Abstract
Recent studies in both humans and experimental rodent models provide new insight into key mechanisms regulating tolerance to self-molecules. These recent advances are bringing about a paradigm shift in our views about tolerance to self-molecules with tissue-restricted expression. There is, indeed, mounting evidence that selected antigen-presenting cells (APCs) have the ability to synthesize and express self-molecules, and that such expression is critical for self-tolerance. Insulin is a key hormone produced exclusively by pancreatic β-cells and a critical autoantigen in type 1 diabetes. It provides an excellent example of a molecule with tissue-restricted expression that is expressed ectopically by APCs. The fact that APCs expressing insulin have been demonstrated in both thymus and peripheral lymphoid tissues suggests that they may play a role in insulin presentation in both the central and peripheral immune system. Experimental mice, in which insulin expression was altered, provide functional data that help to dissect the role of insulin presentation by APCs of the immune system. This review addresses recent literature and emerging concepts about the expression of self-molecules in the thymus and peripheral lymphoid tissues and its relation to self-tolerance.
Introduction
Immunological self-tolerance can be defined as a meta-stable state in which the immune system does not react destructively against self-molecules, cells or tissues. Lack or loss of self-tolerance is likely to result in autoimmune responses, cellular and tissue damage, and eventually the clinical onset of autoimmune disease. Interactions between antigen-presenting cells (APCs) and lymphocytes are critical for self-tolerance, and these are known to take place in both thymus (central tolerance) and peripheral lymphoid tissues (peripheral tolerance).1,2 The mechanisms of positive and negative selection in the thymus are key to the shaping of a self-tolerant T-cell repertoire, especially in early life during the maturation of the immune system. In the thymus, developing lymphocytes with no marked reactivity against self-peptides are positively selected in the thymic cortex and enter the circulation as mature lymphocytes. In contrast, developing lymphocytes with marked reactivity against self-peptides undergo negative selection (deletion) in the thymic medulla.
However, thymic selection has been considered as an effective tolerogenic mechanism only for widely expressed self-molecules. This assumption was based on the consideration that proteins with tissue-restricted expression would not be available for presentation in the thymus. Thus, tolerance to such proteins could only be achieved through mechanisms of peripheral tolerance. Peripheral tolerance mechanisms are indeed operative in extrathymic lymphoid tissues and include deletion, anergy, ignorance and regulatory cells,3 and contribute to maintaining autoreactive lymphocytes under tight control. An important corollary of the above theories is that the presentations of self-molecules that have a tissue-restricted pattern of expression would be mainly a peripheral event and would be dependent on the capture of such self-molecules by APCs, in particular immature dendritic cells (DCs).4
Recent evidence demonstrates that a large number of molecules, including many with tissue-restricted expression (or ‘peripheral’ proteins), are expressed in the thymus.3,5,6 The expression of these proteins correlates, in most cases, with the presence of the corresponding transcript in the thymus. This discovery, together with studies demonstrating the expression of self-molecules also in peripheral lymphoid tissues, offers new insight into the role of self-antigen presentation in determining tolerance. Moreover, the ectopic transcription of genes coding for self-molecules with tissue-restricted expression suggests that capturing may not be the sole mechanism underlying the presentation of self-molecules. This review will focus on studies that have examined the role of insulin expression in lymphoid tissues as a prototypical example of a self-molecule with tissue-restricted expression for which novel findings in humans and animal models offer the opportunity to dissect the role of self-antigen presentation in regulating self-tolerance.
Insulin and other self-molecules with tissue-restricted expression are produced through ectopic gene transcription in both thymus and peripheral lymphoid tissues
A review of the recent literature demonstrates that a large number of self-molecules with tissue-restricted or mainly peripheral expression are expressed in the thymus, at the mRNA and/or protein levels, in both humans and rodents.5 These molecules include pancreatic and thyroid hormones, neuroendocrine molecules and many other proteins.3,6–12 In some cases, these proteins are known target autoantigens in autoimmune disease. Proinsulin/insulin, glutamic acid decarboxylase [both 65 000 and 67 000 molecular weight isoforms (GAD65/67)] and a tyrosine-phosphatase-like protein known as IA-2, are targeted by islet-specific autoimmune responses in type 1 diabetes (T1D or IDDM); thyroid peroxidase and thyroglobulin are autoantigens in autoimmune thyroid diseases (AITD); autoimmune responses to myelin basic protein (MBP) and myelin proteolipid protein (PLP) are typically seen in multiple sclerosis (MS) in humans, as well as in the corresponding mouse model of experimental autoimmune encephalomyelitis (EAE).
Insulin gene transcription in the mouse thymus was originally reported by Jolicouer et al.7 during fetal development and for a few weeks after birth; this was followed by similar reports for human8,9 and rat13 thymus. We surveyed human thymus specimens obtained from aborted fetuses, stillborn babies and children who underwent cardiac surgery (age-range: 20 weeks of fetal development to 13 years of age). The human insulin gene (INS) transcript was observed in almost all of the specimens tested.8 Later studies demonstrated its expression, even in the thymus of a 56-year-old individual.14 Other transcripts coding for T1D autoantigens (GAD, IA-2) were also detected.8,14,15 Sospedra et al.10 performed a more comprehensive survey of the human thymus, which included:
Self-antigens with tissue-restricted expression but present in the circulation at high (albumin), intermediate (thyroglobulin), or low (insulin, glucagon) concentrations.
Self-antigens with tissue-restricted expression that are not detectable in the circulation (thyroid peroxidase, GAD65/67).
Classical sequestered antigens (MBP, the retinal S antigen).
Genes coding for these molecules were all found to be expressed in thymus specimens obtained from children between the ages of 8 days and 13 years. Another comprehensive survey of the mouse thymus provided further evidence that a variety of self-molecules, again including many autoantigens, are expressed in the thymus.16 In this study, the ectopic transcription of several self-molecules was detected in the thymus of C57BL6 mice up to 34 weeks of age.16
We also found that transcripts for the T1D autoantigens insulin, GAD and IA-2, are present in human spleen and lymph nodes throughout life.14,15 This indicates that the expression of genes coding for self-molecules is not limited to the thymus, but also takes place in peripheral lymphoid organs where it may contribute to tolerance. Similar results were obtained in the mouse (D. Hanahan et al., personal communication). These findings may be critical for interpreting the results of some recent studies that are discussed below.
