Abstract
Dexamethasone has been shown to up-regulate human mucin 1 (MUC1) expression in certain types of cancer cell lines in vitro, suggesting that this gluocorticoid may enhance MUC1-based immunotherapies. Here we investigated the effect of dexamethasone on MUC1 expression in the DU145 human prostate cancer cell line in terms of antibody-mediated complement-dependent cell lysis. Cells treated with 1 × 10−8 m dexamethasone in vitro expressed maximal levels of MUC1 after 6 days, with an approximately 3-fold increase over MUC1 levels on untreated cells. DU145 cells were highly resistant to lysis by anti-MUC1 antibody and complement, and their susceptibility to antibody and complement was unaffected by dexamethasone treatment. However, dexamethasone also induced expression of the complement inhibitor decay accelerating factor (DAF) on DU145 cells. Blocking or overcoming the function of DAF resulted in enhanced complement-dependent lysis of dexamethasone-treated cells with anti-MUC1 antibodies, indicating that the failure of dexamethasone to enhance the complement susceptibility of DU145 cells was caused by the up-regulated expression of DAF. We also investigated MUC1 expression in vivo and found that MUC1 expression was significantly up-regulated on tumour cells isolated from immune-deficient mice that had been injected with dexamethasone. However, in contrast to in vitro data, there was no difference between the levels of DAF expressed on tumour-derived DU145 cells isolated from either phosphate buffered saline (PBS)-treated or dexamethasone-treated mice, and tumour cells isolated from dexamethasone-treated mice were more sensitive to complement-mediated lysis. In the broad context of immunotherapy, the in vivo data support the use of dexamethasone as an adjunct treatment. Up-regulated DAF expression would not be a favourable outcome of dexamethasone treatment in terms of complement-dependent antibody therapy, but the in vivo data caution against extrapolation of in vitro data with regard to the modulation of complement inhibitors reported here and elsewhere.
Introduction
Prostate cancer is the most frequently diagnosed noncutaneous cancer in American males and is the second leading cause of cancer death. Although surgery and radiation therapy for primary cancers can be effective, other treatment modalities, including chemotherapy, have less utility. When the cancer is metastatic there are no curative therapies available. Prolongation of life is achieved by chemical or surgical castration, but reoccurrence of androgen-independent cancer almost always occurs, culminating eventually in patient death. Immunotherapeutic approaches to prostate cancer treatment have the potential for a more favourable outcome, particularly for metastatic cancer. Immunotherapeutic approaches might combine a surgical and adjuvant immune system approach or harness the immune system alone. Inducing complement-mediated destruction of cancer cells is one such immune approach.
Human mucin 1 (MUC1), or epsialin, is an epithelial cell-associated mucin normally expressed on the apical surface, but differences in MUC1 expression on cancer cells make MUC1 epitopes tumour-specific (for reviews on MUC1 and cancer, see references7–9). On tumour cells, MUC1 is highly overexpressed and loses its polarized distribution on the cell surface. Tumour-associated MUC1 is also underglycosylated, resulting in the exposure of immunodominant peptide sequences that are normally concealed. MUC1 is expressed by most adenocarcinomas of the breast, lung, stomach, pancreas, colon, ovary, bladder and prostate. MUC1 is thus an important marker of malignancy and is a target for several immunotherapies currently under investigation. With regard to tumour surface antigens, factors that can contribute to the resistance of tumors to immunotherapy include low antigen density, cellular shedding of antigens and antigen variation, including selective down-regulation. It has been shown that dexamethasone up-regulates MUC1 expression in multiple myeloma, prostate and ovarian cancer cell lines in vitro at pharmacologically achievable levels, suggesting that dexamethasone might be a useful adjunct for MUC1-targeting immunotherapies.3,4 Dexamethasone is a synthetic glucocorticoid that regulates gene expression, and there are consensus sequences for steroid response elements for glucocorticoids in the MUC1 promoter.2,3
In this study, we investigated the effect of dexamethasone on MUC1 expression in a human prostate cancer cell line in vitro and in vivo, and addressed the potential consequences of altered MUC1 expression for antibody therapy in terms of complement activation and cell lysis.
Materials and methods
Cells and culture
The DU145 prostate cancer cell line was grown and maintained in RPMI supplemented with 10% fetal calf serum (FCS).
Dexamethasone treatment of cell cultures
For each experimental group, five flasks were plated with cells at approximately 20% confluency. The culture medium was changed every 2 days and freshly prepared dexamethasone (Sigma Aldrich, St. Louis, MO) was added to four of the flasks, on day 0, 2, 4 and 6, respectively, with a final concentration of either 10−5 or 10−8 m. The fifth flask received no dexamethasone and, after 8 days of culture, cells were detached with phosphate-buffered saline (PBS)/0·02% EDTA for analysis. Thus, all cells were in culture for the same length of time (8 days) and all were analysed at the same time. Dexamethasone-containing medium was prepared by dissolving dexamethasone in ethanol with subsequent dilution in culture medium.
