Abstract
The production of inflammatory cytokines from macrophages (Mφ), upon stimulation with plasmid DNA (pDNA) containing CpG motifs, is a critical process for DNA-based therapies such as DNA vaccination and gene therapy. We compared Mφ activation, following stimulation with naked pDNA, based on the production of cytokines from cell lines (RAW264.7 and J774A1) and peritoneal Mφs in primary culture. The Mφ cell lines RAW264.7 and J774A1 produced a significant amount of tumour necrosis factor-α (TNF-α) upon stimulation with naked pDNA and this response required endosomal acidification. On the other hand, peritoneal Mφs (both resident and elicited) in primary culture did not secrete TNF-α or interleukin-6, although they contain the mRNA of toll-like receptor-9 (TLR-9) and are able to respond to CpG oligodeoxynucleotides. This unresponsiveness was not a result of impaired cellular uptake of pDNA because the primary cultured Mφs showed a higher uptake of pDNA than the RAW264.7 and J774A1 cell lines. These findings have important implications for Mφ activation by naked pDNA as it has been generally assumed that pDNA that contains CpG motifs is a potent agent for inducing inflammatory cytokines in vivo, based on evidence from in vitro studies using Mφ cell lines.
Introduction
Bacterial DNA and plasmid DNA (pDNA) contain a relatively high frequency of unmethylated CpG dinucleotides (CpG motifs), which are suppressed and methylated in vertebrate DNA. The immune system has evolved a defence mechanism based on the recognition of these CpG motifs.1,2 For example, bacterial DNA and CpG oligodeoxynucleotides (ODN) can activate cells of the innate immune system, such as macrophages (Mφs) and dendritic cells (DCs), to secrete pro-inflammatory cytokines, including tumour necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6 and IL-12.3–7 This phenomenon appears to be advantageous as far as DNA vaccination is concerned8 because it is crucial in the subsequent development of T-helper 1 (Th1)-biased T-cell lineages in response to CpG DNA.9–11 On the other hand, recent reports have demonstrated that these inflammatory cytokines inhibit transgene expression with pDNA.12,13 Therefore, cytokine production from Mφs upon stimulation with pDNA containing CpG motifs is a critical process in the application of DNA-based therapies, such as DNA vaccination and gene therapy.
Recently it was reported that Toll-like receptor-9 (TLR-9), expressed in Mφs and DCs, recognizes the CpG motifs.14,15 As a first cytosolic event, the adaptor molecule MyD88 is recruited to the receptor complex, followed by engagement of IL-1 receptor-associated kinase (IRAK) and the adapter molecule, TRAF6.16 Oligomerization of TRAF6 leads to the activation of downstream kinases, such as the stress kinase JNK1/2 and the IκB kinase (IKK) complex.17 This, in turn, results in activation of transcription factors such as AP-1 and nuclear factor (NF)-κB. Proof of these phenomena has been obtained using synthetic phosphorothioate CpG ODN (S-ODN), small single-stranded DNA. It is generally assumed that the same mechanism would be involved in cellular activation by pDNA because its backbone contains similar immunostimulatory sequences. In fact, a variety of studies have demonstrated that CpG motifs are required to stimulate immune responses following DNA vaccination.
Although there is a growing body of information reporting Mφ activation by CpG DNA, most studies have been performed using Mφ cell lines derived from the mouse. Few studies have been reported using primary cultured Mφs freshly isolated from animals, which would be a better model than immortalized cells lines as far as reflecting the in vivo situation is concerned.
Previously we have demonstrated that Kupffer cells (liver-resident Mφs) play an important role in the processing of pDNA after intravenous injection into mice.18 In vitro experiments, using mouse peritoneal resident Mφs, have also shown that primary cultured Mφs take up pDNA efficiently via a scavenger receptor-like mechanism and in a specific manner.19,20 In the present study, we evaluated Mφ activation, following stimulation with naked pDNA, using mouse peritoneal resident and elicited cultured Mφs in comparison with the Mφ cell lines RAW264.7 and J774A1. We found that the peritoneal Mφs showed very low or almost no secretion of inflammatory cytokines following stimulation with pDNA, in spite of extensive uptake of CpG DNA. These results contrast with our recent finding that peritoneal Mφs and RAW264.7 cells show similar responsiveness to pDNA complexed with cationic liposomes.21
Materials and methods
Chemicals
RPMI-1640, Dulbecco's modified Eagle's minimal essential medium (DMEM), Hanks' balanced salt solution (HBSS) and thioglycolate broth were obtained from Nissui Pharmaceutical (Tokyo, Japan). Triton X-114 was purchased from Nacalai Tesque (Kyoto, Japan).
Cell cultures
Male ICR mice (5 weeks of age) were purchased from the Shizuoka Agricultural Cooperative Association for Laboratory Animals (Shizuoka, Japan). Resident Mφs were collected (in RPMI-1640) from the peritoneal cavity of unstimulated mice. Cells were washed, suspended in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), penicillin G (100 U/ml), streptomycin (100 µg/ml) and amphotericin B (1·2 µg/ml), and then plated on 24-well culture plates (Falcon; Becton Dickinson, Lincoln Park, NJ), at a density of 5 × 105 cells/well, for the cellular association experiments and activation experiments. For confocal microscopic observations, peritoneal cells were plated on a cover glass, in a 12-well culture plate, at a density of 5 × 105 cells/well. After a 2-hr incubation at 37° in 5% CO2/95% air, adherent Mφs were washed three times with RPMI-1640 to remove non-adherent cells and then cultured under the same conditions for 24 hr. For elicited Mφs, all the processes were the same, except that 1 ml of 2·9% thioglycolate broth was injected intraperitoneally into mice 4 days prior to the collection of Mφs. RAW264.7 or J774A1 cells were cultured in RPMI-1640 supplemented with 10% FBS, penicillin G (100 U/ml) and streptomycin (100 µg/ml). They were then plated on a 24-well culture plate at a density of 2·5 × 105 cells/ml and cultured for 24 hr.