Differential expression of self-molecules and autoimmunity
It may seem paradoxical that autoimmune responses could be observed against self-molecules if these were expressed in the thymus. However, several mechanisms controlling gene expression can influence the quantity and quality of expression of self-molecules in the thymus. Quantitative and qualitative differences in the expression of self-molecules may, in turn, affect the thymic selection and other mechanisms that require self-antigen presentation for achieving and maintaining self-tolerance. The best-characterized examples involve the genes coding for insulin, the expression of which is affected by allelic variation and imprinting in the human thymus,8,9,17 and the islet molecule IA-2,15 which, similarly to the MS autoantigen PLP, is subject to alternative splicing.18,19 These and other genetic effects were collectively examined in a recent review.5 The present article focuses on the regulation of insulin expression in humans and in mouse models in an effort to help to understand the role of its expression in the thymus and peripheral lymphoid tissues.
Regulation of INS transcription in the human thymus
The regulation of INS transcription in the human thymus provides an example of how levels of self-antigen expression may affect tolerance and susceptibility to autoimmune responses against a specific molecule. The levels of insulin mRNA expression in the human thymus correlate with allelic variation at the IDDM2 susceptibility locus. This locus corresponds to a polymorphic variable nucleotide tandem repeat (VNTR) sequence,20–24 which mediates a transcriptional signal in the steady state.25 The transcriptional activity in the thymus was found to be ≈200–300% higher for INS transcripts encoded by IDDM2 alleles that were clinically associated with resistance to the development of diabetes, and conversely it was lower for transcripts encoded by diabetes-predisposing IDDM2 alleles.8,9 Furthermore, there is evidence that parent-of-origin effects may affect the diabetes susceptibility associated with the IDDM2 locus.22–24,26–28 These parent-of-origin effects may be mediated by parental imprinting,29 a mechanism that could suppress the expression of one of the two insulin genes in both thymus and peripheral lymphoid organs. There is indeed evidence that monoallelic expression of the insulin gene takes place in the thymus,8,9 the spleen30 and in the yolk sac.31 Remarkably, the non-expressed allele in thymus and spleen was always the one in cis with the protective IDDM2 alleles. This suggests that imprinting may prevent the protective effect by silencing transcription from these alleles and, in turn, dramatically reduce insulin production in the thymus. Overall, the risk of diabetes that is determined by allelic variation and parent-of-origin effects at the IDDM2 locus appears to be dependent on the levels of INS transcription in the thymus. Differential transcriptional regulation may determine the amounts of insulin produced and, in turn, influence the efficiency of thymic selection processes involving insulin-specific autoreactive T cells. The increased transcription levels detected in thymus fit well with the dominant protective effect associated with certain IDDM2 alleles, as higher insulin levels in the thymus may more efficiently induce the negative selection of insulin-specific T lymphocytes (or improved selection of regulatory T cells). In contrast, homozygosity for diabetes-predisposing alleles results in lower insulin levels, which may be associated with a less efficient deletion of insulin-specific autoreactive T cells (or impaired selection of regulatory T cells). INS transcription in the human thymus also correlates with protein production,8,9 where proinsulin appears to be the main gene product.14 Thymus cells expressing proinsulin are not likely to possess the refined machinery necessary to process proinsulin to mature insulin. Rather, it would seem that proinsulin may be processed into peptides for presentation to developing lymphocytes. Proinsulin expression may be sufficient to obtain tolerance to insulin because most of the known immunodominant epitopes identified as targets of the insulin autoimmune responses in type 1 diabetes are shared by both insulin and proinsulin.32–36
Animal models of insulin expression in the thymus
Besides the work, described above, carried out in humans, and earlier studies demonstrating the generic effects of antigen levels on thymic selection in vitro,1,37–40 studies in transgenic or knockout mice have provided more direct evidence that insulin thymic levels can dramatically affect the development of self-tolerance to insulin. The mouse models studied thus far most often involve manipulations of the non-obese diabetic (NOD) mouse, a spontaneous model of autoimmune diabetes that closely resembles the human disease.41,42 NOD mice develop a lymphocytic infiltrate of the pancreatic islets (insulitis), which leads to β-cell destruction and the development of diabetes in ≈80% of the female and 35–40% of the male mice by 30 weeks of age. The target autoantigens of the autoimmune process in mice overlap with those in humans, as they include proinsulin/insulin, GAD and IA-2. Autoimmune responses to insulin and proinsulin are known to be among the earlier ones to develop during the natural history of the disease,43–50 and both humoral and cellular responses are known to play a key role in diabetes development. In particular, immune responses against a proinsulin epitope encompassing the B chain/C-peptide junction (B24-C33, B24-C36) and a B-chain epitope encompassing the B9-23 residues (B9-23 for CD4 T cells, B15-23 for CD8 T cells) have been associated with diabetes in mice and humans.35,36,44,48,51–54 Responses to other epitopes of proinsulin/insulin have also been reported in patients and prediabetic subjects (C47-A66, C37-A66, C56-A72, B11-C41, B11-27).49,50,55 As with humans, the major histocompatibility complex (MHC) I-A locus, corresponding to the human leucocyte antigen (HLA)-DQ, provides the largest genetic contribution to disease susceptibility.56,57
Unlike humans, however, mice have two non-allelic insulin genes, which are located on two different chromosomes, Ins1 and Ins2.58 They are both expressed in pancreatic β-cells, although they encode slightly different proteins (Table 1). Moreover, allelic variation is not observed in the highly inbred NOD mice, and VNTRs are not known to exist in mice. Chentoufi et al.59 have recently established a very elegant mouse model in which mice lack the expression of either one or two copies of the two insulin genes. While insulin production in pancreatic β-cells is largely unaffected as the result of compensatory mechanisms,59,60 thymic expression is dependent on the number of gene copies present. These studies established that of the two insulin genes, Ins2 is the one that is predominantly, if not almost exclusively, expressed in the thymus. This model of graded insulin deficiency in the thymus also showed a linear correlation with insulin gene copy numbers. Mice expressing low thymic insulin levels presented spontaneous peripheral reactivity to insulin and the C-peptide, whereas mice with normal levels showed no significant response.59 Thus, this work provides functional evidence that thymic insulin levels play a key role in the selection of insulin-specific T cells and supports the concept that variation in INS levels, determined by allelic variation and imprinting phenomena at the IDDM2 locus, is the mechanism by which this locus influences disease risk. Of note, imprinting phenomena affect the expression of both Ins1 ands Ins2 in the yolk sac,61 although it remains to be established whether this phenomenon also occurs in the mouse thymus.
Table 1.