Flow cytometry
Analysis of cell surface expression of MUC1 and complement-inhibitory proteins was performed using appropriate primary antibodies (10 µg/ml) and fluorescein isothiocyanate (FITC) conjugated secondary antibodies as described below. The primary antibodies used were C595 and BCP8 anti-MUC1 monoclonal antibodies (mAbs), kindly provided by Drs M. R. Price (University of Nottingham, Nottingham, UK) and I. F. McKenzie (Austin Research Institute, Heidelberg, Australia), respectively, and 1F5 anti-CD59 mAb, 1H4 anti-DAF mAb and M75 anti-MCP mAb (BD Biosciences Pharmingen, San Diego, CA). For analysis of tumour-derived cells, cell suspensions were prepared by gentle teasing of isolated tumour tissue with scalpels (in RPMI/10% FCS) followed by centrifugation through Ficoll to remove tumour pieces and aggregates. Isolated cells were then washed by centrifugation in RPMI/10% FCS before use in flow cytometry or lysis experiments (see below). For analysis, tubes contained 2–5 × 104 cells and viability was checked by propidium iodide staining.
Complement lysis assays
Complement-mediated cell lysis of cultured cells was determined both by 51Cr release and by trypan blue exclusion staining (the two techniques gave similar results). Briefly, cells were detached with PBS/0·02% EDTA, washed twice and resuspended in culture medium. For 51Cr release assays, cells were preloaded and washed a further Cells (1 × 106/ml final concentration, 100 µl per tube) were incubated in PBS or with C595 or BCP8 anti-MUC1 mAb at varying concentrations for 30 min on ice, spun down, and resuspended in human or rat serum diluted to 40% in culture medium without FCS. Complement-mediated lysis of tumour-derived cells was determined using cells pooled from three mice (each group treated with either PBS or dexamethasone; see below) because of the low numbers of cells available. Tumour-derived cells were prepared as described above and were sensitized to complement with C595 mAb. Heat-inactivated serum (56°/30 min) was used in control experiments. Lysis was determined after 1 hr at 37°. The effect of blocking decay accelerating factor (DAF) function on complement-mediated lysis was determined by including 50 µg/ml 1H4 anti-DAF F(ab′)2 in the anti-MUC1 antibody sensitization incubations. The function blocking activity of 1H4 has been previously characterized. Complement-mediated lysis of dexamethasone-treated cells was determined after 6 days of culture with dexamethasone.
DU145 tumour model and dexamethasone treatment
Four-week-old male Balb/c nude mice (National Cancer Institute, Frederick, MD) were injected subcutaneously into the right chest with 5 × 106 DU145 cells suspended in 0·1 ml PBS. When the tumours reached a volume of approximately 20 mm3 (about day 24), treatment with either dexamethasone or PBS control was initiated. Dexamethasone (1 µg in 50 µl PBS) or PBS was injected subcutaneously at the peritumor site every 3 days for a total of five injections. Principles of laboratory animal care (NIH publication no. 85-23, revised 1985; National Institute of Health, Bethesda, MD) were followed. Dexamethasone was prepared by dissolving in ethanol with subsequent dilution in sterile PBS before injection. Two days following final injection, mice were killed and tumours isolated. Tumour cells were prepared for analysis as described above.
Results
Effect of dexamethasone on MUC1 expression in vitro
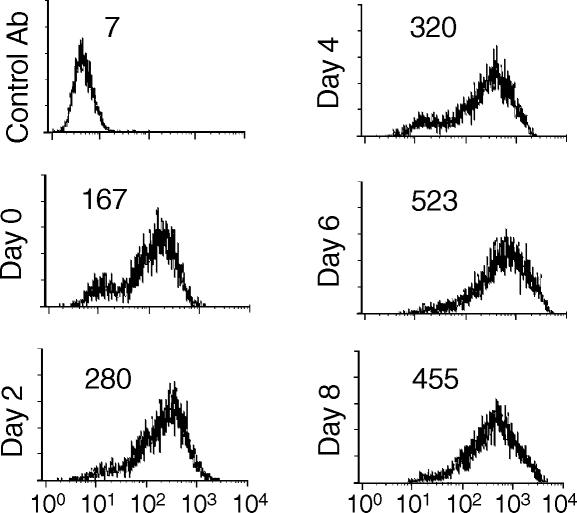
It was previously reported that dexamethasone induced the expression of MUC1 in B-cell lymphoma, ovarian and prostate cancer cell lines in vitro.4 Of these cell lines, the DU145 prostate cancer cell line was chosen to investigate the potential consequences of increased MUC1 expression in terms of antibody therapy. The effect of dexamethasone on MUC1 expression on DU145 prostatic cells in vitro was first confirmed, and the relative levels of MUC1 expression were determined after increasing periods of exposure to dexamethasone (Fig. 1). Cells treated with 1 × 10−8 m dexamethasone (a pharmacologically achievable dose) expressed maximal levels of MUC1 after 6 days with an approximately 3-fold increase over MUC1 levels on untreated cells (Fig. 1). There was no significant difference in levels of MUC1 expression between DU145 cells treated with 1 × 10−8 and 1 × 10−5 m dexamethasone (data not shown). MUC1 expression was heterogeneous, although a more homogeneous pattern of expression was observed after prolonged exposure to dexamethasone and all cells stained positive. In one experiment, MUC1 expression was determined on DU145 after 15 days of dexamethasone treatment; the cells continued to express MUC1 at elevated levels. Dexamethasone also up-regulated MUC1 expression in the prostate cancer cell line PC-3 (1·8-fold increase after 48 hr; data not shown).