Plasmid DNA
The vector pcDNA3 was purchased from Invitrogen (Carlsbad, CA). The vector pCMV-Luc, encoding the firefly luciferase gene, was constructed as described previously.22 pcDNA3 contains 26 5′-Pur-Pur-CpG-Pyr-Pry-3′ sequences, including two GACGTT sequences which have been reported to be the most potent sequences for mice.7 For the cellular-association experiment, pCMV-Luc was radiolabelled with [α-32P]dCTP by nick translation.23 The reaction was performed on 1 µg of pDNA (pCMV-Luc) in a final volume of 40 µl. The incubation buffer was 50 mm Tris–HCl, pH 7·8, 10 mm MgCl2, 0·1 mm dithiothreitol (DTT) and 0·0025% bovine serum albumin (BSA). The reaction was initiated by adding 4 U of Escherichia coli (E. coli) DNA Polymerase I (Takara, Kyoto, Japan) and 0·0004 U of DNAse I (Takara) in the presence of 40 nm unlabelled triphosphate (dATP, dGTP, dTTP) and [α-32P]dCTP (3·7 MBq, 100 µCi). After a 2-hr incubation at 15°, the reaction was terminated by heating at 70° for 10 min. Unincorporated nucleotides were removed using a Sephadex G-50 column. For the confocal microscopy study, pCMV-Luc was labelled using a Fasttag Texas Red labelling kit, according to the manufacturer's instructions (Vector Laboratories, Burlinghame, CA).
Purification of pDNA
To minimize any activation as a result of contaminating lipopolysaccharide (LPS), we used DNA samples extensively purified with Triton X-114, a non-ionic detergent. Extraction of endotoxin from pDNA, methylated-CpG pDNA, E. coli DNA and calf thymus DNA samples was performed according to previously published methods,24,25 with slight modifications. DNA samples were purified by extraction with phenol–chloroform–isoamyl alcohol (25 : 24 : 1; v/v/v) and ethanol precipitation. Ten micrograms of DNA was diluted with 20 ml of pyrogen-free water, then 200 µl of Triton X-114 was added, followed by mixing. The solution was placed on ice for 15 min and then incubated for 15 min at 55°. Subsequently, the solution was centrifuged (20 min, 25°, 600 g). The upper phase was transferred to a new tube, 200 µl of Triton X-114 was added and the previous steps were repeated three or more times. The activity of LPS was measured by the Limulus amebocyte lysate (LAL) assay using the Limulus F Single Test kit (Wako, Tokyo, Japan). After purification using the Endo-free™ plasmid Giga kit, 1 µg/ml pDNA was found to contain 0·01–0·05 endotoxin units (EU)/ml. After extraction using Triton X-114, the endotoxin levels of DNA samples could no longer be determined by the LAL assay, i.e. 1 µg/ml DNA contained less than 0·001 EU/ml. Without extraction of endotoxin by Triton X-114, 100 µg/ml naked pDNA, containing 1–5 EU/ml, was able to stimulate the release of 521 ± 73 pg/ml TNF-α at 24 hr.
ODN
Phosphorothioate ODN was purchased from GENSET (Paris, France). The sequence of CpG S-ODN 1668 (a proven activator of murine immune cells, as previously described) is 5′-TCCATGACGTTCCTGATGCT-3′.5,26 Phosphorothioate non-CpG ODN 1720 (5′-TCCATGAGCTTCCTGATGCT-3′) was used as a control. CG motifs and control GC sequences are underlined. Fluorescein isothiocyanate (FITC)-labelled CpG ODN was purchased from Sawady Technology (Tokyo, Japan).
Reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted from cells using TRIzol (Invitrogen). Five micrograms of RNA was reverse-transcribed to complementary DNA using the SUPERSCRIPT First-Strand Synthesis System for RT–PCR (Invitrogen). Fragments were amplified with Taq polymerase (Takara) using the following primer pairs: mTLR-9, 5′-CCGCAAGACTCTATTTGTGCTGG-3′ and 5′-TGTCCCTAGTCAGGGCTGTACTCAG-3′,15 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TCACCATCTTCCAGGAGCGA-3′ and 5′-ACCAGGAAATGAGCTTGACA-3′. The PCR temperature and cycles were as follows: 60 seconds at 95°, 90 seconds at 56° and 30 seconds at 72°, for 30 cycles. The size of the mTLR-9 and GAPDH products were 260 bp and 720 bp, respectively.
For the fragmentation or denaturation of DNA, pDNA were diluted in endotoxin-free water, to a concentration of 1 mg/ml in a 0·5 ml tube, and sonicated for 2 hr in an ultrasonic automatic washer (Iuchi, Tokyo, Japan). Separately, DNA was heat-denatured at 95° for 10 min. DNA was incubated at 4° before use.
