μg and 2 μg wet weight per ml, respectively. All substances except LPS and S. dublin were tested in the concentrations used or in a 1: 10 dilution (CpG) for contamination with endotoxin, using a turbidimetric kinetic Limulus amoebocyte lysate assay with a detection limit of 0.01 EU/ml. The latter was performed by the quality control division of the University Hospital (Inselspital) Bern, Switzerland, and none of the reagents contained detectable amounts of endotoxin.
Stimulation of cells
TLR ligands and bacteria were added with or without rbo interferon-γ (rboIFN-γ, 10 U/ml; kindly provided by Novartis, Basel, Switzerland) and cells were cultured in a total volume of 200 μl in microtitre plates. For nitrite and cytokine determination, cells were cultured for 24 hr, and supernatants were harvested and either analysed directly (nitrite analysis) or stored at −20° until assayed.
Analysis of bovine cytokines
Capture enzyme-linked immunosorbent assay (ELISA) for bovine cytokines (IL-12, IL-10 and TNF) were performed as described recently,32,33 using black 96-well microtitre plates (Microplate 96-well; Porvair, Shepperton, UK) and the Super Signal ELISA femto maximum sensitivity substrate (Pierce, Oxnard, CA). The relative light unit values were read by an Anthos LUCY 1.0 luminometer (Anthos Labtec, Salzburg, Austria).
Determination of NO
NO synthesis was determined by nitrite accumulation in the medium using the Griess reaction.34 Briefly, 50 μl of Mφ or DC culture supernatants was transferred to wells of new 96-well flat-bottom plates. Fifty microlitres of 1% w/v sulphanilamide (Sigma) and 0.1% w/v naphthylethylenediamine dihydrochloride (Sigma) in 2.5% H3PO4 was added to the supernatants and the absorbance at 540 nm was compared to a NaNO2 standard curve. Preliminary studies demonstrated that measurement by the Griess reaction was optimal after the cells were cultured with the appropriate stimulus for 24 hr, and that the majority of NO is converted into nitrite rather than nitrate under the present culture conditions.
Measurement of superoxide anion
The oxidative burst was determined in cells using lucigenin-enhanced chemiluminescence (LCL) as described.25 Briefly, cells were adjusted to 4 × 105 cells/ml in Hanks' balanced salt solution containing divalent cations (HBSS2+), and 250 μl of this cell suspension was transferred to polystyrene tubes (11 × 47 mm, Berthold Technologies, Wildbad, Germany) and incubated with 50 μm lucigenin (Sigma, St Louis, MO) at 37° for 45 min in the dark. Thereafter, LCL was measured following the addition of the various stimuli, using a LB 950 luminometer (Berthold Technologies) at time-points up to 80 min All samples were measured at least in duplicate and LCL is expressed as counts per minute over time. Test stimuli consisted of TLR agonists, and 4 × 10−7 m phorbol 12-myristate 13-acetate (PMA) or 50 μg/ml zymosan (both Sigma) were used as controls. Zymosan was boiled in distilled water, followed by opsonization with fresh undiluted bovine serum for 30 min at 37°, or left untreated, and was fresh-frozen. All substances were thawed immediately prior to use and diluted when necessary in HBSS2+. The specificity of the LCL reaction for superoxide anion was verified by adding superoxide dismutase (SOD, Sigma) at a concentration of 100 U/ml.
Western blot analysis of PKC and extracellular signal-regulated kinase (ERK) in whole cell lysate
Whole cell lysates from Mφ and DC were incubated for 30 min with or without PMA (5 × 10−7 m) or zymosan (50 μg/ml). Cells were harvested, counted, and washed once with ice cold PBS (300 g, 10 min, 4°), transferred to an Eppendorf tube and pelleted (2000 g, 10 min, 4°). Cell pellets (5 × 106) were lysed in 100 μl of lysis buffer (M-PERM Mammalian Protein Extraction Reagent from Pierce, Oxnard, CA) supplemented with 50 mm sodium fluoride, 1 mm sodium vanadate, 0.5 mm phenylmethylsulphonyl fluoride, 10 μg/ml aprotinin and 10 μg/ml leupeptin (all reagents from Roche Diagnostics, Rotkreuz, Switzerland). The cell material was sonicated for 15 seconds on ice, allowed to sit for 20 min, and then centrifuged at 15 000 g for 10 min The supernatant was removed and boiled for 3 min with 5 × Laemmli buffer. After measuring the protein content, 20 μg of each sample was loaded onto a 10% sodium dodecyl sulphate–polyacrylamide gel for electrophoresis, and run at 100 V for 1.5 hr in a MiniProtean chamber (Bio-Rad, Reinach, Switzerland). Cell proteins were blotted onto nitrocellulose (enhanced chemiluminescence; Amersham, Arlington Heights, IL) at 40 V for 4 hr. The nitrocellulose was blocked with 5% milk powder in PBS with 0.05% Tween-20 (TPBS) overnight, washed, and incubated for 2 hr at room temperature with the primary antibody. These included rabbit antibodies to PKC isoforms α, β, γ, δ, ε, η, ξ (Panvera, Madison, IL; diluted 1: 100 in TPBS), rabbit antibody to phosphorylated PKC (Sigma; diluted 1: 2000 in TPBS), monoclonal antibodies to ERK1, ERK2 (Santa Cruz Biotechnology, Heidelberg, Germany, diluted 1: 500 in TPBS), phosphorylated-ERK (p-ERK; Sigma; diluted 1: 10000 in TPBS), and to β-actin (Sigma, diluted 1: 3000 in TPBS). Blots were washed five times with TPBS and incubated for 1 hr with horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG antibody, respectively (Amersham, diluted 1: 3000 in TPBS). Immunoreactive bands were developed using a chemiluminescent substrate (ECL; Amersham). Antibody specificity was verified by running recombinant PKC isoform proteins (Oxford Biomedical Research, Michigan, IL) on each gel.
Reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR for bovine TLR
Plasmids encoding the full-length products for boTLR2, boTLR4, boTLR9 and the partial sequence for boTLR3, either recently cloned by our group or available in GenBank (Accession numbers AF368419, AY124007, AF310952 and AJ509825, respectively), were used as positive controls. The relative amount of TLR transcribed by Mφ and DC was assessed using the TaqMan™ real-time PCR technology as described recently.35 Briefly, total RNA was extracted from lysed cells using the RNeasy mini kit (Qiagen) and treated with RNase-free DNase I (Amersham Pharmacia Biotech, Uppsala, Sweden) to remove contaminating genomic DNA. The cDNA was synthesized using a first-strand cDNA synthesis kit from Promega (Wallisellen, Switzerland) in a reaction volume of 30 μl on a Gene AMP PCR System 9600 Thermal Cycler (Applied Biosystems, Foster City, CA). The primers and TaqMan probes were designed as described35 using the primer express software (Applied Biosystems). TaqMan PCR for the 18s ribosomal RNA control (Applied Biosystems) and bovine TLR were run as multiplex PCR in the same well and calculated using the comparative CT method (User Manual 2, Applied Biosystems). The PCR reactions contained 300 nm of each primer, 200 nm of the TaqMan probe and commercially available PCR mastermix (TaqMan Universal PCR Mastermix, Applied Biosystems), 1.25 μl of the 18s control, and 2.5 μl of the diluted cDNA sample in a total volume of 25 μl. The samples were placed in 96-well plates and amplified in an automated fluorimeter (ABI Prism 7700 Sequence Detection System, Applied Biosystems). Amplification conditions were 2 min at 50°, 10 min at 95°, 40 cycles of 15 s at 95°, and 60 s at 60°. The primer pairs finally used for RT-PCR and TaqMan PCR are listed in Tables 1 and 2.
Table 1.
Primers for RT-PCR
| TLR2: | forward: | ggctctcccttctgaatgc |
| reverse: | ctaggaccttattgcagctctc | |
| TLR3: | forward: | ctccccaatggaggaagaag |
| reverse: | cctcttcgcaaacagagtgc | |
| TLR4: | forward: | gcccagacagcatttcactc |
| reverse: | gccaccccaggaataaagtc | |
| TLR9: | forward: | tccaagtgctcgacctgagt |
| reverse: | caggttgttccgtgacaggt |
Table 2.
Primers for quantitative PCR (probes labelled 5′ FAM, 3′ TAMRA)
| TLR2: | forward: | acgacgccttcgtgtcctac |
| reverse: | gctcctggaccatgaggtttc | |
| probe: | cgagcgggattcctactgggtgg | |
| TLR4: | forward: | tggaggacatgccagtgct |
| reverse: | caccgacacactgatgatcgt | |
| probe: | agtttcaggaacgccacttgtcagctg |
Statistical analysis
All experiments were performed at least three times, and duplicate or triplicate samples were analysed in each experiment. Data are expressed as mean ±SD and are presented either as means obtained from three to five animals, or as results of a representative experiment.
Results
Differential expression of TLR2 and TLR4 mRNA by bovine Mφ and DC
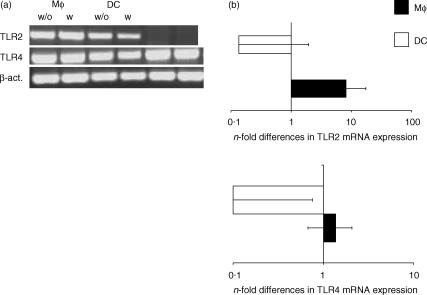
The presence of transcripts of TLR2, TLR3, TLR4, and TLR9 was determined by RT-PCR and real-time PCR in bovine monocytes, Mφ and DC. Highly purified or homogeneous cell populations were used for this purpose. Transcripts for TLR2 and TLR4, but not TLR3 and TLR9, could be detected in Mφ and DC (Fig. 1a), and this expression was not influenced by the presence of rboIFN-γ. However, the amount of transcript varied between the three cell types tested. Using freshly isolated monocytes as reference values, Mφ expressed eight times more TLR2 (Fig. 1b) and 1.5 times more TLR4 transcripts (Fig. 1c) on average than monocytes. In contrast, DC expressed lower amounts of TLR2 and TLR4 transcripts than monocytes (Fig. 1b,c).
Figure 1.
TLR2 and TLR4 mRNA expression in bovine Mφ and DC. The mRNA was extracted from 106 cells, and TLR expression was determined. (a) RT-PCR using primers specific for TLR2 and TLR4 in the absence (w/o) or presence (w) of rboIFN-γ; representative data from one animal are shown. (b) Real-time RT-PCR using primers and probes specific for TLR2 and TLR4 (n-fold differences as compared to freshly isolated monocytes, normalized to 18 seconds). Data from experiments with cells from five different individual donor animals are summarized.
DC differ from Mφ in their ability to produce NO
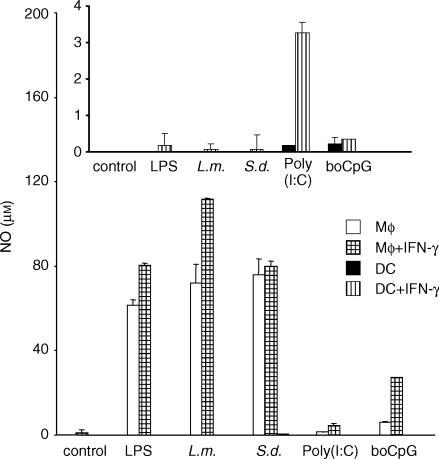
It has been shown for murine Mφ that TLR triggering results in NF-κB activation, which in combination with IFN-γ led to induction/up-regulation of inducible nitric oxide synthase (iNOS), the major NO-generating enzyme of Mφ.36 Important differences were noted between human and murine mononuclear phagocytes. For example, resting murine Mφ or Mφ-like cell lines produce more NO than maximally activated human counterparts.37,38 In addition, varying results on murine DC exist39,40 and bovine Mφ and DC were previously analysed by PCR only.41 Bovine Mφ and DC were exposed to various TLR agonists and to control stimuli with or without costimulation by rboIFN-γ, and their ability to mount a NO response was determined, using the Griess assay 24 hr later. Mφ reacted to most stimuli used with the generation of NO as evidenced by nitrite accumulation in the medium (Fig. 2), and this response was up-regulated by priming Mφ with rboIFN-γ when LPS, L. monocytogenes and the CpG motif were used as stimuli (Fig. 2). In contrast, stimulation of DC did not lead to the production of nitrite exceeding 3 μm, regardless of the stimuli and the presence or absence of rboIFN-γ (Fig. 2, insert). Even when using the strongest trigger combination for Mφ, a combination of heat-killed L. monocytogenes and rboIFN-γ, DC produced, on average, as little as 2 μm nitrite.
