Abstract
Neutrophils are the effector cells in both innate and adaptive immunity, where they perform the functions of phagocytosis and killing of bacteria. They respond to a large number of chemoattractants, but their response to epithelial cell-derived human β-defensins (hBD) has not been investigated. Here we report that hBD-2, but not hBD-1, is a specific chemoattractant for tumour necrosis factor (TNF)-α-treated human neutrophils. The optimal concentration required for maximal chemotactic activity was 5 µg/ml. The effect of hBD-2 on neutrophils was dependent on the G-protein-phospholipase C pathway, as demonstrated by inhibition by pertussis toxin and U-73122. In addition, ligand-receptor analysis indicated that the binding of hBD-2 was markedly inhibited by macrophage inflammatory protein (MIP)-3α, a specific and unique ligand for CCR6. Furthermore, anti-CCR6 antibody could almost completely suppress the cell migration induced by hBD-2, suggesting that hBD-2 mainly utilizes CCR6 as a functional receptor. Thus, our finding that hBD-2 is a potent chemoattractant for human neutrophils through specific receptors provides a novel mechanism by which this peptide contributes to the host defence system by recruiting neutrophils to inflammation/infection sites. This also suggests an important link between epithelial cell-derived antibacterial peptides and neutrophils during infection or inflammation.
Introduction
Antimicrobial peptides participate primarily in the innate immune system and are used as a first line of immune defence in plants, insects and mammals. As effectors of innate immunity, antimicrobial peptides kill a broad spectrum of microbes, including both Gram-positive and Gram-negative bacteria, fungi and certain viruses.1–3
In humans, several antimicrobial peptides have been identified, including salivary histatins, granulysin, lactoferricin, α- and β-defensins, and human cathelicidin hCAP18 (18-kDa human cationic antibacterial protein)-derived LL-37.1 The α- and β-defensin families are the most common human antibacterial peptides, differing from one another in the spacing and connectivity of their six cysteine residues.1,3α-Defensins are found in storage granules of neutrophils and small intestinal Paneth cells, whereas β-defensins are characteristic of epithelial tissues.4,5 To date, six human β-defensins (hBD-1 to -6) have been identified. hBD-1 is constitutively produced by various epithelial tissues, including the urogenital and respiratory tracts.6–8 hBD-2 was originally isolated from extracts of lesional scales from psoriatic skin9 and is mainly present in the skin and the respiratory and gastrointestinal tracts. This peptide is inducibly expressed in inflamed skin lesions and lung tissues upon treatment with bacterial lipopolysaccharide (LPS) and cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-1β.9–11 hBD-3 was also isolated from human lesional psoriatic scales12,13 and is expressed in epithelial and nonepithelial tissues such as the heart, the liver and skeletal muscle. hBD-4 is up-regulated by infection with Gram-positive and Gram-negative bacteria in human respiratory epithelial cells.14 The very recently discovered human defensins hBD-5 and hBD-6 are specifically expressed in the human epididymis. However, these two defensins have, in addition to the conserved six-cysteine motif in all β-defensins, one extra cysteine residue unique to them.15
The neutrophil is the body's first line of defence against microorganisms and is a critical effector cell in both innate and adaptive immunity. Its principal roles in inflammatory and immune responses are phagocytosis and killing of bacteria through both oxidative and nonoxidative mechanisms.16 Neutrophils respond to a large number of chemoattractants to exhibit chemotaxis, activation of integrins, and production of proinflammatory chemokines. The neutrophil chemoattractants include chemokines and classical chemoattractants such as N-formyl methionyl-leucyl-phenylalanine (fMLP), leukotriene B4, activated complement fragment 5 (C5a), and platelet-activating factor.17 Both chemokines and classical chemoattractants act on G-protein-coupled receptors.
Human antibacterial peptides are reported to become chemoattractants for several inflammatory cell types. Previous investigations have shown that hBD-1 and hBD-2 chemoattract T cells and immature dendritic cells through CC-chemokine receptor 6 (CCR6),18 and we have shown that hBD-2 induces the migration of mast cells by activating G-protein-phospholipase C (PL C) coupled receptors,19 resulting in the activation of these cells to release histamine and generate prostaglandin D2·20 hBD-3 and hBD-4 are chemoattractants for monocytes,13,14 while the chemotactic activities of hBD-5 and hBD-6 are not yet known. These findings suggest that defensins serve as an important bridge between the innate and adaptive immune systems by recruiting inflammatory cells.
Recently, some members of the cathelicidin family, including LL-37 and PR-39, have been reported to chemoattract neutrophils.21,22 However, the response of neutrophils to epithelial cell-derived hBDs has not been studied. Therefore, we investigated whether antimicrobial peptides (for example hBDs) other than cathelicidins act on neutrophils. The results of the present study indicate that hBD-2, but not hBD-1, induces the chemotaxis of TNF-α-treated human neutrophils using G-protein-coupled receptors, mainly CCR6. Thus, in addition to its microbicidal activity, hBD-2 could contribute to host defence by recruiting neutrophils to sites of inflammation or microbial invasion.
