Abstract
Secreted aspartic proteinases (Sap) have been described as virulence factors implicated in the mechanisms of host colonization by the yeast Candida albicans in different types of candidiasis. Intraperitoneal inoculation of C. albicans into BALB/c mice rapidly leads to systemic candidiasis, with significant colonization of the kidneys measurable in the following week. In this study we assessed the potential of vaccination with C. albicans secreted aspartic proteinase 2 (Sap2) in preventing systemic candidiasis in BALB/c mice. Intradermal injection of highly purified native Sap2 protein incorporated in alum adjuvant provided efficient immune protection, as indicated by a 20-fold decrease in the colonization of kidneys. The protective effect of Sap2 immunization with alum adjuvant was also observed in mice infected with a lethal inoculum of C. albicans. Immunization with the native Sap2 alone, as well as with a denatured recombinant form of the protein, also conferred protection, albeit to a lesser level. In all cases, protection correlated with an increase in serum antibodies to Sap2. Moreover, passive transfer of anti-Sap2 immunoglobulin G (IgG) significantly decreased the yeast burden in kidneys of C. albicans-infected mice. This result shows that immune protection against systemic candidiasis in mice immunized with Sap2 is antibody-mediated. Taken together, these analyses demonstrate that Sap2 can be successfully used as a vaccination target in systemic candidiasis and reveals the potential immunomodulatory role of Sap2 on C. albicans infection.
Introduction
Candida albicans is usually a harmless commensal in normal hosts. However, in immunodeficient or immunosuppressed patients, invasive candidiasis can become a life-threatening condition.1 Candida spp. and C. albicans, in particular, are among the leading causes of nosocomial infections,2,3 including potentially fatal fungal peritonitis in renal patients undergoing peritoneal dialysis.4
C. albicans expresses several molecules that could account for its ability to evade efficient host protective immune responses and allow invasive processes;5 these include the secretion of aspartyl proteinases (Saps).6,7 The importance of Saps production as a mechanism of C. albicans virulence has been suggested from several studies, which found that Sap-deficient Candida mutants are less virulent than parental strains8,9 and that protease inhibitors reduce Candida virulence.10,11 Moreover, C. albicans isolates obtained from immunocompromised hosts expressed higher levels of Sap activity than those obtained from control patients.12,13 Finally, Saps have been shown to break down several host substrates and to participate in host tissue damage.14,15
Saps are encoded by a multigene family encompassing at least 10 genes16,17 that are differentially regulated at various stages of infection.18 Analyses of secreted Saps composition in cultures of different C. albicans strains revealed that Sap2 is the most abundant19 and preferentially expressed at the late stages of infection in vivo.18 The potential role of Sap2 in controlling the host protective immune response is suggested by the following findings:
The induction of host polyclonal activation and immune-modulation has been ascribed to a 43 000-molecular weight (MW) C. albicans-secreted protein20,21 (a MW similar to that of Sap2).
C. albicans extracts enriched in Sap2 have been successfully used as immunogens to reduce mucosal candidasis in mouse or rat models.22–24
In this work we directly tested whether Sap2 vaccination can protect BALB/c mice from systemic candidiasis. We first provide evidence that Sap2 may account for the B-cell polyclonal activation induced by C. albicans. We further demonstrate that immunization with highly purified native Sap2 before infection dramatically reduced C. albicans colonization. Moreover, injection of either native or recombinant Sap2, in the absence of adjuvant, induced a detectable specific immune response that was associated with reduced C. albicans load upon infection.
Materials and methods
Mice
Male BALB/c and C57BL/6 mice (6–8 weeks old) were purchased from Charles River (Barcelona, Spain). Animals were housed at the animal facilities of the Institute Abel Salazar during the time of the experiments.
C. albicans
C. albicans, identified using API 20 C Aux (Bio-Merieux, Marcy l'Etoile, France), was isolated from a patient with generalized mucocutaneous candidiasis and maintained by passage every 3 weeks in Saboroud dextrose agar (Difco, Detroit, MI). Stocks were made and stored frozen at −80° in 50% glycerol. The pathogenicity was assured by inoculation of C. albicans in C57BL/6 mice every 3 months.
Preparation of C. albicans-produced Sap2
C. albicans was grown in Winge medium (0·3% yeast extract, 0·2% glucose) for 48 hr at 37° in an orbital incubator. Culture supernatant proteins were concentrated by ultrafiltration with a 10 000-MW cut-off membrane in a VivaFlow system (Vivascience, Hanover, Germany), dialysed against Bistris 20 mm buffer, pH 6·0, and separated by ion-exchange chromatrography on a DEAE-cellulose (DE52; Whatman, Maidstone, Kent, UK) column that was eluted with a 0–0·3 m NaCl gradient. Fractions eluted in 0·05–0·15 m NaCl (F0·05–0·15) were found to be enriched in Sap2 and were concentrated by vacuum dialysis using a 14 000-MW cut-off membrane (Sigma, St Louis, MO). Mannoside constituents were removed from this fraction by affinity chromatography in a concanavalin A–sepharose column (Amersham Pharmacia, Uppsala, Sweden) using a buffer of 20 mm Tris/0·5 m NaCl. Sap2 was further purified by a Pepstatin A affinity-chromatography column (Sigma), as described previously.25 As a last purification step, Sap2 preparations were depleted of contaminating endotoxin using a polymixin B column (Pierce, Rockford, IL), and tested by the limulus test (E-toxate; Sigma). All Sap2 preparations used in this study tested endotoxin free.
Cloning of recombinant Sap2 (rSap2)
The full-length mature Sap2-coding sequence was cloned by nested polymerase chain reaction (PCR) using C. albicans genomic DNA as a template. Genomic DNA was prepared as described previously.26 The external and internal primer-pairs were 5′-GTTGATTCCTCTTGGTTGTTGA-3′, 5′-TTTATTCCACCCCTTCATCTTA-3′ and 5′-GTAAAACTCTCGAGAGACAAGC-3′, 5′-TTTATTCCACGAATTCATCTTA-3′, respectively. The first PCR reaction, containing 100 ng of template, 1·5 m MgCl2, 0·2 mm each dNTP (all from Invitrogen, Life Technologies, CA) and 50 pm each primer, in a final volume of 50 µl, was performed as follows: 94° for 2 min 30 seconds, followed by 30 cycles of 30 seconds at 94°, 45 seconds at 53° and 2 min at 72°, and terminated by a 10-min incubation at 72°. The nested PCR reaction was performed using 1 µl of the first PCR product in the same PCR conditions. For both amplifications, the DNA polymerase Expand™ High Fidelity PCR system (Roche, Basel, Switzerland) was used.