Cytokine secretion
Mouse Mφs, resident and elicited peritoneal Mφs from ICR mice, and the Mφ cell lines, RAW264.7 and J774A1, were washed three times with 0·5 ml of RPMI-1640 before use. Naked DNA was diluted in 0·5 ml of Opti-MEM and the cells were incubated for 8 hr with this solution. Then, the cells were washed with RPMI-1640 and incubated with RPMI-1640 containing 10% FBS, continuously, for specified time-periods of up to 48 hr. In the inhibition experiments, cells were incubated with the medium containing an inhibitor alone, at various concentrations, for 30 min, then incubated with the medium containing DNA or liposome formulations together with the inhibitor. At the indicated time-points, the supernatants were collected for enzyme-linked immunosorbent assay (ELISA) and stored at −80°. The levels of TNF-α and IL-6 in the supernatants were determined by using the OptEIA™ set (Pharmingen, San Diego, CA). The detection limit of these sets was 15·6 pg/ml for both cytokines.
Cellular-association experiments
Mouse peritoneal Mφs and RAW264.7 cells were cultured in 24-well plates. The cells were washed twice with HBSS (without phenol red) and then incubated, at 37°, with HBSS containing trypsin (50 µg/ml). Then, the cells were washed with HBSS and incubated with HBSS containing 32P-labelled pDNA (0·1 µg/ml). After a 3-hr incubation at 4°, the cells were washed three times with ice-cold HBSS and then solubilized with 1·0 ml of 0·3 N NaOH containing 0·1% Triton-X-100. Aliquots were taken for the determination of 32P radioactivity using an LSA-500 scintillation counter (Beckman, Tokyo, Japan). The protein content was measured using the modified Lowry method27 with BSA as a standard.
Trichloroacetic acid (TCA) precipitation experiments
After the cellular-association experiments, the medium and cell lysate containing radioactivity derived from 32P-labelled pDNA were subjected to TCA-precipitation experiments to assess the degradation of pDNA by Mφs. A portion of the cell lysate was directly subjected to radioactivity counting and the protein content was measured as described above. The cell lysis solution was neutralized with 1 N HCl, and the same volume of 10 mM Tris-HCl, 1 mM EDTA (TE)-saturated phenol (pH 7·8) was added. The mixture was vortexed and centrifuged at 13 500 g for 10 min. The upper phase was transferred to another tube to which the same volume of ice-cold 10% TCA was added, and the tube was vortexed. After a 10-min incubation on ice, the solution was centrifuged at 13 500 g for 25 min. Supernatants were used for the determination of radioactivity. To quantify the amount of free [α-32P]dCTP in 32P-labelled pDNA, TCA precipitation of a standard sample was performed.
Confocal microscopy
Cells were washed three times with 1·0 ml of HBSS and then incubated, in HBSS, at 4° for 10 min. The cells were then incubated with HBSS containing Texas Red-labelled pDNA and FITC-labelled CpG S-ODN at 4° for 30 min. The cells were washed five times with HBSS and incubated at 37° for 15 min. Then, the cells were fixed with 4% paraformaldehyde for 10 min. The cells were scanned by confocal microscopy (MRC-1024; Bio-Rad, Hercules, CA).
Results
Peritoneal Mφs do not secrete inflammatory cytokines upon stimulation with naked pDNA
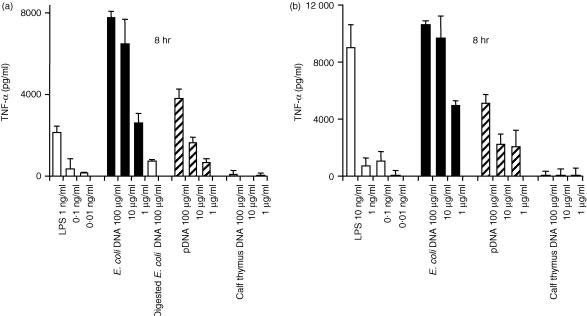
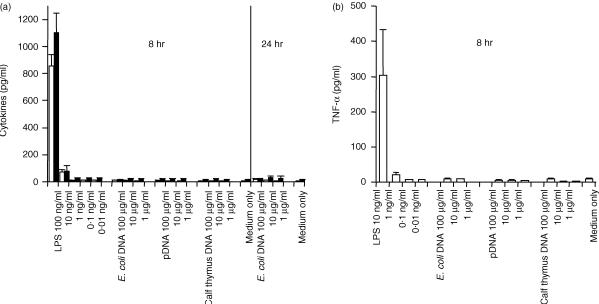
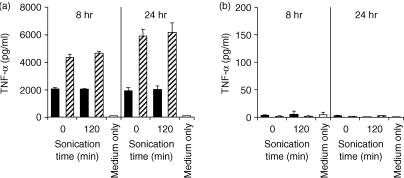
Secretion of inflammatory cytokines, induced by CpG DNA, was examined using mouse Mφ cell lines and peritoneal Mφs. E. coli DNA and pDNA were models of CpG DNA, and calf thymus DNA was used as non-CpG DNA. DNA was extensively purified with phenol and Triton X-114 to avoid contamination with LPS and some bacterial proteins. Two different cell lines – RAW264.7 and J774A1 – secreted a large amount of TNF-α, upon stimulation with naked E. coli DNA and pDNA, in a concentration-dependent manner (Fig. 1). Calf thymus DNA was unable to induce the secretion of TNF-α. Digested E. coli DNA was unable to stimulate the secretion of TNF-α from RAW264.7 cells. These results indicated that, similarly to ODN, naked DNA also activates these Mφ cell lines to produce inflammatory cytokines in a CpG motif-dependent manner. Similar experiments were carried out using two types of mouse peritoneal Mφs – resident and elicited Mφs – isolated from male ICR mice. Surprisingly, CpG DNA, pDNA and E. coli DNA were unable to induce TNF-α release from either resident or elicited Mφs in primary culture, even at a high concentration (100 µg/ml DNA) for up to 24 hr (Fig. 2). Another inflammatory cytokine, IL-6, also failed to be detected in resident Mφs. LPS stimulated all types of Mφs to secrete TNF-α.
