Abstract
Research on CD4+ CD25+ regulatory T cells (Treg) has gathered momentum over the last five years but many aspects of their fundamental biology remain elusive. Treg have been considered to be ‘naturally anergic’ based on their failure to proliferate in response to T-cell receptor ligation in vitro. Several recent studies challenge this view and demonstrate a robust proliferative capacity for CD25+ cells. The significance of this finding for Treg homeostasis and function is considered below.
Anergy or proliferation?
Treg anergy in vitro
CD4 T cells constitute a critical component of the adaptive immune system and are renowned for their capacity to ‘help’ both humoral and cell-mediated responses. However, there is substantial functional diversity within the CD4 compartment and it is becoming clear that certain subpopulations ‘hinder’ rather than ‘help’ immune responses. The most well characterized example is the CD4+ CD25+ population, which appears to play an active role in down-regulating pathogenic autoimmune responses.1 This article will use the term ‘Treg’ to describe the CD4+ CD25+ population although these are clearly not the only lymphocytes with regulatory activity. It was obvious from the early in vitro analysis of CD25+ T cells that their proliferation was not governed by the same rules as that of their CD25− counterparts. In fact, CD25+ cells appeared refractory to T-cell receptor (TCR) -induced proliferation2,3 in some cases even in the presence of antibodies to the costimulatory molecule CD28.4 TCR engagement in such in vitro assays was not a null event, however, because it conferred the ability to suppress the proliferation of cocultured CD25− cells. This is best illustrated by models in which TCR triggering of CD25− cells and CD25+ cells is controlled independently, for example by exploiting TCR transgenics with different peptide specificities.2,5 In fact, far from demonstrating an intrinsic insensitivity to TCR signalling, as might be predicted from their lack of proliferation, CD25+ cells could be induced to acquire suppressive function at peptide concentrations which were 10–100-fold lower than those required to trigger proliferation of CD25− cells.2 In addition, the presence of exogenous cytokines such as interleukin-2 (IL-2)2–4,6–9 or IL-410 released Treg from ‘anergy’ revealing their proliferative potential.
Treg proliferation in vivo
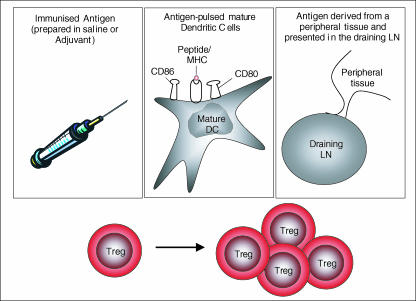
The first studies examining Treg proliferation in vivo documented their ability to divide after adoptive transfer into lymphocyte-deficient hosts11,12 in a major histocompatiblity complex (MHC) class II-dependent manner.13 Such ‘lymphopenia-induced’ proliferation is mechanistically distinct from bona fide antigen-driven proliferation, so the significance of these observations to Treg behaviour in normal animals remained unclear. More recently however, four studies have demonstrated that Treg can proliferate in non-lymphopenic hosts in an antigen-dependent manner.14–17 The ability to interrogate Treg proliferation in response to specific antigens in vivo has been made possible through the use of TCR transgenic Treg, either isolated directly from conventional TCR transgenics16,17 or from TCR transgenics also bearing a source of specific antigen in the thymus.14,15 What we learn from these studies is that, far from being anergic, CD25+ Treg clearly divide when challenged with immunized antigen,14,15 antigen-pulsed dendritic cells (DC),16 or even in response to antigen expressed transgenically on a peripheral tissue14,17 (Fig. 1). This conclusion is at odds with an earlier report suggesting that TCR transgenic Treg failed to proliferate in vivo following immunization.13 Since the latter study examined a single early time-point (and the percentage of undivided cells nevertheless decreased from 84 to 47% following immunization13) it remains possible that this discrepancy reflects a kinetic, rather than a fundamental, difference. Although much of the recent work relies on TCR transgenic mice, data from normal animals support the same conclusions. Accordingly, approximately 10% of the CD4+ CD25+ population in normal mice incorporate the nucleoside analogue, bromodeoxyuridine, during a 3-day pulse18 and interestingly the proliferating cells fall within a subpopulation characterized by high expression of CD44.17 Since contamination by memory CD4 T cells, which would be expected to be CD25low, should be minimal, this suggests that at any given time, a fraction of CD25+ Treg are transiting the S-phase of the cell cycle. Taken together these studies indicate that, rather than being inherently anergic, Treg can and do proliferate in response to antigens in vivo.
Figure 1.