Figure 1.
Effect of dexamethasone on the expression of MUC1 on DU145 cells. Cells were cultured in the absence or presence of 10−8 m dexamethasone for the indicated time period and relative levels of MUC1 expression determined by flow cytometry with anti-MUC1 C595 mAb. All cells were in culture for 8 days, with dexamethasone added at intervals. Day 0 represents culture in the absence of dexamethasone (see ‘Materials and methods’). Staining with a control antibody gave a mean relative fluorescence of <10. The data shown are representative of three separate experiments.
Analysis of MUC1 expression was performed using the anti-MUC1 mAb C595, which recognizes a tumour-associated core protein epitope. Flow cytometry using a second mAb of defined specificity, BCP8, confirmed exposure of MUC1 core protein on DU145 cells (data not shown). Interestingly, the previous study on dexamethasone-induced expression of MUC14 reported that DU145 (and most other cell lines tested) expressed MUC1 glycoforms that were not recognized by antibodies reacting to core protein epitopes. The reason for this discrepancy is not clear.
Effect of dexamethasone on DU145 susceptibility to human complement
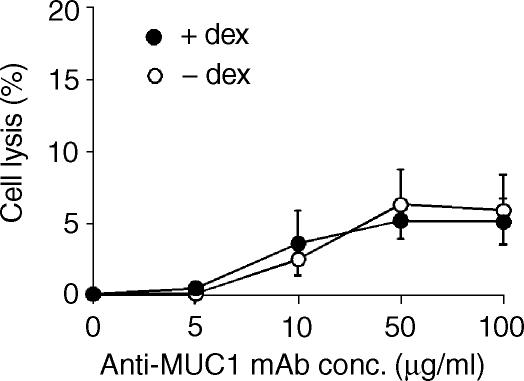
Complement lysis assays of dexamethasone-treated DU145 cells were performed to determine whether increased MUC1 expression translated into enhanced complement sensitivity mediated by anti-MUC1 mAbs. The data in Fig. 2 show that DU145 cells were highly resistant to lysis by anti-MUC1 antibody and complement, and that their susceptibility to anti-MUC1 antibody and complement was unaffected by dexamethasone treatment. Similar results were obtained with two different complement activating antibody isotypes; IgG3 (mAb C595) (Fig. 1) and IgG2b (mAb BCP8) (data not shown).
Figure 2.
Effect of dexamethasone on susceptibility of DU145 cells to antibody and human complement. DU145 cells were treated in culture with or without dexamethasone for 6 days. Cells were isolated and incubated with the indicated concentration of anti-MUC1 C595 mAb in the presence of 40% human serum, and cell lysis was determined after 1 hr at 37°. The data are mean ± SD of three separate experiments.
