Abstract
We have previously shown that human CD3− CD56+ and CD3+ CD56+ cells from some individuals mount vigorous proliferative responses to lipopolysaccharide. Such responses have been blocked by the presence of cytotoxic T-lymphocyte antigen-4 immunoglobulin fusion protein in the cultures, implicating a role for B7-mediated costimulation. Here we confirm this inhibition of natural killer (NK) expansion using antibodies against B7-1 and B7-2. We were unable to specifically detect CD28 on the surface of resting or stimulated human peripheral blood NK cells, however, in either lipopolysaccharide-responsive or non-responsive individuals, using a panel of four different anti-CD28 monoclonal antibodies. T-cell depletion from peripheral blood mononuclear cell cultures resulted in a reduction in the induction of CD25 on activated CD3− CD56hi cells and in the expansion and proliferation of CD3− CD56+ NK cells. Furthermore, reconstitution experiments using peripheral blood dendritic cells and purified NK cells demonstrated that NK expansion could only be achieved in the presence of purified T cells.
Introduction
Human natural killer (NK) cells can be stimulated to proliferate in vitro using combinations of exogenous recombinant cytokines. In general, optimal NK cell proliferation requires the combination of interleukin-12 (IL-12) in addition to a growth factor such as IL-2, IL-7, IL-15, which signal and through the common γ-chain of the IL-2 receptor complex.1–6 Furthermore T-cell-derived IL-21 has recently been shown to induce proliferation in human bone-marrow-derived NK cells.7,8
We have shown that lipopolysaccharide (LPS) induces the proliferation of human peripheral blood CD3− CD56+ cells.9 This proliferative response is dependent on IL-12 and is blocked by cytotoxic T-lymphocyte antigen-4 immunoglobulin fusion protein (CTLA-4Ig), suggesting a role for CD80/86-mediated costimulation.9 Such a role for CD80/86 would seem plausible considering the ability of LPS to augment the expression of these molecules and of monocyte-derived dendritic cells (DCs) to activate human NK cells.9–12 Such costimulation could therefore support NK cell growth by acting on T cells expressing CD28 and driving T-cell-derived growth factor production. Alternatively, up-regulation of CD80/86 could directly engage CD28 expressed on NK cells in the absence of any third party role for T cells.
The CD80/86−CD28 axis is likely to play an important role in NK–DC interactions as cytolytic activity against autologous DCs is lost in NK cells from CD28 knockout mice.13 The induction of interferon-γ (IFN-γ) in murine NK cells is also enhanced by CD80/86–CD28 interaction and murine NK cells express low levels of CD28.14 These studies did not explore, however, whether analogous to its role in T-cell activation CD28-mediated costimulation directly influences NK cell proliferation. Two reports have demonstrated that transfection of target cell lines with human B7-1 (CD80) induces activation markers such as CD69 and cytolytic activity in human NK cells.15,16 Furthermore, CD28, the major costimulatory ligand for CD80/86, has been shown to be present on the surface of human NK cells using one single monoclonal antibody (mAb).15 However, other studies have raised doubts on the expression of CD28 by human NK cells using a series of mAbs recognizing CD28 only on T cells.15–17
In this paper we investigate whether the costimulatory CD80/86−CD28 axis acts directly on NK cells during LPS-induced proliferation or whether NK cells were being stimulated by a bystander effect of T-cell activation.
Materials and methods
Cell preparation and culture
Peripheral blood mononuclear cells (PBMC) were prepared from blood taken with informed consent from healthy adult volunteers. Cell stimulations were performed in bulk cultures using 1 × 106 cells/ml in RPMI-1640 + 10% autologous plasma. LPS (Sigma, Poole, UK) was used at 1 µg/ml unless otherwise stated. For inhibition experiments humanized anti-CD80 and CD86 mAbs (Genetics Institute, Cambridge, MA) were each used at 5 µg/ml.