Conditions that stimulate Treg proliferation in vivo.14–17 Treg proliferate after encounter with immunized antigen, antigen-pulsed mature dendritic cells (DC) and antigen draining from a peripheral tissue.
Which antigen-presenting cells drive Treg Proliferation?
The reluctance of CD25+ cells to proliferate in vitro raises the question of which antigen-presenting cells (APC) have the capacity to drive Treg proliferation and what is the secret of their success? To dissect this issue Steinman's group have interrogated the stimulatory capacity of distinct subsets of APC rather than using splenocytes as a bulk population.16 Two important points arise from this analysis. The first is that freshly isolated B cells or CD11c+ DC are ineffective at triggering the proliferation of TCR transgenic Treg, providing an explanation for the Treg ‘anergy’ observed under standard in vitro culture conditions. The second is that the trick to making Treg divide appears to be to use bone-marrow-derived DC sorted for high CD86 expression, or alternatively to use CD11c+ DC isolated from mice injected with complete Freund's adjuvant (CFA).16 Consistent with the latter observation, CD25+ cells undergoing proliferation in vivo have been shown to be associated with CD11c+ cells at inflammatory sites.19 Are inflammatory signals therefore the key to rendering DC capable of driving Treg proliferation, perhaps as a consequence of CD86 up-regulation? Several lines of evidence suggest that this is an oversimplification. Firstly, culturing CD11c+ cells with lipopolysaccharide (LPS) barely alters their ability to stimulate Treg proliferation, despite endowing them with similar levels of CD80 and CD86 to the bone-marrow-derived DC.16,20 Secondly, injection of antigen in the absence of adjuvant into footpad,16 intraperitoneal cavity (the author's unpublished results) or intravenously15 is sufficient to drive proliferation of Treg in vivo. Thirdly, the steady-state presentation of tissue-derived proteins can stimulate Treg proliferation.14,17 Collectively, these data argue against an obligatory role for inflammation in rendering APC capable of driving Treg proliferation.
The link between CD86 and Treg proliferation remains provocative. Although Treg undergo reduced proliferation in response to CD80/86–/– DC in vitro16 or following anti-CD80/86 blockade in vivo21 the observations described above suggest that provision of CD28 ligands is not the sole determining factor in dictating Treg proliferation. The potential for such ligands to engage cytotoxic T-lymphocyte antigen (CTLA-4), as well as CD28, may be important in this regard. Another curiosity that remains to be explained is why CFA injection, but not LPS stimulation, causes DC to trigger Treg proliferation? One possibility is that this reflects the differential modulation of ligands other than CD80/86 which may hold the key to Treg stimulation. A potential candidate is the ligand for glucocorticoid-induced tumour necrosis factor receptor (GITR), because ligation of GITR permits dramatic proliferation of CD25+ Treg in the presence IL-222 (although signalling through GITR alone is not sufficient to break Treg anergy23,24). An alternative explanation for the discrepancy between LPS activation and CFA injection is that only a fraction of CD11c+ cells have the capacity to trigger Treg proliferation and CFA treatment preferentially recruits this subset.
Does proliferation impair Treg suppressive function?
Treg anergy and suppression have come to be viewed as intimately linked. By and large, treatments that break Treg anergy, such as high-dose anti-CD28 or the provision of exogenous IL-2, also abrogate suppression in cocultures.2–4,7 In addition, there appears to be an inverse relationship between the capacity of different APC types to drive Treg proliferation and to support Treg suppression. For example, responses driven by B cells, which are unable to induce Treg proliferation,16 tend to be the easiest to suppress.20,25 Does proliferation therefore impair Treg suppressive capacity? A closer look at these studies reveals that loss of anergy and loss of suppression can, in fact, be uncoupled. For example, anti-CD282,8 and LPS20 are capable of abolishing suppression without inducing detectable Treg proliferation. Conversely, restoring Treg proliferation in vitro by the addition of exogenous IL-4 does not entail loss of suppressive capacity.10 Evidence from in vivo studies suggests that proliferating Treg are nevertheless competent to suppress their CD25− counterparts14,15 and ameliorate pathology.19 In fact, in a rare consensus in the