The internal primers have been designed to contain an XhoI (sense) and an EcoRI (antisense) sites, allowing for directional and in-frame cloning into the XhoI/EcoRI-digested pRSET-A expression vector (Invitrogen). Integrity and proper insertion of the DNA fragment was verified by sequencing using the Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) and an ABI 373A DNA Automated Sequencer (Applied Biosystems). The Sap2 expression vector was then transfected into Escherichia coli BL21-CodonPLus (DE3) RIL (Stratagene, La Jolla, CA) and expression of recombinant Sap2 was induced by the addition of 1 mm isopropyl-β-d-thiogalactopyranoside (IPTG).
Production of rSap2
E. coli containing pRSET-Sap2 was grown at 37° in 2 × 1·6% tryptone, 1% yeast extract, 0·5% NaCl (YT) medium, supplemented with 2% glucose and ampicillin, to an optical density of 0·8 at 600 nm. IPTG was then added to a final concentration of 1 mm and the culture maintained for an additional 5 hr. Upon analyses, the Sap2 protein was found to be sequestered into insoluble inclusion bodies. For purification, total cell extracts were blotted onto nitrocellulose membrane and the band corresponding to rSap2 was cut out and dissolved in dimethylsulphoxide (DMSO). Control samples were similarly prepared from blotted cell extracts of pRSET-A-transformed E. coli. The amount of blotted rSap2 was estimated by comparison of the rSap2 band intensity with those of blotted bovine serum albumin (BSA) standards.
Challenge infections
Non-lethal infections with C. albicans were evaluated in both kidneys of mice, 6 days after intraperitoneal (i.p.) infection with 5 × 106 viable blastoconidia in 0·5 ml of phosphate-buffered saline (PBS), as previously described.20 Prior to infection, the blastoconidia were incubated in Saboroud glucose broth (Difco) for 18 hr at 37°. The two kidneys were aseptically removed, homogenized in PBS and serially diluted in 1 : 10 dilutions. The colony-forming unit (CFU) values of C. albicans were counted in duplicate cultures of each serial dilution after 48 hr of culture in Mycobiotic agar (Difco).
Alternatively, mice were inoculated i.p. with a lethal dose of C. albicans consisting of 2 × 107C. albicans blastoconidia in 0·5 ml of PBS. Survival of the yeast-inoculated animals was monitored on a daily basis.
Immunizations
Mice were injected intradermically (i.d.), twice with a 3-week intervening period, with 100 µl of the following preparations: 10 µg of native Sap2 in PBS; 10 µg of Sap2 in a 1 : 1 PBS/alum suspension (Aluminium hydroxide Gel; Brenntag, Frederikssund, Denmark, a kind gift of Dr Erik Lindblad, Biosector, Frederikssund, Denmark); or 10 µg of membrane-bound rSap2 dissolved in DMSO. The respective control animals received 100 µl of PBS, 1 : 1 PBS/alum suspension, or DMSO.
Passive immunization was performed by i.p. administration of 100 µg of immunoglobulin G (IgG), purified from the pooled sera of Sap2-immunized mice, or 100 µg of IgG, purified from the pooled sera of sham-immunized mice (prepared as described below), at 24-hr intervals for three consecutive days. The first dose of antibody was administered 6 hr prior to i.p. challenge with C. albicans.
Preparation of C. albicans sonicates (CaS)
Candida blastoconidia were disrupted by sonication (10 cycles of 30 seconds at 100 W) with a Branson cell disrupter, model W 185 D, on ice. The sonicates were successively filtered through 0·45- and 0·2-µm Millipore filters and stored in small aliquots at −80°.
Antibody detection
Specific anti-Sap2 or anti-CaS immunoglobulin in mice sera, collected by retrorbital bleeding, were quantified by enzyme-linked immunosorbent assay (ELISA). Briefly, polystyrene microtitre plates (Nunc, Roskilde, Denmark) were coated with 5 µg/ml of Sap2 or with 10 µg/ml of CaS and incubated overnight at 4°. Wells were then saturated for 1 hr at room temperature with 1% BSA in PBS. Serial dilutions of the serum samples were then plated and incubated for 2 hr at room temperature. After washing, alkaline phosphatase-coupled monoclonal goat anti-mouse IgG or goat anti-mouse immunoglobulin M (IgM) antibody (both from Southern Biotechnology Associates, Birmingham, AL) was added and incubation continued for 30 min at room temperature. After washing, the bound antibodies were detected by development with substrate solution containing p-nitrophenyl phosphate (Sigma) and the reaction was stopped by the addition of 0·1 m EDTA, pH 8·0. The absorbance was measured at 405 nm. The ELISA antibody titres were expressed as the reciprocal of the highest dilution giving an absorbance of 0·1 above that of the control (no serum added).27
The reactivities of anti-Sap2 immunoglobulin were visualized by Western blot analysis. For that purpose, Sap2 contained on 10% sodium dodecyl sulphate (SDS) gels was blotted overnight onto a nitrocellulose membrane (Amersham, Bucks., UK). Transferred proteins were stained with Ponceau S. After destaining with water, the membranes were cut into strips, according to protein migration lanes, and saturated in TBST buffer (0·01 m Tris, 0·15 m NaCl, 0·05% Tween-20, pH 8·0), containing 1% BSA, for 1 hr and further incubated for 4 hr with the individual mice sera diluted in TBST. Sap2-bound antibodies were detected with alkaline-phosphatase-coupled monoclonal goat anti-mouse IgG (Southern Biotechnology Associates), using a solution of 0·33 mg/ml nitroblue tetrazolium dye (Promega, Madison, WI) and 0·17 mg/ml 5-bromo-4-chloroindol-2-yl phosphate (Promega), in AP buffer (0·01 m Tris, 0·01 m NaCl, 0·5 mm MgCl2, pH 9·5), as substrate.
