Abstract
We report on a new approach toward protection against tuberculosis, based on passive inoculation with immunoglobulin A (IgA) antibodies. In a mouse model of tuberculous lung infection, intranasal inoculations of mice with an IgA monoclonal antibody (mAb) against the α-crystallin antigen of Mycobacterium tuberculosis reduced up to 10-fold the lung bacterial counts at nine days after either aerosol- or intranasal challenge. This effect involved synergism between mAb inoculations shortly before and 3 days after infection. Monomeric IgA reduced the colony-forming unit counts to the same extent as the polymeric IgA, suggesting antibody targeting to Fcα, rather than poly-immunoglobulin receptors on infected lung macrophages. The protective effect was of short duration, presumably due to the rapid degradation of the intranasally applied IgA. Our results provide evidence of an alternative approach which could be further developed toward immunoprophylaxis against tuberculosis in immunocompromised subjects.
Introduction
According to currently established concept, immune resistance against tuberculosis (TB) is mediated exclusively by T cells, involving cytokine (mainly interferon-γ)-mediated activation or cytotoxicity of infected macrophages, and this view determines all current strategies of TB vaccine research. However, active tuberculosis develops in the majority of patients despite the presence of abundant T helper 1 immunity1 and T-cell targeted vaccination does not always induce optimal protection either in humans or in experimental animals. Therefore, it is desirable to investigate alternative immune mechanisms of protection. Recently, the potential protective role of antibodies against tuberculosis was re-appraised2 and data demonstrating a protective effect were reported using an immunoglobulin G3 (IgG3) monoclonal antibody (mAb) against lipoarabinomannan (LAM)3 and with antibodies against the heparin-binding haemagglutinin.4 Passive antibody-mediated protection has recently been demonstrated also in respect of a number of non-tuberculous intracellular bacterial infections.5–7 Further indirect supportive data in humans associated serum IgG antibody levels to LAM with milder manifestation of child tuberculosis8 and salivary IgA antibody levels with protection against leprosy.9 Most recently, protection of mice and guinea pigs against TB challenge by vaccination with mycobacterial arabinomannan–protein conjugates, was attributed at least partly to IgG antibodies interfering with the deleterious effects of LAM.10
We focused attention in this study to mAbs of the IgA isotype, in view of a capacity of the poly-IgR-mediated transcytosis of polymeric IgA antibodies to interact with intracellular pathogens11 and of the potent Fcα mediated activation of mononuclear cells for bacterial clearance.12 Interest in the IgA isotype was stimulated also by the finding, that protection against pulmonary tuberculous infection in protein antigen vaccinated mice was associated with elevated IgA antibody forming cells in the lungs.13 Therefore, we recently generated IgA mAbs against surface expressed antigens of Mycobacterium tuberculosis14 and in this paper, we report on the evaluation of their passive protective role in M. tuberculosis-challenged mice.
Materials and methods
Production and fractionation of mAbs
The mouse mAbs against surface expressed antigens of M. tuberculosis were TBA61 (IgA anti-acr; hsp16.3; 16 000 MW homologue of α-crystallin), TB68 (IgG1 of the same epitope specificity as TBA61) and TBA84 (IgA anti PstS-1; 38 000 MW secreted glycolipoprotein).14,15 Ascitic fluids of the above mAbs and the globulin fraction of TB6815 had 1/300 000 antigen-binding titres (dilution giving 30% of plateau OD). Culture supernatants produced in the Integra CL1000 flask (Integra Biosciences, Letchworth, UK) using the protein-free hybridoma medium (Life Technologies, Paisley, UK) were purified by passing through antigen (acr or PstS1)-coupled Affigel-15 columns (Bio-Rad, Hemel Hempstead, UK) and concentrated using Amicon ultrafiltration units (Millipore, Watford, UK). Monomer and polymers of IgA were fractionated by gel-filtration on Superdex-200 columns (Amersham Biosciences, Little Chalfont, UK) and analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) followed by silver staining or immunoblotting, using isotype-specific secondary antibodies (Sigma, Poole, UK). Purified MOPC315 IgA myeloma protein (cat.no. M2046, Sigma) was used as a negative control antibody of unrelated (nitrophenylated proteins) binding specificity.
