Abstract
Tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF superfamily, which is capable of inducing apoptosis in many cell types, including tumour and virus-infected cells, but rarely in normal cells. Expression of TRAIL mRNA and TRAIL receptors has previously been detected in neutrophils; however, the expression of TRAIL protein and the regulation of TRAIL and TRAIL receptor expression in these cells remain unknown. Here we report, for the first time, that neutrophils constitutively express TRAIL protein on their cell surface and that the TRAIL protein is shed during culture. TNF-α is a down-regulator of TRAIL expression, whereas IFN-γ up-regulates the expression of TRAIL. Neutrophils did not express a detectable level of TRAIL-R1 or -R4, but constitutively expressed a low, but substantial, level of TRAIL-R2 and a high level of TRAIL-R3. Although the level of TRAIL-R2 was not significantly altered during culture under different experimental conditions, ≈ 30% of TNF-α-treated cells rapidly lost their high-level TRAIL-R3 expression, whereas the majority of IFN-γ-treated cells retained a high level of TRAIL-R3 expression. Anti-TRAIL neutralizing antibody significantly inhibited neutrophil apoptosis during cultures in medium alone, or in the presence of TNF-α or IFN-γ. Thus, our study identified human neutrophils as a cellular source of TRAIL and suggests that neutrophil-derived TRAIL may play a role in immune surveillance. Our results also suggest a role for the TRAIL/TRAIL receptor system in neutrophil apoptosis.
Introduction
Tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL, also known as Apo2L) is a member of the TNF superfamily1,2 that is capable of inducing apoptosis in a number of cell types, including tumour cells and virus-infected cells, but not in normal cells.3–5 Five TRAIL receptors have been identified: death receptor 4 (DR4/TRAIL-R1) and DR5/TRAIL-R2 have the ability to initiate the apoptosis-signalling cascade after ligation, whereas decoy receptor 1 (DcR1/TRID/TRAIL-R3) and DcR2/TRAIL-R4/TRUNDD lack this ability. The decoy receptors, TRAIL-R3 and -R4, are actually reported to prevent extensive apoptosis in cells and tissues expressing both TRAIL and the death receptors, TRAIL-R1 and -R2. Osteoprotegerin is a soluble receptor for TRAIL and may also act as a soluble decoy receptor. The balance of the expression levels between the death receptors and decoy receptors is an important factor determining the apoptotic effect of TRAIL.3,4
TRAIL is expressed by a wide variety of human cells, including T cells,6,7 monocytes,8 dendritic cells9 and natural killer (NK) cells,10,11 and the expression of TRAIL has been implicated in their cytotoxic activities against tumour cells as well as normal cells such as T cells. In fact, recent studies indicate a role for TRAIL in the immune surveillance of tumour cells by NK cells and T cells12 and also in thymocyte apoptosis and the induction of autoimmune diseases.13
Previous studies suggest that neutrophils may be active in the immune surveillance against tumours. A prominent neutrophil influx is seen with some tumours in vivo and has been correlated with a favourable prognosis in some human studies.14–16 In contrast, depletion of neutrophils has also been shown to be beneficial for inhibiting tumour growth in an animal model.17 While conflicting data exist on the effects of neutrophils on tumour growth, it is apparent that, in some tumour microenvironments, neutrophils can negatively regulate tumour cell growth. Tumour cells transfected with different cytokine cDNAs, including interleukin (IL)-1, -2, -3, -4, -7, -10 or -12, interferon (IFN)-α, -β or -γ, granulocyte–colony-stimulating factor (G-CSF), TNF-α, or FasL, were previously transplanted into mice. Large numbers of leucocytes, including neutrophils, quickly infiltrated the tumours expressing these cytokines, leading to rejection of the tumours. Neutrophils played a key role in all of the cytokine-induced tumour rejection, often in co-operation with CD8-positive T cells.18 However, the underlying mechanisms by which neutrophils play a role in tumour rejection remain unclear.
Expression of TRAIL and TRAIL receptors in human neutrophils have previously been examined, but the findings were not consistent. Renshaw et al. detected constitutive expression of TRAIL mRNA, but not TRAIL protein.19 Renshaw et al. also detected the expression of TRAIL-R2 and -R3 at both mRNA and protein levels and neutrophils were susceptible to recombinant TRAIL.19 In contrast, Daigle & Simon detected the expression of all four TRAIL receptors at mRNA level, but only TRAIL-R1, -R3 and -R4 at the protein level.20 Although stimulation of neutrophils with TRAIL did not induce cell death, it partially blocked granulocyte–macrophage colony-stimulating factor (GM-CSF)-, G-CSF- and IFN-γ-mediated cell survival.21 Additional studies are required to clarify the expression of TRAIL protein and their receptors in these cells.
Here, we report that human neutrophils express cell-surface TRAIL, which is then shed during in vitro cultures. The expression of TRAIL is differentially regulated by cytokines, such as TNF-α or IFN-γ; TNF-α down-regulates, whereas IFN-γ up-regulates. A low, but substantial, level of TRAIL-R2, and a high level of TRAIL-R3, are expressed in freshly isolated neutrophils. The high-level expression of TRAIL-R3 was rapidly down-regulated with TNF-α, but maintained in the presence of IFN-γ. Finally, addition of blocking antibody (Ab) against TRAIL significantly reduced spontaneous and TNF-α-induced neutrophil apoptosis. Thus, our study has determined neutrophils as a cellular source of TRAIL and suggests a role for neutrophil-derived TRAIL in the surveillance against tumours. Our results also suggest that the TRAIL/TRAIL receptor system may contribute to the fate of tissue-infiltrating neutrophils during inflammatory responses.