T-cell depletions were performed using unconjugated anti-CD3 mAb (OKT3, American Type Culture Collection, Rockville, MD) and anti-mouse conjugated magnetic beads (Miltenyi Biotech, Bergish Gladbach, Germany). After depletion less than 1% of contaminating T cells were detected.
Purification of NK cells, T cells and DCs for reconstitution experiments was performed by negative selection using magnetic bead isolation kits (Miltenyi Biotech). These experiments were performed using buffy coats (obtained from North Thames Blood Transfusion Service, London, UK) and all cell fractions were from a single donor for each experiment. NK cells and T cells were >98% pure and total myeloid + lymphoid blood DCs (enriched total HLA-DR high, CD11b negative population) achieved a purity of >78%. Cells were mixed in the following proportions: NK cells 1 × 105, T cells 5 × 105 and DCs 5 × 104 in a total of 1 ml culture volume. Proliferation was measured by the incorporation of [3H]thymidine after 7 or 8 days of culture, as specified.
Flow cytometry
The expression of CD28 on NK cells was monitored using the following combinations of mAbs: anti-CD56 biotin (mouse IgG1, BD Pharmingen, Oxford, UK) together with anti-CD3 fluorescein isothiocyanate (FITC) (mouse IgG1, BD Pharmingen) and phycoerythrin (PE) conjugated anti-CD28 mAb − CD28.2 (mouse IgG1, BD Pharmingen), L293 (mouse IgG1, BD Biosciences, Oxford, UK) or YTH913.12 (rat IgG2b, Serotec, Oxford, UK). Mouse IgG1-PE isotype control reagents (BD Pharmingen) were used for CD28.2 and L293 and rat IgG2b-PE (Serotec) for YTH913.12. Cell stainings were performed for three-colour immunofluorescence by simultaneous addition of anti-CD3, anti-CD56 and either anti-CD28 or an isotype control reagent and incubation on ice for 30 min. Cells were then washed twice and incubated with streptavidin Quantum Red (Sigma, Poole, UK) to detect the anti-CD56 reagent.
Isotype blocking experiments were performed by prior incubation of PBMC with varying concentrations of unconjugated rat IgG2b with irrelevant specificity (Serotec) followed by 10 µg/ml anti-CD28-PE (YTH913.12) along with anti-CD3 and anti-CD56 to identify NK cell and T-cell subpopulations. Mean fluorescence intensities for YTH913.12 binding were then calculated on the entire gated cell populations in the presence or absence of the unconjugated rat IgG antibody.
To assess the proportion of NK cells after culture PBMC were labelled with anti-CD56 biotin (BD Pharmingen) along with the following combinations of mAbs: anti-CD3 Quantum Red and anti-CD94-FITC (mouse IgG1, BD Pharmingen) or anti-CD3-FITC with anti-CD25 Quantum Red (mouse IgG1, Serotec). A total of 20 000 ungated events were acquired for CD28 expression and T-cell depletion experiments and 10 000 ungated events were acquired for all other experiments.
Bromodeoxyuridine labelling for flow cytometry was performed using the BrdU FLUOS kit (Boehringer Mannheim, Heidelberg, Germany) according to the following protocol. PBMC were cultured at 1 × 106/ml in the presence or absence of LPS and cells were labelled with BrdU at a final concentration of 10 µg/ml for the last 16 h of culture. Cells were then washed and first labelled with PE-conjugated anti-CD56 mAb. Subsequently cells were washed in 70% ethanol, 50 mm glycine buffer and DNA denatured intracellularly in 4 m HCl. After neutralization of acid BrdU was detected using an FITC-conjugated anti-BrdU antibody. The specificity of the detection of BrdU was controlled by omission of BrdU in control cultures. Cells were then analysed on a FACScan flow cytometer at 488 nm.
Statistical tests were performed, where possible using a Student's paired t-test.