Effect of dexamethasone on complement inhibitor expression in vitro
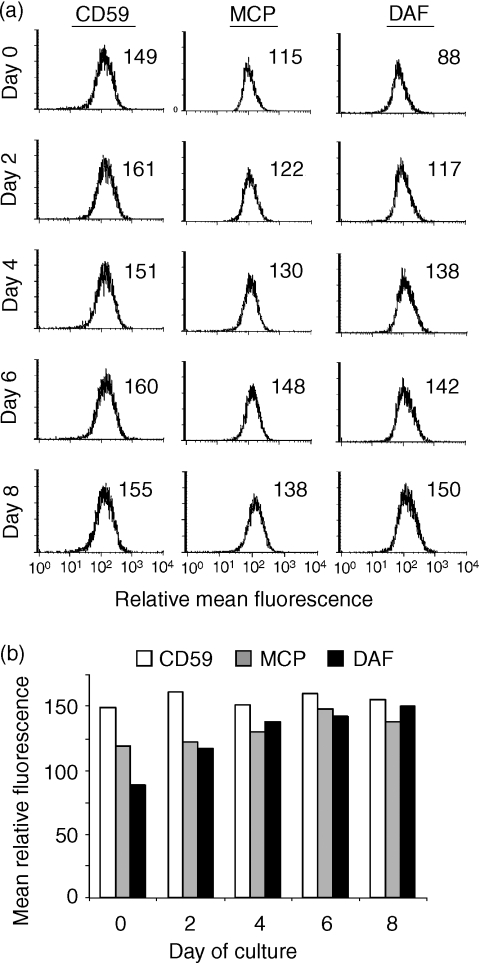
It is well documented that the expression of complement-inhibitory proteins on tumour cells confers protection from antibody-dependent complement-mediated lysis in vitro. Although increased levels of antibody are deposited on dexamethasone-treated DU145 cells, the increase in the complement-activating signal may be insufficient to overcome the effects of complement-inhibitory proteins. We therefore investigated expression of complement-inhibitory proteins on DU145 before and after dexamethasone treatment. DU145 cells express all three membrane-bound complement inhibitors; decay accelerating factor (DAF), membrane cofactor protein (MCP) and CD59 (Fig. 3). Dexamethasone treatment resulted in up-regulated expression of DAF on DU145 in vitro, with a maximal level of expression reached after 4 days of treatment, corresponding to a 75% increase over expression levels on untreated cells. MCP and CD59 expression were unaltered by dexamethasone treatment (Fig. 3). These data raise the possibility that up-regulated DAF expression on dexamethasone-treated DU145 cells counterbalances the effect of increased anti-MUC1 antibody binding. In one experiment, DAF expression was analysed 15 days after dexamethasone treatment and expression of DAF remained elevated.
Figure 3.
Effect of dexamethasone on the expression of complement-inhibitory proteins on DU145 cells. Cells were cultured in the absence (or presence) of dexamethasone for the indicated time periods and the expression of complement-inhibitory proteins examined by flow cytometry. (a) All cells were in culture for 8 days, with dexamethasone added at intervals. Day 0 represents culture in the absence of dexamethasone (see ‘Materials and methods’). (b) Graphical summary of flow cytometry data. Staining with a control antibody gave a mean relative fluorescence of <10. The data shown are representative of three separate experiments.
Effect of complement-inhibitory proteins on complement-mediated lysis
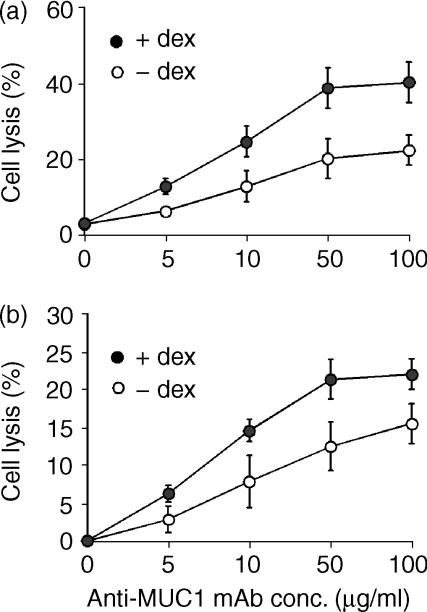
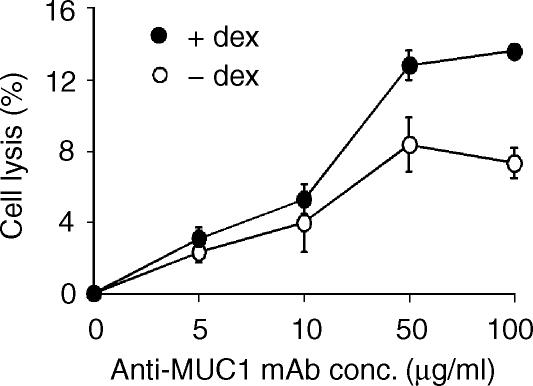
Membrane-bound complement-inhibitory proteins are species selective, and human complement-inhibitory proteins are not very effective against rat or mouse complement.4 Therefore, to investigate the contribution of complement-inhibitory proteins in providing dexamethasone-treated (human) DU145 cells with protection from complement lysis, rat serum was used in lysis assays (mouse serum has poor lytic activity in vitro4,5). Untreated DU145 cells were about 3 times more sensitive to rat complement than to human complement (compare Figs 2 and 4a), indicating the protective role of complement-inhibitory proteins on these cells. However, in contrast to the lack of any effect of dexamethasone on the sensitivity of DU145 cells to human complement (Fig. 2), dexamethasone treatment enhanced the sensitivity of DU145 cells to anti-MUC1 antibody-dependent rat complement-mediated lysis (by about a factor of 2; Fig. 4a). To further investigate whether the increased expression of DAF on dexamethasone-treated DU145 cells was contributing to their resistance to anti-MUC1 ab and human complement, lysis assays were performed in the presence of F(ab′)2 antibody fragments that block DAF function. DU145 cells on which DAF function was blocked were significantly more sensitive to human complement following dexamethasone treatment (P < 0·05 at anti-MUC1 mAb concentrations ≥10 µg/ml) (Fig. 4b). These data indicate that the failure of dexamethasone treatment and increased MUC1 expression to enhance antibody-dependent sensitivity to homologous complement is attributable, at least in part, to the up-regulated expression of DAF. Following dexamethasone treatment there was a greater increase in lysis mediated by rat complement compared to lysis by human complement in the presence of anti-DAF F(ab′)2, suggesting that lysis is also under the control of other complement inhibitors, probably CD59.