Materials and methods
Reagents
Human recombinant TNF-α (2·5 × 107 U/mg); IFN-γ (1 × 107 U/mg); neutralizing Abs against human TRAIL, TNF-α, IFN-γ or GM-CSF; biotinylated goat anti-human TRAIL-R1, -R2, -R3 and -R4 immunoglobulin G (IgG); biotinylated normal goat IgG; and normal mouse IgG1 were purchased from R&D Systems (Minneapolis, MN). Human recombinant GM-CSF (2 × 107 U/mg) was from PeproTech (Rocky Hill, NJ). Biotinylated anti-mouse IgG1, phycoerythrin (PE)-conjugated anti-mouse IgG, and streptavidin-conjugated fluorescein isothiocyanate (FITC) were purchased from BD PharMingen (San Diego, CA). Dextran T500 and Percoll were from Amersham Pharmacia Biotech, Inc. (Piscataway, NJ). [α-32P]dCTP was from ICN (Costa Mesa, CA). Human β-actin cDNA was from Clontech (Palo Alto, CA). Phosphate-buffered saline (PBS), RPMI-1640, protein G–agarose (PGA) and TRIZOL Reagent® were from Life Technologies (Gaithersburg, MD). Fetal calf serum (FCS) was from HyClone (Logan, UT). Phytohaemagglutinin, paraformaldehyde and formamide were from Sigma (St Louis, MO). Accu-prep was from Accurate Chemical & Scientific Corp. (Westbury, NY). Protease inhibitor cocktail tablets, Complete mini, were from Roche (Indianapolis, IN).
Preparation of neutrophils
Human neutrophils were obtained from the heparinized blood of human donors or from granulocytapheresis collections supplied by the Department of Transfusion Medicine, Clinical Center (NIH, Bethesda, MD). One volume of 5% Dextran T500 in PBS was added to three volumes of blood in 50-ml tubes for the sedimentation of red blood cells. After a 30-min incubation at room temperature, the leucocyte-rich plasma was overlaid onto Accu-prep and centrifuged at 800 g for 20 min at room temperature. Neutrophils were separated from erythrocytes by lysis in 0·2% NaCl, washed in complete medium three times at 4°, and resuspended in RPMI containing 10% FCS (complete medium) at a density of 5 × 106 cells/ml. Contamination of mononuclear leucocytes was less than 0·5%, as determined by morphological examination. Peripheral blood mononuclear cell (PBMC) fractions were also collected, and monocytes and lymphocytes were further purified by using an iso-osmotic Percoll gradient. The purity of monocytes and lymphocytes was > 90%.22
Northern blotting
Cells were cultured at a density of 5 × 106 cells/ml in complete medium in six-well cluster tissue culture plates (Costar, Cambridge, MA; 3 ml/well). Total RNA was extracted from each culture by using TRIZOL Reagent®. Northern blot analysis was performed as previously described.23 The following primers were used to obtain human TRAIL, TRAIL-R1, TRAIL-R2, TRAIL-R3 and TRAIL-R4 cDNAs by polymerase chain reaction (PCR) from a human neutrophil cDNA library. TRAIL: forward 5′-GACGAAGAGAGTATGAACAG-3′, reverse 5′-TAGGGTCAGGATAACTTGG-3′; TRAIL-R1: forward 5′-TCGCTGTCCACTTTCGTCTC-3′, reverse 5′-CGTTCCGTCCAGTTTTGTTG-3′; TRAIL-R2: forward 5′-ATGGTCAAGGTCGGTGATTG-3′, reverse 5′-AGGAGTCAAAGGGCACAAAG-3′; TRAIL-R3: forward 5′-GAAAACTCCCCAGAGATGTG-3′, reverse 5′-CATTGATCCCTACGATGGTG-3′; and TRAIL-R4: forward 5′-TTAGCTGTGGTTGTGGTTGG-3′, reverse 5′-TGTCCTTCTTCCAGTGTTGC-3′.
Assay for apoptosis
Two millilitres of neutrophil suspension, at a cell density of 1 × 106 or 5 × 106 cells/ml, was plated into six-well tissue culture plates in the presence or absence of cytokines and/or a neutralizing Ab against human TRAIL. After different intervals of incubation at 37°, the cells were collected and the exposure of phosphatidylserine was detected with an Annexin V–FITC Apoptosis Detection Kit (BD PharMingen) using a FACScan flow cytometer (BD PharMingen).
Flow cytometry analysis
To detect the expression of TRAIL on the cell surface, 5–10 × 105 cells were first washed three times with wash buffer (PBS supplemented with 1% bovine serum albumin and 0·02% NaN3), then incubated with human serum or human IgG for 10 min, and then with anti-TRAIL Ab or control mouse IgG1 (0·5 µg in a 30-µl reaction volume) for 20 min at 4°. The cells were washed with the same buffer and then incubated with biotinylated anti-mouse IgG1. After washing three times with buffer, the cells were incubated with avidin–PE. To detect the expression of TRAIL-R1, -R2, -R3, or -R4, cells were first incubated with biotinylated goat anti-human TRAIL-R1, -R2, -R3, or -R4 IgG or biotinylated normal goat IgG, followed by streptavidin–FITC. Flow cytometry analysis was performed by the FACScan, utilizing cellquest software (BD PharMingen).
Immunoprecipitation and Western blotting
Twenty million neutrophils were incubated for 6 hr in the presence or absence of cytokines, and the cell-free supernatants were obtained. Cells were lysed on ice for 20 min in a buffer containing 50 mm HEPES, 150 mm NaCl, 1% Triton-X-100, 10% glycerol and a cocktail of protease inhibitors. The lysates were spun and the supernatants were collected. The samples were incubated for 1 hr at 4° with a packed volume of ≈ 20 µl of PGA. After centrifugation, the supernatants were collected, mixed with 20 µl of PGA conjugated to 1 µg of normal goat IgG or goat anti-human TRAIL polyclonal IgG, and incubated for 1 hr at 4°. PGA was washed three times with wash buffer containing 50 mm HEPES, 150 mm NaCl, 0·1% Triton-X-100, 10% glycerol and 20 µl of double-strength sample buffer [20% glycerol, 6% sodium dodecyl sulphate (SDS), 10% 2-mercaptoethanol] was added. The samples were boiled for 10 min. Eluted proteins were analysed on 12·5% polyacrylamide gels by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred electrophoretically (at 200 mA for 2 hr) to nitrocellulose membranes (Amersham) using a semidry system. The membranes were incubated with mouse anti-human TRAIL monoclonal IgG, followed by sheep anti-mouse IgG coupled to horseradish peroxidase (Amersham). Peroxidase activity was visualized by the Enhanced Chemiluminescence Detection System (Amersham).
