Abstract
The aim of this study was to examine the mechanism of Epstein–Barr virus (EBV) activation by soluble factors from the inflamed salivary glands of patients with Sjogren's syndrome (SS). Saliva from SS patients was used to examine the regulation of EBV activation by an inflammatory salivary microenvironment. Transient transfection of the EBV-negative salivary gland cell line (HSY) with BZLF1, a trans-activating EBV gene promoter-fusion construct (Zp-luc), was used in this study. The results showed that under conditions where the BZLF1 promoter is activated by potent stimuli, SS saliva (from eight of 12 patients) exerts a significant effect on expression of the luciferase gene. A specific inhibitor of protein kinase C did not affect the SS saliva-induced Zp-luc activity, whereas treatment with inhibitors of calmodulin, calcineurin and IP3, dose-dependently decreased this induction. Transforming growth factor β1 (TGF-β1), which is known to be expressed in SS salivary glands, dose-dependently induced Zp-luc activity. Hence, these results demonstrate the activation of EBV by SS saliva and suggest that EBV activation at the inflammatory site may occur in the presence of TGF-β1 via triggering of the mitogen-activated protein kinase (MAPK) kinase signalling pathway.
Introduction
Sjogren's syndrome (SS) is an organ-specific disorder affecting the salivary and lacrimal glands and leads to clinical symptoms of dryness of the mouth and eyes. Although the pathogenesis of SS remains unclear, there is reportedly a high incidence of Epstein–Barr virus (EBV) reactivation in SS, contributing to the initiation or perpetuation of an immune response in target organs. EBV antigens and EBV DNA have been found in infiltrating lymphocytes and salivary gland epithelial cells of SS patients.1,2 Infectious EBV is present in both the saliva of SS patients3–5 and culture supernatants of cell lines established from SS patients.6 Other defined manifestations of an active EBV infection are the presence of infected B cells that can transform into B-cell lymphomas in the circulation.7 Mariette et al. previously used in situ hybridization to detect EBV DNA in a substantial proportion of lymphoid cells and epithelial cells in salivary glands from patients with SS.2 It has also been shown that antibodies against EBV antigens are elevated in SS sera.8,9 Furthermore, we have recently reported an increase in the enzymatic activity of apoptotic protease, by EBV activation, to be involved in the proteolysis of 120 000 molecular weight (MW) α-fodrin autoantigen during the development of SS.10,11 As EBV is known to induce strong immune responses,12,13 these reports suggest that a reactivated EBV infection may play a role in SS.
EBV is a widely occurring virus of the herpes family that infects epithelial cells of the salivary glands and oropharyngeal tissue, as well as B cells. After the primary infection, the virus remains latent in the host and occasionally becomes reactivated. Reactivation of EBV requires replication of viral genes and transcriptional induction of immediate-early genes mediated by expression of the BZLF1 gene. The BZLF1 gene product, ZEBRA, is considered to first be transcribed in association with viral replication and to be indispensable for the reactivation of EBV.14,15 Expression of the BZLF1 gene that encodes ZEBRA has been reported to be induced by 12-O-tetradecanoylphorbol-13-acetate (TPA),16 the calcium ionophore A23187,17 crosslinking of cell-surface immunoglobulin G (IgG),18 n-butyrate,19 the formation of het DNA associated with P3HR-1 superinfection,20,21 nitric oxide (NO) inhibitor,22 human herpesvirus 6 (HHV6) superinfection in vitro23 and transforming growth factor-β1 (TGF-β1).24 However, the physiological stimuli responsible for the EBV activation in SS have not been characterized.
Moreover, how reactivation of EBV is actually induced in lesions associated with SS, and which signalling pathways are involved in the process of viral reactivation, have not yet been clarified. To elucidate the mechanism underlying the EBV reactivation involved in the pathogenesis and progression of SS, we analysed the contribution of EBV activation by soluble factors in inflammatory saliva using a BZLF1 promoter fusion construct (Zp-luc) transfected into the human salivary gland cell line, HSY, and investigated the signalling pathways that might be involved in EBV reactivation in SS.
Materials and methods
Saliva samples
Saliva samples were collected from 12 patients with primary SS. All patients were seen at the Ichikawa General Hospital of Tokyo Dental College, and diagnosed according to the criteria of Fox & Saito.25 These patients had not received glucocorticoids or immunosuppressive agents for at least 6 months prior to saliva collection.
All of the patients were women (mean age: 56 years). As age-matched controls, saliva samples were also obtained from 10 women who had no clinical evidence of systemic autoimmune disease. The samples were centrifuged at 12 000 g for 45 min and filtered through a 0·22-µm filter to remove cells, virus and particulate debris; aliquots were stored at −80°.