Materials and methods
Reagents hBD-1 and hBD-2 were purchased from the Peptide Institute (Osaka, Japan). Human recombinant macrophage inflammatory protein (MIP)-3α and human recombinant TNF-α were obtained from Genzyme/Techne (London, UK). N-formyl methionyl-leucyl-phenylalanine (fMLP) and U-73122 (1-[6-([(17β)-3-methoxyestra-1,3,5,10-trien-17-yl]amino)hexyl]-1H-pyrrole-2·5-dione) were obtained from Sigma (St. Louis, MO). U-73343 (1-[6-([(17β)-3-methoxyestra-1,3,5,10-trien-17-yl]amino)hexyl]-2,5-pyrrolidine-dione) was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). Pertussis toxin (PTx) was obtained from List Biological Laboratories (Campbell, CA), and Na125I from ICN Biomedicals (Irvine, CA). Monoclonal anti-human CCR6 antibody was purchased from R&D Systems (Minneapolis, MN). BAPTA-AM [1,2-bis(o-amino phenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxy-methyl ester] was purchased from Dojindo Laboratories (Kumamoto, Japan), and carboxy-SNARF-AM (spiro[7H-benzo(c)xanthene-7,1′(3H)isobenzofuran]-ar′-carboxylic acid, 3-(acetyloxy)-10-(dimethylamino)-3′-oxo-, acetoxy-methyl ester) from Molecular Probes (Leiden, the Netherlands). U-73122, U-73343, BAPTA-AM and SNARF-AM were dissolved in dimethyl sulfoxide (DMSO), which itself did not significantly alter the biological assays at the final concentrations employed (<0·1%).
Purification and treatment of peripheral blood neutrophils
Human peripheral blood from healthy volunteers was heparinized, and neutrophils with purity >95% were isolated by Ficoll-Conray density gradient centrifugation after dextran sedimentation of erythrocytes.23 Neutrophils at a final concentration of 5 × 106 cells/ml were resuspended in RPMI 1640 medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS; Sanko Junyaku, Tokyo, Japan), and treated with various concentrations (1–10 ng/ml equivalent to 20–200 units/ml) of human recombinant TNF-α at 37° in 5% CO2 atmosphere for 6 hr. Then, cells were washed twice and resuspended in assay buffer composed of RPMI 1640 containing 0·1% bovine serum albumin (BSA) before assay at a density of 2 × 106 cells/ml.
Chemotaxis assay
The chemotaxis assay was performed using a 48-well microchemotaxis chamber (Neuroprobe, Cabin John, MD). TNF-α-treated neutrophils (50 µl of 2 × 106 cells/ml) were added to the upper wells. The upper wells of the chamber and the lower wells containing chemoattractants were separated by a polycarbonate membrane with 3-µm-diameter pores. After a 60-min incubation, the number of migrated neutrophils adhering to the underside of the filter was counted under a light microscope after fixing the membrane with methanol and staining with DiffQuick (Kokusai Shiyaku, Kobe, Japan). The results were presented as migrated cell number/5 high-power fields (HPF). We confirmed that migrated cells were morphologically neutrophils (>98%) by staining with DiffQuick. Furthermore, cell migration was determined using a Boyden chamber with a cellulose-nitrate filter (pore size 3 µm), and the distance (in µm) from the focal plane on the top of the filter to the farthest two cells in one focal plane was measured, as described previously.24 hBD-2-induced neutrophil chemotaxis was similarly observed using both a microchemotaxis chamber and a Boyden chamber. In some experiments, cells were incubated with anti-human CCR6 antibody or control immunoglobulin G (IgG) (each 50 µg/ml) for 30 min on ice before the chemotaxis assay.
Treatment with PTx, U-73122 and BAPTA-AM
The effects of Gi-protein inhibitor PTx and PL C inhibitor U-73122 were investigated by incubating TNF-α-pretreated neutrophils (2 × 106 cells/ml) with various concentrations of PTx (25–200 ng/ml) for 2 hr or U-73122 (0·01–10 µm) for 1 hr at 37° in assay buffer. Cells were then washed twice and resuspended in the same buffer before the chemotaxis assay.
To investigate the role of intracellular Ca2+ in neutrophil activation, TNF-α-pretreated neutrophils were washed twice with assay buffer and resuspended in it at a concentration of 2 × 106 cells/ml. Thereafter, they were loaded with 100 µm BAPTA-AM (intracellular Ca2+ chelater) for 30 min at 37°. After two washes with assay buffer, the chemotaxis assay was performed as described above. In some experiments, cells were washed twice with phosphate-buffered saline without Ca2+[PBS(–)] supplemented with 0·1% BSA, and loaded with BAPTA-AM. Cells were then washed and resuspended in PBS(–) containing 0·1% BSA for the chemotaxis assay.
In separate experiments, TNF-α-pretreated neutrophils were loaded with Fura-2 acetoxy-methyl ester (Fura-2-AM), and the concentration of intracellular Ca2+ was measured as previously reported.20 The cell viability, assessed by trypan blue dye exclusion after treatment with PTx, U-73122 or BAPTA-AM, was >90%.
Iodination of hBD-2 and MIP-3α
The iodination of hBD-2 and MIP-3α was performed as reported previously.19 Briefly, hBD-2 and MIP-3α were iodinated with 1 mCi and 0·5 mCi Na125I (100 mCi/ml), respectively, using Iodo-Beads (Pierce Chemical, Rockford, IL) at room temperature for 15 min. The 125I-labelled hBD-2 or 125I-labelled MIP-3α was separated from free Na125I by gel filtration through a Sephadex G-10 column (Amersham Pharmacia, Piscataway, NJ) equilibrated with 0·01% acetic acid. The specific activity of the labelled material was 30 Ci/mmol for hBD-2 and 1552 Ci/mmol for MIP-3α. In preliminary experiments, we confirmed that the migration of neutrophils to 125I-labelled hBD-2 or 125I-labelled MIP-3α was the same as that to unlabelled peptides (data not shown). Thus, the chemoattractants used in our study were not degraded during the labelling procedure.