Purification of serum IgG antibodies
Sera of mice inoculated i.d., twice with a 3-week intervening interval, with PBS alone, a 1 : 1 PBS/alum suspension or a 1 : 1 PBS/alum suspension containing 10 µg of Sap2, as described above for immunization protocols, were collected and pooled 20 days after the second i.d. inoculation of any of the three preparations. Purification of IgG from these pooled sera was then performed as follows: pooled serum samples were equilibrated in binding buffer (20 mm sodium phosphate, pH 7·0) by overnight dialysis and 3 ml of these preparations was applied to a Protein G HP affinity column (HiTrap; Amersham Biosciences, Bucks., UK) for each separation. Bound antibodies were eluted with glycine–HCl buffer, pH 2·7, and recovered in 50 µl of 1 m Tris–HCl, pH 9·0, per ml of eluent, according to the manufacturer's instructions. Recovered IgG antibody fractions were further equilibrated in PBS in a VIVAPORE concentrator with a 7500-MW cut-off membrane (Vivascience) and stored at −80° in aliquots. The purified IgG fractions obtained from the sera of PBS-, PBS/alum- or PBS/alum/Sap2-inoculated mice were designated IgG-PBS, IgG-alum and IgG-alumSap2, respectively. Anti-Sap2 titres of these preparations were determined by ELISA, as described above, and were of 28 430 for IgG-alumSap2 and undetectable for IgG-PBS and IgG-alum.
In vitro mononuclear cell cultures
Spleen cells were obtained by gently teasing the organ in RPMI-1640 (Sigma) supplemented with penicillin (100 IU/ml), streptomycin (50 µg/ml), polymixin B (50 µg/ml), 2-mercaptoethanol (0·05 m) and 10% fetal bovine serum (Sigma) (RPMI). Cell suspensions were layered onto 2·5 ml of a polysucrose/sodium ditrizoate solution (Histopaque-1083; Sigma Diagnostics, St Louis, MO) and centrifuged at 800 g for 20 min at room temperature. Cells collected from the medium/Histopaque interface were washed with RPMI, distributed in 96-well plates (5 × 105 cells/well) and cultured for 6 hr at 37° in a humidified atmosphere containing 5% CO2 in air. Plated cells were stimulated with medium alone or with 25 µg of native Sap2 per ml of culture medium.
In vivo treatments with Sap2
Mice were injected i.p. with 50 µg of Sap2 in 0·5 ml of PBS, or with 0·5 ml of PBS alone in control animals.
Flow cytometric analysis
For flow cytometric analysis, spleen cells of BALB/c mice were resuspended in balanced salt solution (BSS), supplemented with 10 mm sodium azide and 1% BSA. The following monoclonal antibodies (mAbs) were used for immunofluorescence cytometric analysis in a FACScan (Becton-Dickinson, San Jose, CA) using cellquest software (Becton-Dickinson): goat anti-mouse IgM fluorescein isothiocyanate (FITC) conjugate (Southern Biotechnology Associates); hamster anti-mouse CD3 FITC conjugate (Southern Biotechnology Associates); and hamster anti-mouse CD69 phycoerythrin (PE) conjugate (PharMingen, San Diego, CA). Dead cells were excluded by propidium iodide incorporation.
Plaque-forming cell (PFC) assays
The number of splenic immunoglobulin-producing cells was assessed by the Protein A PFC assay in agar.28,29 Rabbit antisera against total immunoglobulin were used as developing antibodies and have been previously characterized in detail.30
Statistical analysis
Unless otherwise indicated, the level of significance of the results obtained in immunized mice versus respective control groups was determined using the analysis of variance (anova) single factor, calculated using Microsoft Excel 2000 software.
Results
Immunomodulatory effects of Sap2
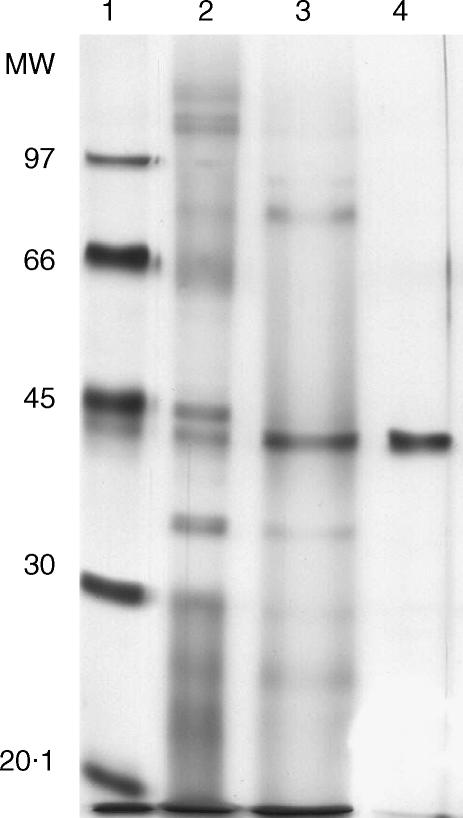
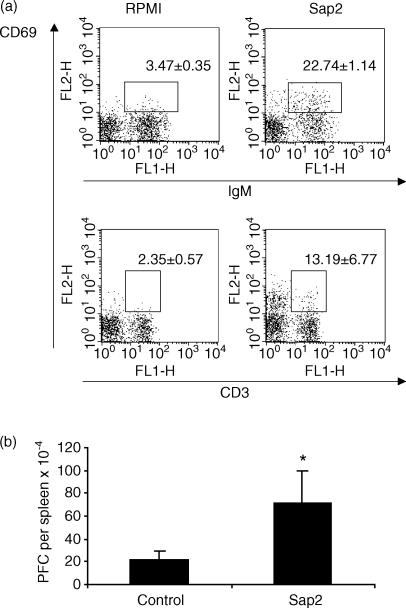
Several immunobiological effects, such as B-cell polyclonal activation and the induction of interleukin (IL)-10 secretion, have been attributed to an unidentified 43 000-MW protein secreted by C. albicans.21 From a similar protein preparation and after N-terminal amino acid sequencing we identified Sap2, a protein of 41 000 MW, as a major component. Serial chromatography columns were applied to C. albicans culture supernatants in order to purify, to homogeneity, a workable quantity of Sap-2 (Fig. 1). To ascertain that the Sap2-mediated biological effects measured in this work were not attributable to contaminating endotoxin, the protein preparation was run through a polymixin B column and confirmed as being endotoxin free using the highly sensitive limulus test. The ability of Sap2 to induce B-lymphocyte activation was tested both in vitro and in vivo. Culture of splenocytes in the presence of Sap2 induced expression of the early activation marker, CD69, on a sizeable population of B lymphocytes and, to a lesser extent, on T lymphocytes (Fig. 2a). Similarly, BALB/c mice that received 50 µg of Sap2 i.p. showed a three-to-fourfold increase in the number of splenic immunoglobulin-secreting cells when compared with control animals (Fig. 2b). However, the serum IL-10 concentration remained constant when tested 2, 6 and 24 hr after Sap2 injection (data not shown). These results indicate that, similarly to p43, Sap2 has a polyclonal immunostimulatory effect but, in contrast to p43, may have a limited immunoregulatory function.