Results
Anti-B7-1 and anti-B7-2 mAbs inhibit NK cell expansion in response to LPS
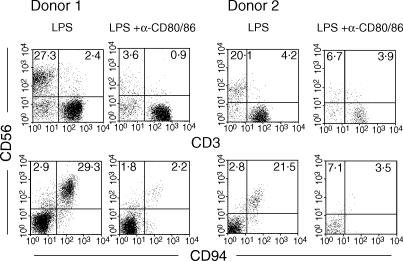
We have previously shown that NK cell expansion in response to LPS can be inhibited by CTLA-4Ig, most probably by blocking costimulation via CD80/86.9 The proportion of NK cells within PBMC after LPS stimulation correlates with their expansion as demonstrated previously by CFSE incorporation.9 Furthermore, increases in the proportion of CD3− CD56+ cells in the presence of LPS are not due to a reduction in the proportions of CD56− T and B cells, which are more resistant to cell death (Goodier and Londei, unpublished results). To confirm the role of CD80/86-mediated costimulation we therefore measured the proportions of CD3− CD56+ cells after LPS stimulation of PBMC in the presence or absence of humanized anti-CD80 and anti-CD86 mAbs to block these molecules directly. Results for two representative donors are shown in Fig. 1. We found that the combination of anti-CD80 and anti-CD86 completely blocked the LPS-mediated expansion of CD3− CD56+ cells as shown by the lack of CD56hi CD94hi cells after 8 days in culture compared to control cultures (Fig. 1). Similar results were observed in a total of four individuals (range CD3− CD56+, LPS = 12·4%−36·8%, LPS + anti-B7 = 3·6%−13·8%, P = 0·031; range CD56+ CD94+, LPS = 15·8%−38·5%; LPS + anti-B7 = 3·6%−13·0%, P = 0·017).
Figure 1.
B7 requirement for NK cell expansion. PBMC were preincubated with anti-B7 and subsequently stimulated with LPS as described in Materials and Methods. Cells were taken after 8 days of stimulation and the proportions of gated CD3− CD56hi CD94hi cells were estimated by flow cytometry. Results for two out of four individuals are shown, all of which gave similar results.
CD28 cannot be specifically detected on NK cells
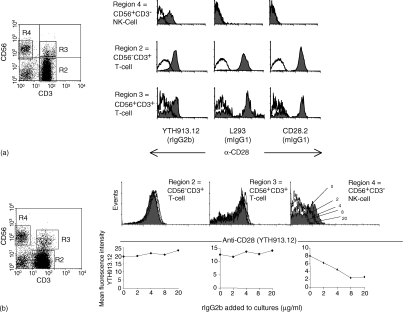
Two reports in human systems have implicated a role for CD28 in human NK cell activation, one of these identifying a variant of this molecule on the cell surface.15,16 We therefore considered the possibility that CD28 was playing a direct role in the stimulation and expansion of human NK cells by LPS and that CTLA-4Ig or anti-B7 reagents were abolishing this interaction. We decided to investigate the presence of a variant CD28 on NK cells compared to T cells.15 CD3− CD56+ cells were counterstained with three anti-CD28 mAbs and isotype-matched control reagents as previously reported.15 Figure 2(a) shows that, as reported previously, only a single mAb, YTH913.12, stains gated CD3− CD56+ NK cells compared to the isotype-matched control. All three mAbs show positive staining on both CD3+ CD56+ and CD3+ CD56− subsets.
Figure 2.
Expression of CD28 on cell subsets. (a) Freshly isolated PBMC were labelled with mAbs to identify the expression of CD28 on gated T-cell and NK subsets (solid fill) by comparison to an isotype-matched control reagent, as described in Materials and Methods. Gates used are shown in the left-hand panel. (b) The binding of YTH913.12 (10 µg/ml) on CD3+ CD56− and CD3+ CD56+ subsets was examined in the presence of an excess of rat IgG2b (three upper panels) and the effect of rat IgG2b on the binding and mean fluorescence intensity for YTH913.12 on gated cell subsets is shown (three lower panels). The concentrations of rat IgG2b added are indicated on the histogram for CD3− CD56+ cells.