Figure 2.
Production of nitric oxide by Mφ and DC exposed to TLR ligands. Purified bovine Mφ or DC were exposed to various TLR ligands or whole bacteria, either alone or with 100 U of rboIFN-γ per ml (pattern bars). Cells treated with medium served as negative controls. levels were determined by the Griess assay. The results are presented as the mean NO concentrations for triplicate cultures and 1 SD, and show representative data from one animal. S.d. = S. dublin; L.m. = L. monocytogenes.
Differential cytokine induction in Mφ and DC
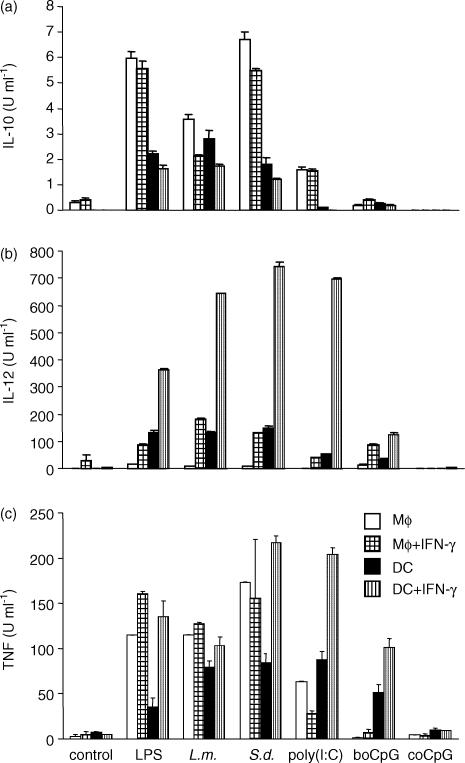
To compare whether bovine DC respond to the TLR ligands used with the release of cytokines, IL-10, IL-12 and TNF were measured in supernatants of bovine Mφ and DC stimulated with a variety of TLR ligands. Mφ and DC were incubated with TLR ligands in the absence or presence of rboIFN-γ as described for the NO assay. After 24 hr incubation, supernatants were harvested and analysed for cytokine content by ELISA. As shown in Fig. 3(a), Mφ, and to a lesser extent, DC, released IL-10 into the supernatant in response to LPS, L. monocytogenes, S. dublin and poly(I:C). In contrast, the CpG motif did not induce the release of IL-10 from either cell type. In all instances, the addition of rboIFN-γ to the cultures diminished the release of IL-10, regardless of the stimuli or the cell-type used. DC released measurable amounts of IL-12 into the supernatant. Similarly stimulated Mφ produced little, if any, IL-12. Mφ that had been costimulated with rboIFN-γ showed a significant amount of IL-12 (Fig. 3b), and costimulation of DC with IFN-γ also up-regulated IL-12 production (Fig. 3b). All TLR ligands induced a TNF response in DC (Fig. 3c); likewise, all TLR ligands induced a TNF response in Mφ, with the possible exception of bovine CpG-stimulated cells. This response was consistently higher in rboIFN-γ costimulated cells (Fig. 3c).
Figure 3.
Cytokine production by Mφ and DC exposed to TLR ligands. Purified bovine Mφ or DC were exposed to various TLR ligands or whole bacteria for 24 hr, either alone or with 100 U of rboIFN-γ per ml (pattern bars). IL-10, IL-12 and TNF were measured in the supernatant by ELISA. Samples were analysed in triplicates, and mean values of three different experiments are shown (means ± SD). S.d. = S. dublin; L.m. = L. monocytogenes.
DC differ from Mφ in their ability to produce superoxide anion
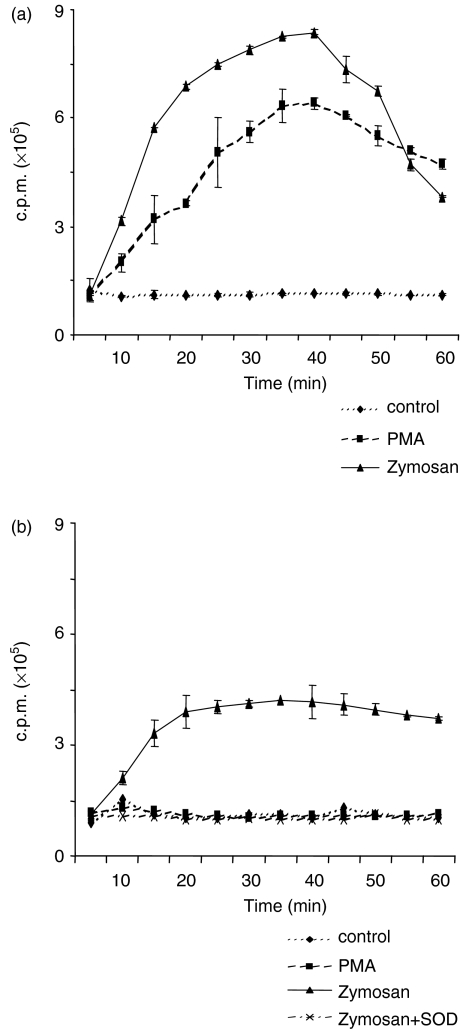
Triggering of an oxidative burst by bacterial lipopeptide and LPS is mediated by TLR2 and TLR4, respectively, in other species.25,42 As TLR ligands or whole bacteria have not been used to determine an oxidative burst in the bovine system, bovine Mφ and DC were exposed to LPS, non-opsonized and opsonized zymosan, and PMA, and the resulting superoxide generation was analysed by LCL. Typically, the LCL response rapidly increased within the first 10 min (for LPS) or 30 min after the addition of PMA and zymosan in Mφ, and declined thereafter to little more than baseline levels about 60 min after stimulation (Fig. 4a). However, Mφ did not respond with a LCL response to L. monocytogenes and S. dublin (data not shown). In contrast, bovine DC reacted with a LCL response to opsonized zymosan and non-opsonized zymosan (data not shown) exclusively, and failed to react to LPS or PMA, the latter being one of the strongest LCL inducers in many cell types including monocytes and Mφ (Fig. 4b,c). To confirm that LCL reactions seen in both zymosan-exposed Mφ and DC monitored for superoxide anion generation, both cell types were stimulated with opsonized zymosan, and LCL was measured in the presence or absence of the superoxide anion scavenger, superoxide dismutase (SOD). In both cases, SOD completely abolished zymosan-induced LCL (Fig. 4a,b).
