Abstract
The activation of dendritic cells (DCs) by microbes is mediated by pattern recognition receptors including the Toll-like receptors (TLR). Bacterial lipopolysaccharide acts via TLR4 whereas peptidoglycan and lipoprotein responses are mediated by TLR2. It is generally accepted that TLR binding to microbes occurs at the cell surface but this has not been directly demonstrated for human DCs. We show here that TLR2 and TLR4 are expressed inside DCs in an abundant tubulovesicular pattern with a focus of intense staining adjacent to the nucleus. In contrast, there was no detectable expression on the cell surface. TLR2 and TLR4 were readily found both intracellularly and on the surface of monocytes. They were shown to be closely associated with the Golgi complex and colocalized with α-tubulin, displaying a high focal concentration at the microtubule organizing centre. Alignment of TLR2 and TLR4 with microtubules was observed, suggesting that microtubules serve as transport tracks for TLR vesicles. Depolymerization of the microtubule network disrupted the intracellular expression of TLR2 and TLR4 and profoundly inhibited interleukin-12 (IL-12) production in response to Neisseria meningitidis but did not prevent phagocytosis. These data are consistent with the bacterial signalling through TLR2 and TLR4 required for IL-12 production occurring inside DCs after phagocytosis.
Introduction
Dendritic cells (DCs) are highly specialized antigen-presenting cells that form a gateway between the innate and adaptive immune systems.1 Immature DCs express surface pattern recognition receptors that bind to microbes or microbial products, which are then internalized and processed by the DCs.1 Whole bacteria, yeasts, protozoa and microbial products have all been found to induce DCs to express surface costimulatory molecules and secrete cytokines required for initiating the T-cell immune response.2 Production of interleukin-12 (IL-12) helps to polarize T helper cells towards the T helper type 1 (Th1) phenotype, which has been shown to be critical in the effector response against many pathogens. Various pattern recognition receptors that recognize pathogen-associated motifs are expressed by immature DCs and drive the maturation process. In particular, the mammalian Toll-like receptor (TLR) family play an important role in pathogen recognition in the innate immune response.3 Of the 10 TLRs that have been described in humans, TLR2 and TLR4 have been the most extensively studied. TLR4, together with MD-2 and CD14, form a signalling complex that responds to the lipopolysaccharide (LPS) of many Gram-negative bacteria.4,5 TLR2 responds to a number of bacterial products including components of Gram-positive bacterial cell walls, peptidoglycan, lipoproteins and lipoteichoic acid.6–8 TLR2 also responds to yeast.9
The Gram-negative bacteria Neisseria meningitidis is a major cause of bacterial meningitis and septicaemia. It is known to engage both TLR2 and TLR4 and it is a potent inducer of tumour necrosis factor (TNF), IL-12 and IL-10 production by monocytes and DCs.10,11 We have recently shown that bacteria must express LPS to stimulate optimal IL-12 and TNF-α cytokine production by DCs.12 Moreover, phagocytosis of the bacteria depended on the expression of LPS and was required for cytokine production, particularly that of IL-12 (Uronen-Hansson et al. submitted for publication). These findings suggested to us that the signalling events required for cytokine production might not occur on the DC surface but rather in the phagosome or lysosome after bacterial internalization and they raised the possibility that TLR2 and/or TLR4 interactions with the bacteria may occur inside the cell. There have been several reports that might support this view. Human TLR2 and TLR6 have been shown to be recruited to macrophage phagosomes where they signal the cell to make cytokines but do not appear to be involved in phagocytosis itself.9,13 Similarly, CpG DNA signals through TLR9 in DC phagosomes14 and LPS has been shown to colocalize with TLR4 in the Golgi complex of epithelial cells.15,16
In the present study, the expression and localization of TLR2 and TLR4 in human DCs was investigated using specific antibodies raised against TLR peptides. TLR2 and TLR4 were not present on the surface of DCs but were readily detected inside the cell, associated with tubulovesicular structures close to the Golgi complex. Colocalization of TLR2 and TLR4 with DC microtubules was observed, suggesting that TLR vesicles move along these structures. Depolymerization of the microtubule network disrupted intracellular TLR2 and TLR4 and inhibited IL-12 production in response to N. meningitidis but did not prevent phagocytosis. These results suggest that the TLR activation by N. meningitidis required for IL-12 production occurs inside DCs and not on the cell surface.