We noted, however, that the reagent binding to the NK cells (CD3− CD56+) was a rat IgG2b antibody, an isotype that is well known to bind to FCγRIII and isoforms of FcγRII present on NK cells in some individuals.18,19 It was therefore important to ensure that the PE-conjugated isotype reagent adequately controlled for Fc receptor binding and the YTH913.12 mAb was not reacting with Fc receptors on the surface of NK cells. We took PBMC from individuals in whom the YTH913.12 mAb ‘labelled’ CD3− CD56+ cells, and titrated an unconjugated rat IgG2b antibody onto these cells prior to the addition of PE-conjugated YTH913.12. Cells were then gated for analysis on CD3− CD56+ NK cells, CD3+ CD56+ T cells and CD3+ CD56− T cells. Titration of rat IgG2b had no effect on the labelling of CD3+ CD56− or CD3+ CD56+ T cells with YTH913.12 (Fig. 2b, two left-hand panels). However, the intensity of YTH913.12 staining on CD3− CD56+ cells reduced progressively with increasing rat IgG2b isotype control concentration (Fig. 2b, right-hand panels). These results show that, in the presence of rat IgG2b, YTH913.12 staining on T cells and NK cells resembles that observed with the L293 and CD28.2 mAbs, and suggests that CD28 is in fact absent from the surface of NK cells.
Removal of T cells results in reduced CD25 expression and proliferation in NK cells
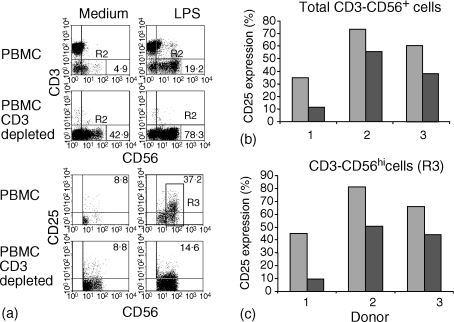
In view of the apparent lack of CD28 on NK cells, we questioned whether factors generated through B7−CD28-mediated costimulation of T cells could be responsible for the activation and expansion of NK cells. To investigate this possibility, we depleted T cells from the PBMC of LPS-responsive individuals using anti-CD3 and magnetic beads prior to LPS stimulation of cultures. The resulting cultures were then assessed for CD25, the expression of which is induced on NK cells by IL-2 and which correlates with proliferative activity in NK cells.20
Substantial CD25 expression was observed on gated CD3− CD56+ NK cells in undepleted cultures following LPS stimulation. T-cell depletion resulted in a decrease in CD25 expression on CD3− CD56+ cells in all individuals tested (Fig. 3a, lower panels). The effect of T-cell depletion on CD25 expression in total CD3− CD56+ cells is shown for three individuals in Fig. 3(b). The proportion of CD3− CD56+ cells expressing CD25 was reduced from 35·0%−73·5% in PBMC to 11·0%−55·4% in CD3-depleted PBMC in the individuals tested (P = 0·007). The decrease in CD25 in CD3-depleted cultures occurred despite enrichment for CD56+ cells and was directly reflected in reduced CD25 expression in the activated CD56hi population (Fig. 3c). The proportion of CD56hi cells expressing CD25 was 45·1%−82% without CD3 depletion and 9·8%−50·8% in depleted cultures for the three individuals tested (P = 0·019).
Figure 3.
T-cell requirement for induction of CD25 on NK cells. PBMC (upper panels) or CD3+ T-cell-depleted PBMC (lower panels) were cultured for 7 days in the presence or absence of LPS. (a) Gated CD3− CD56+ cells (R2, four upper panels) were then assessed for the expression of CD25 (four lower panels). The relative proportions of CD25+ cells were then estimated after LPS stimulation of undepleted or CD3-depleted PBMC in three individual donors (b) as a percentage of total CD3− CD56+ cells and (c) as a percentage of gated CD3− CD56hi cells according to region 3 (R3) shown in (a).