Passive protection studies
mAbs (affinity purified at 4 mg per ml or ascitic fluids) were applied nasally to BALB/c mice (female, aged 8–10 weeks) under light anaesthesia using a halothane/oxygen mixture. 30 µl mAb volumes were inoculated onto the external nares at various dosing times pre- and post-challenge with M. tuberculosis (see below for details). Mice were challenged with suspensions of M. tuberculosis H37Rv bacilli (grown on Middlebrook 7H11 medium) by either the nasal route (6 log10 colony-forming units (CFU) per mouse, applied as above) or by exposure to aerosols using a Henderson apparatus and a Collison 3-jet nebulizer.16 Particles of mean diameter of 2 µm were generated from a water suspension of M. tuberculosis H37Rv containing either log10 7·0 or 7·69 CFU/ml. The aerosol was delivered for 5 min directly to the snouts of animals at 55 l/min air flow rate, resulting in an estimated inhaled dose at time 0 of 100 or 500 CFU/lung. Lungs were harvested 24 hr and 9 days after infection, and 1-ml homogenates in 10-fold serial dilutions were plated on Middlebrook 7H11 agar plates. Visible colonies were counted 2–3 weeks later. In one experiment, viable counts were performed 28 days postchallenge.
Experiments were conducted to evaluate the protective effect of: (i) Ascitic fluids of mAbs delivered intranasally 3 hr before and again 3 and 6 days after aerosol or intranasal challenge with M. tuberculosis; (ii) Affinity-purified TBA61 IgA mAb, delivered either 3 hr prechallenge, or 3 days postchallenge, or both doses combined; and (iii) two doses of either monomeric or polymeric fractions of purified TBA61, delivered 3 hr pre- and 3 days postchallenge. Mice representing the negative control groups were inoculated with phosphate-buffered saline (PBS) or with the MOPC315 IgA which did not result in significantly different lung CFU counts (results not shown). The size of groups ranged from 8 to 17 mice and independent experiments (e.g. in Fig. 2) were conducted to confirm the particular findings.
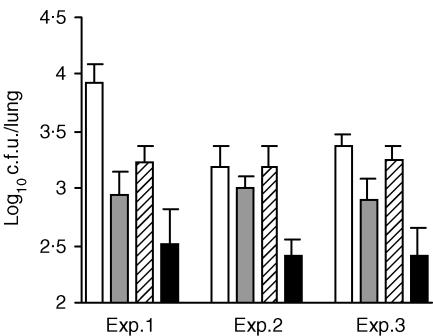
Figure 2.
Synergism between pre- and postinfection inoculated IgA. BALB/c mice were intranasally inoculated with affinity purified TBA61 IgA (4 mg/ml; anti-acr) 3 hr before and 3 days after H37Rv aerosol infection. Columns represent mean values (n = 8) and bars are standard errors of CFU counts 9 days after infection. TBA61 inoculation regime in relation to M. tuberculosis H37Rv challenge: open: MOPC315 IgA myeloma (exp.1) or PBS (exp. 2 and 3) controls; grey, −3 hr; hatched, +3 days; black, −3 hr and +3 days. The data for three independent experiments are shown. The reduction in lung CFU following two doses of TBA61 was significantly different from the control MOPC315 or PBS groups, respectively, in all three experiments (P < 0·01, anova test with multiple comparison of means using Fisher's method).
Modulation of infection of macrophages in vitro
Macrophages from peritoneal exudates of BALB/c mice were allowed to adhere to plastic culture flasks overnight, released by trypsin treatment, re-adhered for 3 hr in 12-well culture plates and then infected with luciferase-tagged M. tuberculosis17 kindly provided by Prof. Douglas Young, Department of Microbiology, Imperial College, London. Purified IgA mAbs or MOPC 315 (an IgA negative control) were added for 4 hr and following washing and killing of extracellular bacilli with amikacin (200 µg/ml for 2 hr), the infected cells were incubated with the same IgAs for 7 days. Cells were lysed in 0·1% Triton-X-100 prior to luminescence assay (Berthold Junior luminometer). The RLU readings have been shown to correlate with the CFU counts.17
Statistical analysis
The effects of antibody treatments were analysed by Student's t-test and by anova test with multiple comparison of means using Fisher's method.