Amino acid sequences encoded by the mouse Ins2 and Ins1 genes
| 1–24 Signal peptide (n = 6; residues 4, 5, 6, 15, 19, 20) | |
| ″Ins2 | MALWMRFLPLLALLFLWESHPTQA |
| ″Ins1 | MALLVHFLPLLALLALWEPKPTQA |
| 25–54 B chain (n = 3; residues 33, 37, 53) | |
| ″Ins2 | FVKQHLCGSHLVEALYLVCGERGFFYTPMS |
| ″Ins1 | FVKQHLCGPHLVKALYLVCGERGFFYTPKS |
| 55–56 | |
| ″Ins2 | RR |
| ″Ins1 | RR |
| 57–87 C-peptide (n = 5; residues 64, 71, 74, 75, 86) | |
| ″Ins2 | EVEDPQVAQLELGGGPGAGDLQTLALEVAQQ |
| ″Ins1 | EVEDPQVEQLELGGSPG**DLQTLALEVARQ |
| 88–89 | |
| ″Ins2 | KR |
| ″Ins1 | KR |
| 90–110 A-chain | |
| ″Ins2 | GIVDQCCTSICSLYQLENYCN |
| ″Ins1 | GIVDQCCTSICSLYQLENYCN |
Amino acid sequences encoded by the Ins2 (top) and Ins1 (bottom) genes are shown.58 Amino acid residues that differ between the two sequences are underlined.
Another important line of support for the hypothesis that levels of insulin expression in the thymus are crucial for thymic selection and diabetes susceptibility came from studies that utilized NOD mice overexpressing the Ins2 gene under the MHC class II promoter.62 The increased levels of Ins2 expression in the thymus of the transgenic mice compared with non-transgenic NOD mice was associated with the complete prevention of insulitis and diabetes. These results are consistent with the notion that increased levels of insulin expression in the thymus could result in a more efficient deletion of insulin-specific T cells in the thymus of NOD mice. The complete prevention of insulitis in this model also suggests that insulin may play a critical role as an early autoantigen.
Using a contrasting approach, Thebault-Baumont et al.63 bred mice with a null Ins2 mutation64 onto the NOD background, so that most of the known susceptibility loci (including the major predisposing MHC locus, I-Ag7) were present in the fourth backcross generation. NOD Ins2−/− mice develop accelerated insulitis and diabetes, and similar results were obtained by Moriyama et al.65 Thus, two independent studies involving NOD Ins2−/− mice show that essentially all mice develop diabetes, regardless of gender, while it is known that male NOD mice are less prone to diabetes development.63,65 Moreover, Ins2−/− NOD mice display increased immune responses to insulin (autoantibodies), and splenocytes from these mice have an increased capacity to transfer diabetes compared with splenocytes from ‘wild-type’ NOD females.63 In contrast, NOD mice lacking expression of the Ins1 gene are protected from the development of diabetes.65 Diabetes and insulitis are markedly reduced in Ins1−/− mice, with virtually no mice developing insulitis and diabetes. Heterozygous mice also show a decreased incidence of diabetes as well as a delayed appearance of symptoms. However, NOD Ins1−/− female mice express insulin autoantibodies at levels similar to those produced by the wild-type NOD female mice, suggesting that the production of insulin antibodies may not be controlled by the expression of insulin in the thymus.
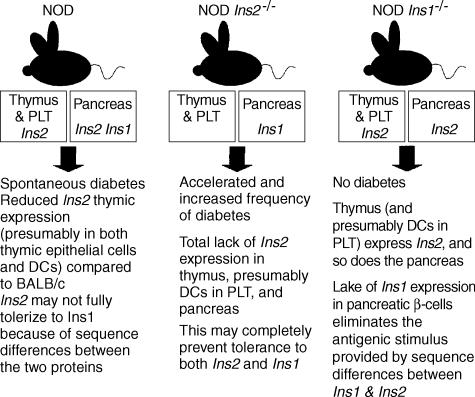
Based on the studies of Chentoufi et al.,59 and the above findings in Ins263,65 and Ins165 knockout mice, one could postulate that the protective effect afforded by the lack of the Ins1 gene product reflects differences between the two insulin proteins produced by the mouse. Moreover, differences in the expression pattern between the thymus and pancreas may be critical for diabetes pathogenesis. Because Ins2 is normally the only insulin gene actively expressed in the thymus, NOD Ins2−/− mice would display accelerated diabetes because they would not have any insulin in the thymus that could be used to delete insulin-specific T cells. On the other hand, NOD mice normally develop diabetes and autoimmune responses to proinsulin/insulin epitopes, despite the expression of Ins2 in the thymus. This suggests that NOD mice may express insufficient amounts of Ins2 in the thymus and, consistent with this assumption, one report has shown that NOD mice express ≈ 50% less insulin in the thymus compared with BALB/c mice.66 However, qualitative differences between Ins2, which is made in the thymus, and Ins1, which is made in the pancreas but not in the thymus, could also be critical for diabetes development. The studies of Moriyama et al.,65 demonstrating protection from diabetes in Ins1 NOD knockout mice, support this hypothesis. In other words, the immune system may not be fully tolerant to Ins1 because the thymus expresses only Ins2. Thus, lack of Ins2 expression completely abolishes tolerance to both insulin variants and accelerates diabetes because Ins1 is still expressed by the pancreas. Lack of Ins1 expression only affects expression in the pancreas, and once Ins1 is not expressed in the pancreas, diabetes does not develop because mice should be tolerant to Ins2 as this is expressed in the thymus. Thus, it would appear that NOD mice are essentially tolerant to Ins2, but are not tolerant to Ins1. These concepts are illustrated in Fig. 1.
Figure 1.
Illustration of the relationship between the expression of insulin in the thymus and peripheral lymphoid tissues (PLT), mediated by thymic epithelial cells and bone marrow-derived dendritic cells (DCs), and diabetes development in the mouse strains. The figure also highlights the different outcomes associated with the manipulation of the Ins2 and Ins1 genes in relation to their expression in the thymus and PLT, as well as in the pancreas. Please see the text for details. NOD, non-obese diabetic.