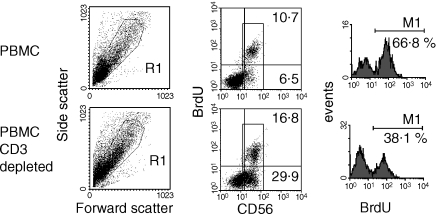
Depleted cultures were then assessed for proliferation. As observed for CD25 expression, a substantial decrease in proliferation occurred after depletion of CD3+ cells and despite enrichment for CD3− CD56+ cells (Table 1). The range of LPS-induced proliferation was 4199–35 503 counts per minute (c.p.m.) prior to depletion and 738–6531 c.p.m. after T-cell depletion in four individuals tested. Furthermore, this reduction in proliferation was reflected in a lower proportion of CD56+ cells incorporating BrdU in CD3-depleted cultures despite enrichment for these cells prior to stimulation (Fig. 4). The proportion of CD56+ cells incorporating BrdU was reduced from 57·0%−63·7% to 34·9%−43% in three individuals tested (P = 0·044).
Table 1.
Depletion of CD3+ cells reduces expansion of CD3− CD56+ cells and proliferation
| Cells | Stimulus | Percentage of total cells CD56+ CD3− | CD56+ CD3+ | Proliferation (c.p.m. ± SD) |
|---|---|---|---|---|
| Experiment 1 | ||||
| Undepleted | Medium | 1·9 | 0·4 | 1896 ± 48 |
| LPS | 18·2 (9·6) | 2·1 | 35503 ± 1242 | |
| CD3 depleted | Medium | 25·5 | <0·1 | 975 ± 25 |
| LPS | 71·2 (2·8) | 0·2 | 6531 ± 94 | |
| Experiment 2 | ||||
| Undepleted | Medium | 3·7 | 1·0 | 621 ± 124 |
| LPS | 7·4 (2·0) | 6·8 | 10089 ± 416 | |
| CD3 depleted | Medium | 37·0 | 0·4 | 278 ± 23 |
| LPS | 56·1 (1·5) | 0·6 | 3106 ± 153 | |
PBMC from two individual donors were depleted of CD3+ cells or left undepleted as described in Materials and Methods. Cells were stimulated with LPS or left in medium alone and cell phenotype and proliferation was tested after 7 days. Consistent results were obtained in two further individuals. Fold changes in the proportion of CD3− CD56+ cells are shown in parentheses.
Figure 4.
Reduced proportion of proliferating CD56+ cells in CD3-depleted cultures. LPS-stimulated PBMC (upper panels) or CD3-depleted PBMC (lower panels) were labelled with BrdU as described in Materials and Methods and the proportion of proliferating NK cells was estimated after gating on CD56+ cells (right-hand panels). Similar results were observed in two further individuals.
Peripheral blood DCs support NK cell expansion only in the presence of T cells
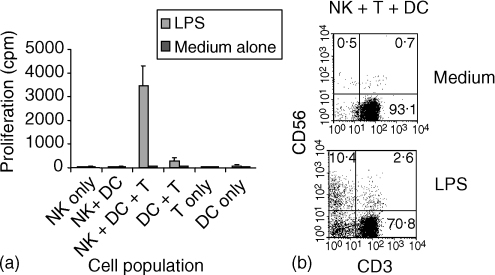
We have shown previously that cell cultures depleted of major histocompatibility complex class II positive cells, i.e. containing NK cells and T cells but not B cells or myeloid cells, do not proliferate in response to LPS.9 Furthermore, reconstitution of such cultures with monocyte-derived DCs leads to a recovery in the proportion of CD3− CD56+ cells and proliferation in response to LPS.9 The presence of T cells in those experiments and the effects of T-cell depletion shown here, however, suggest a possible role for T cells in DC-driven NK expansion. We therefore investigated the role of T cells more closely by purifying peripheral blood DCs and T cells and performing reconstitution experiments to assess the contribution of these cell populations to the expansion of purified NK cells. NK cell proliferation was assessed in cultures stimulated with LPS for 7 days in the presence or absence of DCs and T cells (Fig. 5a). Little or no proliferation was observed in response to LPS for the three cell types when cultured alone. Coculture of purified NK cells and purified DCs also resulted in little proliferation, whereas LPS stimulation resulted in substantial proliferation when all three cell types were present (Fig. 5a). Only marginal proliferation was observed in cocultures of purified DCs and T cells. An increase in the proportion of NK cells after 7 days of culture was also observed by flow cytometry (Fig. 5b). These results confirm a likely requirement for T cells, in addition to DCs, in LPS-driven NK expansion.