Results
Protection with TBA61 against both intranasal and aerosol challenge
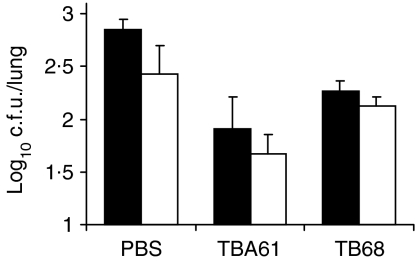
Following delivery of ascitic fluids, mean CFU in the lungs at 9 days post infection showed a statistically significant difference (by Student's t-test) between the mice treated with PBS and the group given TBA61. This was demonstrated in mice challenged with M. tuberculosis by both the aerosol (P ≤ 0·01) and the intransal (P ≤ 0·01) routes (Fig. 1). However, when IgG1 mAb (TB68) targeted against the same epitope of the acr antigen as the mAb TBA61 was delivered by the same inoculation regimen, a much smaller reduction of lung CFU (Fig. 1) was observed. This difference was not statistically different from the PBS control in mice that had been challenged intranasally but in the aerosol-challenged mice the CFUs were significantly lower than the PBS control (P < 0·01). However, the differences between both intranasal and aerosol infected TBA61 and TB68 groups were significant (P < 0·01). Another tested IgA mAb, TBA84, directed against PstS-1 antigen of M. tuberculosis was found to be less effective than TBA61 (result not shown) and therefore omitted from further in vivo analysis. Similar findings, consistent with those demonstrated in Fig. 1 were obtained in other separate experiments (data not shown).
Figure 1.
Reduction of M. tuberculosis CFU in the lungs by intranasally applied IgA mAbs. BALB/c mice were inoculated 3 h before infection and again 3 and 6 days after either intranasal (black columns) or aerosol (white columns) infection with M. tuberculosis H37Rv. Columns represent mean values (PBS and TBA61: n = 15–17; TB68: n = 8) and bars are standard errors of CFU counts in the lungs 9 days after infection. The reduction in lung CFU compared with the PBS control was statistically significant with TBA61 (IgA, anti-acr) following intranasal (P < 0·01) and aerosol (P < 0·01) challenge, and with TB68 (IgG1, antiacr) following aerosol challenge only (P < 0·01). The differences between both intranasal and aerosol infected TBA61 and TB68 groups were significant (P < 0·01).
TBA61 inoculation 3 hr prior to aerosol challenge had no significant influence on the lung CFU counts 24 hr later. The mean log10 CFU counts in the groups of TBA61 inoculated/control mice were 1·37/1·45 or 1·54/1·49 in aerosol or intranasally infected mice, respectively. This lack of reduction of CFU 1 day after infection suggests that the action of mAbs was either not caused by ‘exclusion of infection’ or that the opsonized mycobacteria had not been cleared by that time. We noted greater individual variations of CFU in mAb-inoculated mice compared to control groups and have attributed this to not achieving a sufficient concentration of mAbs in some sites because of the large surface area of the lung. Furthermore, the protective efficacy of IgA appeared to be evanescent, since the significant protection imparted at 9 days after challenge was not sustained at 28 days postchallenge (results not shown).
Synergism between pre- and postinfection inoculation of IgA
The initial schedules of passive IgA treatment involved one application 3 hr prior to infection followed by one or two applications postinfection. As the notion that antibodies might be acting after tuberculous infection seemed contentious, we compared the effect of the combined schedule with that of only single inoculations of affinity-purified TBA61 IgA mAb either before or after M. tuberculosis H37Rv infection in three independent experiments (Fig. 2). These results showed, that a significant reduction of 9-day CFU counts compared with the control groups of an unrelated IgA mAb MOPC315 (in experiment 1) or PBS (in experiments 2 and 3) was achieved in each experiment by a combined pre- and postchallenge treatment with the purified TBA61 antibody (P < 0·01, anova test). In contrast, when only a single dose of TBA61 was given either 3 hr before or 3 days after infection, only a smaller decrease of lung CFU counts was observed which was statistically significant in only one of the three experiments (Fig. 2; Exp. 1).