Materials and methods
DC culture and activation
DCs were generated from peripheral blood mononuclear cells as described previously.17 In brief, monocytes were prepared from peripheral blood mononuclear cells by centrifugation over multistep Percoll gradients. The monocyte fraction was > 95% CD14+ CD3– CD19–. To generate DCs, monocytes were incubated for 5–6 days in RPMI-1640 supplemented with heat-inactivated 5% fetal calf serum, 2·4 mm l-glutamine, 100 U/ml penicillin–streptomycin (all from Gibco, Paisley UK), 100 ng/ml human recombinant granulocyte–macrophage colony-stimulating factor and 50 ng/ml human recombinant IL-4 (Schering-Plough, Welwyn Garden City, Herts UK). Immature DCs prepared in this way were CD14low, CD83–ve, CD86low, CD25–ve. They also expressed human leucocyte antigen (HLA) DR, HLA DQ, HLA Class I, CD40 and CD1a, and were negative for both CD19 and CD3 as described previously.12
In some experiments, DCs were cultured with N. meningitidis H44/76 at a DC to bacteria ratio of 1 : 100 in RPMI-1640 supplemented with 5% heat-inactivated fetal calf serum. When intracellular cytokines were to be measured, the protein transport inhibitor Brefeldin A (Sigma, Poole, UK) was added at 10 μg/ml. To depolymerize microtubules, 100 ng/ml of colcemid (Gibco) was added to cultures as indicated.
Bacteria and phagocytosis assay
Group B N. meningitidis H44/76 were grown on gonococcal agar (Difco, Basingstoke, UK) supplemented with Vitox (Oxoid Ltd, Basingstoke, UK) in an atmosphere of 6% CO2 in air at 36°. The bacteria were used in stationary phase after culture for 18 hr. Suspensions of bacteria were prepared in RPMI-1640 medium without phenol red (Gibco), and their optical density was measured at 540 nm. Bacteria were fixed in 0·5% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 15 min and washed thoroughly in RPMI-1640 medium. Fluorescein isothiocyanate (FITC) -labelled bacteria were prepared by incubation with 0·5 mg/ml FITC (Sigma) for 20 min at 37° followed by extensive washing.
Immunostaining
DCs were stained for surface and intracellular expression of TLR2 and TLR4 with rabbit polyclonal antibodies raised against TLR2 and TLR4 peptides.18 For intracellular staining, DCs were fixed in 4% PFA for 15 min and then permeabilized in Hanks' balanced salt solution with 0·1% saponin (Sigma). To prevent non-specific binding, 10% human serum was added for 30 min. Surface staining was carried out on live cells on ice. TLR antibodies and rabbit immunoglobuin G (IgG) control antibodies (Caltag Medsystems, Silvestone, UK) were added at a final concentration of 10 μg/ml for 1 hr on ice, washed carefully and detected with 1 μg/ml of FITC goat anti-rabbit IgG F(ab)2 (Caltag). Mouse monoclonal antibodies to human Golgin-97 (Molecular Probes, Cambridge Biosciences, Cambridge, UK) were used at 10 μg/ml to stain the Golgi complex. Microtubules were stained with mouse monoclonal TAT-1 antibody to α-tubulin.19 Bound antibodies were detected with Alexa 568-conjugated goat anti-mouse IgG (Molecular Probes). Purified mouse IgG1 was used at 10 μg/ml as an isotype control (Pharmingen). To detect intracellular cytokines, DCs were fixed with 4% PFA, washed in PBS containing 0·1% sodium azide and 0·5% bovine serum albumin (all Sigma) and permeabilized in 50 μl of Permeabilisation solution (Caltag Medsystems). Cells were then incubated with phycoerythrin-conjugated monoclonal antibodies to TNF-α (Becton Dickinson, Oxford UK), IL-12 p40/70 (Pharmingen) or isotype-matched controls for 30 min at room temperature in the dark. The cells were then washed twice in PBS, fixed in CellFix (Becton Dickinson) and analysed on a FACScalibur using cell quest software (Becton Dickinson).