Figure 1.
Cytokine release induced by naked plasmid DNA (pDNA) or other DNAs from macrophage (Mφ) cell lines. RAW264.7 cells (a) or J774A1 cells (b) were incubated with lipopolysaccharide (LPS) (white bar), Escherichia coli (E. coli) DNA (black bar), pDNA (hatched bar), or calf thymus DNA (shaded bar) for 8 or 24 hr. The amount of cytokines released from the Mφs was quantified by enzyme-linked immunosorbent assay (ELISA). The concentration of cytokines present in medium only was subtracted from the cytokine concentration in each sample. Each result represents the mean and standard deviation (n = 3).
Figure 2.
Cytokine release induced by naked plasmid DNA (pDNA) or other DNAs from primary cultured macrophages (Mφs). Resident Mφs (a) or elicited Mφs (b) were incubated with lipopolysaccharide (LPS), Escherichia coli (E. coli) DNA, pDNA, or calf thymus DNA for 8 or 24 hr. The amount of tumour necrosis factor-α (TNF-α) (white bars) and interleukin-6 (IL-6) (black bars) released from the Mφs was quantified by enzyme-linked immunosorbent assay (ELISA). Each result represents the mean and standard deviation (n = 3).
Expression of TLR-9 mRNA and cytokine release induced by CpG DNA from peritoneal Mφs and RAW264.7 cells
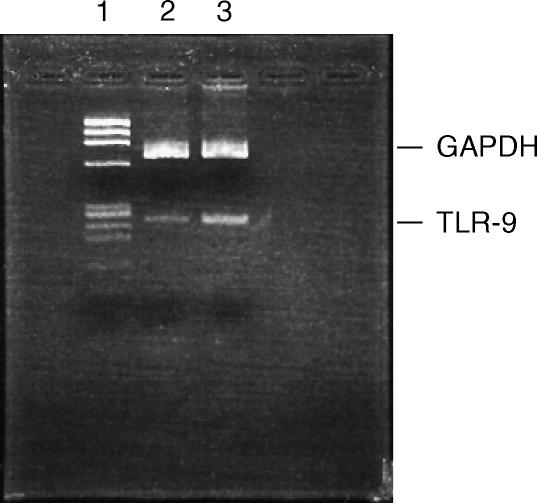
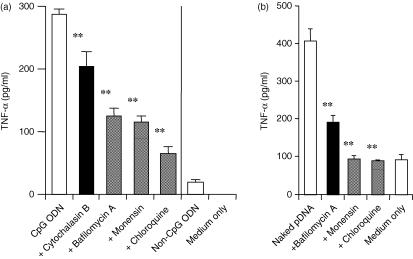
TLR-9 recognizes bacterial CpG motifs. To confirm the reactivity of peritoneal Mφs with CpG DNA, we tested the mRNA expression of TLR-9 in these cells by RT–PCR. Both resident peritoneal Mφs and RAW264.7 cells showed TLR-9 mRNA expression (Fig. 3). Moreover, CpG ODN 1668 was able to induce TNF-α from the resident Mφs (Fig. 4a). CpG DNA requires endocytosis and endosomal acidification to induce inflammatory responses.26,28,29 It was shown that endosomal acidification inhibitors, such as bafilomycin A, chloroquine or monensin, inhibited cytokine secretion by CpG ODN from RAW264.7 cells.28 Therefore, the effect of these inhibitors on cytokine release from resident Mφs was examined. The endocytosis inhibitor, cytochalasin B, inhibited the induction of TNF-α by CpG ODN from the resident Mφs. TNF-α secretion was also significantly reduced by these endosomal acidification inhibitors.
Figure 3.
Expression of Toll-like receptor-9 (TLR-9) mRNA in macrophages (Mφs). Total RNA was extracted from resident Mφs or RAW264.7 cells. mRNA expression of TLR-9 was determined by reverse transcription–polymerase chain reaction (RT–PCR). DNA mobility was analysed by agarose-gel electrophoresis (3·5% gel). Lane 1, Φ-HaeIII marker; lane 2, resident Mφs; lane 3, RAW264.7 cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 4.
Tumour necrosis factor-α (TNF-α) release induced by CpG oligodeoxynucleotides (ODN) or non-CpG ODN from resident macrophages (Mφs) (a) or plasmid DNA (pDNA) from RAW264.7 cells (b). (a) Resident Mφs were incubated with 10 µm CpG ODN or non-CpG ODN, in the presence or absence of inhibitors, at 37° for 8 hr. (b) RAW264.7 cells were incubated with 10 µg/ml CpG pDNA, in the presence or absence of inhibitors, at 37° for 2 hr. Each result represents the mean and standard deviation (n = 3). Differences in the cytokine levels of the samples treated with CpG ODN only and CpG ODN + inhibitors (bafilomycin A, cytochalasin B, chloroquine and monensin) were analysed statistically by using the Student's t-test. **P < 0·01.