Tables 1 and 2 show the sequences of the two proteins and highlight the differences existing between them. It can be noted that with the exception of one epitope encompassing the A chain, the shown target epitopes have sequence differences between Ins2 and Ins1, which may favour the onset of autoimmune responses. Further experiments by Thebault-Baumont et al.63 support this interpretation. They immunized NOD Ins2−/− mice and wild-type NOD mice with a series of Ins2 peptides. Both mouse strains mice showed significant T-cell responses to immunization with the Ins2 peptides 14–30, 20–35, 33–47 and 71–88 (Tables 1 and 2). This would seem logical, as both strains are known to have impaired tolerance to proinsulin/insulin, and immunization with Ins2 peptides will probably induce cross-reactive responses to Ins1. However, NOD Ins2−/− mice showed a significant interleukin-2 (IL-2) response against the Ins2 peptide 88–103, a response that is not seen in the ‘wild-type’ NOD strain67 and that targets a region of complete homology between the A chains of the two mouse insulin proteins. The fact that the lack of Ins2 expression in the thymus is associated with the ability to respond to an additional epitope not normally targeted by NOD mice supports the interpretation presented above. In fact, Ins2 knockout mice would only express Ins1 in the pancreas and would have no ability to tolerize to Ins1 in the thymus; hence, a response against a region that is identical between the two proteins can develop. In contrast, ‘wild-type’ NOD mice express Ins2 in the thymus and do not break tolerance to this epitope, consistent with the expectation that they would be tolerant to a region of the Ins1 protein that is identical to that of the Ins2 expressed in the thymus.
Table 2.
Known proinsulin/insulin target epitopes in autoimmune diabetes
| Epitope 48–62 (B chain/C-peptide junction, aa B24-C36) | |
| ″Ins2 | FFYTPMSEVEDPQ |
| ″Ins1 | FFYTPKSEVEDPQ |
| Epitope 33–47 (B chain aa 9–23, also includes epitope 15–23) | |
| ″Ins2 | SHLVEALYLVCGERG |
| ″Ins1 | PHLVKALYLVCGERG |
| Epitope 14–30 (signal peptide aa 14–20, B chain aa 1–6) | |
| ″Ins2 | LLFLWESHPTQAFVKQHL |
| ″Ins1 | LLALWEPKPTQAFVKQHL |
| Epitope 20–35 (signal peptide aa 20, B chain aa 1–11) | |
| ″Ins2 | HPTQAFVKQHLCGSHL |
| ″Ins1 | KPTQAFVKQHLCGPHL |
| Epitope 71–88 (C-peptide) | |
| ″Ins2 | GPGAGDLQTLALEVAQQK |
| ″Ins1 | SPG**DLQTLALEVARQK |
| Epitope 88–103 (A chain) | |
| ″Ins2 | KRGIVDQCCTSICSLY |
| ″Ins1 | KRGIVDQCCTSICSLY |
Cells expressing self-molecules in thymus and peripheral lymphoid tissues
The evidence discussed so far suggests that the expression of self-molecules in the thymus leads to the deletion of autoreactive T cells and that quantitative and qualitative differences in the expression of self-molecules play a key role in this process. Deletion of autoreactive T cells has been observed both in thymus and peripheral lymphoid tissues.1,68–70 Deletion is mediated by apoptosis and involves those lymphocytes recognizing a self-peptide expressed by a self-MHC molecule on the surface of an APC. Presentation of self-molecules in the thymus may also promote the positive selection of regulatory cells.71,72 Thus, APCs expressing self-molecules may play a key role in inducing immunological tolerance to self-antigens. APCs in thymus and peripheral lymphoid tissues include bone marrow-derived DCs and macrophages, while thymic epithelial cells, including nurse cells, are found only in the thymus. Besides medullar thymic epithelial cells, cortical thymic epithelial cells and nurse cells may also play a role in negative selection.11 Defining the phenotype of the cells expressing self-molecules in the thymus should facilitate their functional characterization, and a number of studies have investigated the nature of these cells.
Initial studies showed that the mouse thymus contains specialized cells expressing peripheral antigens such as insulin and other pancreatic molecules.12 These cells were termed peripheral antigen expressing (PAE) cells.6,12 Thymus transplants involving transgenic mice expressing the Tag antigen (SV40 T antigen) under the rat insulin promoter (RIP-Tag) provided functional evidence that PAE cells can mediate tolerance to the antigens they express. In these experiments, transplanting the thymus of RIP-Tag mice in non-transgenic mice resulted in central tolerance to the Tag antigen.6,12
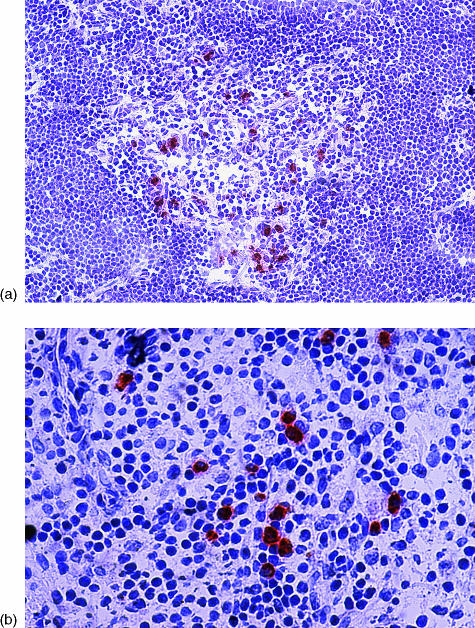
Later studies by Throsby et al.73 showed that PAE cells expressing insulin and other pancreatic hormones (glucagon, somatostatin, pancreatic polypeptide) in the C57BL6 thymus belong to the DC and macrophage lineages. We studied human thymus sections from aborted fetuses, stillborn babies, children who underwent cardiac surgery, or adult organ donors (age-range: 20 weeks of fetal development to 56 years of age).14 Sections were stained using several antibodies to T1D autoantigens, including insulin, GAD and IA-2. We found sparse cells, mostly localized in the thymic medulla or at the cortico-medullary junction, which stained positive for proinsulin (Fig. 2), GAD and IA-2. Using double immunofluorescence, we demonstrated that the same cells expressing proinsulin could co-express GAD and IA-2.14 These cells also co-stained for CD11c, CD83, CD40, CD14, CD80, CD86, CD8α and HLA class II. Among APCs, DCs express CD11c and CD83, while macrophages typically express CD14. These findings indicate that thymic cells expressing islet cell antigens bear surface markers of bone marrow-derived DCs and macrophages. Similarly to the work of Throsby et al.,73 in C57BL6 mice, we were unable to observe co-localization of any of the T1D autoantigens above with cytokeratin, a marker of thymic epithelial cells.14 Thus, studies in both human and mouse thymus provided similar results, even if different methodological approaches were used [double immunofluorescence in tissue sections from human thymus and fluorescence-activated cell sorter (FACS)/reverse transcription–polymerase chain reaction (RT–PCR) in cell fractions isolated from mouse thymus]. We have since confirmed this phenotypic characterization using flow cytometry.74
Figure 2.