Confocal microscopy
Labelled cells were air-dried on poly l-lysine-coated slides and mounted with Citifluor (Citifluor Ltd, Leicester, UK). Confocal images were obtained using a Leica SP2 confocal laser scanning microscope system (Leica, Milton Keynes, UK) fitted with appropriate filter sets. Between 15 and 20 optical sections (0·2–0·5 μm) spanning the entire DC were projected and superimposed with Leica confocal imaging software. All images were acquired in sequential scan mode with a Gain typically no more than 650 V, as recommended by Leica Microsystems, to minimize fluorochrome emission overlap.
Results
Human DCs express TLR2 and TLR4 intracellularly but not on the cell surface
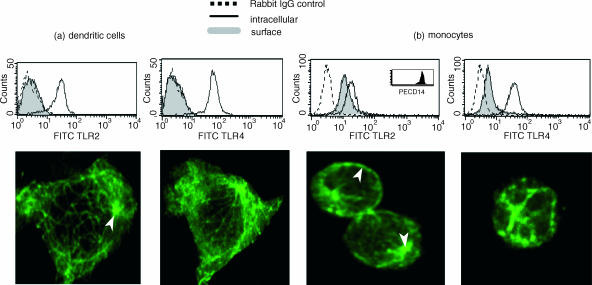
DCs were stained for surface and intracellular expression of TLR2 and TLR4 (Fig. 1a). By fluorescence-activated cell sorter analysis, abundant intracellular staining of both TLR2 and TLR4 was observed but no surface staining could be detected. Confocal microscopy confirmed the absence of TLR2 and TLR4 on the surface but clearly showed intracellular expression of both TLRs with a tubulovesicular staining pattern and a highly concentrated signal in the perinuclear region. Activation of the DCs with N. meningitidis bacteria or with LPS did not induce surface expression of either TLR (data not shown). By comparison, strong cell surface and intracellular expression of TLR2 and TLR4 was found in monocytes (Fig. 1b).
Figure 1.
Surface and intracellular staining of TLR2 and TLR4 in DCs (a) and monocytes (b). For surface staining cells were incubated on ice with TLR antibodies followed by FITC-conjugated F(ab)2 goat anti-rabbit IgG. For intracellullar staining DCs were fixed with 4% PFA, permeabilized in Saponin buffer and then stained. Representative FACS histograms of surface and intracellular staining and confocal images of intracellular staining from five experiments are shown. Arrows point to a highly concentrated signal in the perinuclear region (a,b) as well as surface staining on monocytes (b).
TLR2 and TLR4 are highly expressed in a perinuclear region close to the Golgi complex associated with microtubules
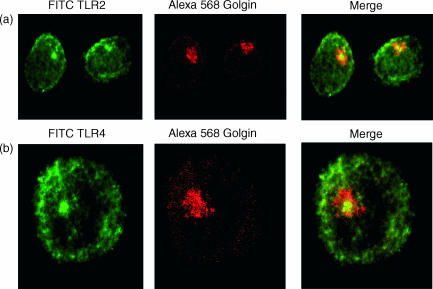
It was reported recently that TLR4 colocalizes with the Golgi apparatus in epithelial cells and monocytes.15,16 Therefore the perinuclear region in DCs that is rich in TLR2 and TLR4 was investigated for colocalization with Golgin-97, a protein expressed in the trans-Golgi network. Confocal images revealed very close association of the Golgi complex with TLR2 (Fig. 2a) and TLR4 (Fig. 2b).
Figure 2.
Association of TLR2 and TLR4 with Golgi apparatus. DCs were fixed with 4% PFA, permeabilized in Saponin buffer and stained with Alexa-568 Golgin-97 and TLR2 (a) and TLR4 (b) antbodies. Merged images show localization of TLR and Golgin at the centre of the Golgi apparatus. Representative images from three experiments are shown.