Treg field, all contributors seem agreed that CD25+ cells that have been induced to proliferate subsequently show an enhanced, rather than reduced, capacity to regulate.5,15,16,22,26,27 Strikingly, this holds true across a broad range of activation stimuli, even extending to Treg that have undergone lymphopenia-induced proliferation in vivo.13 It is tempting to speculate that the act of transiting cell cycle up-regulates the elusive molecule(s) that mediate Treg suppression, although trivial explanations such as the induction of adhesion molecules cannot be ruled out. Since Treg revert to an ‘anergic’ phenotype (based on classical in vitro readouts) following removal of proliferative stimuli2,3,13–15 it also remains possible that suppression is temporarily abolished during Treg proliferation but then is rapidly reinstated, indeed with added potency, as proliferation wanes. However, such a scenario is hard to reconcile with the apparent simultaneous Treg proliferation and suppression observed in vivo.14,15
Antigen specificity of CD4+ CD25+ Treg
Treg thymic selection
Despite an accumulation of evidence to support the existence of CD4+ CD25+ Treg cells, a clear consensus as to whether such cells respond to self or foreign antigens has been lacking. The thymic selection of CD25+ cells with regulatory function is MHC class II dependent,28 indicating that, as for conventional CD25− T cells, a degree of self-reactivity is a prerequisite for effective Treg selection. CD25+ cells may also be subject to negative selection,28–30 again mirroring CD25− T-cell development. In fact, studies using mice in which the diversity of self-peptides available in the thymus could be experimentally controlled suggest that the selection of CD25− and CD25+ cells is tightly linked.29 Altering the diversity of self-peptides available in the thymus changed the absolute number of T cells selected, but intriguingly the ratio of CD25+ cells to CD25− cells remained constant. This suggests that CD4+CD25+ cells might be selected intrathymically at a fixed ratio relative to CD4+ CD25− cells.
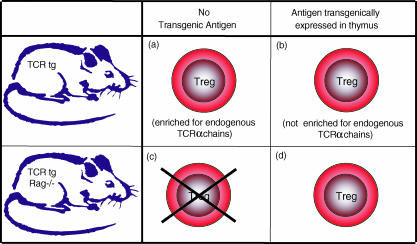
A major step forward in understanding Treg development came from the discovery that TCR transgenic mice which also express the antigen recognized by the transgenic TCR within the thymus generate large numbers of TCR transgenic Treg.31 In contrast, TCR transgenic mice which lack the relevant antigen are unable to generate Treg unless they use endogenous TCRα chains to generate alternative antigen specificities2,3,14,32,33 (Fig. 2). Transgenic expression of thymic antigen can be controlled by a range of different promoters14,31–33 but importantly, not every T cell with the relevant specificity can be induced to undergo Treg differentiation.31 This emphasizes the potential for multiple factors, including avidity, timing and context of antigen presentation, to influence Treg differentiation. Expression of the relevant antigen by thymic epithelium,31,32 perhaps particularly cortical epithelium,28 appears sufficient to mediate the selection of CD25+ Treg. In fact, pioneering studies have revealed that even intrathymic injection of antigen can drive Treg differentiation.34 Under normal circumstances, the requirement for antigen expression in the thymus might appear to impose a serious constraint on the capacity to develop a broad repertoire of Treg, especially to organ-specific antigens. However, the emergence of the idea that tissue-specific proteins are also expressed in the thymus in medullary (but frequently also cortical) epithelial cells35 supports the possibility that the thymus can select Treg which recognize a broad range of peripheral self antigens. A good example is the insulin promoter which has been shown to be functionally active in the thymus, as well as the pancreas36–38 in a manner which can induce Treg differentiation.14
Figure 2.
Treg generation in TCR transgenic mice.2,3,14,31–33 Conventional TCR transgenic (TCR tg) mice generate a small number of CD25+ Treg which are enriched for endogenous TCRα chains (a). If the antigen recognized by the transgenic TCR is expressed in the thymus, the Treg which are produced are not enriched for endogenous TCRα chains (b). TCR transgenic mice on a Rag-deficient background (TCR tg Rag–/–), whose antigen-recognition is stringently restricted to the germ-line encoded specificity, fail to generate CD25+ Treg (c). However, Treg can be produced in TCR tg Rag–/– mice if they are provided with a thymic source of the antigen recognized by the transgenic TCR (d).
Is Treg proliferation driven by self-antigens?