Figure 4.
Generation of superoxide anion by bovine Mφ and DC exposed to PMA, zymosan and LPS. Lucigenin-enhanced chemiluminescence (LCL) activity of Mφ (a) and DC (b) was analysed by incubating cells with 50 μm lucigenin in the dark before adding 5 × 10−7 m PMA, 50 μg/ml non-opsonized zymosan or zymosan opsonized with bovine serum, or LPS. (c) (E. coli O55:B5 1 μg/ml). All samples were measured in duplicate and LCL is expressed as counts per minute (c.p.m.) over time. The specificity of the LCL reaction was tested by adding superoxide dismutase (SOD; 100 U/ml). Representative data obtained from the same donor are shown in (a) and (b).
As it is suggested that murine DC are able to elicit an oxidative burst43 the ability of human Mφ and DC to react by a LCL signal to the same stimuli was determined. Our findings paralleled those made with bovine cells, i.e. human DC strongly reacted to opsonized zymosan, but failed to react to PMA (Fig. 5b), whereas human Mφ reacted to both (Fig. 5a).
Figure 5.
Generation of superoxide anion by human Mφ and DC exposed to PMA and zymosan. Lucigenin-enhanced chemiluminescence (LCL) activity of Mφ (a) and DC (b) analysed by incubating cells with 50 μm lucigenin in the dark before adding 5 × 10−7 m PMA, 50 μg/ml opsonized zymosan. All samples were measured in duplicates and LCL is expressed as counts per minute (c.p.m.) over time. Specificity of LCL reaction was tested by adding superoxide dismutase (100 U/ml).
Mφ and DC differentially express PKC isoforms
Several reports showed that PKC is not only involved in activation of gene transcription after TLR ligation15,16 but also plays an important part in the induction of an oxidative burst by PMA and zymosan.44 Our previous results pointed to differences in the signalling cascade leading to activation of NAD(P)H-dependent oxidase. As a first step, the presence or absence of different PKC isoforms was analysed by immunoblotting using cellular extracts of Mφ and DC stimulated with PMA (LCL inducer in Mφ only) and zymosan (LCL inducer in both cell types tested). To determine the relative amounts of PKC isoforms in Mφ and DC, both cell types were isolated from the same donors and analysed for the presence of various PKC isoforms. Initially, the Ca2+-dependent isoforms PKCα, -β1, -β2 and -γ were assessed. PKCα was present in DC and could not be detected in cellular extracts of Mφ (Fig. 6a). PKCβ1, -β2 and -γ were not detected in either Mφ or DC although the antibodies used reacted strongly, at least in the case of PKCβ1 and -γ, with the recombinant protein. Subsequently, the Ca2+-independent isoforms (novel and atypical) PKCδ, -ε, -η and -ζ were determined. PKCδ was detected in cellular extracts of stimulated DC, but not in extracts of unstimulated DC or Mφ. PKCε, -η and -ζ were present in Mφ and DC, with a tendency of PKCη to be present at a greater amount in cellular extracts of stimulated Mφ. As stimulation of PKC isoforms might subsequently lead to the stimulation of the MAPK pathway, we analysed whether stimulation of Mφ and DC exposed to either PMA or zymosan may lead to differences in ERK1 and ERK2 activation. Both kinases were detected in cellular extracts of Mφ and DC, regardless of whether cells had been stimulated or left untreated. However, cellular extracts of Mφ displayed a different banding pattern of ERK1 compared to DC (Fig. 6b). In addition, only cellular extracts of Mφ stimulated with PMA and zymosan reacted with the antibody to p-ERK (Fig. 6c), whereas no conclusive result could be obtained using the p-PKC antibody.
Figure 6.
Presence of PKC isoforms, ERK1, ERK2 and p-ERK in cellular extracts of Mφ and DC exposed to PMA and zymosan. Cell proteins were extracted from whole cells treated for 30 min at 37° with PMA or opsonized zymosan (oz), or left untreated and analysed for the presence of PKC isoforms as well as ERK1, ERK2 and p-ERK by Western blotting. (a) Presence of different PKC isoforms in Mφ compared to DC. (b) Presence of ERK1, ERK2, proteins in whole cell protein extracts from Mφ or DC incubated with PMA, zymosan, or left untreated. (c) p-ERK detected in whole cell protein extracts from unstimulated and stimulated Mφ or DC. A representative set of data of three independent experiments (two for p-ERK) is shown.
Discussion
Mφ and DC express TLR, allowing a broad range of pathogens or their constituents to be recognized. Although the signalling pathways of TLR have been extensively studied5 it is not known whether Mφ and DC react differently to pathogens. Here, we addressed this issue in the bovine system by exploiting the ability to generate and compare cells derived from the same donor. We investigated whether the two cell types react differentially to established TLR ligands and to whole heat-killed bacteria known to be TLR agonists. As such a difference was observed, it was addressed whether this could be explained by differential TLR expression, or rather by differences in post-receptor-binding events, such as availability of certain signalling molecules. We show that upon triggering by the same set of TLR agonists, Mφ primarily produce IL-10, whereas DC produce primarily IL-12; that Mφ produce 10 times more NO regardless of the stimuli used; and that DC may produce superoxide anion in principle, but fail to react to LPS and PMA. Our data are consistent with the view that monocyte-derived Mφ and DC have a distinct response pattern upon engagement of PRR, most probably TLR.