Statistical analysis
All data are presented as the mean value ± SD. Statistical analysis was performed using the Mann–Whitney U-test.
Results
Regulation of TRAIL mRNA expression in neutrophils
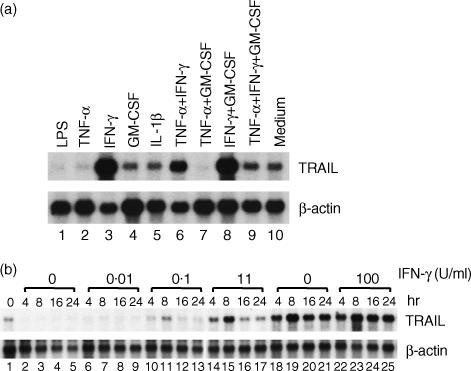
We evaluated the effects of lipopolysaccharide (LPS) and recombinant cytokines on TRAIL mRNA expression in neutrophils (Fig. 1a). In comparison with the cells incubated in medium alone (Fig. 1a, lane 10), LPS- or TNF-α-activated cells contained much lower levels of TRAIL mRNA (Fig. 1a, lanes 1 and 2). Incubation with IFN-γ markedly up-regulated the expression level of TRAIL mRNA, whereas incubation with GM-CSF or IL-1β had no significant effect (Fig. 1a, lanes 3–5). There was no synergistic effect between IFN-γ and GM-CSF (Fig. 1a, lane 8). Addition of TNF-α to IFN-γ, GM-CSF, or IFN-γ+ GM-CSF markedly decreased the levels of TRAIL mRNA expression (Fig. 1a, lanes 6, 7 and 9).
Figure 1.
Expression of tumour necrosis factor-α-related apoptosis-inducing ligand (TRAIL) mRNA in neutrophils. (a) Neutrophils (5 × 106 cells/ml) were cultured for 6 hr in the presence of lipopolysaccharide (LPS) (1 µg/ml), tumour necrosis factor-α (TNF-α) (1 ng/ml), interferon-γ (IFN-γ) (2·5 U/ml), granulocyte–macrophage colony-stimulating factor (GM-CSF) (0·5 ng/ml), interleukin-1β (IL-1β) (0·5 ng/ml), and combinations of these cytokines. (b) Neutrophils were cultured in the presence of different doses of IFN-γ for 4, 8, 16, or 24 hr. Total RNA was extracted and the expression of TRAIL mRNA was analysed by Northern blotting. Representative data of three individual experiments, with similar results obtained on each occasion, are shown.
The kinetics of TRAIL mRNA expression revealed that TRAIL mRNA was constitutively expressed in neutrophils (Fig. 1b, lane 1). After incubation in complete medium, the expression of TRAIL mRNA was down-regulated in neutrophils within 4 hr (Fig. 1b, lanes 2–5). IFN-γ dose-dependently up-regulated the expression of TRAIL mRNA, the peak expression level of which was detected at 8 hr. A concentration of IFN-γ as low as 0·1 U/ml up-regulated the expression of TRAIL mRNA at 8 hr. The maximal effect was achieved with a concentration ranging from 10 to 100 U/ml (Fig. 1b, lanes 6–25).
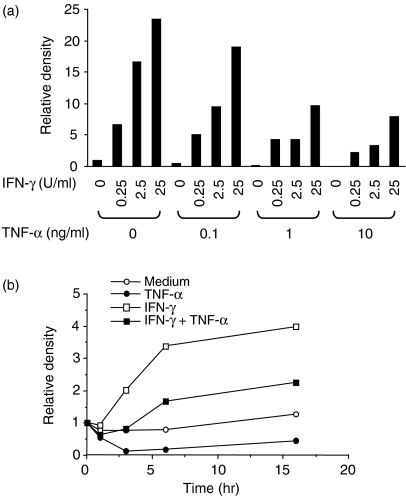
The inhibitory effect of TNF-α on IFN-γ-induced TRAIL mRNA expression was studied in more detail. As shown in Fig. 2a, as little as 0·1 ng/ml of TNF-α inhibited the expression of TRAIL mRNA at 4 hr. TNF-α, at 1 ng/ml, showed as much inhibitory effect as 10 ng/ml of TNF-α. The inhibitory activity of TNF-α was detected for up to 16 hr (Fig. 2b). These results indicated that IFN-γ was an activator of TRAIL mRNA expression in neutrophils, whereas TNF-α was an inhibitor.
Figure 2.
Effects of interferon-γ (IFN-γ) or tumour necrosis factor-α (TNF-α) on the expression of TNF-α-related apoptosis-inducing ligand (TRAIL) mRNA by neutrophils. (a) Neutrophils were cultured in the presence of different doses of interferon-γ (IFN-γ) and/or tumour necrosis factor-α (TNF-α) for 24 hr. (b) Neutrophils were cultured in the presence of 25 U/ml of IFN-γ and/or 1 ng/ml of TNF-α for 1, 3, 6, and 16 hr. Total RNA was extracted and the expression of TRAIL mRNA was analysed by Northern blotting. The blots were hybridized with 32P-labelled human TRAIL or β-actin cDNA probe. Autoradiographic signals were quantified, standardized against the levels of β-actin, and presented as relative density. The level of expression detected in fresh neutrophils equals 1. Representative data of three individual experiments, with similar results obtained on each occasion, are shown.