Protective capacity of purified monomeric and polymeric IgA
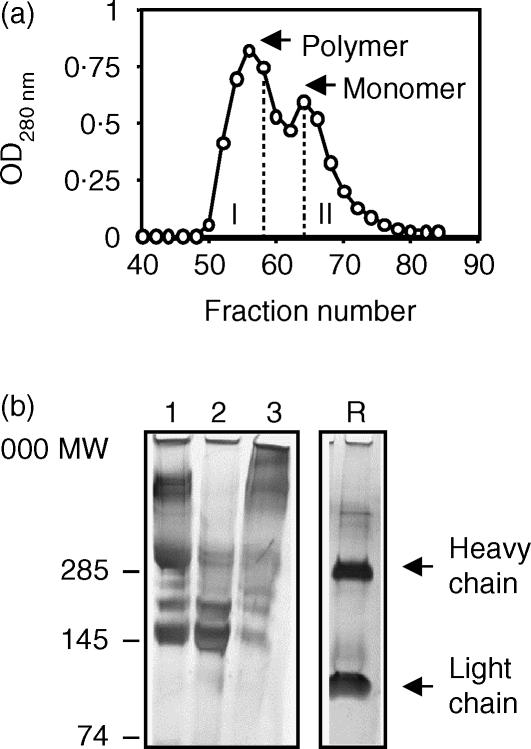
The Superdex-200 column elution profile of purified TBA61 mAb revealed two protein peaks, the smaller (150 000 MW, containing about one third of the total protein) representing the monomeric IgA and the larger fraction containing the dimeric (300 000 MW) and polymeric IgA (Figs 3a,b). The reduced SDS-PAGE pattern of the starting IgA preparation consisted only of heavy and light immunoglobulin chain bands, suggesting that it was of at least 90% purity (Fig. 3b sample R). The IgA preparations, standardized at 1 mg protein/ml had antigen binding titres of 1/50 000 (monomer) and 1/40 000 (polymer).
Figure 3.
Fractionation of purified TBA61: (a) Protein elution profile from Superdex-200 gel column. Eluates were pooled to fractions I and II (excluding the overlapping region) corresponding to polymers and monomer of IgA. (b) Analysis by 4–12% gradient SDS–PAGE and silver staining; 1. Unfractionated IgA, 2. monomer (fraction II), 3. Polymers (fraction I). R. reduced unfractionated TBA61. Heavy and light immunoglobulin chains are indicated.
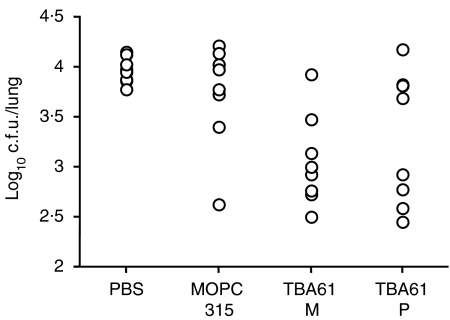
Passive protection using these preparations was tested by two intranasal inoculations 3 hr before and 3 days after H37Rv challenge. This schedule was chosen, because previous experiments had proved it to be equally protective as the earlier used three inoculations of ascites. The results shown in Fig. 4 represent the lung CFU counts in the individual mice. Mean log10 CFU ± SE values for the tested groups showed that the monomeric IgA reduced the lung CFU counts slightly more (3·06 ± 0·16) than the polymeric fraction (3·33 ± 0·23), whereas the MOPC 315 control IgA had no significant effect (3·73 ± 0·18) when compared with the PBS control (3·97 ± 0·05). These results showed, that a reduction of at least two SEs below the mean value of the PBS control was imparted by the monomer in seven of eight mice and by the polymer in five of eight mice. Statistical evaluation of the differences of experimental groups with the PBS control by t-test showed significant reduction of CFU means by treating the mice with either the TBA61 monomer (P = 0·00063) or TBA61 polymers (P = 0·021), although the difference when comparing these two groups with each other was not significant.