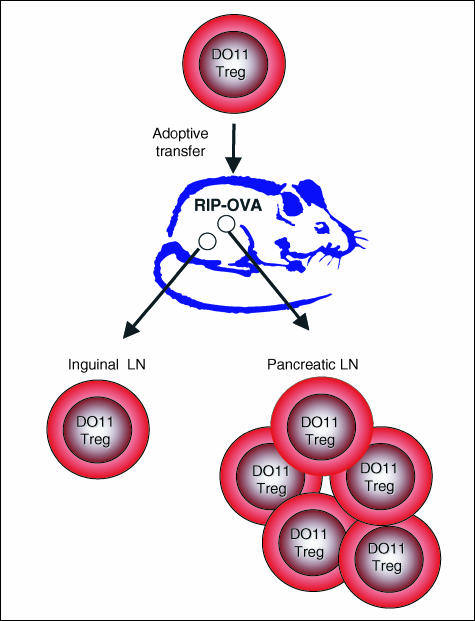
Given that CD25+ Tregs appear ‘self-reactive’, based on their selection in response to thymic antigens, is it fair to conclude that the proliferation of CD25+ Treg observed in the periphery17,18 is driven by interaction with self proteins? Support for this idea comes from studies in which antigen-specific Treg were adoptively transferred into recipient mice expressing the relevant antigen on a peripheral tissue: the transferred Treg proliferated selectively in the lymph nodes draining the antigen-bearing tissue14,17 (Fig. 3). The seminal observation that ablation of a peripheral tissue in utero led to a deficit of functional Treg specific for that tissue (despite their effective thymic selection) is consistent with a role for self antigens in peripheral Treg homeostasis.39 Such a model also fits with the lack of requirement for adjuvant to trigger Treg division in vivo15,16 because self antigens are likely to be presented by APC not exposed to inflammatory stimuli. Thus, it seems likely that at least a portion of the Treg proliferation observed in vivo is driven by encounter with self antigens.
Figure 3.
OVA-specific Treg (DO11 Treg) adoptively transferred into mice expressing OVA in the pancreas proliferate in the pancreatic (but not inguinal) lymph node.14 Similar data have been obtained using a haemagglutinin-specific TCR transgenic system.17
Can Treg respond to foreign antigens?
Since any given TCR is likely to recognize numerous ligands,40 even if the repertoire of CD25+ Treg is biased towards self antigens30 it is inconceivable that it does not encompass cells capable of recognizing foreign determinants. This is supported by the demonstration that T cells specific for alloantigens can be expanded from the CD4+ CD25+ population in vitro.41,42 However, the existence of foreign-reactive Treg does not necessarily equate to their participation in immune responses. In fact, many of the models put forward to explain how Treg suppression is controlled are based on the premise that Treg inhibit responses to self antigens and not foreign antigens. This would allow harmful autoimmune damage to be avoided while permitting defence responses against invading pathogens to proceed uninhibited. The problem with this argument is the steadily increasing list of examples in which Treg modify immune responses to pathogens. Immune responses to Leishmania major,10,43Helicobacter hepaticus,44Pneumocystis carinii,45Listeria monocytogenes46 and Candida albicans47 have all been shown to be modulated by CD4+ CD25+ cells. In fact, many of the early studies that described the regulatory activity of CD25+ Treg in vivo utilized animal models of inflammatory bowel disease48,49 in which the eliciting antigens are thought to derive from gut bacteria rather than self tissues. The ability of CD4+ CD25+ cells to control immune responses elicited by mature DC pulsed with foreign determinants50 also argues against a model in which Treg function is restricted to self antigens. Paradoxically, the participation of CD25+ Treg in pathogen-induced responses may even be beneficial to the host: in an unexpected twist, the ability of Treg to prevent effector cells from eliminating infectious agents can facilitate the development of immunological memory.51 Thus, the available data suggest that CD4+ CD25+ Treg can indeed participate in the regulation of immune responses to foreign proteins.
Can foreign-antigens drive Treg proliferation?
Since the proliferative potential of Treg has only recently been appreciated, few studies have examined this parameter in models of Treg suppression. In one study, CD4+ CD25+ cells were enriched at the site of Leishmania major infection51 and appeared to exhibit a degree of antigen-specificity, based on cytokine production after in vitro challenge with L. major-infected (but not Toxoplamsa gondii-infected) DC, but the contribution of local expansion over selective recruitment was not examined. In relation to C. albicans infection, the proportion of CD4+ CD25+ cells in the mesenteric lymph nodes (but not in Peyer's patches or spleen) was clearly augmented after gastrointestinal infection.47 While it is hard to formally exclude that a fraction of these CD4+ CD25+ cells were activated cells that had up-regulated CD25 in response to infection, at the population level these cells produced IL-10 and showed suppressive activity. It is tempting to speculate that local proliferation of Treg might have contributed to the relative increase in CD4+ CD25+ numbers observed after infection. Curiously, these authors also described an increase in CD4+ CD25+ cells within the thymuses of mice subjected to either gastrointestinal or disseminated infection with C. albicans. Whether this reflects the potential for infections to alter thymic selection of Tregs remains unclear but the data are certainly provocative. Thus, the participation of Treg in immune responses to foreign pathogens could conceivably be facilitated by local Treg proliferation and this is an obvious target for further investigation.