Detection of TRAIL protein on neutrophils
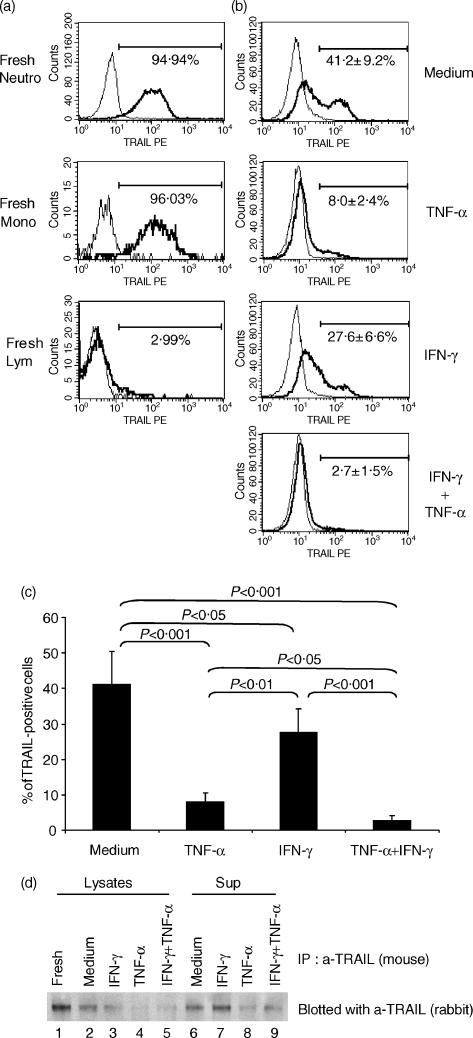
We next analysed, by flow cytometry, the expression of TRAIL protein on neutrophils. As shown in Fig. 3a, TRAIL protein was detected on most freshly isolated neutrophils, as well as on monocytes. The majority of peripheral blood lymphocytes did not express TRAIL, as reported previously.7
Figure 3.
Expression of tumour necrosis factor-α-related apoptosis-inducing ligand (TRAIL) protein in neutrophils. (a) The cell-surface expression of TRAIL on freshly isolated neutrophils, monocytes or lymphocytes was evaluated by flow cytometry. Representative profiles of three individual experiments are shown. (b) The cell-surface expression of TRAIL on neutrophils was evaluated after incubation with tumour necrosis factor-α (TNF-α) (1 ng/ml), interferon-γ (IFN-γ) (100 U), or with a combination of TNF-α (1 ng/ml) and IFN-γ (100 U) by flow cytometry. Representative profiles of three individual experiments are shown. (c) The data of TRAIL expression were quantified and statistical analysis was performed using the Mann–Whitney U-test (n = 3). (d) Neutrophils were cultured in the presence or absence of TNF-α (1 ng/ml), IFN-γ (100 U), or with a combination of TNF-α (1 ng/ml) and IFN-γ (100 U) for 6 hr. Cell lysates and supernatants were subjected to immunoprecipitation with anti-TRAIL mouse monoclonal antibody, and the presence of TRAIL was evaluated by Western blotting with anti-TRAIL rabbit polyclonal Ab. Representative data of two individual experiments, with similar results obtained on each occasion, are shown.
After 24 hr of incubation of neutrophils in complete medium, ≈ 41% of the cells still expressed TRAIL. However, ≈ 59% of the cells were negative (Fig. 3b,3c). When neutrophils were incubated in the presence of TNF-α, the majority of the cells became TRAIL-negative. This was consistent with the results obtained by Northern blotting (Figs 1 and 2). Although IFN-γ up-regulated the level of TRAIL mRNA expression, the percentage of TRAIL-positive cells was significantly lower than that of cells incubated in complete medium. Addition of TNF-α abolished the cell-surface TRAIL protein expressed on IFN-γ-incubated cells (Fig. 3b,3c). Loss of TRAIL expression found after 24 hr of incubation of neutrophils in complete medium or with IFN-γ was not the result of apoptosis of the cells, because TRAIL-negative cells were present in both annexin V-negative and -positive cell fractions (data not shown).
As shown above, IFN-γ up-regulated the expression level of TRAIL mRNA, but did not up-regulate the cell-surface expression of TRAIL protein, suggesting that TRAIL protein synthesized by IFN-γ-treated neutrophils might be shed into the supernatants. To test this hypothesis, we performed Western blotting. As shown in Fig. 3d, cell-associated TRAIL was readily detectable in fresh neutrophils (Fig. 3d, lane 1). The amount of cell-associated TRAIL decreased 6 hr of incubation in complete medium in the presence or absence of IFN-γ (Fig. 3d, lanes 2 and 3), and was almost undetectable in the presence of TNF-α or TNF-α+ IFN-γ (Fig. 3d, lanes 4 and 5). Soluble TRAIL was also readily detectable in the culture supernatant of neutrophils incubated in medium alone (Fig. 3d, lane 6). The amount of soluble TRAIL appeared to increase in the presence of IFN-γ (Fig. 3d, lane 7), and decrease in the presence of TNF-α, or TNF-α+ IFN-γ (Fig. 3d, lanes 8 and 9). These results suggest that a portion of the TRAIL protein synthesized during culture was shed into the culture supernatants, and that the shedding might be enhanced in IFN-γ-treated cells.