Figure 4.
Protective capacity of monomeric and polymeric TBA61 mAb. BALB/c mice were inoculated intranasally 3 h before and 3 days after H37Rv aerosol infection with PBS, or MOPC315 IgA, or monomeric (M) or polymeric (P) fractions of affinity purified TBA61 IgA (4 mg/ml). Columns represent CFU values from individual mice (n = 8) at 9 days after infection. Lung CFU compared to the PBS control were reduced significantly by both the monomeric (P = 0·00063) and polymeric (P = 0·021) fractions.
Effect of IgA mAbs on the infection of macrophages in vitro
We considered that if IgA antibodies could suppress the multiplication of tubercle bacilli in macrophages merely by a simple mechanism, then it should be possible to demonstrate such action in tissue culture. The results of a representative experiment (Table 1) showed no inhibition of M. tuberculosis replication by IgA mAbs.
Table 1.
lack of inhibition of infection of macrophages with IgA mAbs in vitro
| IgA in culture | Mean RLU | Standard error |
|---|---|---|
| TBA61 | 15 041 | 1478 |
| TBA84 | 11 455 | 1853 |
| MOPC 315 | 6618 | 1147 |
| PBS | 7337 | 1865 |
BALB/c peritoneal macrophages (5 × 105 per plastic well) were incubated with luciferase tagged M. tuberculosis at 1 : 10 ratio with 50 µg/ml purified IgA preparations for 4 hr, washed, treated with amikacin and incubated with the same IgAs for another 7 days. MOPC315: IgA myeloma negative control. RLU of lysed cells from triplicate cultures. Background:100 RLU. There were no significant differences among the groups.
Discussion
A significant reduction of CFU counts by TBA61 treatment has been reproducible in several independent experiments when harvesting the lungs 9 days after aerosol infection. The described protection against early tuberculous infection presumably required a sufficiently high concentration of specific IgA antibodies in the lungs. However, the protective effect did not persist for a long period, probably due to the action of powerful proteases against IgA within the respiratory tract fluid.18 Longer lasting effects could possibly be achieved in the future by complexing the IgA molecules with protease inhibitors.19 Ultimately, persisting IgA antibody levels in the lungs induced by prophylactic intranasal vaccination could impart much more stable protection. As intranasal immunization with the mycobacterial PstS-1 antigen in adjuvants led to high numbers of IgA producing cells in the lungs and high IgA antibody levels in the bronchoalveolar lavage13,20 it would be feasible to achieve similar results also for the acr antigen, as it is known to be one of the most immunogenic constituents of tubercle bacilli in man as well as animals.21
The protective effect of IgA mAbs against tuberculous lung infection in our experimental model involved the following two important factors: (1) A role of the IgA isotype, suggested by the finding that an IgG1 mAb of the same specificity was much less effective. The IgA action could have involved a number of mechanisms: e.g. (i) superior homing to the lungs following intranasal (but not intravenous) delivery;14 (ii) antibody-dependent cellular cytotoxicity;22 and (iii) stimulation of antigen presenting cells, required for T-cell activation.23 (2) Binding specificity against the acr antigen, which is present on the surface of tubercle bacilli24 and has enhanced expression in organisms grown within infected macrophages.25 The latter attribute of this antigen could be important, because IgA mAbs against another surface expressed antigen Pst-S1 were much less effective.