Figure 1.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis of secreted aspartic proteinase 2 (Sap2) purification. Protein migration profile on silver nitrate-stained SDS–PAGE of molecular weight (MW) standards (lane 1), crude extracellular products of Candida albicans (lane 2), fractions F0·05–0·15 (lane 3) and of C. albicans-produced Sap2 purified from fractions F0·05–015 by serial concanavalin A and pepstatin A affinity chromatographies (lane 4).
Figure 2.
Lymphocyte stimulatory effect of secreted aspartic proteinase 2 (Sap2). (a) Flow cytometric analysis of CD69 expression on the surface of BALB/c mice B (IgM+) and T (CD3+) cells in spleen mononuclear cell cultures after 6 hr of incubation with Sap2 or medium alone. Numbers represent the mean values + 1 standard deviation (SD) of four samples per group. This is a representative result of four independent experiments. Differences between control and Sap2-treated groups were statistically significant (P < 0·01). (b) Numbers of spleen immunoglobulin-secreting cells in BALB/c mice 5 days after intraperitoneal (i.p.) treatment with phosphate-buffered saline (PBS) or with 50 µg of Sap2. The values represent the mean + 1 SD of four mice per group and are a representative result of two independent experiments.
Immunization with Sap2 confers protection against systemic candidiasis
The immunological properties of Sap2, reported above, prompted us to assess its potential in a vaccination strategy for systemic candidiasis. Upon i.p. injection of C. albicans, BALB/c mice develop systemic candidiasis which can be readily monitored by analysis of the kidney, an organ shown to be preferentially colonized.31 We used this model to investigate whether Sap2 immunization could prevent systemic candidiasis.
In vaccination strategies, the use of recombinant protein may offer several advantages: first, by providing proof that the incriminated protein is solely responsible for the effect monitored; and, second, by offering a safer preparation that excludes potential pathogens. We decided to clone Sap2 into an inducible bacterial expression vector. Sap2 expression by E. coli was, to some extent, toxic to the bacteria and induction of expression had to be limited to the last 5 hr of culture. Although we managed to induce high level of expression, rSap2 was not soluble and remained in inclusion bodies. The rSap2 preparation used in this work was therefore composed of denatured protein.
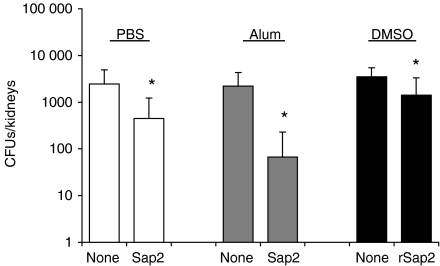
Mice were immunized with Sap2 (two injections i.d. with an intervening 3-week interval) and inoculated i.p. with C. albicans 30 days after the second immunization. The number of C. albicans CFU in the kidney was monitored on day 6 postinfection (Fig. 3). Significant protection against systemic C. albicans infection was observed in mice immunized with Sap2 using alum as adjuvant. Mice that received the protein in the absence of adjuvant, also displayed a significant degree of protection. Taken together with our data above, showing immunoactivation by Sap2, these results suggest that Sap2 has an intrinsic adjuvant-like effect. Finally, a low, but reproducible, reduction in C. albicans colonization was obtained in animals previously injected with rSap2. The low efficiency of the recombinant protein – when compared with native Sap2 – to induce protection may result from the fact that it was administrated in a denatured conformation.
Figure 3.
Decreased colonization of Candida albicans in kidneys from mice immunized with secreted aspartic proteinase 2 (Sap2) or recombinant Sap2 (rSap2). The numbers of C. albicans colony-forming units (CFU) recovered from both kidneys of BALB/c mice treated intradermally (i.d.) with two injections administered with an intervening period of 3 weeks, of Sap2 in phosphate-buffered saline (PBS) (right open bar), Sap2 in alum adjuvant (right grey bar), rSap2 in dimethylsulphoxide (DMSO) (right closed bar) or respective sham-immunized controls (left bars on each of the indicated groups), and infected intraperitoneally (i.p.) with 5 × 106C. albicans blastoconidia 30 days after the second i.d. inoculation. In this figure, and in Figs 5–7, the results shown indicate the mean value + 1 standard deviation (SD) of one representative experiment out of three independent experiments on the Sap2 and rSap2 groups and one representative experiment out of two independent experiments on the Sap2 plus alum group. The number of mice used per group was (from left to right in the figure): 12, 14, 7, 8, 7 and 10. The significance of the results between immunized mice and respective controls is indicated in this figure and in Figs 5–7 (*P < 0·05, **P < 0·01).
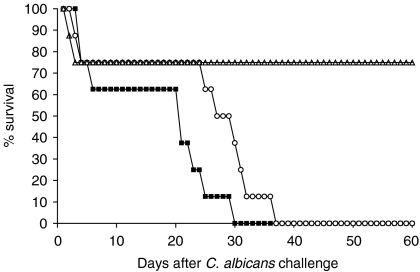
The protective effect of Sap2 immunization against systemic candidiasis was also observed in mice inoculated with a lethal dose of C. albicans. As shown in Fig. 4, mice immunized with Sap2 plus alum showed a better survival rate than mice sham-immunized with PBS or adjuvant alone. The latter showed a median survival time of 21 and 27 days, respectively, and all mice in these groups were dead by day 29 and day 37, respectively, whereas 75% of the Sap2-immunized mice were still alive 60 days after infection. This protective effect of Sap2 immunization was, however, unable to prevent deaths occurring in the first days after yeast inoculation (two animals out of eight), probably because of tumour necrosis factor (TNF)-induced shock.32
Figure 4.
Protective effect of secreted aspartic proteinase 2 (Sap2) immunization against lethal Candida albicans challenge. The survival rates in mice immunized intradermally (i.d.) with two injections, administered with an intervening interval of 3 weeks, of Sap2 in alum adjuvant (open triangles) or sham-immunized controls i.d. inoculated with alum adjuvant only (open circles) or phosphate-buffered saline (PBS) (closed squares), and infected intraperitoneally (i.p.) with 2 × 107C. albicans yeast-form cells 30 days after the second i.d. inoculation. The survival rate of mice immunized with Sap2 plus alum was significantly different (P < 0·05) from that of adjuvant or PBS-inoculated mice, as determined using the Log rank test. Results are one representative experiment out of two, and show the percentage survival of each of the three mice groups monitored daily for 60 days. Eight mice per group were used.