Cell culture, transfections and chemicals
The salivary gland epithelial cell line HSY26 (kindly provided by Dr M. Sato of Tokushima University) was cultured at 37° in minimal essential medium (MEM) containing HEPES (10 mm), penicillin (100 IU/ml), streptomycin (100 µg/ml), and 10% fetal calf serum (FCS). The EBV-positive B-cell line, B95-8, was maintained in RPMI-1640 supplemented with penicillin (100 IU/ml), streptomycin (100 µg/ml) and 10% FCS at 37° in a humidified atmosphere of 5% CO2 in air. We used polymerase chain reaction (PCR) techniques to generate a derivative of a BZLF1 promoter (Zp) construct. The following forward and reverse oligomers were used as primers to create the promoter of the BZLF1 gene, respectively: 5′-CTGCAGCCATGCATATTTCAACTGGG-3′ and 5′-GTCGACGCAAGGTGCAATGTTTAGTG-3. The PCR-amplified promoter fragments, including the KpnI/SalI sites were cloned into the multiple cloning site of the pGL3-basic vector (Promega, Madison, WI), upstream from the luciferase gene. The Zp-luciferase gene (Zp-luc) was transfected into HSY cells using Lipofectin (Life Technologies, Grand Island, NY). The vector pGL3-basic (lacking a promoter) and the vector pGL3-control (Promega) served as negative and positive controls, respectively. Briefly, the transfection medium containing 2 µg of plasmid DNA and 20 µl of Lipofectin reagent in 200 µl of serum-free Dulbecco's modified Eagle's minimal essential medium (DMEM) was incubated for 20 min at room temperature and then diluted with serum-free DMEM to a final volume of 2 ml and added to HSY cells, plated the previous day. The transfection process occurred at 37° for 5 hr after which the DNA-containing medium was replaced with 2 ml of DMEM containing 10% FCS. TPA, calcium ionophore A23187, compound R24571 (calmodulin inhibitor), wortmaninn (IP3-kinase inhibitor), cyclosporin A (calcineurin inhibitor), and 1-(5-isoquinolinyl sulphonyl)-2-methylpiperazine (H-7) [a protein kinase C (PKC) inhibitor] were purchased from Sigma Co. (St Louis, MO); and U0126 [a mitogen-activated protein kinase (MAPK) inhibitor] was purchased from Promega.
Luciferase assay
In order to test the Zp response to various stimuli, we used a plasmid carrying a region of Zp from −221 to +12 bp, which was cloned upstream of the coding sequence of the bacterial luciferase gene (Zp-luc). The Zp region was previously reported to contain TPA response elements. Zp-luc was transfected into the EBV-negative salivary gland cell line, HSY, which was treated with stimuli and assayed for luciferase activity. The transfected cells were incubated for 24 hr followed by stimulation with a 1 : 50 volume of saliva. TGF-β1 (Genzyme Corp., Cambridge, MA) was added to the transfected cells under the same conditions. After rinsing with phosphate-buffered saline (PBS), cells were lysed with reporter lysis buffer (Promega) and the cell lysate was analysed by the luciferase assay with the Promega kit in a Lumat (Bio-Rad, Hercules, CA). To control transfection efficiency, plasmid pRL-TK (Promega) was co-transfected with the luciferase reporter constructs at a ratio of 1 : 4. The results showed the difference in the relative efficiency of transfection between constructs to be negligible. For the assay in the presence of TPA or A23187, cells were also treated with these chemicals after transfection. The inducibility of luciferase activity was defined as the fold activity of the sample to the untreated control. Data shown are from a representative experiment carried out a minimum of three times.
Quantification of TGF-β1 in saliva by enzyme-linked immunosorbent assay
TGF-β1 concentrations in saliva were measured using the Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R & D, Minneapolis, MN). Briefly, saliva was used immediately after thawing from −80° and latent TGF-β1 was activated to immunoreactive TGF-β1. Diluted saliva samples were placed in a 96-well microtitre plate, previously coated with recombinant human TGF-β1 soluble receptor Type II (sRII). After incubation and washing, a horseradish peroxidase-conjugated secondary antibody was added. Hydrogen peroxidase and chromogen were added and colour development was measured at 450 nm using a microplate reader.
Western blot analysis
Cells were harvested, washed briefly with PBS, resuspended in buffer comprising 100 mm Tris–HCl (pH 7·6), 50 mm NaCl, 2 mm EDTA 0·5% Nonidet P-40 (NP-40), 100 µg/ml phenylmethanesulphonyl fluoride and 1 µg/ml of each of leupeptin, pepstatin and aprotinin, and then sonicated; protein concentrations were determined using a modified Lowry assay (Bio-Rad). Equal amounts of protein in loading buffer, heated for 5 min at 100° and separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) on a 10% gel, were transferred (by electroblotting) to a nitrocellulose membrane. The membrane was stained with Ponceau S sodium salt to verify that the same amount of protein had been deposited in each lane. Anti-ZEBRA monoclonal antibody (mAb) (Dako, Carpinteria, CA) was used as a primary antibody, and horseradish peroxidase-conjugated anti-mouse IgG was used as the secondary antibody. The intensity of ZEBRA bands was measured by densitometric analysis using a colour scanner and NIH image 1.6.2. The amount of ZEBRA induced by TPA (25 ng/ml), TGF-β1 (5 ng/ml), and saliva from SS or normal individuals (1 : 50 vol/vol), with or without neutralizing anti-TGF-β1 antibody (Genzyme Corp.), was expressed as follows: [percentage of the relative amount to the negative control (TPA, TGF-β1, saliva from SS or normal individuals) ÷ (untreated)] × 100.