Figure 5.
DC and T-cell requirement for NK expansion. Cell subsets were prepared as described in Materials and Methods and mixed in different combinations prior to stimulation with LPS. Proliferation (a) and NK cell expansion (b) were estimated after 7 days.
Discussion
This paper confirms the ability of reagents interacting with CD80/86, namely anti-B7 antibodies and CTLA-4Ig, to inhibit LPS-mediated expansion of human NK cells. These results are in agreement with previous observations in systems designed to study the early activation of human NK cells and target cell recognition. For example, K562 cells transfected with CD80 stimulated enhanced NK cell CD69 expression and cytolytic activity in syngeneic and xenogeneic systems compared to non-transfected controls.15,16,21
It is uncertain whether CD28 on NK cells plays a direct role in these responses, despite published evidence for the expression of a variant CD28 molecule, recognized by a single mAb.15–17 We show that the anti-CD28 mAb, YTH913.12, no longer binds NK cells in the presence of an excess of unconjugated isotype control mAb. It is therefore likely that the PE-conjugated rat IgG2b isotype-matched control reagent used here (Fig. 2a) and that used in the previous study15 were not as effective in Fc binding. Furthermore, specific binding of the YTH913.12 mAb was confirmed by the staining of T cells, where binding was unaffected by the presence of excess rat IgG2b. A further less likely possibility, but one which could reconcile our observations and those of others,15–17 is that the binding of YTH913.2 to FcR could stabilize its interaction with CD28 on NK cells.
The possibility that CD28 could be transiently induced in NK cells has not been excluded in our study. However, we were unable to detect the binding of all the anti-CD28 mAbs used to NK cells after stimulation with LPS, confirming that this is not stably induced on the cell surface (data not shown). In view of the apparent lack of specific binding of anti-CD28 reagents to human NK cells it was necessary to reappraise our observations on the effect of B7 blockade. These observations, however, supported the possibility that B7 blockade may mediate its effects by acting on CD28-expressing T cells. Indeed, the T-cell depletion experiments performed here specifically resulted in reduced NK cell specific CD25 expression and proliferation.
All human NK cells constitutively express intermediate affinity receptors for IL-2 (CD122 homodimers) and can therefore require high concentrations of exogenous IL-2 for stimulation in vitro.4 However, only the minor CD56hi CD16negative NK cell subset constitutively expresses CD25 to form a high affinity IL-2 receptor complex and this can be induced on the remaining majority of NK cells on activation.22 IL-2 is itself a potent inducer of CD25 on NK cells. This therefore suggests that T-cell-derived IL-2 or other growth factors may be responsible for the LPS-induced CD25 expression on the majority of NK cells in our cultures. The expression of CD25 on NK cells may be critical for NK cell proliferation in response to the low concentrations of IL-2 available in response to LPS. We have observed in some LPS-responsive individuals that neutralizing mAbs to CD25 or to IL-2 itself partially inhibit the expansion of CD3− CD56+ NK cells (by up to 45%) (data not shown). This effect was not achieved by neutralizing other NK growth factors such as IL-15 and IL-18 or their receptors in the same experiments (data not shown).