Figure 4.
Complement-mediated lysis of DU145 cells after dexamethasone treatment. DU145 cells were treated in culture with or without dexamethasone for 6 days. Cells were isolated and sensitized to complement with the indicated concentration of anti-MUC1 C595 mAb.(a) Cell lysis by 40% rat serum (1 hr at 37°).(b) Cell lysis by 40% human serum (1 hr at 37°) following preincubation of cells with anti-DAF F(ab′)2 (50 µg/ml for 30 min). Note the different scales on the y-axis. The data are mean ± SD of three separate experiments.
Effect of dexamethasone on expression of MUC1 and DAF in vivo and susceptibility of tumour-derived cells to complement
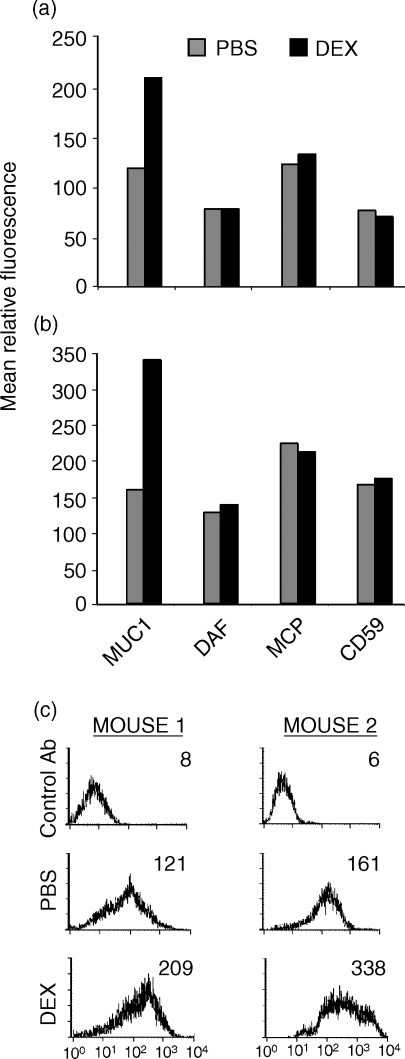
Subcutaneous DU145 tumours growing in nude mice were injected a total of 5 times with either PBS or dexamethasone every 3 days, after which time tumours were removed and cells analysed by flow cytometry. MUC1 expression was about 2-fold higher on tumour-derived cells isolated from mice injected with dexamethasone compared to tumour cells isolated from mice injected with PBS (Fig. 5). Thus, dexamethasone modulates MUC1 expression both in vitro and in vivo. However, in contrast to the effect of dexamethasone on DAF expression in vitro, there was no difference in the level of DAF expression on tumour-derived cells isolated from either the PBS- or dexamethasone-treated mice (compare Figs 3 and 5; there was homogeneous expression of complement inhibitors on tumour-derived cells, as shown in Fig. 3). Thus, from the in vitro complement lysis data, we would predict that up-regulated MUC1 expression without increased DAF expression would result in enhanced sensitivity of tumour cells to anti-MUC1-dependent complement-mediated lysis. This prediction was substantiated in a separate experiment in which it was demonstrated that tumour cells isolated from dexamethasone-treated mice were significantly more sensitive to lysis by anti-MUC1 mAb and human complement than cells isolated from PBS-treated mice (Fig. 6) (P < 0·05 at 50 and 100 µg/ml). For this experiment, tumour cells derived from three mice in each treatment group were pooled for assays because of the limited number of viable cells that could be isolated.
Figure 5.
Effect of injected dexamethasone on the expression of MUC1 and complement-inhibitory proteins on DU145 tumours in nude mice. In two separate experiments (a and b), two mice bearing subcutaneous DU145 tumours each received intratumoral injections of either PBS or 1 µg dexamethasone every 3 days for 2 weeks. Two days after the last dexamethasone injection, tumour cells were isolated and analysed for expression of MUC1 and complement-inhibitory proteins by flow cytometry. (c) Histograms of MUC1 expression data.
Figure 6.
Complement-mediated lysis of tumour-derived DU145 cells. Tumour cells isolated from three mice treated with PBS or from three mice treated with dexamethasone were pooled, sensitized with the indicated concentration of anti-MUC1 C595 mAb, and exposed to 40% human serum. Lysis was determined after 1 hr at 37°. The data are mean ± SD of triplicate determinations.