Results
Activation of the BZLF1 promoter (Zp) in salivary gland cells
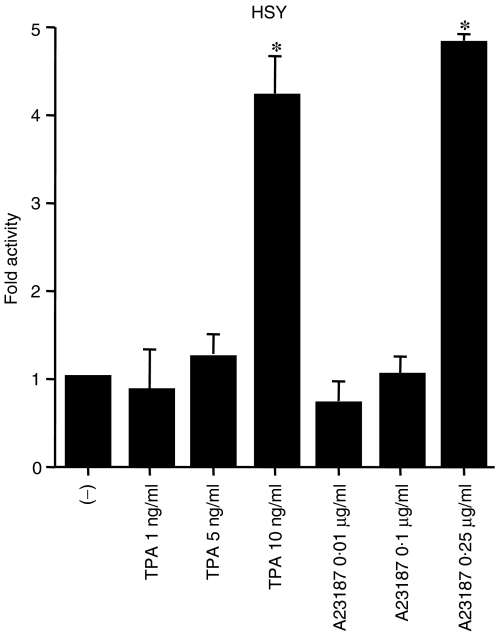
The Zp region has previously been reported to be TPA or A23187 responsive in HeLa cells and B cells.17,27,28 In order to test the Zp activity in salivary glands, we used a plasmid carrying a Zp region (−221 to +12 bp)28 that was cloned into an upstream portion of the luciferase gene (Zp-luc). Zp-luc was transfected into the salivary gland cell line, HSY, which had been treated with TPA or A23187, and a luciferase assay was performed. As shown in Fig. 1, luciferase activity was dose-dependently induced with TPA and A23187, even in the absence of other EBV genes. We found that this region was also responsive to TPA or A23187 in salivary gland epithelial cells.
Figure 1.
Activation of Zp-luc by 12-O-tetradecanoylphorbol-13-acetate (TPA) and A23187 in the Epstein–Barr virus (EBV)-negative salivary gland cell line, HSY. The cells were treated with either TPA (1, 5, or 10 ng/ml) or A23187 (0·01, 0·1, or 0·25 µg/ml), or given no treatment (−). After incubation for 24 hr, the cells were harvested, and luciferase activity was assayed. The bar graph illustrates fold activity. *P < 0·05; Mann–Whitney U-test. The error bars represent standard deviation.
Activation of Zp by saliva from SS patients
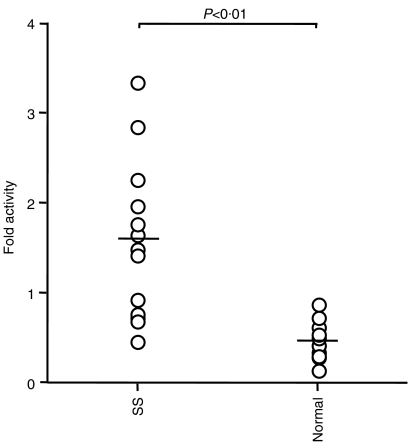
We next investigated whether some soluble factors are involved in the EBV reactivation that occurs in the microenvironment of SS. We collected saliva samples from 12 SS patients and 10 normal subjects, to reproduce the oral environment. As shown in Fig. 2, eight of the 12 saliva samples from SS patients showed increased luciferase activity. In contrast, luciferase activity was not increased in the normal saliva samples. These findings suggest that some humoral factors in SS saliva might play a major role in the reactivation of EBV, which in turn leads to the progression of SS.
Figure 2.
Measurement of luciferase activity in HSY cells transfected with Zp-luc and treated with saliva from the 12 Sjogren's syndrome patients (SS) or 10 normal controls (Normal). P < 0·01; Mann–Whitney U-test.
Effects of PKC inhibitors, calcium/calmodulin-dependent protein kinase, and MAPK kinase on Zp activation by SS saliva
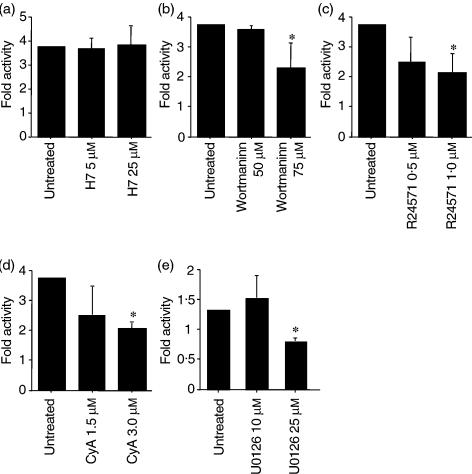
In B cells, the mechanism of EBV reactivation has been analysed and reported,29,30 but the physiological signalling for EBV reactivation in SS has not been characterized. To analyse the signalling pathways occurring downstream of SS saliva stimulation, in our models of SS we further examined whether Zp-luc activation induced by SS saliva could be inhibited by PKC, calcium/calmodulin-dependent protein kinase or MAPK inhibitors. As shown in Fig. 3a, the PKC inhibitor did not affect luciferase activity, but the inhibitors of IP3 (Fig. 3b), calmodulin (Fig. 3c) and calcineurin (Fig. 3d) decreased Zp-luc activity. The SS saliva used was that with the highest fold activity of Zp-luc shown in Fig. 2.