Treg Homeostasis
Homeostasis of Treg in the steady state: a fixed niche for CD25+ cells?
The fact that CD25+ Treg are generated slightly later than CD25− cells permitted the early observation that thymectomy on day 3 after birth allowed the production of CD25− (but not CD25+) cells, leading to autoimmunity.1 The stability of the CD25+ population following thymic export is illustrated by the observation that thymectomy of mice at 4–5 weeks of age fails to perturb the number of CD4+ CD25+ cells in the periphery even when analysed 19 months later.52 In fact, because thymectomy at time-points later than day 3 failed to precipitate overt autoimmunity1 this suggests that a long-lived, potentially self-renewing population of CD25+ cells seeds the periphery within the first week after birth. The recent demonstration that at least a fraction of Treg can exist as a long-lived non-dividing population, while others divide extensively in vivo,17 is of interest in this regard. On a cautionary note, the potential for peripheral Treg generation (see later) to contribute to CD25+ cell numbers after thymectomy should not be overlooked.
In normal mice, the proportion of peripheral CD4 cells expressing CD25 appears to be maintained at a constant value of approximately 10% from 2 weeks of age up until at least 1 year.53 This is consistent with the existence of a fixed niche for CD25+ cells in the periphery. Such a contention is supported by the observation that following the adoptive transfer of Treg to CD3ε–/– recipients, the absolute cell recovery 10–12 weeks later was remarkably constant regardless of the input cell number.12 This suggests that the CD25+ cells expanded to reach a set threshold number. Given the complete absence of T cells in such recipients, this suggests that Treg population size is regulated independently of naïve CD25− T cells. The expansion of Treg after adoptive transfer into mice deficient in CD25+ cells (as a consequence of IL-2Rβ deficiency54 or deficiency of the transcription factor FOXP355) offers circumstantial support for the concept of a fixed niche for Treg.
Given that a niche may exist for CD25+ cells in the periphery, what mechanisms serve to control its size? A definitive answer to this question is not yet possible, but attractive candidates include specific antigen,14,17,39 IL-212,54,56 and CD28 signalling.21,57 One challenge facing investigators is that the role of such factors in the periphery is not easily separable from that played in the thymus. For example, IL-2 impacts on Treg both in the thymus52,54 and periphery.54,56,58 Similarly the transcription factor FOXP3, which appears crucial in directing CD25+ cell differentiation,55,59,60 also seems to play a role in the periphery based on the selective reconstitution of thymic expression in FOXP3-deficient mice.61 Whether the size of the CD25+ peripheral pool is ultimately dictated by a limiting factor (e.g. antigen, APC, or cytokines) or whether CD25+ cells negatively regulate their own homeostasis remains to be determined.
Homeostasis of Treg during inflammation: enhanced Treg function?
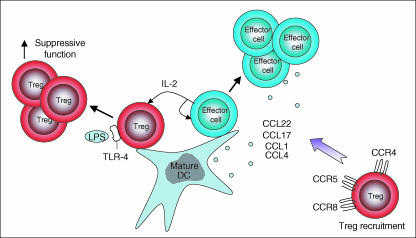
The ability of Treg to down-regulate immune responses to pathogens argues against their function being inhibited by inflammatory signals. On the contrary, local Treg homeostasis and function may even be enhanced following infection. Although capable of little IL-2 production themselves2,5,14,16 CD25+ cells express all the subunits necessary to generate the functional high-affinity IL-2 receptor,54 potentially rendering them exquisitely sensitive to paracrine IL-2 produced by lymphocytes and APC during immune responses. Moreover, Treg are endowed with the capacity to recognize conserved pathogen-derived products, by virtue of Toll-like receptor expression, and exposure to such products, particularly in combination with IL-2, can dramatically augment their suppressive function,26 as can treatment with supernatants taken from LPS-treated DC.62 Curiously, such supernatants (comprising IL-6 plus an unidentified factor) simultaneously render responder T cells less sensitive to Treg suppression.62 Thus, the very same factors that augment immune responses (IL-2, LPS) can concurrently enhance Treg homeostasis and function. Just as conventional T cells proliferate at inflammatory sites, so too can Treg;19 indeed proliferation may augment their suppressive function. Similarly, the recruitment of pathogenic cells to inflammatory sites is likely to be balanced by the simultaneous recruitment of CD25+ Treg attracted by chemokines such as CCL1, CCL17, CCL2263 and CCL4·64 The TRANCE-RANK pathway may also play a key role in the local accumulation of functional Treg in response to short-term inflammation.65 Thus, while inflammation can clearly have conflicting effects on Treg66 one potential outcome is the augmentation of local Treg numbers and function (Fig. 4).