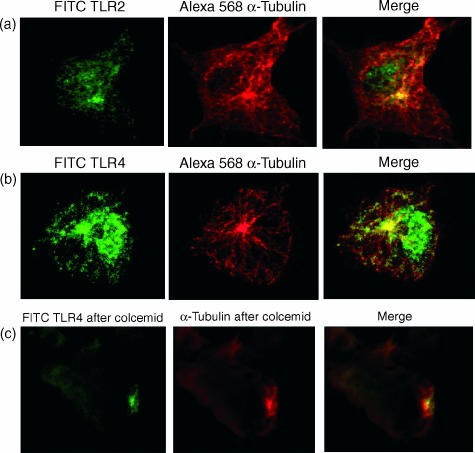
In mammalian cells, the Golgi complex is intimately associated with the cytoskeleton.20 It is centred at the microtubule-organizing centre (MTOC), from which microtubules emanate and serve as tracks for intracellular vesicular transport. As the TLR2 and TLR4 were found very close to the Golgi complex, as well as in cytoplasmic tubulovesicular structures, DCs were stained for α-tubulin to reveal the microtubules. As shown in Fig. 3, the perinuclear region rich in TLR2 (Fig. 3a) and TLR4 (Fig. 3b) colocalized with tubulin, displaying a high focal concentration at the MTOC. Co-alignment of TLR vesicles with microtubules was observed, suggesting that microtubules serve as transport tracks for TLR vesicles.
Figure 3.
Co-localization of TLR2 and TLR4 with α-tubulin. Fixed and permeabilized DCs were stained for TLR2 (a) or TLR4 (b) and α-tubulin. Microtubules were depolymerized for 5 hr with 100 ng/ml of colcemid and then stained for intracellular TLR2 and TLR4 together with α-tubulin (c). Depolymerization of microtubules disrupted the intracellular TLR2 and TLR4 vesicles. Representative confocal images from three experiments are shown.
To examine further the role of microtubules in the transport of TLR vesicles, colcemid was used to depolymerize microtubules in DCs. This drug binds to tubulin leaving only the MTOC intact.21 Figure 3(c) shows a confocal image of colcemid-treated DCs stained for TLR4 and microtubules. As can be seen, the microtubule network has been disrupted together with the TLR vesicles. A focus of TLR4 that colocalizes with the MTOC is still visible. Similar results were obtained with TLR2 (not shown).
Depolymerization of microtubules disrupts IL-12 production by DCs in response N. meningitidis
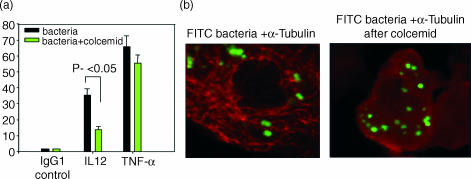
It was shown recently that internalization of the Gram-negative bacterium N. meningitidis by DCs is required for optimal cytokine production, particularly IL-12 (submitted for publication). To explore the role of intracellular TLR expression for DC activation and cytokine production by these bacteria, IL-12 production was determined after disruption of the TLR microtubule association with colcemid. As shown in Fig. 4, treatment with colcemid significantly reduced IL-12 production but had only a marginal effect on TNF-α production. Importantly, colcemid did not prevent phagocytosis of the bacteria (Fig. 4b). These results show that an intact microtubule network together with intracellular expression of TLR2 and TLR4 is required for activation of DCs by the bacteria to produce IL-12 but not for phagocytosis.
Figure 4.
Depolymerization of microtubules disrupts IL-12 production in response to Neisseria meningitidis. DCs were pre-incuabated for 5 hr in the presence or absence of 100 ng/ml colcemid and then stimulated for 14 hr with N. meningitidis bacteria at a ratio of 1 : 100 in the presence of brefeldin A. DCs producing IL-12 and TNF-α were determined by intracellular staining. Mean and SEM of three different experiments with three different donors are shown(a). Phagocytosis after 14 hr with the bacteria is shown by confocal imaging (b).