We have consistently observed that neutralizing antibodies to IL-12 effectively block LPS-induced NK cell expansion.9 Similarly to the high affinity IL-2 receptor, the functional receptor for IL-12 is constitutively expressed on only the minor CD3− CD56hi NK subset.22 Exogenous IL-2 has been shown to induce the expression of functional IL-12 receptors and signal transducer and activator of transcription type 4 activation in NK cells and could in this way prime these cells to respond to IL-12 induced by LPS in myeloid cells.23,24
Monocyte-derived and peripheral blood DCs have been shown to support the early activation of purified human NK cells.11,12 In our previous studies we also showed that the depletion of HLA-DR+ cells from PBMC resulted in a loss of NK cell proliferation and expansion and that this could be reconstituted with autologous monocyte-derived DCs.9 We show here that the LPS-driven expansion and proliferation of purified NK cells cannot be driven by peripheral blood DCs alone and requires T cells. It is therefore possible that the early activation of NK cells can be achieved by DC/myeloid cell-derived factors alone but that T cells provide the necessary growth factors for subsequent proliferation and expansion. This is supported by our observations in T-cell-depleted cultures where, although a higher proportion of NK cells have an activated phenotype (CD56hi) upon LPS stimulation, proportionally fewer of these express CD25. Others have shown that monocyte-derived DCs pulsed with intact bacteria can drive enhanced proliferation of purified NK cells.25 This would seem to contrast with our results where proliferation and expansion of NK cells occurs only in the presence of T cells. However, these and other studies contrast with ours in so far as the NK cells used have been polyclonally expanded prior to resting and restimulation. In these studies, a higher proportion of NK cells may have acquired some of the regulated components of cytokine receptor chains, in particular IL-12Rβ and CD25.12,25 In this way, previously activated NK cells could respond to DCs via the IL-12 pathway, which could synergize with autocrine IL-2 production for the enhancement of proliferation in that study.25 It is further possible that components of intact/live bacteria activate distinct signals to purified LPS.
The ability of target cells transfected with B7-1 to induce enhanced CD69 expression and cytolytic activity in primary human NK cells or NK cell lines compared to non-transfected targets, however, remains to be explained.15,16 It is possible that an unknown receptor on NK cells interacts with B7 and provides sufficient signals for CD69 expression and cytolytic activity but not for CD25 expression and expansion. In this respect, and in view of the observed exclusive expression of CD28 on CD3+ CD56− and CD3+ CD56+ T cells in our cultures, we propose that costimulation blockade in LPS-stimulated PBMC is affecting these populations in the first instance, with subsequent consequences for NK cell proliferation.
The importance of T cells for NK cell maintenance and expansion could have consequences for innate NK immunity in diseases where the T-cell compartment is compromised. In chronic untreated human immunodeficiency virus type 1 infection, for example, the loss of CD4 T cells and cytokine-driven help could explain the parallel diminishment in NK cell numbers, particularly in the CD56lo D16hi population.26,27
Acknowledgments
This study was supported by European Community Grant QLK1-CT-1999-00037, Arthritis Research Campaign, UK, and the Wellcome Trust Grant 050453.