In contrast to primary cultured Mφs, RAW264.7 cells and J774A1 cells could be stimulated by pDNA to produce TNF-α (Fig. 1). The effect of inhibitors of endocytosis or endosomal acidification on the cytokine responses induced in RAW264.7 cells was examined. Cytochalasin B inhibited the TNF-α release that was stimulated by pDNA. Monensin, bafilomycin A and chloroquine, inhibitors of endosomal acidification, suppressed the induction of TNF-α (Fig. 4), suggesting that similar mechanisms are involved in cytokine induction by pDNA in Mφs.
Cellular uptake and subsequent degradation of pDNA are not major factors in the unresponsiveness of peritoneal Mφs to pDNA
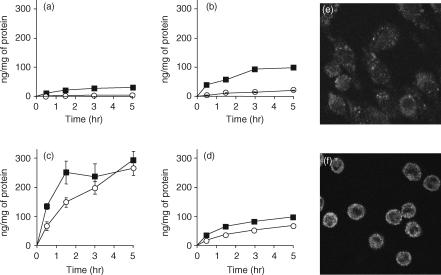
It has been reported that TLR-9 is an intracellular protein.30 To determine whether the difference in activation induced by CpG DNA (other than CpG S-ODN) is the result of a difference in the cellular uptake of DNA, we compared the degree of binding and uptake of 32P-labelled pDNA in all the Mφ types used in this study. The cellular association of 32P-labelled pDNA in the RAW264.7 and J774A1 cells was time- and temperature-dependent (Fig. 5). The resident and elicited Mφs showed a significantly higher cellular association of 32P-labelled pDNA at both 4° and 37° than observed in the RAW264.7 and J774A1 cell lines. In the confocal microscopy study, fluorescein-labelled pDNA was also taken up by both RAW264.7 cells and resident Mφs. The latter took up pDNA more efficiently (Fig. 5).
Figure 5.
Cellular association time-course experiments of 32P-labelled plasmid DNA (pDNA) in RAW264.7 cells (a), J774A1 cells (b), and resident (c) or elicited (d) macrophages (Mφs); and cellular localization of pDNA in RAW264.7 cells (e) or resident Mφs (f). (a–d) Cells were incubated with 32P-labelled pDNA (0·1 µg/ml) at 37° (closed square) or 4° (open circle). Each point represents the mean ± standard deviation (n = 3). (e and f) Cells were incubated at 4° for 30 min in the presence of 5 µg/ml Texas Red-labelled pDNA. After washing, the cells were warmed to 37° to allow internalization for 15 min. Images represent laser-scanning confocal microscopy sections.
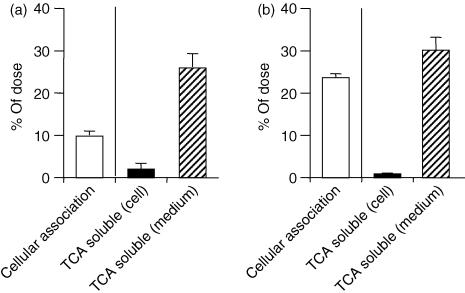
After endocytosis, DNA is degraded by deoxyribonuclease II (DNAse II) in the lysosomal compartment31 and this event may affect the recognition of CpG motifs by TLR-9 in Mφs. Therefore, the difference in degradation efficiency may account for the distinct responsiveness between primary Mφs and cell lines. To explore the effect of degradation of pDNA on immunoactivation, we measured the amount of 32P-labelled pDNA degraded by RAW264.7 cells and resident Mφs by the TCA precipitation method. The TCA-soluble degradation products will be small DNA fragments (short oligodeoxynucleotides), as 50% precipitation occurs with the 16-mer oligodeoxynucleotides.32 Both RAW264.7 cells and resident Mφs degraded the pDNA and released its degradation products into the medium to a similar extent (Fig. 6).
Figure 6.
Degradation of 32P-labelled plasmid DNA (pDNA) by RAW264.7 cells (a) or resident macrophages (Mφs) (b). The cells were incubated with 32P-labelled pDNA (0·1 µg/ml) at 37° or 4° for 3 hr. The degree of cellular association and degradation of 32P-labelled pDNA was measured by the trichloroacetic acid (TCA) precipitation method. Each result represents the mean and standard deviation (n = 6).
Single-stranded DNA or smaller DNA was unable to induce TNF-α production from peritoneal Mφs
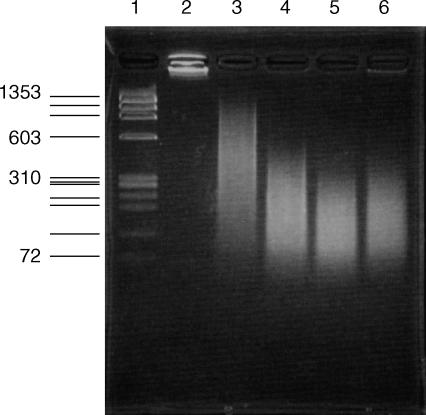
Resident peritoneal Mφs were found to induce inflammatory cytokines by a small single-stranded CpG ODN (Fig. 4a), although they were unable to produce TNF-α following stimulation with larger double-stranded pDNA (Fig. 1). E. coli DNA is often used after sonication and heat denaturation.3,5,7,33,34 We prepared small DNA fragments by sonication to examine whether the physicochemical properties of DNA affect the activation of Mφs. pDNA fragments were reduced to less than 600 bp after sonication for 120 min (Fig. 7). In addition, pDNA, or its fragments, were denatured at 90° to prepare single-stranded DNA. In RAW264.7 cells, the amount of TNF-α released by DNA fragments was almost the same as that released by intact pDNA (Fig. 8). On the other hand, heat-denatured pDNA significantly increased TNF-α production from the Mφ cell line, indicating that single-stranded DNA is a more potent cell activator. However, resident Mφs could not secrete TNF-α upon stimulation with small-size pDNA or heat-denatured pDNA.