Binding of 125I-hBD-2 or 125I-MIP-3α to neutrophils
Aliquots of 50 µl of neutrophil suspensions (2 × 106 cells/ml) were incubated with 2 µl of 125I-hBD-2 (final concentration 0·329–84·3 nm) or 125I-MIP-3α (0·00648–1·66 nm) in 100 µl RPMI 1640 containing 1% heat-inactivated BSA for 30 min at 25°. After incubation, the mixture was layered over 500 µl of 10% sucrose/PBS in a 1·5-ml polypropylene tube, and then centrifuged at 400 g for 5 min at 4°. After aspirating the supernatant, the tube was cut 2–3 mm above the cell pellet, and the cell-associated 125I-hBD-2 or 125I-MIP-3α was counted using a gamma counter (model 1270 Rack Gamma II; Amersham Pharmacia). Nonspecific binding was determined in parallel experiments in the presence of a 100-fold excess of unlabelled peptide hBD-2 or MIP-3α. Specific binding was calculated by subtracting the nonspecific binding from total counts. The data were curve-fitted with the computer program ligand to determine the affinity and number of binding sites.25 To evaluate the competitive binding of 125I-hBD-2, neutrophils (1 × 106 cells/ml) were incubated with 10 nm125I-hBD-2 for 30 min at 25° in the presence of various concentrations (from 4 to 1000 nm) of unlabelled hBD-1, hBD-2 or MIP-3α in 100 µl RPMI 1640 containing 1% heat-inactivated BSA. For the competitive binding of 125I-MIP-3α, neutrophils were incubated with 0·41 nm of 125I-MIP-3α in the presence of 0·2–41 nm of unlabelled hBD-1, hBD-2 or MIP-3α. The specific binding was determined as described above.
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of CCR6 mRNA expression by neutrophils
Total RNA was extracted from TNF-α-treated or untreated neutrophils using Trizol reagent (BRL, Life Technologies, Rockville, MD) according to the manufacturer's instructions. First-strand cDNA was synthesized from 3 µg of total RNA with oligo(dT)12−18 primers using Superscript II RNase H– reverse transcriptase (Life Technologies). To exclude the possibility of genomic DNA contamination, we performed control reactions in which the reverse transcriptase was omitted. Furthermore, to remove RNA complementary to the cDNA, 1 µl (2 units) of RNase H (Life Technologies) was added to the reactions mixtures, which were then incubated at 37° for 20 min. PCR reactions were performed in a final volume of 25 µl of a mixed solution containing 10 U AmpliTaq enzyme (Applied Biosystems, Foster City, CA) and 0·8 µm of each forward and reverse primer. The CCR6 forward primer was 5′-ATTTCAGCGATGTTTTCGACTC-3′ and the reverse primer was 5′-GGAGAAGCCTGAGGACTTGTA-3′;26 the GAPDH forward primer was 5′-ACCACAGTCCATGCCATCAC-3′ and the reverse primer was 5′-TCCACCACCCTGTTGCTGTA-3′.27 The reaction mixture was subjected to 29 cycles of PCR with the following conditions: 94° for 1 min, 61·5° for 2 min, and 72° for 3 min. The PCR products were analysed by electrophoresis in a 1·5% agarose gel stained with ethidium bromide.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (anova) and P < 0·05 was considered to be significant. The results are shown as mean ± standard deviation (SD).
Results
hBD-2 induces the chemotaxis of TNF-α-pretreated neutrophils
To investigate the action of hBD-2 on neutrophils, we first evaluated the ability of hBD-2 to induce the migration of neutrophils. When neutrophils were pretreated with TNF-α, a cytokine known to effectively prime neutrophil functions such as chemotaxis,28 the migration of neutrophils towards hBD-2 as well as MIP-3α was enhanced (Fig. 1). In contrast, neutrophils cultured in the absence of TNF-α did not exhibit chemotactic activity when incubated with hBD-2 or MIP-3α, whereas fMLP could chemoattract these cells. Pretreatment of neutrophils with increasing concentrations of TNF-α (1–10 ng/ml) resulted in the chemotaxis of neutrophils towards various concentrations of hBD-2 (1–10 µg/ml); this migration was dose-dependent and bell-shaped, with 5 µg/ml hBD-2 being the optimal chemotactic concentration, and neutrophils treated with 2·5 ng/ml TNF-α demonstrated maximal migration towards hBD-2. However, at concentrations of 5 and 10 ng/ml of TNF-α, a slight inhibition of chemotaxis towards hBD-2 was noted (Fig. 1). Therefore, for further studies, the concentration of 2·5 ng/ml TNF-α was used. In separate experiments, we found that TNF-α itself at concentrations of 1–10 ng/ml was not chemotactic for neutrophils, which was in accordance with the findings of previous studies.28 However, TNF-α-preincubated cells showed a slight increase in migration without hBD-2.
Figure 1.
Effect of TNF-α treatment on the migration of neutrophils towards hBD-2. Neutrophils treated with increasing concentrations of TNF-α for 6 hr were placed into the upper wells of the microchamber and allowed to migrate towards 1–10 µg/ml hBD-2, 4 µg/ml MIP-3α and 10−8 m fMLP for 1 hr at 37°. Neutrophil chemotaxis was assessed by counting the number of migrated cells through the polycarbonate membrane. Each bar represents the mean of seven separate experiments.
Both hBD-1 and hBD-2 have been shown to induce the migration of dendritic cells and T cells with similar potency and efficacy.18 However, in the present study, we found that hBD-1 could only weakly elicit the migration of TNF-α-treated neutrophils. This relatively weak activity was observed at concentrations of 10 and 20 µg/ml, as shown in Fig. 2. In separate experiments, we confirmed that the hBD-1 used in this study was active, as it exhibited bactericidal activity against Escherichia coli at the same potency as hBD-2 (unpublished observations). MIP-3α was used as a positive control because it has been reported to chemoattract TNF-α-treated neutrophils.29 In our experimental conditions, MIP-3α induced the migration of TNF-α-treated neutrophils in a dose-dependent manner (Fig. 2).
Figure 2.