Figure 3.
Effects of a protein kinase inhibitor, calmodulin antagonists, and a MAPK kinase (MEK) inhibitor on Sjogren's syndrome (SS) saliva-induced Zp-luc activation. HSY cells were pretreated for 1 hr with H7 (a), Wortmaninn (b), R24571 (c), cyclosporin A (d), or U0126 (e) before the addition of SS saliva. Cells were harvested 24 hr later, and the cell extracts were assayed for luciferase activity. This result is representative of three independent experiments. *P < 0·05; Mann–Whitney U-test.
Moreover, a specific inhibitor of MAPK also reduced Zp-luc activity (Fig. 3e), suggesting that the effects of SS saliva on BZLF1 expression require calcium/calmodulin and the MAPK pathway.
Zp activation by TGF-β1
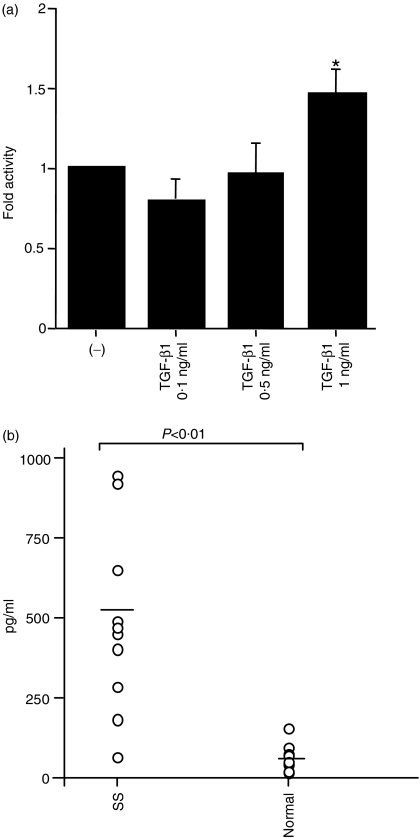
Finally, we assumed that cytokines, which are expressed in SS, might play a role in the reactivation of EBV. It is known that (in contrast to normal salivary glands) SS salivary glands express increased levels of cytokines,31,32 but the relationship between these cytokines and EBV reactivation is not well characterized. TGF-β1, which is known to be expressed in SS, can further induce EBV reactivation in the EBV-positive B-cell lines P3HR-1, Akata24 and B95-8.33 To investigate cytokine efficiency in Zp activation, we conducted a luciferase assay using TGF-β1. The result indicated that 1 ng/ml of TGF-β1 increases Zp-luc activity (Fig. 4a). Next, to address the issue of the presence of TGF-β1 in saliva, we measured TGF-β1 levels using an ELISA. Figure 4(b) shows TGF-β1 concentrations to be significantly elevated in SS saliva compared with the saliva of healthy volunteers. A significant correlation was observed between the level of fold activity and the concentration of TGF-β1 in each saliva sample from SS patients (r = 0·75, P < 0·01). A concentration of 1 ng/ml TGF-β1, higher than the highest concentration in SS saliva, had 1·4-fold Zp-luc activity, whereas the SS saliva stimulated Zp-luc activity by 3·5-fold. These results demonstrate that TGF-β1 alone is not sufficient to stimulate Zp-luc, suggesting the possibility of a synergistic additional factor(s) in SS saliva. We speculate that TGF-β1 is not the sole activation factor, but a major factor of ZEBRA expression by SS saliva because TGF-β1-specific antibody inhibited the expression of ZEBRA in SS saliva, as shown by Western blotting.
Figure 4.
Effect of transforming growth factor-β1 (TGF-β1) on Zp activation. Luciferase assay of the BZLF1 promoter activities in HSY cells stimulated with TGF-β1 after transfection. Cells were harvested 24 hr later, and the cell extracts were assayed for luciferase activity. *P < 0·05; Mann–Whitney U-test (a). TGF-β1 concentrations in saliva were measured by enzyme-linked immunosorbet assay (ELISA) (b). Saliva was used immediately after thawing from −80°. The treated samples were measured at 450 nm using a microplate reader.