(a) Proinsulin-positive cells in human thymus, stained on a frozen section using streptavidin–biotin–peroxidase and the AEC substrate (red), counterstained with haematoxylin (×64 magnification). (b) Proinsulin-positive cells in human spleen, stained on a frozen section using streptavidin–biotin–peroxidase and the AEC substrate (red), counterstained with haematoxylin (×128 magnification).
Given the phenotype established by these studies, it should be no surprise that insulin, GAD and IA-2 transcripts, as well as cells expressing these molecules, were also found in human peripheral lymphoid tissues, such as spleen (Fig. 2) and lymph nodes.14 These cells expressed the same phenotypic markers of their thymus counterparts, suggesting that bone marrow-derived APCs can express T1D autoantigens also in peripheral lymphoid organs. Another consistent feature of these cells in both thymus and spleen was the formation of ‘rosettes’ in which lymphocytes surrounded proinsulin-positive cells. Both the localization within the thymic medulla and the rosettes are typical of DCs and, to a lesser extent, of macrophages.75 Negative selection is compartmentalized in the thymic medulla and is mostly mediated by DCs,76 and our findings suggest that self-APCs include DCs that may be involved in the clonal deletion of self-reactive lymphocytes. This hypothesis is strengthened by the observation, in both human thymus and spleen, of rosettes consisting of proinsulin-positive cells in intimate contact with apoptotic cells.14 This is consistent with reports that regulatory DCs kill T cells by inducing apoptosis1 and that T-cell apoptosis also takes place in the spleen.68–70 DCs are known to be capable of stimulating the immune response or mediating tolerance, depending on their maturation status and phenotype.4,77 Thymic expression of self-molecules can provide a strong tolerogenic signal, but this signal may be incomplete. Although tolerance may be initiated in the thymus, peripheral mechanisms may be necessary to complete the tolerogenic process or, more likely, to provide the continuous exposure to self that is necessary to control emerging cells with autoreactive potential.4
Other investigators, however, produced evidence that thymic epithelial cells express self-molecules in the thymus, including insulin and other T1D autoantigens. Sospedra et al.10 used RT–PCR to detect the presence of transcripts encoding several self-molecules in human thymic cell fractions, and found transcripts of several autoantigens in both fractions enriched for either DCs or thymic epithelial cells. Recent studies by Derbinski et al.,16 in the thymus of C57BL6 mice, demonstrated that medullary thymic epithelial cells express self-molecules with tissue-restricted expression. These authors detected the transcripts of a number of self-molecules, including insulin, GAD, IA-2, MBP, PLP, thyroglobulin, etc., in sorted populations of medullar thymic epithelial cells but not in cortical epithelial cells, DCs and macrophages.
Thus, there is evidence that both bone marrow-derived APCs and thymic epithelial cells express self-antigens with tissue-restricted expression in the thymus. However, the relative importance of self-molecule expression by bone marrow-derived APCs and thymic epithelial cells is not completely defined. PAE cells with a DC phenotype exist not only in human thymus but also in peripheral lymphoid tissues, such as spleen and lymph nodes.14 These tissues, similarly to the thymus, also expressed transcripts for self-molecules such as the T1D autoantigens insulin, GAD and IA-2.14,15 These findings support the concept that DCs are involved in the production of self-molecules because thymic epithelial cells do not exist in peripheral lymphoid tissues. Given the phenotypic identity of cells expressing proinsulin and other T1D autoantigens in the human thymus and peripheral lymphoid tissues, it would seem reasonable that bone marrow-derived APCs would be able to transcribe genes coding for self-molecules. Consistent with this interpretation, we have detected insulin mRNA from CD11c-positive DCs after sorting.78 Functional evidence for a tolerogenic function of bone marrow-derived APCs derives from the studies of Steptoe et al.79 who demonstrated that syngeneic transplantation of haematopoietic stem cells encoding Ins2 transgenically targeted to APCs totally prevents the development of spontaneous autoimmune diabetes in NOD mice. Thus, bone marrow-derived APCs expressing self-molecules have tolerogenic function in a stringent autoimmune model of diabetes. Recent studies have also investigated the role of the autoimmune regulator protein, encoded by the AIRE gene, in determining susceptibility to the autoimmune polyendocrinopathy candidiasis ectodermal distrophy (APECED) syndrome.80 The AIRE protein is a transcription factor that is primarily expressed in thymic medullary epithelial cells and monocyte DCs in the thymus, but also in a rare subset of cells in the lymph nodes, spleen and fetal liver.81 The APECED syndrome is characterized by the presence of autoimmune polyendocrinopathy, candidiasis and ectodermal dystrophy. Variable combinations of autoimmune endocrine diseases such as Addison's disease, hypoparathyroidism and type 1 diabetes are often seen in patients with APECED.82 The APECED syndrome is caused by mutations in the AIRE gene.83 Two lines of AIRE knockout mice have been generated in order to dissect the role of AIRE in the autoimmune manifestations of the APECED syndrome.84,85 These mice develop normally, have a normal distribution of B and T cells, normal thymic maturation and T-cell activation. However, the T-cell receptor (TCR)-Vβ repertoire is altered in peripheral T cells and, when mice are immunized, the peripheral T cells have a three- to fivefold increased proliferation, suggesting a role for AIRE in maintaining homeostatic regulation of the immune system.84 These mice also display variable degrees of the autoimmune features of APECED, including multiorgan lymphocytic infiltration, circulating autoantibodies and infertility.84,85 Another striking feature observed in the AIRE knockout mice is the lack of expression of a variety of genes coding for self-molecules, including the insulin gene, in thymic epithelial cells.85 While more studies are needed to assess the consequences of the lack of self-antigen expression by thymic epithelial cells in AIRE-deficient mice, these findings collectively underscore the importance of both DCs and thymic epithelial cells in expressing self-molecules and mediating tolerogenic signals.