Regulation of TRAIL receptor expression in neutrophils
We also evaluated the expression of TRAIL-R1, -R2, -R3 and -R4 by Northern blotting. As shown in Fig. 4, the expression of TRAIL-R2 and -R3 mRNA was most readily detectable in freshly isolated neutrophils. During culture in complete medium, the expression of TRAIL-R2 mRNA rapidly increased, reached a peak by 6 hr, and the expression level was sustained at 12 hr. The expression of TRAIL-R3 mRNA also slightly increased at 6 hr, but decreased thereafter. IFN-γ inhibited the early up-regulation of TRAIL-R2 mRNA expression detected during culture in complete medium. In contrast, TRAIL-R3 mRNA expression had markedly increased in the presence of IFN-γ at 6 hr. TNF-α had a completely opposite effect. In TNF-α-treated cells, the expression of TRAIL-R2 mRNA rapidly increased and peaked by 6 hr, whereas the expression of TRAIL-R3 mRNA markedly decreased. The R2/R3 ratio dramatically increased in TNF-α-treated cells. Thus, changes in the level of TRAIL-R2 and -R3 mRNA expression occur rapidly in cultured neutrophils, with TNF-α promoting the expression of signal-transducing TRAIL receptors, while IFN-γ favours the expression of decoy receptors.
Figure 4.
Expression of mRNA for tumour necrosis factor-α-related apoptosis-inducing ligand (TRAIL)-R1, -R2, -R3 and -R4 in neutrophils. Neutrophils (5 × 106 cells/ml) were incubated in 3 ml of complete medium or with 1 ng/ml of tumour necrosis factor-α (TNF-α) or 100 U/ml of interferon-γ (IFN-γ) for the indicated time-points. Total RNA was isolated and the expression of mRNA for TRAIL-R1, -R2, -R3 and -R4 was analysed by Northern blotting. Autoradiographic signals were quantified, standardized against the levels of β-actin, and are presented as relative density. The level of expression detected in fresh neutrophils equals 1. The ratios of TRAIL-R2 mRNA expression to R3 mRNA expression (R2/R3) were also calculated.
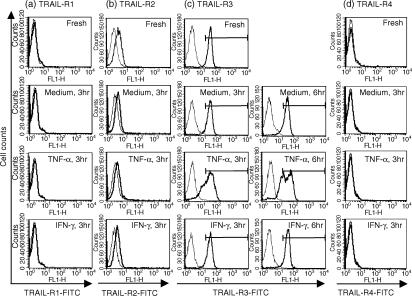
We next evaluated the cell-surface expression of TRAIL-R1, -R2, -R3 and -R4 on neutrophils by flow cytometry (Fig. 5). We focused on early time-points because cytokine effects on TRAIL receptor mRNA expression were maximal by 6 hr (Fig. 4). Freshly isolated neutrophils did not express a detectable level of TRAIL-R1 or -R4 (Figs 5a and 6d). However, they expressed a low, but significant, level of TRAIL-R2. The expression of cell-surface TRAIL-R2 appeared to decrease, but was still detectable after 3 hr of incubation of cells in complete medium. Neither TNF-α nor IFN-γ affected TRAIL-R2 expression (Fig. 5b). Unlike TRAIL-R2, TRAIL-R3 was highly expressed on most freshly isolated neutrophils (Fig. 5c). The expression level of TRAIL-R3 was approximately sevenfold higher than that of TRAIL-R2, based on their mean fluorescence values (35 vs. 5). Approximately 94% of cells incubated in complete medium and 93% of cells incubated in the presence of IFN-γ continued to express high levels of TRAIL-R3 at 3 hr. At 6 hr, ≈ 13% of cells incubated in complete medium became negative for TRAIL-R3 (from 96·5% for fresh cells to 83·6%, Table 1), whereas ≈ 95% of cells incubated with IFN-γ still expressed a high level of TRAIL-R3. Approximately 27% and 24% of cells treated with TNF-α became negative for TRAIL-R3 expression at 3 and 6 hr, respectively. These results suggest that TNF-α and IFN-γ can greatly influence the susceptibility of neutrophils to TRAIL by down- or up-regulating the expression of TRAIL receptors.
Figure 5.
Cell-surface expression of tumour necrosis factor-α-related apoptosis-inducing ligand (TRAIL)-R1, -R2, -R3 and -R4. Neutrophils were incubated in the presence or absence of 1 ng/ml of TNF-α or 100 U/ml of interferon-γ (IFN-γ) for 3 or 6 hr. Cells were harvested and the expression of TRAIL-R1, -R2, -R3, or -R4 was evaluated by flow cytometry. (a) TRAIL-R1. (b) TRAIL-R2. (c) TRAIL-R3. (d) TRAIL-R4. Representative profiles of three individual experiments are shown.
Figure 6.
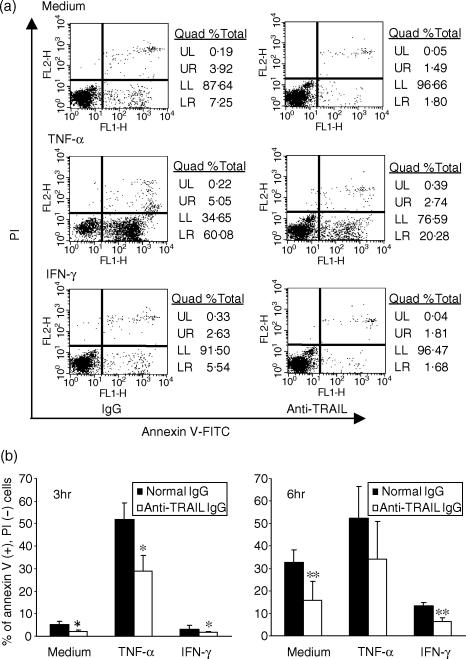
Effects of anti-tumour necrosis factor-α-related apoptosis-inducing ligand (TRAIL) neutralizing antibody (Ab) on neutrophil apoptosis. Neutrophils (1 × 106 cells/ml) were incubated in complete medium or with 1 ng/ml TNF-α or 100 U/ml interferon-γ (IFN-γ) in the presence of 1 µg of normal mouse immunoglobulin G (IgG) or anti-human TRAIL neutralizing Ab, for 3 or 6 hr. The externalization of phosphatidylserine on plasma membranes was analysed by flow cytometry using an Annexin V–fluorescein isothiocyanate (FITC) apoptosis detection kit. (a) Representative quadrant plots of four different experiments are shown. (b) The percentages of propidium iodide negative [PI(–)], annexin V positive [annexin V(+)] cells shown in Table 2 are presented as a graph. Statistical significance was evaluated by the Mann–Whitney U-test. *P < 0·05, n = 4. **P < 0·05, n = 3.