Both Mφ and DC expressed substantial amounts of TLR2 and TLR4 mRNA transcripts, but the levels of expression decreased markedly during differentiation into DC, as described for TLR-agonist-matured DC.9 In contrast, we were not able to detect transcripts for TLR3 and TLR9. Whereas the lack of mRNA transcripts for TLR9 is in accordance with other reports on TLR9 expression in myeloid DC,45 the lack of TLR3 transcripts may be because of either a lack in sensitivity of the PCR reaction or maturation processes leading to the down-regulation of TLR3.9,11 Interestingly, both Mφ and DC still reacted to TLR3 and TLR9 ligands, mainly in the presence of IFN-γ, suggesting that other pathways of stimulation may account for the observed production of NO and cytokines. Internalized poly(I:C) could lead to the activation of protein kinase R or IFN-response factor 146,47 subsequently leading to the production of NO and cytokines. Murine monocytes, Mφ and DC have been described as being directly activated by CpG-ODN, whereas the corresponding human cells lack TLR9 expression and are not activated by CpG-ODN.48,49 However, human monocytes have been described to react to CpG-ODN50 but only in the presence of either PBMC51 or plasmacytoid DC via cytokines secreted by these cells.10 Thus, one possible explanation for the reaction of bovine Mφ and DC in the present study might be that the cultures contained a small percentage of PBMC or plasmacytoid DC-like cells, subsequently leading to the production of NO by bovine Mφ and DC.
Since high-quality antibodies are still not available for TLR, and are completely lacking for the bovine system, reports rely on mRNA expression profiles, and thus data obtained have to be interpreted with caution. However, a reduction of surface-expressed CD14, and a reduction in the amount of TLR2 and TLR4 mRNA transcripts point to decreased sensitivity of DC to bacteria-derived PAMP when compared with macrophages. On the other hand, it was earlier shown that bacterial PAMP-induced NO synthesis is a differentiation-dependent trait associated with the maturation of bovine monocytes to Mφ.26 Nevertheless, the observation that regardless of TLR specificity, distinct effector functions were preferentially observed in Mφ or DC is difficult to explain with a general modulation of TLR expression and points to cell-differentiation stage-dependent expression of signalling pathways. This is not without precedent as myeloid DC and plasmacytoid DC were shown to react differentially to a TLR7 agonist.52
Remarkable species variability exists for the ability of APC to express iNOS and synthesize NO. At least in vitro, maximally activated human Mφ produce less NO than unstimulated murine counterparts.38,53 Cultured bovine Mφ and Mφ in vivo may be induced to synthesize at least 10-fold higher amounts of NO than counterparts from small ruminants.54 Furthermore, at least some murine DC were reported to synthesize NO in similar amounts as murine Mφ55 whereas human DC neither expressed iNOS nor produced high amounts of NO.55–57 Here, we provide evidence that bovine monocyte-derived Mφ, but not bovine monocyte-derived DC, produced high amounts of NO in response to TLR ligation. Whereas the involvement of an autocrine IFN-β loop, as shown for the murine system,23,58 is not known in the bovine system, a clear up-regulation of NO synthesis by IFN-γ was shown for the putative TLR2 ligand, heat-killed L. monocytogenes59 and the TLR9 ligand, bovine CpG.7,31
We also observed differences between the APC types studied in their cytokine response to TLR ligands or whole heat-killed bacteria. Bovine Mφ and DC released similar amounts of TNF into the supernatant in response to nearly all TLR ligands tested, similar to observations in other species.60,61 In accordance with the finding that the main source of IL-12 in vivo are DC but not Mφ62,63 we found considerably higher concentrations in supernatants of TLR agonist-exposed DC than Mφ, particularly when costimulated with IFN-γ (Fig. 3b). In contrast, bovine Mφ produced high levels of IL-10 but little IL-12. This essentially proved that DC are able to react to TLR ligands, and points to post-recognition deficiencies when effector functions other than TNF production were studied. This difference between Mφ and DC in their response to bacterial triggers may be a differentiation-dependent process.64 IL-4, one of the factors used in DC differentiation, is known to down-regulate bacterially triggered NO synthesis by bovine Mφ.65 This down-regulation may be required for an optimal IL-12 signal elicited in DC (this paper), as NO was shown to curtail an IL-12 response.66 Thus, the inhibition of IL-12 production, but not TNF, in Mφ may be the result of the NO production66 whereas bovine and human DC, lacking iNOS and NO production,55,57 are not affected.
Murine DC responded to opsonized and non-opsonized zymosan with an oxidative burst43 whereas human DC were not capable of eliciting an oxidative burst to PMA.67 The conflict whether or not DC are able to elicit an oxidative burst can now easily be solved in the light of our data. In the present study, both bovine and human DC failed to react to PMA with an oxidative burst, thus confirming data published by other groups.67,68 Similarly, murine bone marrow-derived DC responded to opsonized and non-opsonized zymosan, but not to PMA with an LCL signal (Jungi, unpublished observation). This inability may depend on the presence of IL-4, leading to the absence of cytochrome b558 subunits.68 However, DC of both species show a strong oxidative burst response to non-opsonized and opsonized zymosan, similar to that described for murine DC.43 This suggests that zymosan acts via a mechanism that is different from that of PMA. This mechanism does not involve Fc receptors, as the reaction was independent of opsonization. Although pathogens have been shown to enter DC, and even induce a weak oxidative burst69 there is as yet no example of a pathogen shown to be eliminated by DC by the ROI pathway. Thus, it may be concluded that DC possess the cellular machinery to generate ROI, but this is activated in a far more restricted manner than in Mφ.
Engagement of TLR results in downstream activation of ECSIT18 bridging TNF receptor-associated factor 6 (TRAF6) with the activation of the MAPK signalling cascade. This results in activation of NF-κB and AP-1 via c-jun N-terminal kinase, p38 and ERK17 subsequently leading to the production of cytokines and the generation of ROI.5,70 As both Mφ and DC express similar subsets of TLR, fine-tuning of the inflammatory response to a pathogen at the intracellular signalling level appears to play an important role in the generation of pathogen-specific responses.