Conclusions
The research discussed here provides evidence that self-molecules with tissue-restricted expression are expressed in the thymus, where such expression may promote the development of self-tolerance. Genetic factors modulate the expression of specific genes and, in turn, influence susceptibility to autoimmune responses against the molecules encoded by these genes. In addition, some of the self-molecules studied, and in particular islet cell molecules that are targets of autoimmunity in T1D, are also expressed in peripheral lymphoid tissues. Transgenic and knockout mouse models, in which the expression of the insulin genes is altered, exemplify the tolerogenic role of self-antigen expression in the thymus and peripheral lymphoid tissues. These models also help in understanding the mechanisms underlying insulin autoimmune responses. Progress has been made in the characterization of the cells expressing self-molecules in the thymus. There is evidence that both bone marrow-derived APCs and thymic epithelial cells express self-molecules. Unlike thymic epithelial cells, bone marrow-derived APCs are abundantly represented in both thymus and peripheral lymphoid tissues, and may also contribute to self-tolerance in the periphery. The existence of cells, capable of expressing self-molecules, in both the central and peripheral compartments of the immune system, suggests that both thymic and peripheral tolerance mechanisms are likely to be affected by the ectopic expression of self-molecules in DCs and thymic epithelial cells. Given the property of DCs to autonomously express self-antigens, the expression of self-antigens by APC does not seem to be entirely dependent on antigen capture. Further studies should address whether there are differences between naturally expressed, processed and presented epitopes and those that are captured and then presented, and whether these differ in their ability to stimulate or inhibit the immune response. Most studies addressing responses to specific epitopes have used synthetic peptides; only a few have studied naturally processed and presented epitopes.86 Essentially no studies have yet addressed responses to naturally expressed, processed and presented epitopes, and it is plausible that the epitopes which are expressed naturally by APCs, either by autonomous production or capture, may be more likely to influence tolerance or immune responses. It also remains to be tested whether the expression of self-antigens by DCs in the peripheral immune system can only induce tolerance or, under particular conditions, result in the activation of immune responses. Further investigations are required to fully establish the phenotype and function of APCs expressing self-molecules, the nature of the epitopes expressed, and how these factors influence the immune system responsiveness towards self.
Acknowledgments
Studies by the author discussed in this article were supported by grants from the National Institutes of Health (NIAID AI-44456), the Juvenile Diabetes Research Foundation (JDRF, CDA no. 296117), the American Diabetes Association, and the Diabetes Research Institute Foundation. Tissue samples were provided by Dr C. K. Petito and I. Logvisnki, University of Miami Brain and Fetal Tissue Bank (a joint effort with the University of Maryland Brain and Tissue Banks through NICHD contract no. NO1-HD-8-3284).
References
- 1.Sprent J, Webb SR. Intrathymic and extrathymic clonal deletion of T cells. Curr Opin Immunol. 1995;7:196–205. doi: 10.1016/0952-7915(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. Immunological tolerance. In: Paul WE, editor. Fundamental Immunology. New York: Raven Press, Ltd; 1993. pp. 677–731. [Google Scholar]
- 3.Klein L, Kyewski B. ‘Promiscuous’ expression of tissue antigens in the thymus: a key to T-cell tolerance and autoimmunity? J Mol Med. 2000;78:483–94. doi: 10.1007/s001090000146. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 5.Pugliese A. Peripheral antigen-expressing cells and autoimmunity. Endocrinol Metab Clin North Am. 2002;31:411–30. doi: 10.1016/s0889-8529(01)00014-7. , viii. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D. Peripheral antigen-expressing cells in thymic medulla: factors in self- tolerance and autoimmunity. Curr Opin Immunol. 1998;10:656–62. doi: 10.1016/s0952-7915(98)80085-x. [DOI] [PubMed] [Google Scholar]
- 7.Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci USA. 1994;91:6707–11. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugliese A, Zeller M, Fernandez A, Jr, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–7. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 9.Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–92. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 10.Sospedra M, Ferrer-Francesch X, Dominguez O, Juan M, Foz-Sala M, Pujol-Borrell R. Transcription of a broad range of self-antigens in human thymus suggests a role for central mechanisms in tolerance toward peripheral antigens. J Immunol. 1998;161:5918–29. [PubMed] [Google Scholar]
- 11.Werdelin O, Cordes U, Jensen T. Aberrant expression of tissue-specific proteins in the thymus: a hypothesis for the development of central tolerance. Scand J Immunol. 1998;47:95–100. doi: 10.1046/j.1365-3083.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith KM, Olson DC, Hirose R, Hanahan D. Pancreatic gene expression in rare cells of thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol. 1997;9:1355–65. doi: 10.1093/intimm/9.9.1355. [DOI] [PubMed] [Google Scholar]
- 13.Heath VL, Moore NC, Parnell SM, Mason DW. Intrathymic expression of genes involved in organ specific autoimmune disease. J Autoimmun. 1998;11:309–18. doi: 10.1006/jaut.1998.0210. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese A, Brown D, Garza D, et al. Self-antigen-presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. J Clin Invest. 2001;107:555–64. doi: 10.1172/JCI10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diez J, Park Y, Zeller M, et al. Differential splicing of the IA-2 mRNA in pancreas and lymphoid organs as a permissive genetic mechanism for autoimmunity against the IA-2 IDDM autoantigen. Diabetes. 2001;50:895–900. doi: 10.2337/diabetes.50.4.895. [DOI] [PubMed] [Google Scholar]
- 16.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–9. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 17.Vafiadis P, Ounissi-Benkalha H, Palumbo M, Grabs R, Rousseau M, Goodyer CG, Polychronakos C. Class III alleles of the variable number of tandem repeat insulin polymorphism associated with silencing of thymic insulin predispose to type 1 diabetes. J Clin Endocrinol Metab. 2001;86:3705–10. doi: 10.1210/jcem.86.8.7733. [DOI] [PubMed] [Google Scholar]