Table 1.
Effects of tumour necrosis factor-α (TNF-α) or interferon-γ (IFN-γ) on the cell-surface expression of TNF-related apoptosis-inducing ligand (TRAIL)-R3
| TRAIL-R3- positive cells (%) | |
|---|---|
| Fresh | 96·5 ± 2·1 |
| 3 hr | |
| ″Medium | 94·2 ± 1·6 |
| ″TNF-α | 70·1 ± 1·6* |
| ″IFN-γ | 93·1 ± 3·0 |
| 6 hr | |
| ″Medium | 83·6 ± 2·1** |
| ″TNF-α | 72·8 ± 1·1* |
| ″IFN-γ | 94·8 ± 1·1 |
Data are presented as the mean ± standard deviation (SD) of three independent experiments with three different donors.
Statistical significance was evaluated by the Mann–Whitney U-test.
P < 0·01
P < 0·05.
Effects of anti-TRAIL neutralizing Ab on neutrophil apoptosis
The detection of TRAIL and TRAIL receptor expression by neutrophils led us to formulate the hypothesis that neutrophil-generated TRAIL might play a role in their own apoptosis. To test this hypothesis, we added neutralizing anti-TRAIL Ab to neutrophil cultures and evaluated the percentage of apoptotic cells [annexin V-positive, propidium iodide (PI)-negative] by flow cytometry (Fig. 6, Table 2). About 5% of neutrophils were apoptotic after 3 hr of incubation in complete medium. In the presence of 100 U/ml of IFN-γ, only 3% of the cells were apoptotic, whereas ≈ 52% of cells were apoptotic in the presence of 1 ng/ml of TNF-α. The percentage of apoptotic cells found in TNF-α-treated cells in this study was higher than that of previous studies.24,25 This may be, in part, a result of the different detection methods for apoptotic cells and because of a more strict gating for annexin V-negative cells, as shown in Fig. 6(a). Interestingly, the addition of anti-TRAIL neutralizing Ab significantly decreased the percentage of apoptotic neutrophils incubated in complete medium, TNF-α-containing medium and IFN-γ-containing medium. At 6 hr, ≈ 32% of cells incubated in complete medium and 13% of cells incubated with IFN-γ were apoptotic. Addition of anti-TRAIL Ab inhibited the apoptosis of neutrophils by ≈ 50%. Although the effect of anti-TRAIL was not statistically significant for neutrophils incubated with TNF-α for 6 hr, anti-TRAIL Ab partly inhibited the TNF-α-induced apoptosis of neutrophils in all experiments. Incubation with anti-TRAIL Ab also increased the percentage of annexin V-negative, PI-negative cells. The effect of anti-TRAIL Ab on neutrophil apoptosis at 24 hr was much smaller (data not shown), indicating that TRAIL is involved in an early stage of neutrophil apoptosis in vitro.
Table 2.
Effects of anti-tumour necrosis factor-α-related apoptosis-inducing ligand (TRAIL) neutralizing antibody (Ab) on polymorphonuclear (PMN) cell apoptosis
| % of PI− Annexin V− | % of PI+ Annexin V− | % of PI− Annexin V+ | % of PI+ Annexin V+ | |
|---|---|---|---|---|
| 3 hr | ||||
| ″Medium + IgG | 92·9 ± 2·8 | 0·3 ± 0·2 | 5·2 ± 1·5 | 1·6 ± 1·6 |
| ″Medium + Ab | 96·6 ± 0·4 | 0·1 ± 0·1 | 2·2 ± 0·6* | 1·0 ± 0·4 |
| ″TNF-α + IgG | 44·0 ± 9·2 | 0·1 ± 0·1 | 52·0 ± 7·0 | 3·8 ± 2·3 |
| ″TNF-α + Ab | 67·8 ± 9·2* | 0·4 ± 0·3 | 28·7 ± 7·2* | 3·2 ± 2·7 |
| ″IFN-γ + IgG | 94·9 ± 2·4 | 0·2 ± 0·2 | 3·2 ± 1·5 | 1·6 ± 0·9 |
| ″IFN-γ + Ab | 97·2 ± 0·6 | 0·1 ± 0·1 | 1·7 ± 0·2* | 1·0 ± 0·5 |
| 6 hr | ||||
| ″Medium + IgG | 65·6 ± 6·3 | 0·1 ± 0·1 | 32·6 ± 5·6 | 1·7 ± 0·6 |
| ″Medium + Ab | 82·3 ± 10·4** | 0·1 ± 0·1 | 15·7 ± 8·5** | 1·9 ± 1·8 |
| ″TNF-α + IgG | 42·7 ± 16·7 | 0·1 ± 0·1 | 52·3 ± 14·2 | 4·9 ± 2·5 |
| ″TNF-α + Ab | 62·1 ± 19·0 | 0·2 ± 0·2 | 34·2 ± 16·7 | 3·5 ± 2·4 |
| ″IFN-γ + IgG | 84·8 ± 2·4 | 0·1 ± 0·1 | 13·3 ± 1·7 | 1·8 ± 1·4 |
| ″IFN-γ + Ab | 92·3 ± 2·3** | 0·1 ± 0·1 | 6·5 ± 1·7** | 1·2 ± 0·9 |
Data are presented as mean ± standard deviation (SD) of four individual experiments with four different donors for 3 hr, and of three experiments with three different donors for 6 hr. Statistical significance was evaluated by the Mann–Whitney U-test.
P < 0·05, n = 4.
P < 0·05, n = 3.
IgG, immunoglobulin G; PI, propidium iodide.