We conclude that immunization with Sap2 confers protection against systemic candidiasis in mice.
Anti-Sap2 immmunoglobulin production is increased in treated mice
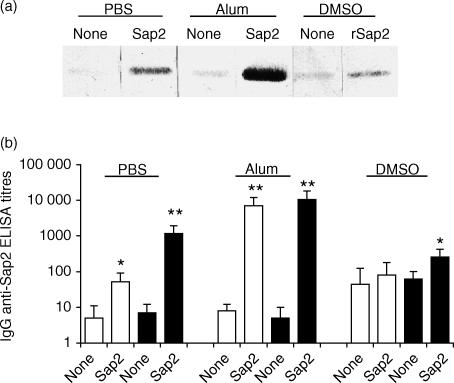
To determine the immunogenic efficiency of the different Sap2 preparations, sera from the animals analysed above were tested for the presence of antibodies specific for this protein. Western blot analysis was used to reveal IgG reactivity against blotted Sap2 in sera collected 10 days after the second antigenic stimulation (Fig. 5a). Specific reactivity was readily detectable in mice immunized with native Sap2 alone or with alum, but much reduced in animals that had received denatured rSap2. For each group of mice, the signal was always stronger than the background anti-Sap2 reactivity detected in sera from control mice.
Figure 5.
Immunoglobulin G (IgG) antibodies to secreted aspartic proteinase 2 (Sap2) are elicited in BALB/c mice treated intradermally (i.d.) with this protein. (a) Western blot analysis of anti-Sap2 IgG reactivities of 50-fold diluted sera collected 10 days after the second i.d. immunization from mice immunized with Sap2 (lane 2), Sap2 plus alum (lane 4) or recombinant Sap2 (rSap2) (lane 6) and respective controls (lanes 1, 3, 5). Serum samples from individual mice, representative of each group, are shown.(b) Serum IgG enzyme-linked immunosorbent assay (ELISA) titres of anti-Sap2 IgG in sera collected 10 days after the second i.d. immunization (open bars) or when the mice were killed, 6 days after C. albicans challenge (closed bars). In this figure, and in Fig. 6, the vehicle used on the i.d. inoculations of sham-immunized (none) or immunized (Sap2, rSap2) mice is indicated in underlined labels above bars. DMSO, dimethylsulphoxide; PBS, phosphate-buffered saline.
In a second step, ELISAs were performed for the quantitative measurement of anti-Sap immunoglobulins in the sera collected before and after infection (Fig. 5b). The titres of anti-Sap2 IgM did not differ significantly in the sera from any mice group (control and immunized) (data not shown). However, anti-Sap2 IgG titres were significantly higher in mice immunized with native Sap2 than in the controls. Immunizations performed in the absence, rather than the presence, of adjuvant were less potent, but significant. This result supports the notion of an intrinsic adjuvant effect of Sap2. The specific Sap2 humoral response induced by immunization is dramatically enhanced after infection, although infection per se does not elicit the production of anti-Sap2 immunoglobulin. Finally, the ELISA titres of anti-Sap2 immunoglobulins in rSap2-immunized mice were only significantly different, as compared to controls, after C. albicans infection. Taken together, these results and those presented above, indicate that the production of anti-Sap2 IgG elicited by immunization with Sap2 correlates with protection against systemic C. albicans infection.
The humoral response to C. albicans structural antigens is not affected by Sap2 or rSap2 immunization
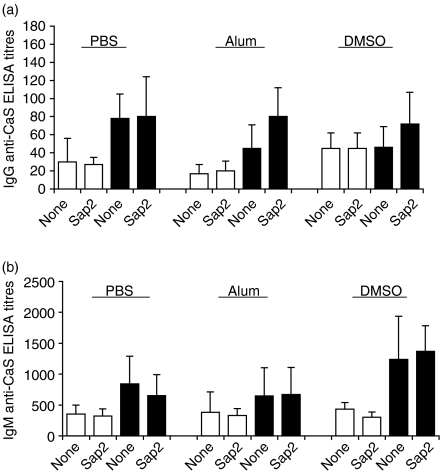
Our results strongly suggest that protection against C. albicans colonization by previous Sap2 immunization results from the specific inhibition of Sap2 activities. As these activities may encompass inhibition of the host immune responses against the fungus itself, we next monitored antibody reactivities against C. albicans structural antigens (CaS) in the sera of the various experimental groups (Fig. 6). The titres of anti-CaS IgG or IgM were not significantly different between groups of immunized mice and their respective controls. Anti-CaS IgM were detected in all groups of mice and increased after infection, as expected in a primary immune response elicited by the inoculated Candida (Fig. 6b). Anti-CaS IgG were also detected, although at lower levels. The low titres of anti-CaS reactivity detected in sera from non-infected animals probably reflected the binding of polyreactive natural antibodies.33 We conclude that the production of anti-Candida immunoglobulin is non-protective and not significantly affected by Sap2 immunization.
Figure 6.
Titres of antibody to Candida albicans sonicates (CaS) in secreted aspartic proteinase 2 (Sap2)- or recombinant Sap2 (rSap2)-immunized BALB/c mice. Serum immunoglobulin G (IgG) (a) and immunoglobulin M (IgM) (b) titres of anti-CaS, before (10 days after the second antigenic intradermal stimulation, open bars) and 6 days after C. albicans challenge (closed bars), on the indicated mice groups. DMSO, dimethylsulphoxide; PBS, phosphate-buffered saline.
Passive transfer of anti-Sap2 IgG has a protective effect against systemic candidiasis
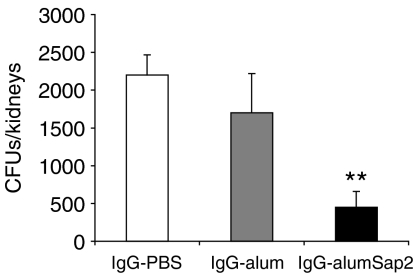
In order to demonstrate the potential protective role of anti-Sap2 immunoglobulin in systemic candidiasis, IgG purified from the pooled sera of Sap2-immunized mice (IgG-alumSap2), or IgG purified from the pooled sera of sham-immunized mice (IgG-PBS and IgG-alum), as controls, were passively administered to C. albicans-infected mice. As shown in Fig. 7, a significant reduction in the number of C. albicans CFUs in kidneys was observed in mice passively transferred with IgG-alumSap2 compared to mice transferred with IgG-alum or IgG-PBS. Moreover, the IgG-alumSap2 preparation showed an inhibitory effect on the expression of CD69 induced in vitro on B cells by Sap2 stimulation, whereas IgG-PBS and IgG-alum had no detectable effect (data not shown).