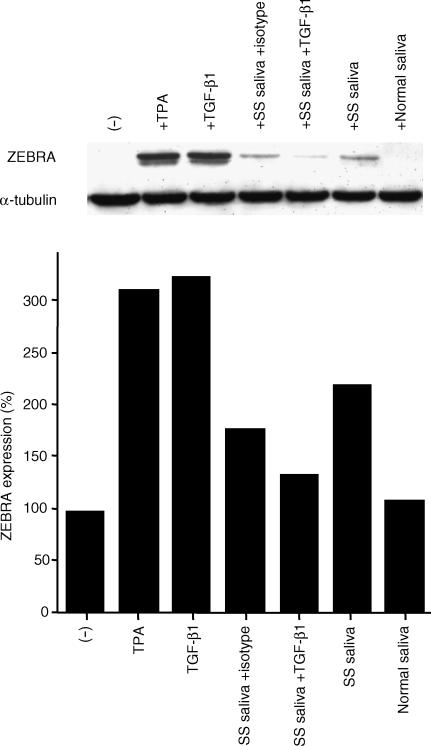
Furthermore, to investigate the effect of SS saliva on expression of ZEBRA protein, we used EBV-positive B95-8 cells in which ZEBRA protein can be induced under several conditions (i.e. by TPA and TGF-β1). B95-8 cells were exposed to SS saliva, which had a high TGF-β1 concentration of 920 pg/ml. As shown in Fig. 5, ZEBRA protein was detected following treatment with SS saliva, suggesting that SS saliva contains factors that induce EBV reactivation. Neutralizing anti-TGF-β1 inhibited the induction of ZEBRA by SS saliva, in contrast to isotype-matched control antibody (Fig. 5). These results suggest that TGF-β1 may play a role in the EBV reactivation of a subpopulation in SS.
Figure 5.
Effect of Sjogren's syndrome (SS) saliva on the expression of ZEBRA in Epstein–Barr virus (EBV)-positive B95-8 cells. B95-8 cells were treated for 48 hr with 25 ng/ml of 12-O-tetradecanoylphorbol-13-acetate (TPA) or 5 ng/ml of transforming growth factor-β1 (TGF-β1), or cells were cultured with SS saliva and a normal control for 48 hr. The neutralizing anti-TGF-β1 (10 µg/ml) was added at the start of the culture. ZEBRA bands were quantified by densitometric analysis. The results were calculated and the amount of ZEBRA was expressed as described in the Materials and methods. Tubulin was used for normalizing the amount of protein loaded. The results shown are representative of three independent experiments.
Discussion
In the pathogenesis of SS, a role for EBV reactivation has been suggested.1–4,6 EBV is a ubiquitous human herpesvirus that replicates in the salivary glands during primary infection34 and the virus remains latent at this site in normal adults.35 Initiation of the EBV lytic cycle is dependent on transcription of the BZLF1 gene. Although the viral lytic cycle can be induced by various reagents in vitro, including TPA,16 calcium ionophore,17 anti-IgG,18 n-butyrate19 and NO inhibitor,22 it remains unclear how EBV reactivation is induced in SS. In this study, in order to clarify the association of EBV reactivation in SS pathogenesis and further analyse the mechanism of EBV reactivation, we modelled the reactivation of EBV, which latently infected the salivary gland, using plasmid DNA containing the BZLF1 promoter upstream from the luciferase reporter gene.
The Zp-luc(−221) reporter system has been widely used as a marker of EBV reactivation, even in EBV-negative cells.36 However, it is well known that the induction of promoter activity by TPA, or TGF-β1, in EBV-negative cells is relatively low.33,36 These results might be largely a result of the absence of ZEBRA in these assay systems. Regulation of ZEBRA is known to be achieved by two steps.27 Initial activation of the BZLF1 promoter is weak, but this level of ZEBRA sufficiently induces full activation of the promoter by means of self-promoter bindings. Therefore, the weak fold-induction of Zp-luc seen in our system might correspond to the initial ‘weak’ activations prior to the occurrence of full activation.
We demonstrated the EBV reactivation model in SS and found that expression of the BZLF1 gene in glandular epithelial cells might be induced via MAPK and calcium/calmodulin dependent-kinase pathways, as previously demonstrated in B lymphocytes.29,30 However, the physiological stimuli responsible for the EBV activation in SS have not yet been characterized.
Our results showed that treatment with saliva from SS patients increases the reactivation ability of EBV as compared with normal saliva samples. This suggests the existence of factors that are able to induce the reactivation of EBV. It has been reported that infectious EBV is present in the saliva of SS patients1 and in HHV6 superinfection in vitro,23 but we considered that these viruses were not present in the saliva samples used in the present study, as a result of the saliva processing technique used (centrifugation for 45 min at 12 000 g to remove cells and particulate debris, followed by filtering through a 0·22-µm filter). We thus assumed that some soluble factors play a major role in the reactivation of EBV.