Discussion
In the present study we demonstrated that human neutrophils express TRAIL. Both TRAIL mRNA and protein were constitutively expressed in freshly isolated neutrophils. As noted above, Renshaw et al. previously detected constitutive expression of TRAIL mRNA in human neutrophils by using the RNase protection assay (RPA); however, they failed to detect the expression of TRAIL protein.19 Thus, our study is the first to demonstrate the expression of TRAIL mRNA by Northern blotting and TRAIL protein by Western blotting in human neutrophils. IFN-γ is a potent inducer of TRAIL mRNA expression in neutrophils, as well as in other types of cells, including monocytes,8 dendritic cells9 and NK cells.26 Although IFN-γ up-regulated the expression of TRAIL mRNA in neutrophils, a concomitant increase in TRAIL protein levels was not seen on the cell surface. Increased levels of a soluble form of TRAIL were subsequently detected in the culture supernatants of IFN-γ-treated cells. It was previously reported that a biologically active soluble form of TRAIL could be secreted from LPS-activated monocytes and macrophages.27 As FasL is processed by matrix metalloproteinases (MMPs) to yield a soluble form of FasL,28 we hypothesized that MMPs might also be involved in the cleavage of TRAIL on neutrophils. However, the addition of the MMP inhibitor, KB8301, to neutrophil cultures only slightly increased the level of TRAIL cell-surface expression, as measured by flow cytometry, implicating the involvement of other enzymes in this process.
In contrast to IFN-γ, TNF-α down-regulated the expression levels of TRAIL mRNA. This effect was observed even at low concentrations of TNF-α and has not been demonstrated in other cell types. TNF-α is one of the most potent neutrophil activators and has been shown to up-regulate the expression of many genes, including cytokines and chemokines.29,30 The precise mechanism of the TNF-α-induced down-regulation of TRAIL mRNA expression is, at present, unclear. The expression of TRAIL mRNA was also down-regulated by LPS. As LPS induces the production of TNF-α in neutrophils,31 the negative effect of LPS on TRAIL mRNA expression may be a result of the TNF-α secreted from LPS-activated neutrophils.
We detected very low levels of TRAIL-R1 and TRAIL-R4 mRNA, moderate levels of TRAIL-R2 mRNA and high levels of TRAIL-R3 mRNA, in freshly isolated neutrophils by Northern blotting. In agreement with the Northern blot data, we also detected a low level of cell-surface TRAIL-R2 and a high level of cell-surface TRAIL-R3 by flow cytometry. Daigle & Simon previously detected the expression of mRNA for all four TRAIL receptors by reverse transcription (RT)–PCR, and TRAIL-R1, -R3 and -R4, but not -R2, by flow cytometry in freshly isolated neutrophils, suggesting that TRAIL-R1 was the major signal transducing receptor.20 In contrast, Renshaw et al. detected the expression of TRAIL-R2 and -R3, but not TRAIL-R1 or -R4, by both RPA and flow cytometry.19 Therefore, our data support the results reported by Renshaw et al. High levels of TRAIL-R3 were maintained on > 90% of cells incubated with IFN-γ for 6 hr, whereas ≈ 13% of cells incubated in complete medium lost a high level of TRAIL-R3 expression. The consistent TRAIL-R3 expression in IFN-γ-treated cells was associated with the high-level expression of TRAIL-R3 mRNA in cells, suggesting a role for IFN-γ as a positive regulator of TRAIL-R3 expression in neutrophils. As IFN-γ down-regulates TRAIL-R3 expression in monocytes,8 IFN-γ appears to have a differential effect on TRAIL-R3 expression in different leucocyte populations.
Unlike IFN-γ, TNF-α acted as a negative regulator of TRAIL-R3. Flow cytometry analyses of TNF-α-treated neutrophils revealed that TNF-α down-regulated TRAIL-R3 expression in ≈ 30% of cells within 3 hr. As TRAIL-R1 and -R2 contain the death domain and the TRAIL-R3 receptor acts as a decoy, the data led us to propose that TNF-α promotes apoptosis in neutrophils by down-regulating TRAIL-R3. This hypothesis was supported by the finding that a neutralizing Ab against TRAIL dramatically inhibited TNF-α-induced apoptosis in neutrophils. Soluble TRAIL released into the culture supernatants of TNF-α-treated neutrophils appeared to be sufficient to induce neutrophil apoptosis. As for the death receptors, a significant level of TRAIL-R2 was expressed on the cells. Neutrophils treated with IFN-γ for 6 hr were less sensitive to TRAIL than those incubated in medium alone or with TNF-α, despite their increased TRAIL production, suggesting that the level of TRAIL-R3 on IFN-γ-treated cells was high enough to overcome the threat of the increased amounts of TRAIL produced by the same cells. Inhibition of apoptosis by anti-TRAIL Ab was much lower, at 24 or 40 hr; thus, TRAIL-mediated apoptosis appears to be less important in a later stage of incubation and other apoptotic processes must be occurring. These include Fas/FasL, or TNF-α/TNF-R interactions, or a decrease or increase in the amounts of anti-apoptotic or pro-apoptotic members of the Bcl-2 family.
Previous studies addressing the sensitivity of neutrophils to recombinant TRAIL reached differing conclusions. Daigle et al. reported that although 100 ng/ml of recombinant TRAIL with enhancer that causes oligomerization of soluble TRAIL and enhances its apoptotic effect did not increase the rate of cell death after 40 hr incubation in vitro, it partially inhibited survival signals provided by GM-CSF, G-CSF, or IFN-γ.20 In contrast, Renshaw et al. found that neutrophil apoptosis could be specifically accelerated by exposure to a leucine zipper-tagged form of TRAIL, which mimics cell-surface TRAIL.19 We used commercially available recombinant TRAIL (up to 100 ng/ml) with enhancer and tested its effect on neutrophil apoptosis, but there was no significant effect (data not shown). As neutrophils express high levels of TRAIL and release them into culture supernatants, evaluating an additional effect of recombinant TRAIL might be difficult. The role of TRAIL in neutrophil apoptosis needs to be clarified using cells from TRAIL-deficient mice.