Discussion
Bacteria and other microbes that bind to pattern recognition receptors on DCs are internalized and processed for presentation to T cells. The activated DCs also release cytokines and chemokines to alert other immune cells to the site of infection and to provide a second signal to the responding T cells. As immature DCs constantly internalize antigens from their microenvironment, activation of the cells via pattern recognition receptors must be tightly regulated to prevent the migration of DCs and subsequent cytokine production in the absence of infection. We show here that immature DCs express substantial amounts of intracellular TLR2 and TLR4 whereas cell surface expression could not be detected by either flow cytometry or confocal microscopy. In most reports, TLR expression by DCs has been detected by messenger RNA analysis and not direct staining.22–24 In one study however, TLR4 was readily detected on the surface of monocytes but its expression on immature DCs was estimated as less than 100 molecules per cell, which did not increase on maturation.23 Surface expression of TLR4 on human DCs has not been described. Although the presence of very few TLR molecules on DCs cannot be excluded by our experiments, it is clear that expression is very much less than the levels found on other cells such as monocytes, granulocytes and cell lines and may be absent all together.16,23,25 Interestingly, messenger RNA levels of TLR2 and TLR4 decrease markedly during DC generation from monocytes indicating different requirements for TLRs by these cells.23
Double staining and colocalization studies by confocal microscopy showed that intracellular TLR2 and TLR4 in DCs and monocytes are expressed in close association with microtubules (Fig. 3) suggesting that microtubules may serve as a transport network for the TLRs. TLR4 has been reported to traffic between the Golgi apparatus and the cell surface in TLR4-transfected epithelial cells, consistent with this suggestion but in DCs they remain intracellular. Although TLR2 and TLR4 were associated with microtubules in DCs (Fig. 3) it was not clear whether they were indeed moving and if so in which direction. In macrophages, TLR2 and TLR6 have been found to localize around phagosomes containing yeast.9,13 Similarly, intracellular TLR9 has been found to form complexes with MyD88 around macrophage lysosomes following the uptake of CpG DNA.14 Transport of bacterial LPS to the Golgi apparatus has been shown.26,27 In epithelial cells, LPS together with TLR4 has been found to reside around the Golgi complex in a paranuclear location15 and rapid recycling of a TLR4/MD-2/CD14 complex between the Golgi and plasma membrane has been described.16 In this system, monocytes and HEK293 cell lines were used, both of which clearly express surface TLR4 and it was concluded that the signalling events were initiated at the cell membrane. In contrast, the absence of detectable TLR2 or TLR4 on the DC surface may suggest that DC activation by LPS expressed by bacteria occurs either inside the cell after phagocytosis or through some other receptor. Other studies have shown that pro-inflammatory cytokine production in response to N. meningitidis depends on TLR2 and TLR4.10 In addition, phagocytosis of N. meningitidis requires the expression of LPS on the bacterial outer membrane and is necessary for maximal IL-12 production (Uronen-Hansson et al. submitted for publication). In this report we showed that that wild-type N. meningitidis were readily and rapidly phagocytosed by the DCs, whereas isogenic LPS-deficient mutant bacteria were not. This mutant completely lacks LPS, whereas other surface antigens are unaltered.28,29,29 Disruption of microtubules, and therefore the intracellular expression of TLR2 and TLR4, was shown in the current paper to inhibit IL-12 production, but not phagocytosis, by DCs activated with N. meningitidis (Fig. 4).