Figure 7.
Decreased colonization, by Candida albicans, in kidneys of mice passively transferred with immunoglobulin G (IgG) to secreted aspartic proteinase 2 (Sap2). Numbers are given of C. albicans colony-forming units (CFU) recovered from both kidneys of BALB/c mice passively immunized by three intradermal (i.d.) inoculations with 100 µg of IgG purified from the pooled sera of Sap2-immunized mice (IgG-alumSap2) or with the same dose of IgG purified from the pooled sera of mice sham-immunized with phosphate-buffered saline (PBS) (IgG-PBS) or alum (IgG-alum), and infected intraperitoneally (i.p.) with 5 × 106C. albicans blastoconidia on the day of the first antibody treatment. Results represent the mean value + 1 standard deviation (SD) of one representative experiment of two carried out. Four mice were used per group. The statistical difference between anti-Sap2-treated mice and controls is indicated (**P < 0·01). No significant difference between the control groups was observed.
This result indicates that anti-Sap2 immunoglobulins have a protective role against systemic candidiasis.
Discussion
We report here that immunization with the C. albicans extracellular protein, Sap2, decreases the severity of murine systemic candidiasis. This protection correlates with the production of anti-Sap2 IgG and did not affect the humoral immune response against total C. albicans proteins. Sap2 protein has polyclonal lymphocyte activation and adjuvant properties that may explain its role in C. albicans virulence and the efficiency of its inhibition in limiting infection.
Our analyses indicate that the anti-Sap2 humoral immune response induces protection against C. albicans systemic candidiasis. Using three different immunization protocols, we generated three groups of animals displaying various titres of anti-Sap2 immunoglobulin and found that the higher the titre, the more efficient the protection. Thus, classical immunization with native Sap2 and alum as adjuvant induced a strong specific humoral response associated with a 20-fold decrease in C. albicans load in the kidney upon infection when compared with unimmunized animals. Similar treatment, without adjuvant, induced a 100-fold lower amount of specific antibodies and a fivefold lower protection upon infection. Finally, denatured recombinant Sap2 is a poor immunogen and its injection is associated with a limited decrease in the infection score. Immunization with Sap2 plus alum adjuvant also markedly increased the survival rate of mice inoculated with a lethal dose of C. albicans although did not prevent death at early time-points after systemic yeast challenge, which were probably caused by shock.32 This lack of protective effect conferred by Sap2 immunization in the first 24–72 hr after i.p. C. albicans challenge might be a consequence of late expression of this protein, as previously reported to occur in BALB/c mice inoculated i.p. with C. albicans.18 Therefore, other described vaccination approaches targeting C. albicans structural antigens, which are more effective in preventing early acute responses to this yeast,34 probably avoid the production of toxic amounts of TNF elicited by the binding of Candida to pattern recognition receptors on cells of the innate immune system.35,36
The protective role of anti-Sap2 immunoglobulin against systemic candidiasis was also evidenced by the decrease of C. albicans fungal load in kidneys observed in mice passively transferred with anti-Sap2 IgG. However, as this decrease was less marked than the one observed in mice immunized with Sap2, this could indicate that cellular immune responses, well known to mediate protection against C. albicans infection,37 also contribute to the protective mechanisms elicited by Sap2 immunization. Previous reports have indicated that antibodies against Candida Sap confer protection in experimental rodent models of C. albicans vaginitis,22,23,34 and nasal vaccination with Sap2 protected from C. albicans colonization of the gastrointestinal tract.24 The present work therefore extends the protective effect of Sap2 immunization from mucosal to systemic forms of candidiasis.
The protection to systemic candidiasis induced by Sap2 immunization does not result from enhanced host protective immune responses against structural C. albicans proteins. Hence, the production of antibodies against C. albicans structural antigens upon infection is equally low in all groups of mice, independently of previous immunization with Sap2. These results also reveal that mice developing mild or severe systemic candidiasis show a similar low humoral response to C. albicans structural antigens. This apparent paradox is in agreement with previous reports indicating that transfer of these antibodies does not confer protection to infection and may rather facilitate host colonization.38–40 In turn, these findings suggest that neutralization of Sap2 by specific antibodies must directly affect C. albicans virulence.
Immunization with extracellular proteins has been successfully used to control infections caused by different bacteria, such as as Mycobacteria,41,42 Legionella pneumophila,43 Streptococcus mutans,44 Yersinia spp.,45 Salmonella enteritidis,46 the protozoan Trypanosoma cruzi47 and the fungus C. albicans.40 Such strategies have shown potent immune protection in cases where immunization with microbial structural antigens had failed.40 Similarly to Sap2, some of these microbial extracellular proteins were enzymes47,48 and it is tempting to propose that neutralization of the enzymatic activities by specific antibodies could account, at least in part, for the immunoprotection achieved with these protocols. Alternatively, and as described for several other microbial extracellular proteins, the direct activity of these compounds may be to facilitate pathogen colonization by exerting direct effects on the host immune system, such as polyclonal lymphocyte activation,21,47,49,50 induction of host IL-10 and IL-4 production,21,45,49,50 or interference with major histocompatibility complex (MHC) class II-dependent T-cell responses.51 We show that Sap2 shares some of these activities: it induces polyclonal activation of B cells both in vivo and in vitro and, to some extent, of T cells, and has an intrinsic adjuvant effect. Sap2 may thus be included in the group of multifunctional proteins generally designated as ‘moonlighting proteins’.52
In a previous report, an unidentified 43 000-MW C. albicans-secreted protein has been described as a potent immunomodulatory molecule, inducing lymphocyte polyclonal activation20 and host IL-10 production.21 C. albicans Sap2 is a 41 000-MW secreted protein that has been shown, in this study, to mediate some of these effects. While readily detectable, the number of lymphocytes responding to Sap2 stimulation was far lower than that reported for p43,53 and IL-10 production was induced neither in vivo nor in vitro. We propose that although an immunomodulatory role for Sap2 on C. albicans colonization cannot be excluded, it is unlikely that p43 activities are caused by Sap2 alone. Finally, our immunization protocol efficiently reduced, but did not prevent, systemic candidiasis. As other Saps (namely Sap4, Sap5 and Sap6) have been shown to promote the invasive process at early stages of C. albicans infection,9 it is conceivable that immunogenic preparations, including these Saps together with Sap2, would improve the protective effect revealed here. However, sequence similarities between different Saps17 raise the possibility that immunization with Sap2 could induce the production of antibodies that are cross-reactive with other C. albicans-secreted proteases.