References
- 1.Gately MK, Desai BB, Wolitzky AG, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874. [PubMed] [Google Scholar]
- 2.Naume B, Gately M, Espevik T. A comparative study of IL-12 (cytotoxic lymphocyte maturation factor) -, IL-2-, and IL-7-induced effects on immunomagnetically purified CD56+ NK cells. J Immunol. 1992;148:2429. [PubMed] [Google Scholar]
- 3.Naume B, Johnsen AC, Espevik T, Sundan A. Gene expression and secretion of cytokines and cytokine receptors from highly purified CD56+ natural killer cells stimulated with interleukin-2, interleukin-7 and interleukin-12. Eur J Immunol. 1993;23:1831. doi: 10.1002/eji.1830230815. [DOI] [PubMed] [Google Scholar]
- 4.Warren HS, Kinnear BF, Kastelein RL, Lanier LL. Analysis of the costimulatory role of IL-2 and IL-15 in initiating proliferation of resting (CD56dim) human NK cells. J Immunol. 1996;156:3254. [PubMed] [Google Scholar]
- 5.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen KB, Salazar-Mather TP, Dalod MY, et al. Coordinated and distinct roles for IFN-αβ, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 7.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 8.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol. 2002;72:856. [PubMed] [Google Scholar]
- 9.Goodier MR, Londei M. Lipopolysaccharide stimulates the proliferation of human CD56+ CD3− NK cells: a regulatory role of monocytes and IL-10. J Immunol. 2000;165:139. doi: 10.4049/jimmunol.165.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919. [PubMed] [Google Scholar]
- 11.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geldhof AB, Moser M, Lespagnard L, Thielemans K, De Baetselier P. Interleukin-12-activated natural killer cells recognize B7 costimulatory molecules on tumor cells and autologous dendritic cells. Blood. 1998;91:196. [PubMed] [Google Scholar]
- 14.Walker W, Aste-Amezaga M, Kastelein RA, Trinchieri G, Hunter CA. IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-γ. J Immunol. 1999;162:5894. [PubMed] [Google Scholar]
- 15.Galea-Lauri J, Darling D, Gan SU, Krivochtchapov L, Kuiper M, Gaken J, Souberbielle B, Farzaneh F. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol. 1999;163:62. [PubMed] [Google Scholar]
- 16.Wilson JL, Charo J, Martin-Fontecha A, et al. NK cell triggering by the human costimulatory molecules CD80 and CD86. J Immunol. 1999;163:4207. [PubMed] [Google Scholar]
- 17.Lang S, Vujanovic NL, Wollenberg B, Whiteside TL. Absence of B7.1−CD28/CTLA-4-mediated co-stimulation in human NK cells. Eur J Immunol. 1998;28:780. doi: 10.1002/(SICI)1521-4141(199803)28:03<780::AID-IMMU780>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Haagen IA, Geerars AJ, Clark MR, van de Winkel JG. Interaction of human monocyte Fcγ receptors with rat IgG2b. A new indicator for the FcγRIIa (R-H131) polymorphism. J Immunol. 1995;154:1852. [PubMed] [Google Scholar]
- 19.Metes D, Ernst LK, Chambers WH, Sulica A, Herberman RB, Morel PA. Expression of functional CD32 molecules on human NK cells is determined by an allelic polymorphism of the FcγRIIC gene. Blood. 1998;91:2369. [PubMed] [Google Scholar]
- 20.Clausen J, Vergeiner B, Enk M, Petzer AL, Gastl G, Gunsilius E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology. 2003;207:85. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 21.Costa C, Barber DF, Fodor WL. Human NK cell-mediated cytotoxicity triggered by CD86 and Galα 1,3-Gal is inhibited in genetically modified porcine cells. J Immunol. 2002;168:3808. doi: 10.4049/jimmunol.168.8.3808. [DOI] [PubMed] [Google Scholar]
- 22.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang KS, Ritz J, Frank DA. IL-2 induces STAT4 activation in primary NK cells and NK cell lines, but not in T cells. J Immunol. 1999;162:299. [PubMed] [Google Scholar]
- 24.Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000;95:3183. [PubMed] [Google Scholar]
- 25.Ferlazzo G, Morandi B, D'Agostino A, Meazza R, Melioli G, Moretta A, Moretta L. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33:306. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 26.Mansour I, Doinel C, Rouger P. CD16+ NK cells decrease in all stages of HIV infection through a selective depletion of the CD16+ CD8+ CD3− subset. AIDS Res Hum Retroviruses. 1990;6:1451. doi: 10.1089/aid.1990.6.1451. [DOI] [PubMed] [Google Scholar]
- 27.Hu PF, Hultin LE, Hultin P, et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+ CD56+ cells and expansion of a population of CD16dim CD56− cells with low lytic activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:331. [PubMed] [Google Scholar]