Figure 7.
Electrophoresis of plasmid DNA (pDNA) fragments prepared by sonication. DNA mobility was analysed by agarose-gel electrophoresis (3·5% gel). Lane 1, Φ-HaeIII marker; lane 2, control pCMV-Luc; lanes 3–6, pCMV-Luc fragments produced by sonication for 30, 60, 90 and 120 min, respectively.
Figure 8.
Cytokine release induced by plasmid DNA (pDNA) fragments or single-stranded DNA from RAW264.7 cells (a) or resident macrophages (Mφs) (b). The cells were incubated with double-stranded DNA (black bar) or single-stranded DNA (hatched bar) for 8 hr. Cytokine release from Mφs was quantified by enzyme-linked immunosorbent assay (ELISA). Each result represents the mean and standard deviation (n = 6).
Discussion
The important role of immunostimulatory effects mediated by the CpG motif in gene therapy and DNA vaccination has been well defined. However, most in vitro studies, focusing on the mechanisms of Mφ activation mediated by CpG DNA, have been carried out using CpG ODN and bacterial genomic DNA in Mφ cell lines.28,35,36 Only a few reports have investigated immune responses induced by pDNA or bacterial DNA from Mφs or monocytes in primary culture.37–39
In the present study, we used Triton X-114 to remove LPS from DNA samples. Naked pDNA and E. coli genomic DNA containing unmethylated CpG motifs were able to stimulate the Mφ cell lines RAW264.7 and J774A1 to produce a significant amount of TNF-α (Fig. 2), while mammalian calf thymus DNA, which does not contain immunostimulatory CpG motifs, could not stimulate TNF-α production from these cell lines. Inhibitors of endocytosis and endosomal acidification prevented the pDNA-stimulated release of TNF-α from RAW264.7 cells (Fig. 4b). Therefore, it is probable that pDNA activates RAW264.7 cells to secrete cytokines by the same mechanism as that reported for CpG ODN.28
On the other hand, the resident and elicited Mφs from male ICR mice did not show any TNF-α or IL-6 induction by naked pDNA or E. coli DNA, even when exposed to a very high concentration of these DNAs (Fig. 1). Similar results were observed in the resident Mφs isolated from female ICR and male C3H/HeJ (LPS non-responders) and C3H/HeN mice (data not shown). Peritoneal Mφs primed by interferon-γ (IFN-γ) also showed similar results, although increased TNF-α secretion induced by LPS was observed in the primed cells (data not shown). This unresponsiveness of primary Mφs disagreed with previous reports.5,38 Bone marrow-derived Mφs can respond to pDNA,38 and only 1 µg/ml pDNA can induce NF-κB activation and TNF-α production in such Mφs. It may be that peritoneal Mφs and bone marrow-derived Mφs exhibit different responses to pDNA. Another report shows that DNA from Gram-positive Staphylococcus aureus can activate peritoneal Mφs of C3H/HeJ mice to produce TNF-α.5 S. aureus DNA may contain higher numbers or more potent CpG motifs than Gram-negative E. coli DNA or the pDNA that we used. Further studies are required to explain these contradictions. The very low, almost negligible, induction of cytokine upon stimulation with naked DNA was in complete contrast to our recent findings on DNA/cationic liposome complexes.21 Both the resident peritoneal Mφs and RAW264.7 cells secreted a large amount of TNF-α following incubation with pDNA and E. coli DNA complexed with LipofectAMINE plus. Also of note, similar cytokine induction was evoked in both resident peritoneal Mφs and RAW264.7 cells by the complexes prepared with calf thymus DNA and methylated-CpG pDNA, indicating that the Mφ activation was a CpG motif-independent process.
The restricted induction of cytokines by naked pDNA was not a result of the lack of TLR-9 expression in the peritoneal Mφs (Fig. 3), which is responsible for recognition of the bacterial CpG DNA.14,15 Indeed, the Mφ can release TNF-α in a CpG motif-dependent manner (Fig. 4), as previously reported.4,38 Therefore, the resident Mφs have the ability to respond to CpG DNA.
CpG DNA should be taken up by Mφs and, thereafter, be transported to the endosomal/lysosomal compartment for recognition by TLR-9.30 pDNA was efficiently taken up and degraded into smaller DNA fragments by the resident peritoneal Mφs (Figs 5 and 6). The pDNA degradation would be mediated by DNAse II in the lysosomal compartment of the Mφs.31,40,41 However, the apparent uptake efficiency of pDNA by the resident Mφs appeared to be higher that that of RAW264.7 cells. Therefore, the very low responsiveness to pDNA was not a result of impaired cellular uptake of the CpG DNA by the peritoneal Mφs.