In the light of our observations, a number of possible mechanisms of protection have been identified, while others can possibly be discounted. It may be argued, that IgA antibodies are not acting by ‘exclusion of infection’ on the grounds of: (1) failure of their 24-hr prechallenge inoculation to reduce consistently the 9-day lung infection; (2) failure to inhibit the infection of macrophages in vitro; and (3) lack of any reduction of lung CFUs 1 day following aerosol infection of mice, although the latter result could also be interpreted, that clearance of opsonized mycobacteria may take longer than 24 hr. Antibody-mediated agglutination of tubercle bacilli seems unlikely to play a role, given the low infective inocula that would make it practically impossible for two bacteria to come into contact with one another, considering the volume/surface area of the lungs. The finding that the monomeric form of TBA61 was protective suggests targeting of IgA to an Fcα receptor on infected murine lung macrophages.26 The slightly lesser protection with polymeric TBA61 could have been because of its attachment to the secretory component within the bronchoalveolar lavage13 leading to its sequestration by epithelial cells en route to the infected lung alveoli. This outcome following intranasal application of IgAs is the opposite to the superior antiviral protection by polymeric versus monomeric IgA in experiments involving immunoglobulin transcytosis from blood into mucosal secretions.27
The observed synergistic action between pre- and postchallenge inoculated IgA antibody could also suggest the possible role of Fcα receptor mediated killing of organisms inside infected cells12 involving activation-dependent apoptosis of macrophages. Organisms released from apoptotic macrophages could be newly opsonized and re-targeted via the same Fcα receptor phagocytic pathway as uninfected cells, thus resulting in additional killing of the bacteria. Such possibility offers an explanation for the beneficial effect of IgA inoculation postinfection. Alternatively, IgA mAbs could act on bacilli released from macrophages by the early cytotoxic action of γδ T cells.28
In conclusion, our results support the recent increased interest in the role of antibodies in controlling intracellular microbial infections,5–7 and particularly for exploiting the IgA isotype for protection.12 At present, our results provide evidence for a novel mode of defence against tuberculosis. This has potential for application, particularly for immunoprophylaxis in immunocompromised hosts at risk of tuberculous infection, when vaccination is not possible. The protective mechanisms operating in vivo, but not in vitro, which need further study are: changes in the localization of the infected macrophages or in lung histopathology and analysis of lymphocyte subsets as well as other cells of the lung inflammatory infiltrate. Our data suggest, that further analysis of mechanisms of antibody action on intracellular infections is emerging as a fertile field for future research.
Note added in proof
The IgA mediated protection against Mtb infection may involve mechanisms described in the following paper: Reljic R, Crawford C, Challacombe S, Ivanyi J. Mouse IgA inhibits cell growth by stimulating TNFα and apoptosis of macrophage cell lines. International Immunology, in press.
Acknowledgments
This investigation was supported by the grant QLK2-1999-00367 from the EU Commission. The studies at CAMR were supported in part also by the U.K. Department of Health. The views expressed in the publication are those of the authors and not necessarily those of the funding bodies.
Abbreviations
- LAM
lipoarabinomannan
- polyIgR
polyIg receptor
- RLU
relative luminescence units
- PBS
phosphate-buffered saline
- CFU
colony-forming units
- mAbs
monoclonal antibodies.
References
- 1.Surcel HM, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G, et al. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–32. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95:15688–93. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–4. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–7. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 6.Winslow GM, Yager E, Shilo K, Volk E, Reilly A, Chu FK. Antibody-mediated elimination of the obligate intracellular bacterial pathogen ehrlichia chaffeensis during active infection. Infect Immun. 2000;68:2187–95. doi: 10.1128/iai.68.4.2187-2195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–12. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 8.Costello AM, Kumar A, Narayan V, Akbar MS, Ahmed S, Abou-Zeid C, et al. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg. 1992;86:686–92. doi: 10.1016/0035-9203(92)90192-f. [DOI] [PubMed] [Google Scholar]