Discussion
Glucocorticoids represent a treatment modality for various types of cancer, and they are often administered in conjunction with other types of therapy. In the context of immunotherapy, the previous finding that dexamethasone up-regulated MUC1 expression in multiple myeloma, prostate and ovarian cancer cell lines in vitro suggested that there may be clinical benefit in combining dexamethasone with therapeutic approaches that target MUC1.4 Anti-MUC1 mAbs represent one type of immunothepeutic approach currently under investigation, and complement-dependent effector mechanisms have been implicated in the successful outcome of antibody therapy of cancer in both animal models and patients.3,6,12,13 Here we investigated the influence of dexamethasone-mediated up-regulation of MUC1 expression on anti-MUC1 antibody effector function with regard to complement activation and lysis of the DU145 prostate cancer cell line, and determined the effect of dexamethasone on MUC1 expression on DU145 cells in vivo.
Dexamethasone caused up-regulated expression of MUC1 on DU145 cells in vitro, and elevated expression levels were maintained with prolonged exposure to dexamethasone. However, dexamethasone treatment did not result in increased susceptibility of anti-MUC1 antibody sensitized DU145 cells to complement-mediated lysis, despite a 3-fold increase in the level of MUC1 expression. Dexamethasone also up-regulated the expression of the complement-inhibitory protein DAF, and our data indicate that augmented DAF expression directly contributed to the failure of dexamethasone to enhance the complement sensitivity of DU145 cells. With regard to humoral immune mechanisms, up-regulated DAF expression is an unfavourable response of tumour cells to dexamethasone, but, significantly, we did not observe this response in vivo. Dexamethasone treatment of tumour-bearing mice enhanced MUC1 but not DAF expression on tumour cells, resulting in enhanced sensitivity of tumour-derived cells to anti-MUC1 mAb and complement. Of course, the down-regulation of complement inhibitors is of potential benefit during therapy with complement-activating mAb, and there are numerous reports of cytokine-mediated modulation of complement inhibitor expression in tumour cell lines in vitro. Nevertheless, cytokines that down-regulate complement inhibitors can also down-regulate expression of tumour-associated antigens, as recently shown in vitro with renal tumour cell lines.6
To our knowledge, this is the first report of dexamethasone directly modulating the expression of a membrane-bound complement inhibitor. Dexamethasone treatment has, however, been shown to inhibit tumour necrosis factor (TNF)-α and interferon (IFN)-γ-mediated up-regulation of DAF in lung cancer cell lines in vitro.3 TNF-α and IFN-γ are proinflammatory cytokines that can be produced within a tumour microenvironment. Consistent with its known anti-inflammatory properties, dexamethasone has also been shown to up-regulate expression of the soluble complement inhibitors factor H and C1-inhibitor, and to down-regulate expression of C3 in various cell lines.2,8 It is not clear if these effects of dexamethasone would be of consequence with regard to the potential impact of complement-dependent immune mechanisms in cancer therapy, although it is noteworthy that the secretion and/or binding of factor H by certain kinds of cancer cell lines provides tumour cells with increased resistance to complement.6,11,22 In contrast to our finding that dexamethasone did not affect the sensitivity of DU145 prostate cancer cells to anti-MUC1 antibody and complement in vitro, an earlier study found that dexamethasone enhanced complement-mediated lysis of B-cell lymphoma cell lines sensitized with rituximab, an anti-CD20 mAb approved for the treatment of B-cell lymphoma.5 The mechanism by which dexamethasone enhanced sensitivity to complement-mediated lysis was not determined, but dexamethasone did not affect the expression of complement-inhibitory proteins in B-cell lymphoma cell lines.
In broader terms, our finding that dexamethasone up-regulated MUC1 expression, but not complement inhibitor expression, on DU145 cells in vivo strengthens the hypothesis that there will be potential benefit in combining dexamethasone with MUC1-targeted immunotherapies. In any case, the effect of dexamethasone on complement inhibitor expression is unlikely to be of consequence for immunotherapies based on radiolabelled or toxin conjugated antibodies, or on cytotoxic T lymphocyte (CTL) effector mechanisms. However, the expression of inhibitors of complement activation (DAF and MCP) can potentially affect antibody-dependent cell-mediated cytotoxicity (ADCC), as complement can enhance ADCC via coligation of complement receptors on effector cells. At least in some mouse models, it has been shown that ADCC can play a critical role in the cytotoxicity of antibodies against tumors,2,4 and for complement-resistant tumour targets it seems likely that Fc receptor-dependent mechanisms would dominate. Inhibitors of complement activation may also affect complement-dependent cell cytotoxicity (CDCC), an effective mechanism of tumour cell destruction in the presence of β-glucan, a biological response modifier that activates effector cells via complement receptor 3 (CR3).1–3 In the context of antibody and complement effector mechanisms, it is noteworthy that DAF was not up-regulated on DU145 tumours in mice treated with dexamethasone and that the tumour-derived cells displayed enhanced sensitivity to complement. This was in contrast to the effect of dexamethasone on DAF expression and complement sensitivity in vitro. It is possible that, in vivo, dexamethasone may be regulating cytokine-mediated modulation of DAF expression (see above). In any case, the data serve as a caution against the extrapolation of in vitro data to an in vivo situation with regard to the modulation of complement inhibitor expression. The data do, however, support further investigation into the utility of dexamethasone with immunotherapeutic protocols in appropriate models. In humans, the expression of MUC1 has been shown to vary among individuals with prostate cancer,3 and the same may be true for DAF expression.1 Consideration will also have to be given to the immunosuppressive effects of dexamethasone, which could potentially compromise some immunotherapies, and to the fact that up-regulated MUC1 expression may influence tumour progression, as MUC1 is implicated in metastasis (cell migration and adhesion), angiogenesis and immune regulation (reviewed in references4,7–9).