In cellular activation experiments, E. coli DNA is often used after sonication and heat denaturation.3,5,7,33,34 Heat-denatured (single-stranded) E. coli DNA was 10–30% more mitogenic than double-stranded DNA as far as B cells were concerned.34 In fact, in the present study, RAW264.7 cells were stimulated to produce about twice as much TNF-α by single-stranded pDNA compared with double-stranded pDNA (Fig. 8a). On the other hand, DNA fragments prepared by sonication resulted in a similar level of TNF-α secretion compared with control pDNA. This was in agreement with a previous report that mycobaterial DNA fragments generated by digestion with restriction enzyme or the fragments prepared by sonication do not modify IL-12 induction by THP-1 monocytes.42 Heat-denatured single-stranded pDNA, which exhibited a greater ability to induce TNF-α in RAW264.7 cells, could not stimulate resident Mφs to induce inflammatory cytokines (Fig. 8a).
The findings of the present study suggest that peritoneal Mφs, or other Mφs such as Kupffer cells, may play an insignificant role in cytokine production through direct activation by naked pDNA in vivo. pDNA is efficiently taken up by the liver when it is injected into mice, and hepatic accumulation has been found to occur preferentially in the non-parenchymal cells, including Kupffer cells (liver Mφs).18 When mice are sensitized with d-galactosamine they suffer from lethal toxic shock as a result of TNF-α, induced by bacterial DNA, producing fulminant apoptosis of liver cells.5 However, a very large dose (300 µg/mouse) is required to produce this shock. Moreover, bacterial DNA alone is less toxic and cannot induce lethal shock without LPS or d-galactosamine treatment.43 These observations are in agreement with our speculation involving restricted cytokine production by direct pDNA stimulation from Mφs in vivo.
The restricted cytokine induction by naked pDNA also contrasts with our recent observation of DCs in culture. Significant production of inflammatory cytokines, such as TNF-α, IL-6 and IL-12, was induced by naked pDNA and E. coli DNA, but not by calf thymus DNA, from both bone marrow-derived DCs in primary culture and a DC cell line, DC2.4.44 Neither type of DC displayed CpG motif-independent cytokine secretion upon stimulation with DNA/cationic liposome complexes. Therefore, DCs, another important cell population for DNA-based therapies, show CpG motif-dependent cyokine production against pDNA, regardless of whether the cells are in primary culture or of a cell line. Mφs have features distinct from DCs in terms of cytokine induction by pDNA.
In conclusion, the present study demonstrated that primary cultured mouse peritoneal Mφs and Mφ cell lines exhibit significantly different responses to pDNA as far as inflammatory cytokine induction is concerned. In contrast to the cell lines, the peritoneal Mφs secreted almost no inflammatory cytokines (TNF-α, IL-6) upon stimulation with pDNA, in spite of extensive uptake of the CpG DNA. These findings have important implications for Mφ activation by naked pDNA in DNA-based therapies because it has been generally assumed that pDNA-containing CpG motifs are potent agents for inducing inflammatory cytokines in vivo based on information from in vitro studies using Mφ cell lines.
Acknowledgments
This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Sciences and Technology, Japan.
Abbreviations
- DC
dendritic cell
- FBS
fetal bovine serum
- IL
interleukin
- LPS
lipopolysaccharide
- Mφ
macrophage
- ODN
oligodeoxynucleotides
- pDNA
plasmid DNA
- TCA
trichloroacetic acid
- TLR
toll-like receptor
- TNF-α
tumour necrosis factor-α.
References
- 1.Krieg AM, Kline JN. Immune effects and therapeutic applications of CpG motifs in bacterial DNA. Immunopharmacology. 2000;48:303. doi: 10.1016/s0162-3109(00)00228-9. [DOI] [PubMed] [Google Scholar]
- 2.Wagner H. Immunobiology of Bacterial CpG-DNA. In: Wagner H, editor. Current Topics in Microbiology and Immunology. Vol. 247. Berlin: Springer; 1999. [Google Scholar]
- 3.Yi AK, Klinman DM, Martin TL, Matson S, Krieg AM. Rapid immune activation by CpG motifs in bacterial DNA. Systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J Immunol. 1996;157:5394. [PubMed] [Google Scholar]
- 4.Sparwasser T, Miethke T, Lipford G, Borschert K, Hacker H, Heeg K, Wagner H. Bacterial DNA causes septic shock. Nature. 1997;386:336. doi: 10.1038/386336a0. [DOI] [PubMed] [Google Scholar]
- 5.Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, Heeg K, Wagner H. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur J Immunol. 1997;27:1671. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 6.Krieg AM, Love HL, Yi AK, Harty JT. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161:2428. [PubMed] [Google Scholar]
- 7.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh M, O'Hagan D. Advances in vaccine adjuvants. Nat Biotechnol. 1999;17:1075. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 9.Weeratna RD, McCluskie MJ, Xu Y, Davis HL. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine. 2000;18:1755. doi: 10.1016/s0264-410x(99)00526-5. [DOI] [PubMed] [Google Scholar]