- 18.Anderson AC, Nicholson LB, Legge KL, Turchin V, Zaghouani H, Kuchroo VK. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice. Mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–70. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 20.Bennett ST, Todd JA. Human type 1 diabetes and the insulin gene: principles of mapping polygenes. Annu Rev Genet. 1996;30:343–70. doi: 10.1146/annurev.genet.30.1.343. [DOI] [PubMed] [Google Scholar]
- 21.Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984;33:176–83. doi: 10.2337/diab.33.2.176. [DOI] [PubMed] [Google Scholar]
- 22.Pugliese A, Awdeh ZL, Alper CA, Jackson RA, Eisenbarth GS. The paternally inherited insulin gene B allele (1,428 FokI site) confers protection from insulin-dependent diabetes in families. J Autoimmun. 1994;7:687–94. doi: 10.1006/jaut.1994.1053. [DOI] [PubMed] [Google Scholar]
- 23.Bennett ST, Lucassen AM, Gough SC, et al. Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nat Genet. 1995;9:284–92. doi: 10.1038/ng0395-284. [DOI] [PubMed] [Google Scholar]
- 24.Bennett ST, Wilson AJ, Cucca F, et al. IDDM2-VNTR-encoded susceptibility to type 1 diabetes: dominant protection and parental transmission of alleles of the insulin gene-linked minisatellite locus. J Autoimmun. 1996;9:415–21. doi: 10.1006/jaut.1996.0057. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy GC, German MS, Rutter WJ. The minisatellite in the diabetes susceptibility locus IDDM2 regulates insulin transcription. Nat Genet. 1995;9:293–8. doi: 10.1038/ng0395-293. [DOI] [PubMed] [Google Scholar]
- 26.Julier C, Hyer RN, Davies J, et al. Insulin-IGF2 region on chromosome 11p encodes a gene implicated in HLA-DR4-dependent diabetes susceptibility. Nature. 1991;354:155–9. doi: 10.1038/354155a0. [DOI] [PubMed] [Google Scholar]
- 27.Bui MM, Luo DF, She JY, Maclaren NK, Muir A, Thomson G, She JX. Paternally transmitted IDDM2 influences diabetes susceptibility despite biallelic expression of the insulin gene in human pancreas. J Autoimmun. 1996;9:97–103. doi: 10.1006/jaut.1996.0012. [DOI] [PubMed] [Google Scholar]
- 28.Polychronakos C, Kukuvitis A, Giannoukakis N, Colle E. Parental imprinting effect at the INS-IGF2 diabetes susceptibility locus. Diabetologia. 1995;38:715–9. doi: 10.1007/BF00401845. [DOI] [PubMed] [Google Scholar]
- 29.Sleutels F, Barlow DP, Lyle R. The uniqueness of the imprinting mechanism. Curr Opin Genet Dev. 2000;10:229–33. doi: 10.1016/s0959-437x(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 30.Pugliese A, Miceli D. The insulin gene in diabetes. Diabetes Metab Res Rev. 2002;18:13–25. doi: 10.1002/dmrr.261. [DOI] [PubMed] [Google Scholar]
- 31.Moore GE, Abu-Amero SN, Bell G, Wakeling EL, Kingsnorth A, Stanier P, Jauniaux E, Bennett ST. Evidence that insulin is imprinted in the human yolk sac. Diabetes. 2001;50:199–203. doi: 10.2337/diabetes.50.1.199. [DOI] [PubMed] [Google Scholar]
- 32.Kuglin B, Gries FA, Kolb H. Evidence of IgG autoantibodies against human proinsulin in patients with IDDM before insulin treatment. Diabetes. 1988;37:130–2. doi: 10.2337/diab.37.1.130. [DOI] [PubMed] [Google Scholar]
- 33.Castano L, Ziegler AG, Ziegler R, Shoelson S, Eisenbarth GS. Characterization of insulin autoantibodies in relatives of patients with type I diabetes. Diabetes. 1993;42:1202–9. doi: 10.2337/diab.42.8.1202. [DOI] [PubMed] [Google Scholar]
- 34.Keilacker H, Rjasanowski I, Besch W, Kohnert KD. Autoantibodies to insulin and to proinsulin in type 1 diabetic patients and in at-risk probands differentiate only little between both antigens. Horm Metab Res. 1995;27:90–4. doi: 10.1055/s-2007-979915. [DOI] [PubMed] [Google Scholar]
- 35.Rudy G, Stone N, Harrison LC, et al. Similar peptides from two beta cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Mol Med. 1995;1:625–33. [PMC free article] [PubMed] [Google Scholar]
- 36.Alleva DG, Crowe PD, Jin L, et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173–80. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–63. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 38.Sebzda E, Wallace VA, Mayer J, Yeung RS, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–8. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 39.Oehen SU, Ohashi PS, Burki K, Hengartner H, Zinkernagel RM, Aichele P. Escape of thymocytes and mature T cells from clonal deletion due to limiting tolerogen expression levels. Cell Immunol. 1994;158:342–52. doi: 10.1006/cimm.1994.1281. [DOI] [PubMed] [Google Scholar]
- 40.Liblau RS, Tisch R, Shokat K, Yang X, Dumont N, Goodnow CC, McDevitt HO. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci USA. 1996;93:3031–6. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makino S, Kunimoto D, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Exp Anim (Tokyo) 1980;29:1. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 42.Wong FS, Janeway CAJ. Insulin-dependent diabetes mellitus and its animal models. Curr Opin Immunol. 1999;11:643–7. doi: 10.1016/s0952-7915(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 43.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA. 2000;97:1701–6. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, Bergerot I, Elliott JF, Harrison LC, Abiru N, Eisenbarth GS, Delovitch TL. Evidence that a peptide spanning the B-C junction of proinsulin is an early autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol. 2001;167:4926–35. doi: 10.4049/jimmunol.167.9.4926. [DOI] [PubMed] [Google Scholar]
- 45.Eisenbarth GS, Moriyama H, Robles DT, et al. Insulin autoimmunity: prediction/precipitation/prevention type 1A diabetes. Autoimmun Rev. 2002;1:139–45. doi: 10.1016/s1568-9972(02)00035-6. [DOI] [PubMed] [Google Scholar]
- 46.Eisenbarth GS, Kotzin BL. Enumerating autoreactive T cells in peripheral blood: a big step in diabetes prediction. J Clin Invest. 2003;111:179–81. doi: 10.1172/JCI17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trudeau JD, Kelly-Smith C, Verchere CB, Elliott JF, Dutz JP, Finegood DT, Santamaria P, Tan R. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest. 2003;111:217–23. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narendran P, Mannering SI, Harrison LC. Proinsulin – a pathogenic autoantigen in type 1 diabetes. Autoimmun Rev. 2003;2:204–10. doi: 10.1016/s1568-9972(03)00009-0. [DOI] [PubMed] [Google Scholar]
- 49.Narendran P, Williams AJ, Elsegood K, Leech NJ, Dayan CM. Humoral and cellular immune responses to proinsulin in adults with newly diagnosed type 1 diabetes. Diabetes Metab Res Rev. 2003;19:52–9. doi: 10.1002/dmrr.332. [DOI] [PubMed] [Google Scholar]
- 50.Schloot NC, Willemen S, Duinkerken G, de Vries RR, Roep BO. Cloned T cells from a recent onset IDDM patient reactive with insulin B-chain. J Autoimmun. 1998;11:169–75. doi: 10.1006/jaut.1997.0183. [DOI] [PubMed] [Google Scholar]