In conclusion, the immunoprotection to murine systemic candidiasis obtained by immunization with a C. albicans extracellular protein we report here highlights the interest to explore further, similar strategies, to the management of other systemic fungal infections.
Acknowledgments
We are most grateful for the assistance of Julia Lobato at the IGC sequencing facility. This work was supported by Fundação para a Ciência e a Tecnologia (FCT), grant no. POCTI//33570/MGI/99. This grant also financed a BIC fellowship for Luzia Teixeira. Iris Caramalho received a fellowship from the FCT GGP XXI/BIC J/3459/96.
References
- 1.Kullberg BJ, Oude-Lashof AML. Epidemiology of opportunistic invasive mycoses. Eur J Med Res. 2002;7:183–91. [PubMed] [Google Scholar]
- 2.Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220:751–8. doi: 10.1097/00000658-199412000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, Gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136:834–44. doi: 10.7326/0003-4819-136-11-200206040-00013. [DOI] [PubMed] [Google Scholar]
- 4.Bibashi E, Memmos D, Kokolina E, Tsakiris D, Sofianou D, Papadimitrou M. Fungal peritonitis complicationg peritoneal dialysis during an 11-year period: report of 46 cases. Clin Infect Dis. 2003;36:927–31. doi: 10.1086/368210. [DOI] [PubMed] [Google Scholar]
- 5.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–35. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 6.Cutler JE. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 7.De Bernardis F, Sullivan PA, Cassone A. Aspartyl proteinases of Candida albicans and their role in pathogenicity. Med Mycol. 2001;39:303–13. doi: 10.1080/mmy.39.4.303.313. [DOI] [PubMed] [Google Scholar]
- 8.Hube B, Sanglard D, Odds FC, Hess D, Monod M, Schäfer W, Brown AG, Gow NA. Gene disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 in Candida albicans attenuates virulence. Infect Immun. 1997;65:3529–38. doi: 10.1128/iai.65.9.3529-3538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanglard D, Hube B, Monod M, Odds FC, Gow NAR. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–46. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rüchel R, Ritter B, Schaffrinski M. Modulation of experimental systemic murine candidosis by intravenous pepstatin. Zentralbl Bakteriol. 1990;273:391–403. doi: 10.1016/s0934-8840(11)80443-3. [DOI] [PubMed] [Google Scholar]
- 11.Korting HC, Schaller M, Eder G, Hamm G, Böhmer U, Hube B. Effects of the human immunodeficiency virus (HIV) proteinase inhibitors saquinavir and indinavir on in vitro activities of secreted aspartyl proteinases of Candida albicans isolates from HIV-infected patients. Antimicrob Agents Chemother. 1999;43:2038–42. doi: 10.1128/aac.43.8.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ollert MW, Wende C, Gorlich M, McMullan-Vogel CG, Borg-von Zepelin M, Vogel C-W, Korting HC. Increased expression of Candida albicans secretory proteinase, a putative virulence factor, in isolates from human immunodeficiency virus-positive patients. J Clin Microbiol. 1995;33:2543–9. doi: 10.1128/jcm.33.10.2543-2549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bernardis F, Chiani P, Ciccozzi M, et al. Elevated aspartic proteinase secretion and experimental pathogenicity of Candida albicans isolated from oral cavities of subjects infected with human immunodeficiency virus. Infect Immun. 1996;64:466–71. doi: 10.1128/iai.64.2.466-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colina AR, Aumont F, Deslauriers N, Belhumeur P, de Repentigny L. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Infect Immun. 1996;64:4514–9. doi: 10.1128/iai.64.11.4514-4519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaller M, Korting HC, Schäfer W, Bastert J, Chen W, Hube B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol. 1999;34:169–80. doi: 10.1046/j.1365-2958.1999.01590.x. [DOI] [PubMed] [Google Scholar]
- 16.Monod M, Togni G, Hube B, Sanglard D. Multiplicity of genes encoding secreted aspartic proteinases in Candida species. Mol Microbiol. 1994;13:357–68. doi: 10.1111/j.1365-2958.1994.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 17.Monod M, Hube B, Hess D, Sanglard D. Differential regulation of SAP8 and SAP9, which encode two new members of the secreted aspartic proteinase family in Candida albicans. Microbiology. 1998;144:2731–7. doi: 10.1099/00221287-144-10-2731. [DOI] [PubMed] [Google Scholar]
- 18.Staib P, Kretschmar M, Nichterlein T, Hof H, Morschhäuser J. Differential activation of a Candida albicans virulence gene family during infection. Proc Natl Acad Sci USA. 2000;97:6102–7. doi: 10.1073/pnas.110031497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusek M, Smith EA, Monod M, Dunn BM, Foundling SI. Extracellular aspartic proteinases from Candida albicans, Candida tropicalis, and Candida parapsilosis yeasts differ substantially in their specificities. Biochemistry. 1994;33:9791–9. doi: 10.1021/bi00198a051. [DOI] [PubMed] [Google Scholar]
- 20.Tavares D, Salvador A, Ferreira P, Arala-Chaves M. Immunological activities of a Candida albicans protein which plays an important role in the survival of micoorganism in the host. Infect Immun. 1993;61:1881–8. doi: 10.1128/iai.61.5.1881-1888.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavares D, Ferreira P, Arala-Chaves M. Increased resistance to systemic candidiasis in athymic or interleukin-10-depleted mice. J Infect Dis. 2000;182:266–73. doi: 10.1086/315674. [DOI] [PubMed] [Google Scholar]
- 22.Cassone A, Boccanera M, Adriani D, Santoni G, De Bernardis F. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect Immun. 1995;63:2619–24. doi: 10.1128/iai.63.7.2619-2624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bernardis F, Boccanera M, Adriani D, Girolamo A, Cassone A. Intravaginal and intranasal immunizations are equally effective in inducing vaginal antibodies and conferring protection against vaginal candidiasis. Infect Immun. 2002;70:2725–9. doi: 10.1128/IAI.70.5.2725-2729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suenobu N, Quejón M, Kiyono H. Nasal vaccination induces the ability to eliminate Candida colonization without influencing the pre-existing antigen-specific IgE Abs: a possibility for the control of Candida-related atopic dermatitis. Vaccine. 2002;20:2972–80. doi: 10.1016/s0264-410x(02)00218-9. [DOI] [PubMed] [Google Scholar]