Acknowledgments
The work was supported by grants DAMD17-99-1-9325 and DAMD17-01-0393 (Department of the Army).
References
- 1.Agrawal B, Gendler SJ, Longenecker BM. The biological role of mucins in cellular interactions and immune regulation: prospects for cancer immunotherapy. Mol Med Today. 1998;4:397–403. doi: 10.1016/s1357-4310(98)01322-7. [DOI] [PubMed] [Google Scholar]
- 2.Denda-Nagai K, Irimura T. MUC1 in carcinoma–host interactions. Glycoconj J. 2000;17:649–58. doi: 10.1023/a:1011039013134. [DOI] [PubMed] [Google Scholar]
- 3.Rye PD, Price M, Petrakou E, et al. International workshop on monoclonal antibodies against MUC1. Tumor Biol. 1998;19:1–152. [Google Scholar]
- 4.Treon SP, Mollick JA, Urashima M, et al. Muc-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood. 1999;93:1287–98. [PubMed] [Google Scholar]
- 5.Schut IC, Waterfall PM, Ross M, O'Sullivan C, Miller WR, Habib FK, Bayne CW. MUC1 expression, splice variant and short form transcription (MUC1/Z, MUC1/Y) in prostate cell lines and tissue. Brit J Urol Int. 2003;91:278–83. doi: 10.1046/j.1464-410x.2003.03062.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsarfaty I, Hareuveni M, Horev J, et al. Isolation and characterization of an expressed hypervariable gene coding for a breast-cancer-associated antigen. Gene. 1990;93:313–8. doi: 10.1016/0378-1119(90)90242-j. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster CA, Peat N, Duhig T, Wilson D, Taylor-Papadimitriou J, Gendler SJ. Structure and expression of the human polymorphic epithelial mucin gene: an expressed VNTR unit. Biochem Biophys Res Commun. 1990;173:1019–29. doi: 10.1016/s0006-291x(05)80888-5. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Abagyan RA, Dong S, Gilbert A, Nussenzweig V, Tomlinson S. Mapping the active site of CD59. J Exp Med. 1997;185:745–53. doi: 10.1084/jem.185.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada N, Harada R, Fujita T, Okada H. Monoclonal antibodies capable of causing hemolysis of neuraminidase-treated human erythrocytes by homologous complement. J Immunol. 1989;143:2262–6. [PubMed] [Google Scholar]
- 10.Coyne KE, Hall SE, Thompson S, et al. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992;149:2906–13. [PubMed] [Google Scholar]
- 11.Yu J, Caragine T, Chen S, Morgan BP, Frey AF, Tomlinson S. Protection of human breast cancer cells from complement-mediated lysis by expression of heterologous CD59. Clin Exp Immunol. 1999;115:13–8. doi: 10.1046/j.1365-2249.1999.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Dong S, Rushmere NK, Morgan BP, Abagyan R, Tomlinson S. Mapping the regions of the complement inhibitor CD59 responsible for its species selectivity. Biochemistry. 1997;36:9423–8. doi: 10.1021/bi970832i. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Caragine T, Cheung NK, Tomlinson S. CD59 expressed on a tumor cell surface modulates DAF expression and enhances tumor growth in a rat model of human neuroblastoma. Cancer Res. 2000;60:3013–118. [PubMed] [Google Scholar]
- 14.Harris CL, Spiller OB, Morgan BP. Human and rodent decay-accelerating factors (CD55) are not species restricted in their complement-inhibiting activities. Immunology. 2000;100:462–70. doi: 10.1046/j.1365-2567.2000.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong GL, Mattes MJ. Mouse strains with typical mammalian levels of complement activity. J Immunol Meth. 1989;125:147–58. doi: 10.1016/0022-1759(89)90088-4. [DOI] [PubMed] [Google Scholar]
- 16.Bergman I, Basse PH, Barmada MA, Griffin JA, Cheung NK. Comparison of in vitro antibody-targeted cytotoxicity using mouse, rat and human effectors. Cancer Immunol Immunother. 2000;49:259–66. doi: 10.1007/s002620000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manches O, Lui G, Chaperot L, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–54. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 18.Treon SP, Mitsiades C, Mitsiades N, Young G, Doss D, Schlossman R, Anderson KC. Tumor cell expression of CD59 is associated with resistance to CD20 serotherapy in patients with B-cell malignancies. J Immunother. 2001;24:263–71. [PubMed] [Google Scholar]