IFN-γ is a product of T cells and NK cells and regulates immune-cell function. In mice that are unresponsive to IFN-γ, such as IFN-γ-receptor knockout or STAT 1 knockout mice, tumours developed more rapidly than in wild-type mice when induced with chemical carcinogens.32 Therefore, IFN-γ plays an important role in tumour regression and one of the ways that this may be accomplished is through the up-regulation of TRAIL in leucocytes. Recently, it was reported that TRAIL, in conjunction with anti-CD3, could augment IFN-γ secretion by human lymphocytes in vitro.33 This may suggest an amplification loop whereby neutrophil-derived TRAIL stimulates lymphocytes to secrete IFN-γ, which further up-regulates TRAIL release and enhances tumour cell killing. Here, we have presented evidence indicating a new role for IFN-γ in the expression of TRAIL and TRAIL receptors in neutrophils. The fate, as well as functions, of neutrophils can be greatly influenced by IFN-γ and the increased generation of TRAIL by IFN-γ-activated neutrophils may be one of the mechanisms that regulate neutrophil-mediated tumour rejection previously observed in a number of animal tumour models.
Acknowledgments
The authors are grateful to Dr Joost J. Oppenheim for reviewing this manuscript.
References
- 1.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 2.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Dixit V. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 4.Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–63. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 5.Sedger LM, Shows DM, Blanton RA, Peschon JJ, Goodwin RG, Cosman D, Wiley SR. IFN-γ mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999;163:920–6. [PubMed] [Google Scholar]
- 6.Thomas WD, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195–200. [PubMed] [Google Scholar]
- 7.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells. A novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–60. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–53. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Exp Med. 1999;190:1155–64. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–80. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnsen AC, Haux J, Steinkjer B, Nonstad U, Egeberg K, Sundan A, Ashkenazi A, Espevik T. Regulation of APO-2 ligand/trail expression in NK cells – involvement in NK cell-mediated cytotoxicity. Cytokine. 1999;11:664–72. doi: 10.1006/cyto.1999.0489. [DOI] [PubMed] [Google Scholar]
- 12.Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature's TRAIL – on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- 13.Lamhamedi-Cherradi S-E, Zheng S-J, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat Immunol. 2003;4:255–60. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- 14.Hamlin IME. Possible host resistance in carcinoma of the breast: a histological study. Br J Cancer. 1968;22:383–401. doi: 10.1038/bjc.1968.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black MM, Barclay THC, Hankey BF. Prognosis in breast cancer utilizing histological characteristics of the primary tumor. Cancer. 1975;36:2048–55. doi: 10.1002/cncr.2820360919. [DOI] [PubMed] [Google Scholar]
- 16.Bassler T, Dittman AM, Dittrich M. Mononuclear stromal reactions in mammary carcinoma, with reference to medullary carcinomas with a lymphoid infiltrate. Virchow Arch A Pathol Anat Histol. 1981;393:75–91. doi: 10.1007/BF00430872. [DOI] [PubMed] [Google Scholar]
- 17.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–40. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Carto E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–45. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 19.Renshaw SA, Parmar JS, Singleton V, et al. Acceleration of human neutrophil apoptosis by TRAIL. J Immunol. 2003;170:1027–33. doi: 10.4049/jimmunol.170.2.1027. [DOI] [PubMed] [Google Scholar]
- 20.Daigle I, Simon H-U. Alternative functions for TRAIL receptors in eosinophils and neutrophils. Swiss Med Wkly. 2001;131:231–7. doi: 10.4414/smw.2001.09707. [DOI] [PubMed] [Google Scholar]
- 21.Daigle I, Yousefi S, Colonna M, Green DR, Simon H-U. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med. 2002;8:61–7. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- 22.Xu LL, McVicar DW, Ben-Baruch A, Kuhns DB, Johnston J, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur J Immunol. 1995;25:2612–7. doi: 10.1002/eji.1830250931. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura T. cDNA cloning of guinea pig monocyte chemoattractant protein-1 and expression of the recombinant protein. J Immunol. 1993;150:5025–32. [PubMed] [Google Scholar]
- 24.Murray J, Barbara JAJ, Dunkley SA, et al. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha. Requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–83. [PubMed] [Google Scholar]
- 25.Salamone G, Giordano M, Trevani AS, Gamberale R, Vermeulen M, Schettinni J, Geffner JR. Promotion of neutrophil apoptosis by TNF-α. J Immunol. 2001;166:3476–83. doi: 10.4049/jimmunol.166.5.3476. [DOI] [PubMed] [Google Scholar]
- 26.Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon-α-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–70. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halaas O, Vik R, Ashkenazi A, Espevik T. Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scand J Immunol. 2000;51:244–50. doi: 10.1046/j.1365-3083.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 28.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 30.Yamashiro S, Kamohara H, Yoshimura T. MCP-1 is selectively expressed in the late phase by cytokine-stimulated human neutrophils: TNF-α plays a role in the maximal MCP-1 mRNA expression. J Leukoc Biol. 1999;65:671–9. doi: 10.1002/jlb.65.5.671. [DOI] [PubMed] [Google Scholar]
- 31.Dubravec DB, Spriggs DR, Mannick JA, Rodrick ML. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor. Proc Natl Acad Sci USA. 1990;87:6758–61. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou AH, Tsai HF, Lin LL, Hsieh SL, Hsu PI, Hsu PN. Enhanced proliferation and increased IFN-gamma production in T cells by signal transduced through TNF-related apoptosis-inducing ligand. J Immunol. 2001;167:1347–52. doi: 10.4049/jimmunol.167.3.1347. [DOI] [PubMed] [Google Scholar]