Superoxide anion production depends, at least partially, on the activation of PKC which contributes to the activation of the NADPH-dependent oxidase. This enzyme is activated in human and murine monocytes and Mφ with PMA and the TLR2/TLR6 heterodimer ligand zymosan.71–73 Furthermore, differentiation towards Mφ was reported to require sustained PKC activation in different systems.74–76 Therefore, the observed changes in the PKC isoform profile seen in protein extracts of bovine Mφ and DC could represent maturation-dependent differences that lead to an altered function of the cells. In addition, phosporylated ERK was detected only in Mφ, but not in DC. Activation of PKC isoforms stimulates the ERK pathway that is also involved in the activation of the NADPH oxidase.77,78 Thus, our observation is in-line with others showing that some TLR agonists, such as LPS and CpG-ODN, activate ERK in Mφ, but not in DC. This pathway was reported to induce the up-regulation of TNF production, but the down-regulation of IL-12 production.79 Thus, we suggest that the differential induction of cytokines and possibly iNOS by TLR agonists as seen in the present study is the result of differential intracellular signalling pathways expressed in the respective cells. It remains to be seen which differentiation-stage-specific molecules restrict the response of DC to stimuli of reactive oxygen generation.
We are aware of several limitations in this type of experiment. Firstly, because of a lack of blocking antibodies, studies of TLR triggering currently rest on the use of established ligands for a single TLR. The pathogens used could engage more than one PRR, including PRR other than TLR. For example, co-receptors such as dectin-1 were shown to be necessary for reactive oxygen generation.80 Evidence has been provided that zymosan interacts with heterodimers of TLR2 and TLR6, and possibly dectin-170,81 and heat-killed L. monocytogenes selectively interacts with TLR2.59 The TLR agonists used in this study encompassed highly purified poly(I:C), chromatographically pure CpG and highly purified LPS shown to be TLR4-dependent in a C3H/HeJ Mφ system (Remer and Jungi, unpublished), and the presence of contaminating endotoxin was thoroughly ruled out in the non-LPS samples. The second restriction concerns the cells investigated. Given the versatility of the monocytoid cell system, Mφ and DC generated in vitro have to be regarded as prototypes of two differentiation forms, but the in vivo correlate of these cells is not yet clear and has not yet been identified in the bovine system. Whereas the signal promoting monocyte differentiation towards Mφ is still controversial,82,83 the differentiation of DC is governed by IL-4 and GM-CSF.
In summary, our comparison of the ability of bovine Mφ and DC to express TLR and to respond to a variety of TLR ligands suggests that these two monocyte-derived cell types show differences in the level of expression of TLR, particularly of TLR2. These two cell types also differ in the expression of CD14, the binding site for a variety of ligands of bacterial origin. Moreover, beyond receptor expression, these two cell types respond to putative TLR agonists in a distinct and differentiation-stage-specific manner suggesting that post-receptor-binding signalling events are distinct. It will be of interest to study the sites in the respective signalling cascades at which these two cell types differ.
Acknowledgments
We thank Dr I. Roditi (Institute of Cell Biology, University of Bern) for providing rboIL-4 and Dr S. Inamura (National Institute of Animal Health, Tsukuba, Japan) for boGM-CSF. We are grateful to M. Brcic (Institute of Veterinary Virology) for the excellent technical assistance and Dr E. Peterhans (Institute of Veterinary Virology) for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation Grant 32-65036.02 (to T.W.J.)
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, Nutman TB. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Yrlid U, Svensson M, Johansson C, Wick MJ. Salmonella infection of bone marrow-derived macrophages and dendritic cells. influence on antigen presentation and initiating an immune response. FEMS Immunol Med Microbiol. 2000;27:313. doi: 10.1111/j.1574-695X.2000.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 5.Dalpke A, Heeg K. Signal integration following Toll-like receptor triggering. Crit Rev Immunol. 2002;22:217. [PubMed] [Google Scholar]
- 6.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 9.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 10.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 11.Muzio M, Bosisio D, Polentarutti N, D'Amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 12.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 13.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 14.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Lafuse WP, Zwilling BS. Regulation of toll-like receptor 2 expression by macrophages following Mycobacterium avium infection. J Immunol. 2000;165:6308. doi: 10.4049/jimmunol.165.11.6308. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Jacinto R, McCall C, Li L. Regulation of IL-1 receptor-associated kinases by lipopolysaccharide. J Immunol. 2002;168:3910. doi: 10.4049/jimmunol.168.8.3910. [DOI] [PubMed] [Google Scholar]
- 17.Hacker H, Mischak H, Hacker G, Eser S, Prenzel N, Ullrich A, Wagner H. Cell type-specific activation of mitogen-activated protein kinases by CpG-DNA controls interleukin-12 release from antigen-presenting cells. Embo J. 1999;18:6973. doi: 10.1093/emboj/18.24.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway CA, Ghosh S. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166:2444. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 20.Ozinsky A, Smith KD, Hume D, Underhill DM. Co-operative induction of pro-inflammatory signaling by Toll-like receptors. J Endotoxin Res. 2000;6:393. [PubMed] [Google Scholar]