Figure 4.
Treg homeostasis during inflammation.19,26,63,64,67 Mature dendritic cells (DC) stimulate the proliferation of both effector T cells and Treg. Treg which have divided may have increased suppressive capacity. Paracrine IL-2 and pathogen-derived products (e.g. LPS) stimulate Treg through the high-affinity IL-2 receptor and Toll-like receptors (e.g. TLR-4). This may enhance suppressor function. Chemokines produced from mature DC and activated T cells may recruit Treg via the chemokine receptors CCR4, CCR8 and CCR5.
Once activated by TCR signalling, the effector activity of Treg is not antigen-specific, at least in vitro.5 This raises the possibility that Treg recruited by inflammatory signals would not necessarily have to recognize antigens derived from an invading pathogen to exert local suppression. In fact, Treg responding to self-antigens could conceivably participate in the regulation of an immune response to a pathogen, especially given the potential for IL-2 signalling and Toll-like receptor ligation to enhance their numbers and function. Consistent with the idea that infection may augment Treg function, CD25+ cells isolated from animals infected with herpes simplex virus (HSV) showed enhanced regulatory activity in vitro and this was not restricted to responses to HSV antigens.67 As highlighted by the authors, HSV is probably directly recognized by only a minute fraction of the CD25+ population, rendering it likely that the global enhancement of Treg activity constitutes a ‘bystander’ effect of infection. In a separate study, the ability of Treg from the pancreatic lymph node, but not other lymph nodes, to control diabetes might similarly reflect the fact that these Treg have been exposed to local short-term inflammation, resulting in augmented suppressor function.65
Taken together, these observations suggest that as inflammation escalates, so too does the capacity of Treg to bring it back under control by virtue of their expansion, augmented function and local recruitment. Immune responses may be initiated not because Treg are somehow ‘switched off’, but simply because they are insufficiently represented locally to quell the response. Initially, the pathogenic T cells win the ‘numbers game’, but as the response proceeds, the tide begins to turn with the local augmentation of Treg numbers and function. The capacity of Treg to inhibit aspects of innate immunity44 in addition to dampening the T-cell response may be central to their anti-inflammatory activities. Why would inflammatory sites form the focus for Treg recruitment concomitantly with attracting immune effectors? One possibility is that this allows immune responses to proceed within an ‘allowable’ window such that they are vigorous enough to provide adequate host defence, without necessarily eliminating the pathogen (which could limit immune memory) or causing an unacceptable level of host damage.
Induction of Treg in the periphery: the plot thickens
While this review has focused on the Treg that are selected to be CD4+ CD25+ cells in the thymus, these cells should be viewed as one of several regulatory T-cell subsets. It is becoming increasingly clear that while CD4+ CD25+ Treg emerging from the thymus can exhibit immunosuppressive function, so too can regulatory T cells that are induced in the periphery. Confusingly, such cells may also up-regulate CD2568–70 although this is by no means a universal marker of regulatory T cells.11,32,71–74 A bewildering array of protocols can induce regulatory T cells, including intravenous70 or oral68,70 antigen administration, anti-CD4 antibodies,75 costimulatory blockade,76,77 or the targeting of antigens to immature DC using antibodies to DEC-205.69 Treg may even arise as part of a normal immune response to infection.78In vitro, T cells with regulatory properties can be generated by repetitive stimulation with immature DC79 or by activation in the presence of IL-10 alone80 or in combination with transforming growth factor-β.81
Notwithstanding the diverse conditions under which regulatory T cells are induced, the phenotypic parallels with thymic-derived CD25+ Treg are striking. Examples include the expression of CTLA-468,69,73 and failure to produce IL-2,68–70 both of which are highly characteristic of thymic-derived CD25+ cells.2,4,14,15,82,83 There is evidence that CD25− regulatory T cells express GITR84 and it has even been suggested that the small amount of FOXP3 mRNA expressed in CD4+ CD25− cells may identify a regulatory subset55 strengthening the links between thymic-derived and inducible Treg. Along similar lines, it is tempting to speculate that the mRNA for Toll-like receptors detected in CD4+ CD45RBlow CD25− cells (but absent from CD4+ CD45RBhi cells)26 derives from a regulatory CD25− subpopulation. Short of being a cruel divine joke designed (with some success) to create a confusing literature, this suggests that similar pathways may underpin the biology of distinct subsets of regulatory lymphocytes. Future studies are necessary to delineate the extent to which the functions of such subsets are overlapping.