- 19.Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol. 2000;51:634–41. doi: 10.1046/j.1365-3083.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 20.Ollert MW, David K, Vollmert C, Juhl H, Erttmann R, Bredehorst R, Vogel CW. Mechanisms of in vivo anti-neuroblastoma activity of human natural IgM. Eur J Cancer. 1997;33:1942–8. doi: 10.1016/s0959-8049(97)00285-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Zhang S, Cheung NK, Ragupathi G, Livingston PO. Antibodies against GD2 ganglioside can eradicate syngeneic cancer micrometastases. Cancer Res. 1998;58:2844–9. [PubMed] [Google Scholar]
- 22.Blok VT, Gelderman KA, Tijsma OH, Daha MR, Gorter A. Cytokines affect resistance of human renal tumour cells to complement-mediated injury. Scand J Immunol. 2003;57:591–9. doi: 10.1046/j.1365-3083.2003.01265.x. [DOI] [PubMed] [Google Scholar]
- 23.Varsano S, Rashkovsky L, Shapiro H, Radnay J. Cytokines modulate expression of cell-membrane complement inhibitory proteins in human lung cancer cell lines. Am J Respir Cell Mol Biol. 1998;19:522–9. doi: 10.1165/ajrcmb.19.3.3181. [DOI] [PubMed] [Google Scholar]
- 24.Lappin DF, Whaley K. Modulation of complement gene expression by glucocorticoids. Biochem J. 1991;280:117–23. doi: 10.1042/bj2800117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dauchel H, Julen N, Lemercier C, Daveau M, Ozanne D, Fontaine M, Ripoche J. Expression of complement alternative pathway proteins by endothelial cells. Differential regulation by interleukin 1 and glucocorticoids. Eur J Immunol. 1990;20:1669–75. doi: 10.1002/eji.1830200808. [DOI] [PubMed] [Google Scholar]
- 26.Friese MA, Hellwage J, Jokiranta TS, Meri S, Muller-Quernheim HJ, Peter HH, Eibel H, Zipfel PF. Different regulation of factor H and FHL-1/reconectin by inflammatory mediators and expression of the two proteins in rheumatoid arthritis (RA) Clin Exp Immunol. 2000;121:406–15. doi: 10.1046/j.1365-2249.2000.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junnikkala S, Jokiranta TS, Friese MA, Jarva H, Zipfel PF, Meri S. Exceptional resistance of human H2 glioblastoma cells to complement-mediated killing by expression and utilization of factor H and factor H-like protein 1. J Immunol. 2000;164:6075–81. doi: 10.4049/jimmunol.164.11.6075. [DOI] [PubMed] [Google Scholar]
- 28.Fedarko NS, Fohr B, Robey PG, Young MF, Fisher LW. Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J Biol Chem. 2000;275:16666–72. doi: 10.1074/jbc.M001123200. [DOI] [PubMed] [Google Scholar]
- 29.Jurianz K, Ziegler S, Donin N, Reiter Y, Fishelson Z, Kirschfink M. K562 erythroleukemic cells are equipped with multiple mechanisms of resistance to lysis by complement. Int J Cancer. 2001;93:848–54. doi: 10.1002/ijc.1406. [DOI] [PubMed] [Google Scholar]
- 30.Rose AL, Smith BE, Maloney DG. Glucocorticoids and rituximab in vitro: synergistic direct antiproliferative and apoptotic effects. Blood. 2002;100:1765–73. [PubMed] [Google Scholar]
- 31.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 32.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci USA. 1998;95:652–6. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross GD, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999;42:61–74. doi: 10.1016/s0162-3109(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 34.Freedland SJ, Seligson DB, Liu AY, et al. Loss of CD10 (neutral endopeptidase) is a frequent and early event in human prostate cancer. Prostate. 2003;55:71–80. doi: 10.1002/pros.10202. [DOI] [PubMed] [Google Scholar]
- 35.von Mensdorff-Pouilly S, Snijdewint FG, Verstraeten AA, Verheijen RH, Kenemans P. Human MUC1 mucin: a multifaceted glycoprotein. Int J Biol Markers. 2000;15:343–56. doi: 10.1177/172460080001500413. [DOI] [PubMed] [Google Scholar]