The expression of ZEBRA can be accomplished, in vitro, by treatment of latent EBV-positive B cells with various activating agents, including TGF-β1, TPA, butyrate, calcium ionophores and anti-IgG. These treatments trigger a variety of cellular signalling pathways, resulting in the activation of cellular transcription factors stimulating transcription from the BZLF1 promoter, Zp.27,30,37,38 Zp can be activated through PKC and calcium/calmodulin-dependent protein kinase directly cross-linking via anti-IgG.39 Anti-IgG also induced rapid phosphorylation of MAPK in Akata cells.30 Moreover, MAPK was involved in the activation of BZLF1 induced by TGF-β1 in Raji and B95-8 cells.33 In P3HR-1 and Rael cells, however, MAPK was not involved in the activation of Zp-luc by TGF-β1.40 This discrepancy might be explained by different characteristics of cell lineage. The signal transduction of Zp-luc activation in salivary gland cells has not been reported. We investigated the SS saliva signal in EBV reactivation in our models by using specific inhibitors of intracellular signals. A specific inhibitor of PKC did not affect the SS saliva-induced Zp-luc activity, whereas treatment with inhibitors of calmodulin, calcineurin, IP3 and MAPK, dose dependently decreased this induction. This implies that the effect of SS saliva on BZLF1 expression requires calcium/calmodulin and/or the MAPK pathway.
TGF-β1 has been shown to exert its effects through a wide range of intracellular routes. Recent studies from several laboratories have reported that Smads are intermediate effector proteins which transduce the TGF-β1 signal from the plasma membrane to the nucleus.41 TGF-β1 can induce gene expression via c-Jun N-terminal kinase (JNK) activation42,43 or p38 MAPK.44 The role of MAPK/ERK in the ΤGF-β1 signalling pathway has been described.45 PKC has also been shown to be involved in TPA- and anti-IgG-induced EBV reactivation30,46 and could play an important role in the signal transduction by TGF-β1.47,48 This suggests that SS saliva might contain some soluble factor(s), such as TGF-β1, which can induce virus reactivation.
It is known that, in contrast to normal salivary glands, SS salivary glands express increased levels of cytokines.31,49 Furthermore, recent reports have suggested that in latently infected B cells, the lytic cycle can be induced by TGF-β124 and that salivary TGF-β1 concentrations are elevated in SS.31 In our experiments, TGF-β1 increased Zp-luc activity, and TGF-β1 concentrations were significantly elevated in SS saliva as compared to saliva from healthy volunteers (Fig. 4). Furthermore, we investigated the effect of SS saliva on Zp activation in B cells by Western blot analysis, using an SS saliva sample with a high concentration of TGF-β1. ZEBRA was detected upon exposure to SS saliva (Fig. 5), suggesting that SS saliva contains factors which induce viral reactivation. As shown in Fig. 5, neutralizing anti-TGF-β1 inhibited ZEBRA induction by SS saliva. In SS saliva, TGF-β1 may play a major role in viral reactivation.
In conclusion, we have shown that SS saliva induces Zp-luc activity and that calcium/calmodulin and/or the MAPK pathway is required for this induction, as demonstrated through the use of specific signalling inhibitors. These findings may be of importance for developing new strategies to inhibit EBV reactivation in SS.
Acknowledgments
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, and Culture of Japan.
Abbreviations
- EBV
Epstein–Barr virus
- ELISA
enzyme-linked immunosorbent assay
- HHV6
human herpesvirus 6
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- PBS
phosphate-buffered saline
- PKC
protein kinase C
- SS
Sjogren's syndrome
- TGF-β1
transforming growth factor-β1
- TPA
12-O-tetradecanoylphorbol-13-acetate
- ZEBRA
BamHI-Z-DNA fragment of Epstein–Barr replication activator
References
- 1.Saito I, Servenius B, Compton T, Fox RI. Detection of Epstein–Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989;169:2191–8. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariette X, Gozlan J, Clerc D, Bisson M, Morinet F. Detection of Epstein–Barr virus DNA by in situ hybridization and polymerase chain reaction in salivary gland biopsy specimens from patients with Sjogren's syndrome. Am J Med. 1991;90:286–94. [PubMed] [Google Scholar]
- 3.Saito I, Nishimura S, Kudo I, Fox RI, Moro I. Detection of Epstein–Barr virus and human herpes virus type 6 in saliva from patients with lymphoproliferative diseases by the polymerase chain reaction. Arch Oral Biol. 1991;36:779–84. doi: 10.1016/0003-9969(91)90026-q. [DOI] [PubMed] [Google Scholar]
- 4.Fox RI, Pearson G, Vaughan JH. Detection of Epstein–Barr virus-associated antigens and DNA in salivary gland biopsies from patients with Sjogren's syndrome. J Immunol. 1986;137:3162–8. [PubMed] [Google Scholar]
- 5.Fox RI, Bumol T, Fantozzi R, Bone R, Schreiber R. Expression of histocompatibility antigen HLA-DR by salivary gland epithelial cells in Sjogren's syndrome. Arthritis Rheum. 1986;29:1105–11. doi: 10.1002/art.1780290908. [DOI] [PubMed] [Google Scholar]