- 9.Cree IA, Sharpe S, Sturrock NDC, Cochrance IH, Dawlako EC, Smith WCS, et al. Mucosal immunity to mycobacteria in leprosy patients and their contacts. Lepr Rev. 1988;59:309–16. doi: 10.5935/0305-7518.19880038. [DOI] [PubMed] [Google Scholar]
- 10.Hamasur B, Haile M, Pawlowski A, Schroder U, Williams A, Hatch G, et al. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine. 2003;21:4081–93. doi: 10.1016/s0264-410x(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri FM, Johansen K, Basile G, Kraehenbuhl JP, Svensson L. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J Virol. 1998;72:2708–14. doi: 10.1128/jvi.72.4.2708-2714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellwig SM, van Spriel AB, Schellekens JF, Mooi FR, van de Winkel JG. Immunoglobulin A-mediated protection against Bordetella pertussis infection. Infect Immun. 2001;69:4846–50. doi: 10.1128/IAI.69.8.4846-4850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falero-Diaz G, Challacombe S, Banerjee D, Douce G, Boyd A, Ivanyi J. Intranasal vaccination of mice against infection with Mycobacterium tuberculosis. Vaccine. 2000;18:3223–9. doi: 10.1016/s0264-410x(00)00134-1. [DOI] [PubMed] [Google Scholar]
- 14.Falero-Diaz G, Challacombe S, Rahman D, Mistry M, Douce G, Dougan G, et al. Transmission of IgA and IgG monoclonal antibodies to mucosal fluids following intranasal or parenteral delivery. Int Arch Allergy Immunol. 2000;122:143–50. doi: 10.1159/000024370. [DOI] [PubMed] [Google Scholar]
- 15.Ivanyi J, Morris JA, Keen M. Studies with monoclonal antibodies to mycobacteria. In: Macario AJL, Macario EC, editors. Monoclonal Antibodies Against Bacteria. New York: Academic Press; 1985. pp. 59–90. [Google Scholar]
- 16.Williams A, Davies A, Marsh PD, Chambers MA, Hewinson RG. Comparison of the protective efficacy of bacille Calmette–Guérin vaccination against aerosol challenge with Mycobacterium tuberculosis and Mycobacterium bovis. Clin Infect Dis. 2000;30(Suppl. 3):S299–301. doi: 10.1086/313878. [DOI] [PubMed] [Google Scholar]
- 17.Snewin VA, Gares MP, Gaora PO, Hasan Z, Brown IN, Young DB. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect Immun. 1999;67:4586–93. doi: 10.1128/iai.67.9.4586-4593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds HY. Identification and role of immunoglobulins in respiratory secretions. Eur J Respir Dis Suppl. 1987;153:103–16. [PubMed] [Google Scholar]
- 19.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285–96. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez A, Troye-Blomberg M, Lindroth K, Ivanyi J, Singh M, Fernandez C. B- and T-cell responses to the mycobacterium surface antigen PstS-1 in the respiratory tract and adjacent tissues. Role of adjuvants and routes of immunization. Vaccine. 2003;21:458–67. doi: 10.1016/s0264-410x(02)00478-4. [DOI] [PubMed] [Google Scholar]
- 21.Ivanyi J, Thole J. Specificity and function of T and B cell recognition in tuberculosis. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection and Control. Washington DC: ASM Press; 1994. pp. 437–58. [Google Scholar]
- 22.Tagliabue A, Boraschi D, Villa L, Keren DF, Lowell GH, Rappuoli R, et al. IgA-dependent cell-mediated activity against enteropathogenic bacteria: distribution, specificity, and characterization of the effector cells. J Immunol. 1984;133:988–92. [PubMed] [Google Scholar]
- 23.Arulanandam BP, Raeder RH, Nedrud JG, Bucher DJ, Le J, Metzger DW. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J Immunol. 2001;166:226–31. doi: 10.4049/jimmunol.166.1.226. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham AF, Spreadbury CL. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J Bacteriol. 1998;180:801–8. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Y, Crane DD, Simpson RM, Zhu YQ, Hickey MJ, Sherman DR, et al. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci USA. 1998;95:9578–83. doi: 10.1073/pnas.95.16.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauldie J, Richards C, Lamontagne L. Fc receptors for IgA and other immunoglobulins on resident and activated alveolar macrophages. Mol Immunol. 1983;20:1029–37. doi: 10.1016/0161-5890(83)90044-5. [DOI] [PubMed] [Google Scholar]
- 27.Renegar KB, Small PA., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–8. [PubMed] [Google Scholar]
- 28.Dieli F, Ivanyi J, Marsh PD, Williams A, Naylor I, Sireci G, et al. Characterization of lung γδT dells following intranasal infection with Mycobacterium bovis Bacillus Calmette–Guérin. J Immunol. 2003;170:463–9. doi: 10.4049/jimmunol.170.1.463. [DOI] [PubMed] [Google Scholar]