- 21.Thoma-Uszynski S, Kiertscher SM, Ochoa MT, Bouis DA, Norgard MV, Miyake K, Godowski PJ, Roth MD, Modlin RL. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000;165:3804. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 22.Hume DA, Underhill DM, Sweet MJ, Ozinsky AO, Liew FY, Aderem A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2:11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 24.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 25.Remer KA, Brcic M, Jungi TW. Toll-like receptor-4 is involved in eliciting an LPS-induced oxidative burst in neutrophils. Immunol Lett. 2002;85:75. doi: 10.1016/s0165-2478(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 26.Jungi TW, Thony M, Brcic M, Adler B, Pauli U, Peterhans E. Induction of nitric oxide synthase in bovine mononuclear phagocytes is differentiation stage-dependent. Immunobiology. 1996;195:385. doi: 10.1016/S0171-2985(96)80054-4. [DOI] [PubMed] [Google Scholar]
- 27.Werling D, Hope JC, Chaplin P, Collins RA, Taylor G, Howard CJ. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]
- 28.Sopp P, Kwong LS, Howard CJ. Identification of bovine CD14. Vet Immunol Immunopathol. 1996;52:323. doi: 10.1016/0165-2427(96)05583-3. [DOI] [PubMed] [Google Scholar]
- 29.Furger A, Jungi TW, Salomone JY, Weynants V, Roditi I. Stable expression of biologically active recombinant bovine interleukin-4 in Trypanosoma brucei. FEBS Lett. 2001;508:90. doi: 10.1016/s0014-5793(01)03031-9. [DOI] [PubMed] [Google Scholar]
- 30.Hirai T, Oikawa M, Inumaru S, Yokomizo Y, Kusakari N, Mori K. Effects of recombinant bovine granulocyte-macrophage colony-stimulating factor on bovine peripheral blood neutrophil functions in vitro and in vivo. J Vet Med Sci. 1999;61:1249. doi: 10.1292/jvms.61.1249. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Shoda LK, Brayton KA, Estes DM, Palmer GH, Brown WC. Induction of interleukin-6 and interleukin-12 in bovine B lymphocytes, monocytes, and macrophages by a CpG oligodeoxynucleotide (ODN 2059) containing the GTCGTT motif. J Interferon Cytokine Res. 2001;21:871. doi: 10.1089/107999001753238123. [DOI] [PubMed] [Google Scholar]
- 32.Kwong LS, Hope JC, Thom ML, Sopp P, Duggan S, Bembridge GP, Howard CJ. Development of an ELISA for bovine IL-10. Vet Immunol Immunopathol. 2002;85:213. doi: 10.1016/s0165-2427(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 33.Hope JC, Kwong LS, Entrican G, Wattegedera S, Vordermeier HM, Sopp P, Howard CJ. Development of detection methods for ruminant interleukin (IL) -12. J Immunol Meth. 2002;266:117. doi: 10.1016/s0022-1759(02)00113-8. [DOI] [PubMed] [Google Scholar]
- 34.Adler H, Frech B, Thony M, Pfister H, Peterhans E, Jungi TW. Inducible nitric oxide synthase in cattle. Differential cytokine regulation of nitric oxide synthase in bovine and murine macrophages. J Immunol. 1995;154:4710. [PubMed] [Google Scholar]
- 35.Werling D, Collins RA, Taylor G, Howard CJ. Cytokine responses of bovine dendritic cells and T cells following exposure to live or inactivated bovine respiratory syncytial virus. J Leukoc Biol. 2002;72:297. [PubMed] [Google Scholar]
- 36.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184. [PubMed] [Google Scholar]
- 38.Schneemann M, Schoedon G. Species differences in macrophage NO production are important. Nat Immunol. 2002;3:102. doi: 10.1038/ni0202-102a. [DOI] [PubMed] [Google Scholar]
- 39.Blank C, Bogdan C, Bauer C, Erb K, Moll H. Murine epidermal Langerhans cells do not express inducible nitric oxide synthase. Eur J Immunol. 1996;26:792. doi: 10.1002/eji.1830260410. [DOI] [PubMed] [Google Scholar]
- 40.Qureshi AA, Hosoi J, Xu S, Takashima A, Granstein RD, Lerner EA. Langerhans cells express inducible nitric oxide synthase and produce nitric oxide. J Invest Dermatol. 1996;107:815. doi: 10.1111/1523-1747.ep12330572. [DOI] [PubMed] [Google Scholar]
- 41.Norimatsu M, Harris J, Chance V, Dougan G, Howard CJ, Villarreal-Ramos B. Differential response of bovine monocyte-derived macrophages and dendritic cells to infection with Salmonella typhimurium in a low-dose model in vitro. Immunology. 2003;108:55. doi: 10.1046/j.1365-2567.2003.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aliprantis AO, Weiss DS, Zychlinsky A. Toll-like receptor-2 transduces signals for NF-kappa B activation, apoptosis and reactive oxygen species production. J Endotoxin Res. 2001;7:287. [PubMed] [Google Scholar]
- 43.Marcinkiewicz J, Nowak B, Grabowska A, Bobek M, Petrovska L, Chain B. Regulation of murine dendritic cell functions in vitro by taurine chloramine, a major product of the neutrophil myeloperoxidase-halide system. Immunology. 1999;98:371. doi: 10.1046/j.1365-2567.1999.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Downey GP, Chan CK, Lea P, Takai A, Grinstein S. Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J Cell Biol. 1992;116:695. doi: 10.1083/jcb.116.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colonna M, Krug A, Cella M. Interferon-producing cells. on the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14:373. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 46.Der SD, Lau AS. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc Natl Acad Sci USA. 1995;92:8841. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blair LA, Maggi LB, Jr, Scarim AL, Corbett JA. Role of interferon regulatory factor-1 in double-stranded RNA-induced iNOS expression by mouse islets. J Biol Chem. 2002;277:359. doi: 10.1074/jbc.M109819200. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann G, Weiner GJ, Krieg AM. CpG DNA. a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci USA. 1999;96:9305. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci United States America. 2001;98:9237. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer M, Redecke V, Ellwart JW, Scherer B, Kremer JP, Wagner H, Lipford GB. Bacterial CpG-DNA triggers activation and maturation of human CD11c-, CD123+ dendritic cells. J Immunol. 2001;166:5000. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- 51.Hartmann G, Krieg AM. CpG DNA and LPS induce distinct patterns of activation in human monocytes. Gene Ther. 1999;6:893. doi: 10.1038/sj.gt.3300880. [DOI] [PubMed] [Google Scholar]