Effects of hBD-1 and hBD-2 on neutrophil migration. Neutrophils treated with 2·5 ng/ml TNF-α for 6 hr (hatched bars) or untreated (closed bars) were placed into the upper wells of the microchamber and allowed to migrate towards 5–40 µg/ml hBD-1, 1–10 µg/ml hBD-2, 1–8 µg/ml MIP-3α, 10−8 m fMLP or medium alone (Med) for 1 hr at 37°. Each bar represents the mean ± SD of four separate experiments.
Checkerboard analysis of hBD-2-induced neutrophil migration
The ability of hBD-2 to stimulate the directional (chemotaxis) or random (chemokinesis) migration of TNF-α-pretreated neutrophils was investigated by employing a checkerboard analysis. As shown in Table 1(a), the presence of various concentrations of hBD-2 in the lower compartments demonstrated the gradient-dependent migration of neutrophils. The presence of this peptide in only the upper compartment did not induce any substantial increase in cell migration. However, a slight dose-dependent increase in migration (chemokinesis), with a bell-shaped curve, was observed when equal concentrations of peptide were added to both the upper and the lower chambers. Thus, we concluded that the migration of neutrophils towards hBD-2 was based predominantly on chemotaxis rather than chemokinesis. Interestingly, the addition of MIP-3α to the upper chambers substantially inhibited cell migration towards hBD-2 (Table 1b), suggesting that hBD-2 and MIP-3α probably share the same receptor on neutrophils.
Table 1.
Checkerboard analysis of hBD-2-induced neutrophil migration
| (a) | |||||
|---|---|---|---|---|---|
| hBD-2 in upper chamber (mg/ml) | |||||
| hBD-2 in lower chamber (mg/ml) | 0 | 1 | 2·5 | 5 | 10 |
| 0 | 41 ± 6 | 32 ± 5 | 39 ± 9 | 51 ± 4 | 26 ± 7 |
| 1 | 76 ± 4 | 44 ± 2 | 26 ± 4 | 39 ± 8 | 61 ± 9 |
| 2·5 | 169 ± 33 | 107 ± 27 | 76 ± 11 | 38 ± 6 | 53 ± 4 |
| 5 | 286 ± 62 | 223 ± 39 | 157 ± 17 | 114 ± 23 | 59 ± 5 |
| 10 | 158 ± 17 | 99 ± 16 | 73 ± 11 | 82 ± 8 | 79 ± 7 |
| (b) | |||||
|---|---|---|---|---|---|
| MIP-3α in upper chamber (μg/ml) | |||||
| hBD-2 in lower chamber (μg/ml) | 0 | 1 | 2 | 4 | 8 |
| 0 | 41 ± 6 | 52 ± 2 | 37 ± 7 | 53 ± 5 | 36 ± 7 |
| 1 | 76 ± 4 | 73 ± 9 | 46 ± 6 | 37 ± 9 | 44 ± 5 |
| 2·5 | 169 ± 33 | 152 ± 26 | 109 ± 21 | 68 ± 12 | 69 ± 5 |
| 5 | 286 ± 62 | 263 ± 43 | 188 ± 37 | 103 ± 8 | 67 ± 5 |
| 10 | 158 ± 17 | 134 ± 17 | 99 ± 25 | 70 ± 6 | 82 ± 3 |
Various concentrations of hBD-2 were added to the lower chambers, and TNF-α-treated neutrophils in the upper chambers containing either hBD-2 (a) or MIP-3α (b) at indicated concentrations were allowed to migrate. The migratory response was measured after a 60 min-incubation by counting the number of cells through the polycarbonate membrane. Each value represents the mean ± SD of three separate experiments.
Competitive binding between hBD-2 and hBD-1 or MIP-3α to neutrophils
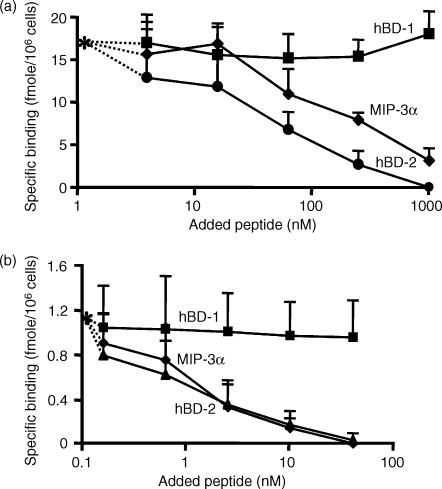
It has been reported that hBD-1 and hBD-2 share the same functional receptor, CCR6, in inducing the migration of dendritic cells and T cells.18 Furthermore, MIP-3α, a sole chemokine ligand for CCR6,30 has been recently reported to chemoattract TNF-α-treated neutrophils through this receptor.29 Thus, we sought to determine whether CCR6 is a receptor for hBD-2 on neutrophils by performing competitive binding assays between 125I-hBD-2 and unlabelled hBD-1 or MIP-3α. As shown in Fig. 3(a), the binding of 125I-hBD-2 to neutrophils was competitively and completely inhibited by the addition of increasing amounts of unlabelled hBD-2, and unlabelled MIP-3α could substantially suppress this binding (at approximately 80%). Although hBD-1 showed a very weak chemotactic activity against neutrophils, as shown above in Fig. 2, this peptide could not compete with the binding of hBD-2 at all. Furthermore, we evaluated the competitive binding of 125I-MIP-3α on neutrophils. Figure 3(b) shows that hBD-2 completely inhibited the binding of 125I-MIP-3α, as did unlabelled MIP-3α. As expected, hBD-1 could not inhibit the binding of MIP-3α. Our results suggest that hBD-2 and MIP-3α share CCR6 as their main receptor on neutrophils; however, neutrophils may possess an additional receptor(s) for hBD-2, which would explain the incomplete inhibition of the binding of hBD-2 by MIP-3α, as shown in Fig. 3(a).
Figure 3.