- 25.Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981;659:99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- 26.Magee BB, D'Souza TM, Magee PT. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J Bacteriol. 1987;169:1639–43. doi: 10.1128/jb.169.4.1639-1643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okahashi N, Takahashi I, Nakai M, Senpuku H, Nisizawa T, Koga T. Identification of antigenic epitopes in an alanine-rich repeating region of a surface protein antigen of Streptococcus mutans. Infect Immun. 1993;61:1301–6. doi: 10.1128/iai.61.4.1301-1306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundkvist I, Holmberg D, Ivars F, Coutinho A. The immune response to bacterial dextrans. III. Oncogenic development and strain distribution of specific clonal precursors. Eur J Immunol. 1986;16:357–62. doi: 10.1002/eji.1830160814. [DOI] [PubMed] [Google Scholar]
- 29.Gronowicz E, Coutinho A, Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976;6:588–90. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- 30.Forni L. Reagents for immunofluorescence and their use for studying lymphoid cell products. In: Lefkovitz I, Pernis B, editors. Immunological Methods. New York: Academic Press Inc.; 1979. pp. 151–7. [Google Scholar]
- 31.Lischewski A, Kretschmar M, Hof H, Amann R, Hacker J, Morschhäuser J. Detection and identification of Candida species in experimentally infected tissue and human blood by rRNA-specific fluorescent in situ hybridization. J Clin Microbiol. 1997;35:2943–8. doi: 10.1128/jcm.35.11.2943-2948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allendoerfer R, Magee DM, Smith JG, Bonewald L, Graybill JR. Induction of tumor-alpha in murine Candida albicans infection. J Infect Dis. 1993;167:1168–72. doi: 10.1093/infdis/167.5.1168. [DOI] [PubMed] [Google Scholar]
- 33.Klinman DM. Cross-reactivity of IgM-secreting B cells from normal BALB/c mice. J Immunol. 1992;149:3569–73. [PubMed] [Google Scholar]
- 34.Bromuro C, Torosantucci A, Chiani P, Conti S, Polonelli L, Cassone A. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated candidiasis in recipients of a Candida albicans vaccine. Infect Immun. 2002;70:5462–70. doi: 10.1128/IAI.70.10.5462-5470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garner RE, Rubanowice K, Sawyer RT, Hudson JA. Secretion of TNF-α by alveolar macrophages in response to Candida albicans mannan. J Leukoc Biol. 1994;55:161–8. doi: 10.1002/jlb.55.2.161. [DOI] [PubMed] [Google Scholar]
- 36.Brown GD, Herre J, Williams DL, Willment JA, Marshell ASJ, Gordon S. Dectin-1 mediates the biological effects of β-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romani L. Immunology of invasive candidiasis. In: Calderone RA, editor. Candida and Candididasis. Washington DC: ASM Press; 2002. pp. 223–42. [Google Scholar]
- 38.De Bernardis F, Boccanera M, Adriani D, Spreghini E, Santoni G, Cassone A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect Immun. 1997;65:3399–405. doi: 10.1128/iai.65.8.3399-3405.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–8. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavares D, Ferreira P, Vilanova M, Videira A, Arala-Chaves M. Immunoprotection against systemic candidiasis in mice. Int Immunol. 1995;5:785–96. doi: 10.1093/intimm/7.5.785. [DOI] [PubMed] [Google Scholar]
- 41.Pal PG, Horwitz MA. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–92. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva RA, Pais TF, Appelberg R. Effects of interleukin-12 in the long-term protection conferred by a Mycobacterium avium subunit vaccine. Scand J Immunol. 2000;52:531–3. doi: 10.1046/j.1365-3083.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 43.Blander SJ, Horwitz MA. Vaccination with the major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of legionnaire's disease. J Exp Med. 1989;169:691–705. doi: 10.1084/jem.169.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares R, Ferreira P, Santarém MMG, Silva MT, Arala-Chaves M. Low T- and B-cell reactivity is an apparently paradoxical request for murine immunoprotection against Streptococcus mutans. Scand J Immunol. 1990;31:361–6. doi: 10.1111/j.1365-3083.1990.tb02779.x. [DOI] [PubMed] [Google Scholar]
- 45.Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, Aepfelbacher M, Heesemann J. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med. 2002;196:1017–24. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strindelius L, Degling Wikingsson L, Sjöholm I. Extracellular antigens from Salmonella enteritidis induce effective immune response in mice after oral vaccination. Infect Immun. 2002;70:1434–42. doi: 10.1128/IAI.70.3.1434-1442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reina-San-Martin B, Degrave W, Rougeot C, et al. A B-cell mitogen from a pathogenic trypanosome is a eukariotic proline racemase. Nat Med. 2000;6:890–7. doi: 10.1038/78651. [DOI] [PubMed] [Google Scholar]
- 48.Smith DJ, King WF, Barnes LA, Trantolo D, Wise DL, Taubman MA. Facilitated intranasal induction of mucosal and systemic immunity to mutans streptococcal glucosyltransferase peptide vaccines. Infect Immun. 2001;69:4767–73. doi: 10.1128/IAI.69.8.4767-4773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferreira P, Brás A, Tavares D, Vilanova M, Ribeiro A, Videira A, Arala-Chaves M. Purification, and biochemical and biological characterization of an immunosuppressive and lymphocyte mitogenic protein secreted by Streptococcus sobrinus. Int Immunol. 1997;9:1735–43. doi: 10.1093/intimm/9.11.1735. [DOI] [PubMed] [Google Scholar]
- 50.Jahreis A, Beckheinrich P, Haustein UF. Effects of two novel cationic staphylococcal proteins (NP-tase and p70) and enterotoxin B on IgE synthesis and interleukin-4 and interferon-gamma production in patients with atopic dermatitis. Br J Dermatol. 2000;142:680–7. doi: 10.1046/j.1365-2133.2000.03412.x. [DOI] [PubMed] [Google Scholar]
- 51.Lee LY, Miyamoto YJ, McIntyre BW, Höök M, McCrea KW, McDevitt D, Brown E. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J Clin Invest. 2002;110:1461–71. doi: 10.1172/JCI16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jefferey CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- 53.Vilanova M, Tavares D, Ferreira P, Oliveira L, Nóbrega A, Appelberg R, Arala-Chaves M. Role of monocytes in the up-regulation of the early activation marker CD69 on B and T murine lymphocytes induced by microbial mitogens. Scand J Immunol. 1996;43:155–63. doi: 10.1046/j.1365-3083.1996.d01-25.x. [DOI] [PubMed] [Google Scholar]