In view of these findings, we suggest that DC binding to N. meningitidis via LPS and the subsequent signalling for IL-12 production are two independent, but closely related, events. Recent data from our laboratory suggest that phagocytosis of wild-type bacteria requires LPS binding protein (LBP) and can occur by binding CR3 (CD11b/CD18) (Osman et al. manuscript in preparation). This is consistent with previous findings that CR3 binds LPS.30 Interestingly, TNF-α production by DCs was only marginally affected after disruption of microtubules. Experiments with cytochalasin D as well as with transwells (Uronen-Hansson et al. submitted for publication), in which phagocytosis of bacteria was inhibited or the bacteria were physically separated from DCs by a porous membrane, showed a more significant reduction in IL-12 production compared to TNF-α. These findings suggested that production of IL-12 depends on internalization of whole bacteria whereas TNF-α production can be more easily induced by soluble bacterial components, such as LPS, released by the bacteria. Some signalling from the cell surface is likely to occur as our previously published data showed that purified meningococcal LPS can induce some TNF-α production, but not IL-12, by DCs. Only whole bacteria induced significant levels of IL-12 production.12 Interestingly, TNF-α production in response to fungal particles required recognition by Dectin-1 and TLR2, but could occur in the absence of phagocytosis.31,32 As TLRs other than TLR2 and TLR4, such as TLR9, are expressed intracellularly, the possibility that they also participate in the response cannot be excluded. It is suggested that the intracellular expression of TLRs in DCs may ensure internalization of antigen for processing and presentation to T cells before activation and migration. This would ensure that DCs activated by surface binding of microbial products to pattern recognition receptors do not arrive in the draining lymph nodes empty handed.
References
- 1.Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 2.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 3.Muzio M, Mantovani A. The Toll receptor family. Allergy. 2001;56:103–8. doi: 10.1034/j.1398-9995.2001.056002103.x. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B. Toll-like receptors: how they work and what they do. Curr Opin Hematol. 2002;9:2–10. doi: 10.1097/00062752-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–6. [PubMed] [Google Scholar]
- 7.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 8.Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166:2444–50. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 9.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–15. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 10.Pridmore AC, Wyllie DH, Abdillahi F, Steeghs L, van der Ley P, Dower SK, Read RC. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR4/MD2. J Infect Dis. 2001;183:89–96. doi: 10.1086/317647. [DOI] [PubMed] [Google Scholar]
- 11.Ingalls RR, Lien E, Golenbock DT. Differential roles of TLR2 and TLR4 in the host response to Gram-negative bacteria: lessons from a lipopolysaccharide-deficient mutant of Neisseria meningitidis. J Endotoxin Res. 2000;6:411–15. [PubMed] [Google Scholar]
- 12.Dixon GL, Newton PJ, Chain BM, et al. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect Immun. 2001;69:4351–7. doi: 10.1128/IAI.69.7.4351-4357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–68. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–70. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–43. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenhalls G, Squires GR, Stevens-Muller L, Bezuidenhout J, Amphlett G, Duncan K, Lukey PT. Associations between Toll-like receptors and IL-4 in the lungs of patients with tuberculosis. Am J Respir Cell Mol Biol. 29:28–38. doi: 10.1165/rcmb.2002-0163OC. [DOI] [PubMed] [Google Scholar]
- 19.Woods A, Sherwin T, Sasse R, MacRae TH, Baines AJ, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 2002;93:491–500. doi: 10.1242/jcs.93.3.491. 1989. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson JG, Lippincott-Schwartz J. Sorting and signaling at the Golgi complex. Cell. 2000;101:693–6. doi: 10.1016/s0092-8674(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 21.Hagiwara H, Takata K. Depolymerization of microtubules by colcemid induces the formation of elongated and centriole-nonassociated striated rootlets in PtK(2) cells. Cell Tissue Res. 2002;309:287–92. doi: 10.1007/s00441-002-0560-9. [DOI] [PubMed] [Google Scholar]
- 22.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 23.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 24.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 25.Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR) 2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 26.Thieblemont N, Wright SD. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J Exp Med. 1999;190:523–34. doi: 10.1084/jem.190.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thieblemont N, Wright SD. Mice genetically hyporesponsive to lipopolysaccharide (LPS) exhibit a defect in endocytic uptake of LPS and ceramide. J Exp Med. 1997;185:2095–100. doi: 10.1084/jem.185.12.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steeghs L, den Hartog R, den Boer A, Zomer B, Roholl P, van der Ley P. Meningitis bacterium is viable without endotoxin. Nature. 1998;392:449–50. doi: 10.1038/33046. [DOI] [PubMed] [Google Scholar]
- 29.Steeghs L, de Cock H, Evers E, Zomer B, Tommassen J, Van Der LP. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 2001;20:6937–45. doi: 10.1093/emboj/20.24.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright SD, Levin SM, Jong MT, Chad Z, Kabbash LG. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989;169:175–83. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]