Competitive binding of hBD-1, hBD-2 and MIP-3α on neutrophils. TNF-α-treated neutrophils were incubated in RPMI 1640 containing 1% heat-inactivated BSA with 125I-hBD-2 for 30 min at 25° in the presence of 4–1000 nm unlabelled hBD-1 (▪), hBD-2 (•) or MIP-3α (⋄) (a). For the competition binding between 125I-MIP-3α and unlabelled peptides (b), hBD-1 (▪), hBD-2 (▴) or MIP-3α (⋄) was used at concentrations of 0·2–41 nm. The specific binding of 125I-hBD-2 without unlabelled peptide was 17·6 ± 4·6 fmol/106 cells, whereas that of 125I-MIP-3α was 1·17 ± 0·13 fmol/106 cells (*). Values are the mean ± SD of four separate experiments.
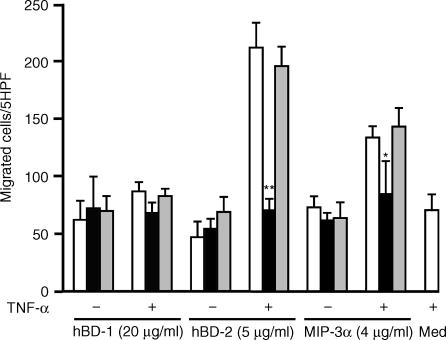
Effects of anti-CCR6 antibody on hBD-2-induced neutrophil chemotaxis
Further, the effects of anti-CCR6 antibody on hBD-2-induced neutrophil chemotaxis were examined. Figure 4 indicates that, when TNF-α-treated neutrophils were incubated with anti-CCR6 antibody (50 µg/ml), hBD-2-induced chemotaxis was almost completely inhibited. This inhibitory effect was also observed for MIP-3α-stimulated neutrophils, used as a positive control. Interestingly, a slight but not significant decrease was observed for migration towards hBD-1. The control IgG had no effect on cell migration. These findings confirm that hBD-2 utilizes CCR6 as a functional receptor to chemoattract neutrophils. The inhibitory activity of anti-CCR6 antibody was specific to both hBD-2 and MIP-3α, as the treatment of neutrophils with this antibody did not affect fMLP-induced chemotaxis (data not shown).
Figure 4.
Inhibition of hBD-2-induced neutrophil chemotaxis by anti-CCR6 antibody. Neutrophils treated with 2·5 ng/ml TNF-α (+) or untreated (–) were further incubated for 30 min with 50 µg/ml anti-CCR6 (hatched bars) or control IgG (closed bars), and then allowed to migrate towards 20 µg/ml hBD-1, 5 µg/ml hBD-2 and 4 µg/ml MIP-3α. Open bars represent neutrophils without anti-CCR6 or control IgG, and stippled bars represent cells treated with TNF-α and used for the chemotaxis assay without peptides (med, medium alone). Values are compared for neutrophils with and without anti-CCR6 antibody (* P < 0·05; ** P < 0·005). Each bar represents the mean ± SD of four separate experiments.
Effects of PTx and U-73122 on hBD-2-induced chemotaxis
To investigate possible pathways for hBD-2 signalling in neutrophils, the effects of the Gi-type G protein inhibitor PTx and the PL C inhibitor U-73122 were examined. When TNF-α-treated neutrophils were incubated with various concentrations of PTx (25–200 ng/ml), a dose-dependent inhibition of hBD-2-induced chemotaxis was observed (Fig. 5). This confirms that hBD-2 utilizes Gi protein-coupled receptors to activate neutrophils. Further, U-73122 (0·1–10 µm) abolished the migration of TNF-α-treated neutrophils towards hBD-2 in a dose-dependent manner. Inhibition of hBD-2-induced neutrophil chemotaxis was unaffected by identical treatment with U-73343, an inactive analogue of U-73122 (Fig. 5) used as a control, showing that the inhibitory effect of U-73122 on hBD-2-mediated chemotaxis is specific. Together, these observations suggest the involvement of a G protein-coupled PL C pathway in the action of hBD-2 on neutrophils.
Figure 5.
Effects of PTx and U-73122 on hBD-2-induced chemotaxis. TNF-α-treated neutrophils were further incubated with PTx for 2 hr, and U-73122 or U-73343 for 1 hr at 37° at the indicated concentrations before the chemotaxis assay was performed using 5 µg/ml hBD-2 or medium alone without peptide (Med). Values are compared for neutrophils without and with PTx or U-73122 treatment (* P < 0·05; ** P < 0·005). Values are the mean ± SD of four separate experiments.
Effects of BAPTA-AM on hBD-2-induced chemotaxis
It is known that the activation of G protein-coupled PL C induces the generation of inositol 1,4,5 triphosphate (IP3) leading to Ca2+ mobilization from intracellular stores.31 Thus, we investigated whether hBD-2 could induce intracellular Ca2+ mobilization from TNF-α-treated/Fura-2 AM-loaded neutrophils. Unfortunately, for all concentrations tested, neither hBD-2 nor MIP-3α induced detectable Ca2+ mobilization from TNF-α-treated neutrophils, whereas fMLP did so (data not shown). Thus, we investigated whether intracellular Ca2+ was actually involved in hBD-2-induced neutrophil chemotaxis by treating cells with BAPTA-AM, an intracellular Ca2+ chelater. As shown in Fig. 6(a), the chemotactic activity of hBD-2 against neutrophils was suppressed by treatment with BAPTA-AM in both the presence and the absence of extracellular Ca2+ (P < 0·05). To exclude the possibility that the acetoxymethyl ester itself exerts a toxic effect on neutrophil chemotaxis, TNF-α-treated neutrophils were preincubated with 100 µm carboxy-SNARF-AM, an intracellular dye for measurement of intracellular pH.32 This treatment, unlike BAPTA-AM at the same concentration, did not affect hBD-2-induced cell migration (Fig. 6b), suggesting that the inhibitory effect of BAPTA-AM was attributable not to nonspecific inhibition of the acetoxymethyl ester, but to its chelating of intracellular Ca2+. This finding suggests that intracellular Ca2+ plays a crucial role in the induction of neutrophil chemotaxis by hBD-2, although this peptide was incapable of inducing detectable Ca2+ flux.