- 51.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–62. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 52.Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcuteneous administration of insulin peptide B-(9–23) Proc Natl Acad Sci USA. 1996;93:956–60. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong FS, Karttunen J, Dumont C, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–31. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 54.Martinez NR, Augstein P, Moustakas AK, Papadopoulos GK, Gregori S, Adorini L, Jackson DC, Harrison LC. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest. 2003;111:1365–71. doi: 10.1172/JCI17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durinovic-Bello I, Boehm BO, Ziegler AG. Predominantly recognized proinsulin T helper cell epitopes in individuals with and without islet cell autoimmunity. J Autoimmun. 2002;18:55–66. doi: 10.1006/jaut.2001.0566. [DOI] [PubMed] [Google Scholar]
- 56.Wicker LS, Miller BJ, Coker LZ, McNally SE, Scott S, Mullen Y, Appel MC. Genetic control of diabetes and insulitis in the nonobese diabetic (NOD) mouse. J Exp Med. 1987;165:1639–54. doi: 10.1084/jem.165.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chao CC, Sytwu HK, Chen EL, Toma J, McDevitt HO. The role of MHC class II molecules in susceptibility to type I diabetes: identification of peptide epitopes and characterization of the T cell repertoire. Proc Natl Acad Sci USA. 1999;96:9299–304. doi: 10.1073/pnas.96.16.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wentworth BM, Schaefer IM, Villa-Komaroff L, Chirgwin JM. Characterization of the two nonallelic genes encoding mouse preproinsulin. J Mol Evol. 1986;23:305–12. doi: 10.1007/BF02100639. [DOI] [PubMed] [Google Scholar]
- 59.Chentoufi AA, Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes. 2002;51:1383–90. doi: 10.2337/diabetes.51.5.1383. [DOI] [PubMed] [Google Scholar]
- 60.Leroux L, Desbois P, Lamotte L, et al. Compensatory responses in mice carrying a null mutation for Ins1 or Ins2. Diabetes. 2001;50(Suppl. 1):S150–3. doi: 10.2337/diabetes.50.2007.s150. [DOI] [PubMed] [Google Scholar]
- 61.Giddings SJ, King CD, Harman KW, Flood JF, Carnaghi LR. Allele specific inactivation of insulin 1 and 2, in the mouse yolk, indicates imprinting. Nat Genet. 1994;6:310–3. doi: 10.1038/ng0394-310. [DOI] [PubMed] [Google Scholar]
- 62.French MB, Allison J, Cram AS, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1997;46:34–9. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 63.Thebault-Baumont K, Dubois-Laforgue D, Krief P, et al. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest. 2003;111:851–7. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duvillie B, Cordonnier N, Deltour L, et al. Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci USA. 1997;94:5137–40. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moriyama H, Abiru N, Paronen J, et al. Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc Natl Acad Sci USA. 2003;100:10376–81. doi: 10.1073/pnas.1834450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brimnes MK, Jensen T, Jorgensen TN, Michelsen BK, Troelsen J, Werdelin O. Low expression of insulin in the thymus of non-obese diabetic mice. J Autoimmun. 2002;19:203–13. doi: 10.1006/jaut.2002.0616. [DOI] [PubMed] [Google Scholar]
- 67.Halbout P, Briand JP, Becourt C, Muller S, Boitard C. T cell response to preproinsulin I and II in the nonobese diabetic mouse. J Immunol. 2002;169:2436–43. doi: 10.4049/jimmunol.169.5.2436. [DOI] [PubMed] [Google Scholar]
- 68.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–96. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–8. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 70.Ducoroy P, Lesourd M, Padros MR, Tournefier A. Natural and induced apoptosis during lymphocyte development in the axolotl. Dev Comp Immunol. 1999;23:241–52. doi: 10.1016/s0145-305x(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 71.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 72.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4+ CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 73.Throsby M, Homo-Delarche F, Chevenne D, Goya R, Dardenne M, Pleau JM. Pancreatic hormone expression in the murine thymus: localization in dendritic cells and macrophages. Endocrinology. 1998;139:2399–406. doi: 10.1210/endo.139.5.5989. [DOI] [PubMed] [Google Scholar]
- 74.Pugliese A, Diez J. Lymphoid organs contain diverse cells expressing self-molecules. Nat Immunol. 2002;3:335–6. doi: 10.1038/ni0402-335b. [DOI] [PubMed] [Google Scholar]
- 75.Kyewski BA, Fathman CG, Rouse RV. Intrathymic presentation of circulating non-MHC antigens by medullary dendritic cells. An antigen-dependent microenvironment for T cell differentiation. J Exp Med. 1986;163:231–46. doi: 10.1084/jem.163.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fowlkes BJ, Ramsdell F. T-cell tolerance. Curr Opin Immunol. 1993;5:873–9. doi: 10.1016/0952-7915(93)90099-e. [DOI] [PubMed] [Google Scholar]
- 77.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoyd organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 78.Diez J, Allende G, Zeller M, Pugliese A. A proinsulin epitope expressed on the surface of CD11c+/CD14+ cells in human blood. Diabetes/Metabolism Res Rev. 2002;18(Suppl. 4):S14. [Google Scholar]
- 79.Steptoe RJ, Ritchie JM, Harrison LC. Transfer of hematopoietic stem cells encoding autoantigen prevents autoimmune diabetes. J Clin Invest. 2003;111:1357–63. doi: 10.1172/JCI15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pitkanen J, Peterson P. Autoimmune regulator: from loss of function to autoimmunity. Genes Immun. 2003;4:12–21. doi: 10.1038/sj.gene.6363929. [DOI] [PubMed] [Google Scholar]
- 81.Heino M, Peterson P, Kudoh J, et al. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257:821–5. doi: 10.1006/bbrc.1999.0308. [DOI] [PubMed] [Google Scholar]
- 82.Perheentupa J. APS-I/APECED: the clinical disease and therapy. Endocrinol Metab Clin North Am. 2002;31:295–320. doi: 10.1016/s0889-8529(01)00013-5. , vi. [DOI] [PubMed] [Google Scholar]
- 83.Heino M, Peterson P, Kudoh J, Shimizu N, Antonarakis SE, Scott HS, Krohn K. APECED mutations in the autoimmune regulator (AIRE) gene. Hum Mutat. 2001;18:205–11. doi: 10.1002/humu.1176. [DOI] [PubMed] [Google Scholar]
- 84.Ramsey C, Winqvist O, Puhakka L, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 85.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 86.Peakman M, Stevens EJ, Lohmann T, et al. Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J Clin Invest. 1999;104:1449–57. doi: 10.1172/JCI7936. [DOI] [PMC free article] [PubMed] [Google Scholar]