Concluding remarks
The discovery that thymus-derived CD4+ CD25+ Treg have a greater proliferative capacity than previously appreciated14–17 forces us to revisit some of our preconceptions regarding this specialized subset. On the one hand, Treg proliferation can proceed in the absence of overt inflammation15,16 and may reflect recognition of local tissue-derived antigens.14,17 Continuous stimulation by self proteins is consistent with the well-established role of this subset in preventing autoimmunity. On the other hand, inflammation may promote Treg recruitment and function, and DC from inflammatory sites are implicated in Treg proliferation both in vitro16 and in vivo.19 This argues for an additional role for Treg in tuning immune responses initiated by foreign antigens. The potential of CD25+ Treg to modulate immune responses to both self and foreign antigens makes understanding the biology of this population a crucial objective.
Acknowledgments
I am grateful to D. Sansom, P. Lane, A. Chodos and G. Anderson for commenting on the manuscript and A. Abbas and E.A. Green for helpful discussions. L.S.K.W. is funded by The Wellcome Trust.
References
- 1.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 3.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity. Production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 4.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 6.Jordan MS, Riley MP, von Boehmer H, Caton AJ. Anergy and suppression regulate CD4+ T cell responses to a self peptide. Eur J Immunol. 2000;30:136–44. doi: 10.1002/1521-4141(200001)30:1<136::AID-IMMU136>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Kuniyasu Y, Takahashi T, Itoh M, Shimizu J, Toda G, Sakaguchi S. Naturally anergic and suppressive CD25(+) CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol. 2000;12:1145–55. doi: 10.1093/intimm/12.8.1145. [DOI] [PubMed] [Google Scholar]
- 8.Ermann J, Szanya V, Ford GS, Paragas V, Fathman CG, Lejon K. CD4+CD25+ T cells facilitate the induction of T cell anergy. J Immunol. 2001;167:4271–5. doi: 10.4049/jimmunol.167.8.4271. [DOI] [PubMed] [Google Scholar]
- 9.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;4:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2002;170:394–9. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 11.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–18. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 12.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells. IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–60. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 13.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+) CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–44. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 14.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–58. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–91. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–47. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon B. Continuous activation of autoreactive CD4+CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–46. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori S, Haury M, Lafaille JJ, Demengeot J, Coutinho A. Peripheral expansion of thymus-derived regulatory cells in anti-myelin basic protein T cell receptor transgenic mice. Eur J Immunol. 2002;32:3729–35. doi: 10.1002/1521-4141(200212)32:12<3729::AID-IMMU3729>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4(+) CD25(+) regulatory T cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 20.George TC, Bilsborough J, Viney JL, Norment AM. High antigen dose and activated dendritic cells enable Th cells to escape regulatory T cell-mediated suppression in vitro. Eur J Immunol. 2003;33:502–11. doi: 10.1002/immu.200310026. [DOI] [PubMed] [Google Scholar]
- 21.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge. CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–52. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 22.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+) CD25(+) immunoregulatory T cells. Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 24.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4+CD25+ (high) regulatory T cell function. J Immunol. 2002;169:6210–17. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 25.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 26.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–46. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+) 25(+) immunoregulatory T cells. J Exp Med. 2001;194:427–38. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacholczyk R, Kraj P, Ignatowicz L. Peptide specificity of thymic selection of CD4+CD25+ T cells. J Immunol. 2002;168:613–20. doi: 10.4049/jimmunol.168.2.613. [DOI] [PubMed] [Google Scholar]
- 30.Romagnoli P, Hudrisier D, van Meerwijk JP. Preferential recognition of self antigens despite normal thymic deletion of CD4+CD25+ regulatory T cells. J Immunol. 2002;168:1644–8. doi: 10.4049/jimmunol.168.4.1644. [DOI] [PubMed] [Google Scholar]
- 31.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 32.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–63. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 33.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4(+) CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 34.Trani J, Moore DJ, Jarrett BP, et al. CD25+ immunoregulatory CD4 T cells mediate acquired central transplantation tolerance. J Immunol. 2003;170:279–86. doi: 10.4049/jimmunol.170.1.279. [DOI] [PubMed] [Google Scholar]
- 35.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–9. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 36.Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci USA. 1994;91:6707–11. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–30. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–45. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–82. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason D. A very high level of crossreactivity is an essential feature of the T- cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 41.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4+CD25+ immunoregulatory T cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–6. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang S, Camara N, Lombardi G, Lechler RI. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood. 2003;102:2180–6. doi: 10.1182/blood-2003-04-1164. [DOI] [PubMed] [Google Scholar]