Figure 6.
Effects of BAPTA-AM on hBD-2-induced neutrophil chemotaxis. Neutrophils treated with 2·5 ng/ml TNF-α were further incubated with 100 µm BAPTA-AM (a) and 100 µm BAPTA-AM or SNARF-AM (b) for 30 min at 37°, washed, placed into the upper wells of the microchamber and allowed to migrate towards hBD-2 (5 µg/ml), MIP-3α (4 µg/ml) or fMLP (10−8 m). Values are compared for neutrophils without and with BAPTA-AM or SNARF-AM treatment (* P < 0·05). Values are the mean ± SD of four separate experiments.
Expression of CCR6 by neutrophils
We examined whether neutrophils could express CCR6 using fluorescence-activated cell sorter (FACS) and RT-PCR analysis. The expression of CCR6 could not be detected on TNF-α-treated or untreated neutrophils by FACS analysis (data not shown). This is probably attributable to the lower number of CCR6 receptors on the neutrophil surface. Interestingly, RT-PCR results on neutrophil mRNA showed that CCR6 transcripts were expressed in TNF-α-treated neutrophils but not in untreated neutrophils (Fig. 7).
Figure 7.
RT-PCR analysis of CCR6 mRNA expression. Total RNA (3 µg) isolated from 2·5 ng/ml TNF-α-treated [TNF-α(+)] or untreated neutrophils [TNF-α(–)] was analysed for expression of CCR6 mRNA by RT-PCR. Lanes 1 and 3 are control reactions where reverse transcriptase was omitted before subjection to PCR. Lanes 2 and 4 represent reactions using cDNA after RT of samples from TNF-α-untreated and -treated cells, respectively. Arrowheads show the anticipated molecular sizes of PCR products of CCR6 (1021 bp) and GAPDH (447 bp).
Discussion
Neutrophils constitute more than half of the circulating white blood cells in humans and are the predominant inflammatory cells initially recruited to sites of infection or inflammation. In response to stimuli, they immediately migrate to infected or inflamed tissues.
The current study was designed to investigate whether epithelial cell-derived antimicrobial peptides could chemoattract human neutrophils, which would demonstrate that, in addition to their microbicidal activity, antimicrobial peptides could contribute to host defence by recruiting neutrophils to sites of inflammation. We showed that hBD-2 is a potent and specific chemoattractant for TNF-α-treated neutrophils through the G protein-PL C-dependent pathway, and CCR6 is one of the functional receptors involved in this activation.
Reports of the effects of TNF-α on neutrophil chemotaxis have been conflicting. Some investigators have indicated that TNF-α is chemotactic for neutrophils at concentrations as low as 100 units/ml,33 while other investigators were unable to demonstrate TNF-α chemotactic activity.34 Other studies showed that preincubation of neutrophils with TNF-α inhibited chemotaxis to other chemoattractants.35 In our study, TNF-α itself did not induce significant migration of neutrophils at any of the concentrations tested (20–200 units/ml). At those concentrations, the treatment of cells with TNF-α enhanced hBD-2- as well as MIP-3α-induced cell migration in a dose-dependent manner. These findings were in accordance with those of a recent study showing the enhancement of MIP-3α-induced neutrophil migration by TNF-α.29
While the precise mechanism of TNF-α priming of neutrophils is unclear, it may involve alteration of ligand–receptor coupling. Changes in both fMLP receptor number and affinity have been previously proposed: an increase in receptor numbers was demonstrated using nanomolar concentrations of TNF-α,36 whereas picomolar concentrations produced a change in the affinity of fMLP receptors.37 The same phenomenon was reported in a study showing that TNF-α increases the number and affinity of the C5a receptor on neutrophils.38 In this study, we observed that TNF-α treatment increased the binding of 125I-hBD-2 to neutrophils (data not shown) and increased expression of CCR6 mRNA transcripts, which is consistent with the finding that TNF-α enhanced hBD-2-induced neutrophil migration.
In this study, the competitive binding assays indicated that hBD-2 could completely inhibit the binding of MIP-3α, a sole ligand for CCR6, on neutrophils. Furthermore, the addition of MIP-3α to upper chambers inhibited cell migration towards hBD-2, and anti-CCR6 antibody could almost completely suppress the chemotactic activity of hBD-2. These observations suggest that hBD-2 probably utilizes CCR6 as a functional receptor.
We could detect neither MIP-3α-induced intracellular Ca2+ mobilization (data not shown) nor expression of CCR6 on neutrophils by FACS analysis; this was probably attributable to the low number of CCR6 receptors on the surface of the neutrophils. However, MIP-3α could induce neutrophil migration. Similarly, hBD-2 could not induce detectable intracellular Ca2+ mobilization, but enhanced neutrophil migration. Interestingly, we could only detect the expression of CCR6 transcripts in TNF-α-treated neutrophils by employing RT-PCR. We tried to reveal the presence of the receptors for hBD-2 on neutrophils by performing Scatchard analysis. However, we could detect specific binding for 125I-hBD-2 125I-MIP-3α at 25° but not at 4° (data not shown). These observations suggest that the binding is influenced by factors such as receptor internalization and recycling, which occur during assays at 25°.