- 6.Tateishi M, Saito I, Yamamoto K, Miyasaka N. Spontaneous production of Epstein–Barr virus by B lymphoblastoid cell lines obtained from patients with Sjogren's syndrome. Possible involvement of a novel strain of Epstein–Barr virus in disease pathogenesis. Arthritis Rheum. 1993;36:827–35. doi: 10.1002/art.1780360614. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan JL. Epstein–Barr virus and lymphoproliferative disorders. Semin Hematol. 1988;25:269–79. [PubMed] [Google Scholar]
- 8.Inoue N, Harada S, Miyasaka N, Oya A, Yanagi K. Analysis of antibody titers to Epstein–Barr virus nuclear antigens in sera of patients with Sjogren's syndrome and with rheumatoid arthritis. J Infect Dis. 1991;164:22–8. doi: 10.1093/infdis/164.1.22. [DOI] [PubMed] [Google Scholar]
- 9.Yamaoka K, Miyasaka N, Yamamoto K. Possible involvement of Epstein–Barr virus in polyclonal B cell activation in Sjogren's syndrome. Arthritis Rheum. 1988;31:1014–21. doi: 10.1002/art.1780310812. [DOI] [PubMed] [Google Scholar]
- 10.Inoue H, Tsubota K, Ono M, et al. Possible involvement of EBV-mediated alpha-fodrin cleavage for organ-specific autoantigen in Sjogren's syndrome. J Immunol. 2001;166:5801–9. doi: 10.4049/jimmunol.166.9.5801. [DOI] [PubMed] [Google Scholar]
- 11.Haneji N, Nakamura T, Takio K, et al. Identification of alpha-fodrin as a candidate autoantigen in primary Sjogren's syndrome. Science. 1997;276:604–7. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 12.Rickinson AB, Moss DJ, Wallace LE, et al. Long-term T-cell-mediated immunity to Epstein–Barr virus. Cancer Res. 1981;41:4216–21. [PubMed] [Google Scholar]
- 13.Slaughter L, Carson DA, Jensen FC, Holbrook TL, Vaughan JH. In vitro effects of Epstein–Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. J Exp Med. 1978;148:1429–34. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Countryman J, Miller G. Activation of expression of latent Epstein–Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–9. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooney CM, Rowe DT, Ragot T, Farrell PJ. The spliced BZLF1 gene of Epstein–Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989;63:3109–16. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.zur Hausen H, O'Neill FJ, Freese UK, Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978;272:373–5. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]
- 17.Faggioni A, Zompetta C, Grimaldi S, Barile G, Frati L, Lazdins J. Calcium modulation activates Epstein–Barr virus genome in latently infected cells. Science. 1986;232:1554–6. doi: 10.1126/science.3012779. [DOI] [PubMed] [Google Scholar]
- 18.Takada K. Cross-linking of cell surface immunoglobulins induces Epstein–Barr virus in Burkitt lymphoma lines. Int J Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 19.Luka J, Kallin B, Klein G. Induction of the Epstein–Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979;94:228–31. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- 20.Meuller-Lantzsch N, Georg B, Yamamoto N, zur Hausen H. Epstein–Barr virus-induced proteins. II. Analysis of surface polypeptides from EBV-producing and -superinfected cells by immunoprecipitation. Virology. 1980;102:401–11. doi: 10.1016/0042-6822(80)90107-5. [DOI] [PubMed] [Google Scholar]
- 21.Mueller-Lantzsch N, Georg B, Yamamoto N, zur Hausen H. Epstein–Barr virus-induced proteins. III. Analysis of polypeptides from P3HR-1-EBV-superinfected NC37 cells by immunoprecipitation. Virology. 1980;102:231–3. doi: 10.1016/0042-6822(80)90087-2. [DOI] [PubMed] [Google Scholar]
- 22.Mannick JB, Asano K, Izumi K, Kieff E, Stamler JS. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein–Barr virus reactivation. Cell. 1994;79:1137–46. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 23.Flamand L, Stefanescu I, Ablashi DV, Menezes J. Activation of the Epstein–Barr virus replicative cycle by human herpesvirus 6. J Virol. 1993;67:6768–77. doi: 10.1128/jvi.67.11.6768-6777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.di Renzo L, Altiok A, Klein G, Klein E. Endogenous TGF-beta contributes to the induction of the EBV lytic cycle in two Burkitt lymphoma cell lines. Int J Cancer. 1994;57:914–9. doi: 10.1002/ijc.2910570623. [DOI] [PubMed] [Google Scholar]
- 25.Fox RI, Saito I. Criteria for diagnosis of Sjogren's syndrome. Rheum Dis Clin North Am. 1994;20:391–407. [PubMed] [Google Scholar]
- 26.Yanagawa T, Hayashi Y, Nagamine S, Yoshida H, Yura Y, Sato M. Generation of cells with phenotypes of both intercalated duct-type and myoepithelial cells in human parotid gland adenocarcinoma clonal cells grown in athymic nude mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51:187–95. doi: 10.1007/BF02899028. [DOI] [PubMed] [Google Scholar]