- 10.Raz E, Tighe H, Sato Y, et al. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman M, Martin OE, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 12.Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum Gene Ther. 1997;8:2019. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- 13.Ghazizadeh S, Carroll JM, Taichman LB. Repression of retrovirus-mediated transgene expression by interferons: implications for gene therapy. J Virol. 1997;71:9163. doi: 10.1128/jvi.71.12.9163-9169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 15.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacker H, Vabulas RM, Takeuchi O, Hoshino K, Akira S, Wagner H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF) 6. J Exp Med. 2000;192:595. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud V, Liu ZG, Bennett B, Suzuki N, Xia Y, Karin M. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawabata K, Takakura Y, Hashida M. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm Res. 1995;12:825. doi: 10.1023/a:1016248701505. [DOI] [PubMed] [Google Scholar]
- 19.Takagi T, Hashiguchi M, Mahato RI, Tokuda H, Takakura Y, Hashida M. Involvement of specific mechanism in plasmid DNA uptake by mouse peritoneal macrophages. Biochem Biophys Res Commun. 1998;245:729. doi: 10.1006/bbrc.1998.8521. [DOI] [PubMed] [Google Scholar]
- 20.Takakura Y, Takagi T, Hashiguchi M, et al. Characterization of plasmid DNA binding and uptake by peritoneal macrophages from class A scavenger receptor knockout mice. Pharm Res. 1999;16:503. doi: 10.1023/a:1018842210588. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda K, Ogawa Y, Kishimoto M, Takagi T, Hashida M, Takakura Y. Plasmid DNA activates murine macrophages to induce inflammatory cytokines in a CpG motif-independent manner by complex formation with cationic liposomes. Biochem Biophys Res Commun. 2002;293:344. doi: 10.1016/S0006-291X(02)00210-3. [DOI] [PubMed] [Google Scholar]
- 22.Nomura T, Yasuda K, Yamada T, Okamoto S, Mahato RI, Watanabe Y, Takakura Y, Hashida M. Gene expression and antitumor effects following direct interferon (IFN)-gamma gene transfer with naked plasmid DNA and DC-chol liposome complexes in mice. Gene Ther. 1999;6:121. doi: 10.1038/sj.gt.3300792. [DOI] [PubMed] [Google Scholar]
- 23.Rigby PW, Dieckmann M, Rhodes C, Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977;113:237. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- 24.Cotten M, Baker A, Saltik M, Wagner E, Buschle M. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Ther. 1994;1:239. [PubMed] [Google Scholar]
- 25.Hartmann G, Krieg AM. CpG DNA and LPS induce distinct patterns of activation in human monocytes. Gene Ther. 1999;6:893. doi: 10.1038/sj.gt.3300880. [DOI] [PubMed] [Google Scholar]
- 26.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Smith RL. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975;63:414. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- 28.Yi AK, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg AM. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755. [PubMed] [Google Scholar]
- 29.Hacker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Krieser RJ, Eastman A. The cloning and expression of human deoxyribonuclease II. A possible role in apoptosis. J Biol Chem. 1998;273:30909. doi: 10.1074/jbc.273.47.30909. [DOI] [PubMed] [Google Scholar]
- 32.Cleaver JE, Boyer HW. Solubility and dialysis limits of DNA oligonucleotides. Biochim Biophys Acta. 1972;262:116. doi: 10.1016/0005-2787(72)90224-9. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz DA, Quinn TJ, Thorne PS, Sayeed S, Yi AK, Krieg AM. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. 1997;100:68. doi: 10.1172/JCI119523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S, Beard C, Jaenisch R, Jones P, Sprent J. Mitogenicity of DNA from different organisms for murine B cells. J Immunol. 1997;159:3119. [PubMed] [Google Scholar]
- 35.Sester DP, Stacey KJ, Sweet MJ, Beasley SJ, Cronau SL, Hume DA. The actions of bacterial DNA on murine macrophages. J Leukoc Biol. 1999;66:542. doi: 10.1002/jlb.66.4.542. [DOI] [PubMed] [Google Scholar]
- 36.Gao JJ, Zuvanich EG, Xue Q, Horn DL, Silverstein R, Morrison DC. Cutting edge: bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW264.7 macrophages. J Immunol. 1999;163:4095. [PubMed] [Google Scholar]
- 37.Sweet MJ, Stacey KJ, Kakuda DK, Markovich D, Hume DA. IFN-gamma primes macrophage responses to bacterial DNA. J Interferon Cytokine Res. 1998;18:263. doi: 10.1089/jir.1998.18.263. [DOI] [PubMed] [Google Scholar]
- 38.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116. [PubMed] [Google Scholar]
- 39.Bauer M, Heeg K, Wagner H, Lipford GB. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology. 1999;97:699. doi: 10.1046/j.1365-2567.1999.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odaka C, Mizuochi T. Role of macrophage lysosomal enzymes in the degradation of nucleosomes of apoptotic cells. J Immunol. 1999;163:5346. [PubMed] [Google Scholar]
- 41.McIlroy D, Tanaka M, Sakahira H, et al. An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 2000;14:549. [PMC free article] [PubMed] [Google Scholar]
- 42.Filion MC, Filion B, Reader S, Menard S, Phillips NC. Modulation of interleukin-12 synthesis by DNA lacking the CpG motif and present in a mycobacterial cell wall complex. Cancer Immunol Immunother. 2000;49:325. doi: 10.1007/s002620000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowdery JS, Chace JH, Yi AK, Krieg AM. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570. [PubMed] [Google Scholar]
- 44.Yoshinaga T, Yasuda K, Ogawa Y, Takakura Y. Efficient uptake and rapid degradation of plasmid DNA by murine dendritic cells via a specific mechanism. Biochem Biophys Res Commun. 2002;299:389. doi: 10.1016/s0006-291x(02)02648-7. [DOI] [PubMed] [Google Scholar]