The chemotactic activity of hBD-2 was inhibited by both PTx and U-73122, indicating that the G-protein-PL C pathway is involved in hBD-2-induced neutrophil migration. Because signalling by PL C results in the generation of IP3, leading to the mobilization of Ca2+ from intracellular stores, it was of interest to investigate the role of intracellular Ca2+ in the activity of hBD-2. Our results showed that, although hBD-2 was unable to induce detectable Ca2+ flux, intracellular Ca2+ is important for the activity of this peptide, because when cells were treated with an intracellular Ca2+ chelater, BAPTA-AM, neutrophil migration towards hBD-2 was almost completely abolished. However, the role of cytosolic Ca2+ in neutrophil chemotaxis remains controversial and difficult to understand. Conflicting studies have reported the intracellular and extracellular Ca2+ requirements for neutrophil chemotaxis. However, several studies have shown that the chelation of extracellular or intracellular Ca2+ inhibits the migration of neutrophils.39–41 Marks and Maxfield revealed that the treatment of human neutrophils with BAPTA-AM caused inhibition of cell migration.41 In addition, U-73122 has been reported to suppress fMLP-induced neutrophil chemotaxis.42 However, some investigators have proposed that cytosolic Ca2+ and the PL C pathway are not required for chemotaxis in neutrophils.43,44 Further work is necessary to clarify discrepancies in the role of cytosolic Ca2+ in neutrophil migration.
The lifespan of infiltrating neutrophils is much longer than that of circulating neutrophils. After the onset of an inflammatory reaction, large numbers of neutrophils are still present at the inflamed site long after the cessation of neutrophil influx.45 These infiltrating cells are activated by a wide variety of stimuli, including chemotactic factors and cytokines. Neutrophils express various chemokine receptors, and these receptors play a role in the mobilization of neutrophils.46 For instance, the expression of CC-chemokine receptors involved in neutrophil migration, including CCR1, CCR2, CCR3, CCR5 and CCR6,27,47,48 has been reported to be up-regulated in activated neutrophils. Despite the apparent induction of functional CCR6 on TNF-α-treated neutrophils,29 the biological significance of CCR6 expression in neutrophils was poorly understood. The present study revealed that hBD-2 was able to exert chemotactic activity against neutrophils, but failed to induce other neutrophil functions such as superoxide generation (data not shown).
The antibacterial peptide hBD-2 is highly concentrated in human epithelial tissues. It has been estimated at 2·3 µm in airway surface liquid,49 ∼16 µm in IL-1α-stimulated epidermis,50 and ∼157 µm in psoriatic skin lesions.51 Assuming that the expression of hBD-2 is increased at infection or inflammation sites, hBD-2 may potentially reach its optimal chemotactic concentration to chemoattract cytokine-primed neutrophils.
As neutrophils are known to participate in innate and adaptive immunity, our finding that hBD-2 is a potent chemoattractant of human neutrophils through specific receptors provides a novel mechanism by which this peptide contributes to host defence, by recruiting neutrophils to inflammation or infection sites. The current study also suggests an important link between epithelial cell-derived antibacterial peptides and neutrophils.
Acknowledgments
This work was supported in part by grants from the Promotion and Mutual Aid Corporation for Private Schools of Japan, the Atopy (Allergy) Research Center, Juntendo University, Tokyo, Japan, and the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
References
- 1.Lehrer IR, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11:23–7. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 2.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- 4.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–9. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 5.Lehrer RI, Ganz T, Selsted ME. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991;64:229–30. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- 6.Fulton C, Anderson GM, Zasloff M, Bull R, Quinn AG. Expression of natural peptide antibiotics in human skin. Lancet. 1997;350:1750–1. doi: 10.1016/S0140-6736(05)63574-X. [DOI] [PubMed] [Google Scholar]
- 7.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–60. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 8.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr, Ganz T. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–42. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 10.Schröder JM. Epithelial peptide antibiotics. Biochem Pharmacol. 1999;57:121–34. doi: 10.1016/s0006-2952(98)00226-3. [DOI] [PubMed] [Google Scholar]
- 11.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson JM. Human β-defensin-2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–80. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harder J, Bartels J, Christophers E, Schröder JM. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 13.García JRC, Jaumann F, Schultz S, et al. Identification of a novel, multifunctionnal β-defensin (human β-defensin 3) with specific antimicrobial activity. Cell Tissue Res. 2001;306:257–64. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- 14.García JRC, Krause A, Schultz S, et al. Human β-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–21. [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Nagase T, Makita R, et al. Identification of multiple novel epididymis-specific β-defensin isoforms in humans and mice. J Immunol. 2002;169:2516–23. doi: 10.4049/jimmunol.169.5.2516. [DOI] [PubMed] [Google Scholar]
- 16.Lehrer RI, Ganz T, Selsted ME, Babior BM, Curnutte JT. Neutrophils and host defense. Ann Intern Med. 1988;109:127–42. doi: 10.7326/0003-4819-109-2-127. [DOI] [PubMed] [Google Scholar]
- 17.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 18.Yang D, Chertov O, Bykovskaia SN, et al. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 19.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human β-defensin-2 acts as chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol. 2002;14:421–6. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 20.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang HJ, Ross CR, Blecha F. Chemoattractant properties of PR-39, a neutrophil antibacterial peptide. J Leukoc Biol. 1997;61:624–9. doi: 10.1002/jlb.61.5.624. [DOI] [PubMed] [Google Scholar]