- 27.Flemington E, Speck SH. Autoregulation of Epstein–Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1227–32. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flemington E, Speck SH. Identification of phorbol ester response elements in the promoter of Epstein–Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1217–26. doi: 10.1128/jvi.64.3.1217-1226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellinghoff I, Daibata M, Humphreys RE, Mulder C, Takada K, Sairenji T. Early events in Epstein–Barr virus genome expression after activation. regulation by second messengers of B cell activation. Virology. 1991;185:922–8. doi: 10.1016/0042-6822(91)90574-u. [DOI] [PubMed] [Google Scholar]
- 30.Daibata M, Speck SH, Mulder C, Sairenji T. Regulation of the BZLF1 promoter of Epstein–Barr virus by second messengers in anti-immunoglobulin-treated B cells. Virology. 1994;198:446–54. doi: 10.1006/viro.1994.1056. [DOI] [PubMed] [Google Scholar]
- 31.Fox PC, Grisius MM, Bermudez DK, Sun D. Cytokine mRNA expression in labial salivary glands and cytokine secretion in parotid saliva in Sjogren's syndrome. Adv Exp Med Biol. 1998;438:909–15. doi: 10.1007/978-1-4615-5359-5_129. [DOI] [PubMed] [Google Scholar]
- 32.Fox PC, Brennan M, Di Sun P. Cytokine expression in human labial minor salivary gland epithelial cells in health and disease. Arch Oral Biol. 1999;44(Suppl. 1):S49–52. doi: 10.1016/s0003-9969(99)90018-3. [DOI] [PubMed] [Google Scholar]
- 33.Fahmi H, Cochet C, Hmama Z, Opolon P, Joab I. Transforming growth factor beta 1 stimulates expression of the Epstein–Barr virus BZLF1 immediate-early gene product ZEBRA by an indirect mechanism which requires the MAPK kinase pathway. J Virol. 2000;74:5810–8. doi: 10.1128/jvi.74.13.5810-5818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan DG, Niederman JC, Miller G, Smith HW, Dowaliby JM. Site of Epstein–Barr virus replication in the oropharynx. Lancet. 1979;2:1154–7. doi: 10.1016/s0140-6736(79)92384-5. [DOI] [PubMed] [Google Scholar]
- 35.Chang RS, Lewis JP, Abildgaard CF. Prevalence of oropharyngeal excreters of leukocyte-transforming agents among a human population. N Engl J Med. 1973;289:1325–9. doi: 10.1056/NEJM197312202892501. [DOI] [PubMed] [Google Scholar]
- 36.Niller HH, Salamon D, Uhlig J, et al. Nucleoprotein structure of immediate-early promoters Zp and Rp and of oriLyt of latent Epstein–Barr virus genomes. J Virol. 2002;76:4113–8. doi: 10.1128/JVI.76.8.4113-4118.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrell PJ, Rowe DT, Rooney CM, Kouzarides T. Epstein–Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989;8:127–32. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu N, Takada K. Analysis of the BZLF1 promoter of Epstein–Barr virus: identification of an anti-immunoglobulin response sequence. J Virol. 1993;67:3240–5. doi: 10.1128/jvi.67.6.3240-3245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daibata M, Mellinghoff I, Takagi S, Humphreys RE, Sairenji T. Effect of genistein, a tyrosine kinase inhibitor, on latent EBV activation induced by cross-linkage of membrane IgG in Akata B cells. J Immunol. 1991;147:292–7. [PubMed] [Google Scholar]
- 40.Liang CL, Chen JL, Hsu YP, Ou JT, Chang YS. Epstein–Barr virus BZLF1 gene is activated by transforming growth factor-beta through cooperativity of Smads and c-Jun/c-Fos proteins. J Biol Chem. 2002;277:23345–57. doi: 10.1074/jbc.M107420200. [DOI] [PubMed] [Google Scholar]
- 41.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 42.Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–56. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Zhou G, Hu MC, Yao Z, Tan TH. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor beta (TGF-beta)-activated kinase (TAK1), a kinase mediator of TGF beta signal transduction. J Biol Chem. 1997;272:22771–5. doi: 10.1074/jbc.272.36.22771. [DOI] [PubMed] [Google Scholar]
- 44.Hannigan M, Zhan L, Ai Y, Huang CK. The role of p38 MAP kinase in TGF-beta1-induced signal transduction in human neutrophils. Biochem Biophys Res Commun. 1998;246:55–8. doi: 10.1006/bbrc.1998.8570. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi K, Shirakabe K, Shibuya H, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–11. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 46.Davies AH, Grand RJ, Evans FJ, Rickinson AB. Induction of Epstein–Barr virus lytic cycle by tumor-promoting and non-tumor-promoting phorbol esters requires active protein kinase C. J Virol. 1991;65:6838–44. doi: 10.1128/jvi.65.12.6838-6844.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halstead J, Kemp K, Ignotz RA. Evidence for involvement of phosphatidylcholine-phospholipase C and protein kinase C in transforming growth factor-beta signaling. J Biol Chem. 1995;270:13600–3. doi: 10.1074/jbc.270.23.13600. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki M, Asplund T, Yamashita H, Heldin CH, Heldin P. Stimulation of hyaluronan biosynthesis by platelet-derived growth factor-BB and transforming growth factor-beta 1 involves activation of protein kinase C. Biochem J. 1995;307:817–21. doi: 10.1042/bj3070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol. 1994;152:5532–9. [PubMed] [Google Scholar]