- 43.Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol. 2002;169:3232–41. doi: 10.4049/jimmunol.169.6.3232. [DOI] [PubMed] [Google Scholar]
- 44.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T (R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–19. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–91. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Kursar M, Bonhagen K, Fensterle J, Kohler A, Hurwitz R, Kamradt T, Kaufmann SH, Mittrücker HW. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J Exp Med. 2002;196:1585–92. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montagnoli C, Bacci A, Bozza S, Gaziano R, Mosci P, Sharpe AH, Romani L. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol. 2002;169:6298–308. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- 48.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB (low) CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4+CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–66. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 52.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Regulatory CD4 T cells. expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371–8. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 53.Suri-Payer E, Amar AZ, McHugh R, Natarajan K, Margulies DH, Shevach EM. Post-thymectomy autoimmune gastritis. fine specificity and pathogenicity of anti-H/K APTase-reactive T cells. Eur J Immunol. 1999;29:669–77. doi: 10.1002/(SICI)1521-4141(199902)29:02<669::AID-IMMU669>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 54.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 55.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–36. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 56.Furdado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196:851–7. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 58.Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci USA. 2002;99:8832–7. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hori S, Nomura N, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 60.Khattri R, Cox T, Yasayko S-A, Ramsdell F. An essential role for scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 61.Khattri R, Kasprowicz D, Cox T, Mortrud M, Appleby MW, Brunkow ME, Ziegler SF, Ramsdell F. The amount of scurfin protein determines peripheral T cell number and responsiveness. J Immunol. 2001;167:6312–20. doi: 10.4049/jimmunol.167.11.6312. [DOI] [PubMed] [Google Scholar]
- 62.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 63.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+) CD25(+) regulatory T cells. J Exp Med. 2001;194:847–53. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 65.Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4+CD25+ Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002;16:183–91. doi: 10.1016/s1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- 66.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci USA. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25+CD4+ regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–53. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 69.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–9. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 70.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+ CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–95. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 71.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25– subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 72.Olivares-Villagomez D, Wensky AK, Wang Y, Lafaille JJ. Repertoire requirements of CD4+ T cells that prevent spontaneous autoimmune encephalomyelitis. J Immunol. 2000;164:5499–507. doi: 10.4049/jimmunol.164.10.5499. [DOI] [PubMed] [Google Scholar]
- 73.Sundstedt A, O'Neill EJ, Nicolson KS, Wraith DC. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J Immunol. 2003;170:1240–8. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 74.Bynoe MS, Evans JT, Viret C, Janeway CA. Epicutaneous immunization with autoantigenic peptides induces T suppressor cells that prevent experimental allergic encephalomyelitis. Immunity. 2003;19:317–28. doi: 10.1016/s1074-7613(03)00239-5. [DOI] [PubMed] [Google Scholar]
- 75.Hara M, Kingsley CI, Niimi M, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–96. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 76.Taylor PA, Noelle RJ, Blazar BR. CD4+CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–8. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Maurik A, Herber M, Wood KJ, Jones ND. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J Immunol. 2002;169:5401–4. doi: 10.4049/jimmunol.169.10.5401. [DOI] [PubMed] [Google Scholar]
- 78.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–31. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 81.Chen ZM, O'Shaughnessy MJ, Gramaglia I, Panoskaltsis-Mortari A, Murphy WJ, Narula S, Roncarolo MG, Blazar BR. IL-10 and TGF-beta induce alloreactive CD4+CD25- T cells to acquire regulatory cell function. Blood. 2003;101:5076–83. doi: 10.1182/blood-2002-09-2798. [DOI] [PubMed] [Google Scholar]
- 82.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+) CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Urashihara K, Kanai T, Ko K, Totsuka T, Makita S, Iiyama R, Nakamura T, Watanabe M. Regulation of murine inflammatory bowel disease by CD25+ and CD25– CD4+ glucocorticoid-induced TNF receptor family-related gene+ regulatory T cells. J Immunol. 2003;171:708–16. doi: 10.4049/jimmunol.171.2.708. [DOI] [PubMed] [Google Scholar]