- 23.Nagaoka I, Hirata M, Sugimoto K, Tsutsumi-Ishii Y, Someya A, Saionji K, Igari J. Evaluation of the expression of human CAP18 gene during neutrophil maturation in the bone marrow. J Leukoc Biol. 1998;64:845–52. doi: 10.1002/jlb.64.6.845. [DOI] [PubMed] [Google Scholar]
- 24.Yomogida S, Nagaoka I, Saito K, Yamashita T. Evaluation of the effects of defensins on neutrophil functions. Inflamm Res. 1996;45:62–7. doi: 10.1007/BF02265117. [DOI] [PubMed] [Google Scholar]
- 25.Munson PJ, Rodbard D. LIGAND. A versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–39. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 26.Dieu M-C, Vanbervliet B, Vicari A, et al. Selective recruitment of immature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsutsumi-Ishii Y, Nagaoka I. Modulation of human β-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J Immunol. 2003;170:4226–36. doi: 10.4049/jimmunol.170.8.4226. [DOI] [PubMed] [Google Scholar]
- 28.Bajaj MS, Kew RR, Webster RO, Hyers TM. Priming of human neutrophil functions by tumor necrosis factor. Enhancement of superoxide anion generation, degranulation, and chemotaxis to chemoattractants C5a and F-Met-Leu-Phe. Inflammation. 1992;16:241–50. doi: 10.1007/BF00918813. [DOI] [PubMed] [Google Scholar]
- 29.Yamashiro S, Wang JM, Yang D, Gong WH, Kamohara H, Yoshimura T. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958–63. [PubMed] [Google Scholar]
- 30.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mousli M, Hugli TE, Landry Y, Bronner C. Peptidergic pathway in human skin and rat peritoneal mast cell activation. Immunopharmacology. 1994;27:1–11. doi: 10.1016/0162-3109(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 32.Elsner J, Dichmann S, Dobos GJ, Kapp A. Actin polymerization in human eosinophils, unlike human neutrophils, depends on intracellular calcium mobilization. J Cell Physiol. 1996;167:548–55. doi: 10.1002/(SICI)1097-4652(199606)167:3<548::AID-JCP18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Figari IS, Mori NA, Palladino MA., Jr Regulation of neutrophil migration and superoxide production by recombinant tumor necrosis factor-α and -β: comparison to recombinant interferon-α and interleukin-1α. Blood. 1987;70:979–84. [PubMed] [Google Scholar]
- 34.Newman I, Wilkinson PC. Chemotactic activity of lymphotoxin and tumor necrosis factor alpha for human neutrophils. Immunology. 1989;66:318–20. [PMC free article] [PubMed] [Google Scholar]
- 35.Mrowietz V, Schroder JM, Christophers E. Recombinant tumor necrosis factor alpha lacks chemotactic activity for human peripheral blood neutrophils and monocytes. Biochem Biophys Res Commun. 1988;153:1223–8. doi: 10.1016/s0006-291x(88)81358-5. [DOI] [PubMed] [Google Scholar]
- 36.Tenneberg SD, Solomkin JS. Activation of neutrophils by cachectin/tumor necrosis factor. Priming of N-formyl-methionyl-leucyl-phenylalanine-induced oxidative responsiveness via receptor mobilization without degranulation. J Leukoc Biol. 1990;47:217–23. doi: 10.1002/jlb.47.3.217. [DOI] [PubMed] [Google Scholar]
- 37.Atkinson YH, Marasco WA, Lopez AF, Vadas MA. Recombinant human tumor necrosis factor-α. Regulation of N-formyl-methionyl-leucyl-phenylalanine receptor affinity and function on human neutrophils. J Clin Invest. 1988;81:759–65. doi: 10.1172/JCI113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger M, Wetzler ME, Wallis RS. Tumor necrosis factor is the major monocyte product that increases complement receptor expression on mature human neutrophils. Blood. 1988;71:151–8. [PubMed] [Google Scholar]
- 39.Elferink JGR, Deierkauf M. The effect of quin2 on chemotaxis by polymorphonuclear leukocytes. Biochim Biophys Acta. 1985;846:364–9. doi: 10.1016/0167-4889(85)90007-2. [DOI] [PubMed] [Google Scholar]
- 40.Meshulam T, Proto P, Diamond RD, Melnick DA. Calcium modulation and chemotactic response: divergent stimulation of neutrophil chemotaxis and cytosolic calcium response by the chemotactic peptide receptor. J Immunol. 1986;137:1954–60. [PubMed] [Google Scholar]
- 41.Marks PW, Maxfield FR. Transient increases in cytosolic free calcium appear to be required for the migration of adherent human neutrophils. J Cell Biol. 1990;110:43–52. doi: 10.1083/jcb.110.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferretti ME, Nalli M, Biondi C, Calamussi ML, Pavan B, Traniello S, Spisani S. Modulation of neutrophil phospholipase C activity and cyclic AMP levels by fMLP-OMe analogues. Cell Signal. 2001;13:233–40. doi: 10.1016/s0898-6568(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Jiang H, Xie W, Zhang Z, Smarcka AV, Wu D. Roles of PLC-β2 and β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science. 2000;287:1046–9. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 44.Perez HD, Elfman F, Marder S, Lobo E, Ives HE. Formyl peptide-induced chemotaxis of human polymorphonuclear leukocytes does not require either marked changes in cytosolic calcium or specific granule discharge. Role of formyl peptide receptor reexpression (or recycling) J Clin Invest. 1989;83:1963–70. doi: 10.1172/JCI114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coxon A, Tang T, Mayadas N. Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo: a role for granulocytes/macrophage colony-stimulating factor. J Exp Med. 1999;190:923–34. doi: 10.1084/jem.190.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashiro S, Kamohara H, Wang JM, Yang D, Gong WH, Yoshimura T. Phenotypic and functional change of cytokine-activated neutrophils: inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J Leukoc Biol. 2001;69:698–704. [PubMed] [Google Scholar]
- 47.Bonecchi R, Polentarutti N, Luini W, et al. Up-regulation of CCR1 and CCR3 and induction of chemotaxis to CC chemokines by IFN-gamma in human neutrophils. J Immunol. 1999;162:474–9. [PubMed] [Google Scholar]
- 48.Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest. 1999;103:1269–76. doi: 10.1172/JCI5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 50.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, Ganz T. Human β-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentation. J Invest Dermatol. 2002;118:275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 51.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DYM. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]