- 52.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 53.Weinberg JB. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med. 1998;4:557. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfister H, Remer KA, Brcic M, Fatzer R, Christen S, Leib S, Jungi TW. Inducible nitric oxide synthase and nitrotyrosine in listeric encephalitis: a cross-species study in ruminants. Vet Pathol. 2002;39:190. doi: 10.1354/vp.39-2-190. [DOI] [PubMed] [Google Scholar]
- 55.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577. [PubMed] [Google Scholar]
- 56.Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168:2997. doi: 10.4049/jimmunol.168.6.2997. [DOI] [PubMed] [Google Scholar]
- 57.Paolucci C, Rovere P, De Nadai C, Manfredi AA, Clementi E. Nitric oxide inhibits the tumor necrosis factor alpha -regulated endocytosis of human dendritic cells in a cyclic GMP-dependent way. J Biol Chem. 2000;275:19638. doi: 10.1074/jbc.M000511200. [DOI] [PubMed] [Google Scholar]
- 58.Weinstein SL, Finn AJ, Dave SH, Meng F, Lowell CA, Sanghera JS, DeFranco AL. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-beta. J Leukoc Biol. 2000;67:405. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- 59.Flo TH, Halaas O, Lien E, Ryan L, Teti G, Golenbock DT, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 60.Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, Fenton MJ. Different Toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol. 2001;69:1036. [PubMed] [Google Scholar]
- 61.Qi H, Denning TL, Soong L. Differential induction of interleukin-10 and interleukin-12 in dendritic cells by microbial toll-like receptor activators and skewing of T-cell cytokine profiles. Infect Immun. 2003;71:3337. doi: 10.1128/IAI.71.6.3337-3342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Medical. 1997. pp. 186–1819. [DOI] [PMC free article] [PubMed]
- 64.Jungi TW, Adler H, Adler B, Thony M, Krampe M, Peterhans E. Inducible nitric oxide synthase of macrophages. Present knowledge and evidence for species-specific regulation. Vet Immunol Immunopathol. 1996;54:323. doi: 10.1016/s0165-2427(96)05690-5. [DOI] [PubMed] [Google Scholar]
- 65.Jungi TW, Brcic M, Sager H, Dobbelaere DA, Furger A, Roditi I. Antagonistic effects of IL-4 and interferon-gamma (IFN-gamma) on inducible nitric oxide synthase expression in bovine macrophages exposed to gram-positive bacteria. Clin Exp Immunol. 1997;109:431. doi: 10.1046/j.1365-2249.1997.4891384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang FP, Niedbala W, Wei XQ, Xu D, Feng GJ, Robinson JH, Lam C, Liew FY. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur J Immunol. 1998;28:4062. doi: 10.1002/(SICI)1521-4141(199812)28:12<4062::AID-IMMU4062>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 67.Fanger NA, Voigtlaender D, Liu C, Swink S, Wardwell K, Fisher J, Graziano RF, Pfefferkorn LC, Guyre PM. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor Fc gamma RI (CD64) expressed on human blood dendritic cells. J Immunol. 1997;158:3090. [PubMed] [Google Scholar]
- 68.Yu D, Imajoh-Ohmi S, Akagawa K, Kanegasaki S. Suppression of superoxide-generating ability during differentiation of monocytes to dendritic cells. J Biochem (Tokyo) 1996;119:23. doi: 10.1093/oxfordjournals.jbchem.a021211. [DOI] [PubMed] [Google Scholar]
- 69.Newman SL, Holly A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect Immun. 2001;69:6813. doi: 10.1128/IAI.69.11.6813-6822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Underhill DM. Mini-review Toll-like receptors: networking for success. Eur J Immunol. 2003. pp. 33–1767. [DOI] [PubMed]
- 71.Sakata A, Ida E, Tominaga M, Onoue K. Arachidonic acid acts as an intracellular activator of NADPH-oxidase in Fc gamma receptor-mediated superoxide generation in macrophages. J Immunol. 1987;138:4353. [PubMed] [Google Scholar]
- 72.Larsen EC, DiGennaro JA, Saito N, Mehta S, Loegering DJ, Mazurkiewicz JE, Lennartz MR. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J Immunol. 2000;165:2809. doi: 10.4049/jimmunol.165.5.2809. [DOI] [PubMed] [Google Scholar]
- 73.Gozal E, Forman HJ, Torres M. ADP stimulates the respiratory burst without activation of ERK and AKT in rat alveolar macrophages. Free Radic Biol Med. 2001;31:679. doi: 10.1016/s0891-5849(01)00630-x. [DOI] [PubMed] [Google Scholar]
- 74.Aihara H, Asaoka Y, Yoshida K, Nishizuka Y. Sustained activation of protein kinase C is essential to HL-60 cell differentiation to macrophage. Proc Natl Acad Sci USA. 1991;88:11062. doi: 10.1073/pnas.88.24.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mischak H, Pierce JH, Goodnight J, Kazanietz MG, Blumberg PM, Mushinski JF. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-alpha and – delta and not by protein kinase C-beta II-epsilon-zeta, and – eta. J Biol Chem. 1993;268:20110. [PubMed] [Google Scholar]
- 76.Davis TA, Saini AA, Blair PJ, Levine BL, Craighead N, Harlan DM, June CH, Lee KP. Phorbol esters induce differentiation of human CD34+ hemopoietic progenitors to dendritic cells: evidence for protein kinase C-mediated signaling. J Immunol. 1998;160:3689. [PubMed] [Google Scholar]
- 77.El Benna J, Faust RP, Johnson JL, Babior BM. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. J Biol Chem. 1996;271:6374. doi: 10.1074/jbc.271.11.6374. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. [DOI] [PubMed] [Google Scholar]
- 78.El Benna J, Han J, Park JW, Schmid E, Ulevitch RJ, Babior BM. Activation of p38 in stimulated human neutrophils. phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys. 1996;334:395. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 79.Hacker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, Heeg K, Lipford GB, Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. Embo J. 1998;17:6230. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akiyama Y, Griffith R, Miller P, Stevenson GW, Lund S, Kanapa DJ, Stevenson HC. Effects of adherence, activation and distinct serum proteins on the in vitro human monocyte maturation process. J Leukoc Biol. 1988;43:224. doi: 10.1002/jlb.43.3.224. [DOI] [PubMed] [Google Scholar]
- 83.Kreutz M, Andreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood. 1990;76:2457. [PubMed] [Google Scholar]