Abstract
The signalling pathways leading to CXCL8/IL-8-induced human neutrophil migration have not been fully characterized. The present study demonstrates that CXCL8 induces tyrosine phosphorylation as well as enzymatic activity of proline-rich tyrosine kinase 2 (Pyk2), a non-receptor protein tyrosine kinase (PTK), in human neutrophils. Induction of Pyk2 tyrosine phosphorylation by CXCL8 is regulated by Src PTK activation, whereas it is unaffected by phosphatidylinositol 3-kinase activation. Inhibition of Pyk2 activation by PP1, a Src PTK inhibitor, is paralleled by the inhibition of CXCL8-mediated neutrophil chemotaxis. Among CXCL8 receptors, Src protein tyrosine kinase activation selectively regulates CXCR1-mediated polymorphonuclear neutrophil (PMN) chemotaxis. Overexpression of PykM, the kinase-dead mutant of Pyk2, blocks CXCL8-induced chemotaxis of HL-60-derived PMN-like cells, thus pinpointing the key role of Pyk2 in CXCL8-induced chemotaxis.
Introduction
Polymorphonuclear neutrophils (PMNs) respond to a variety of inflammatory mediators by enhancing margination to the endothelium and migrating from the bloodstream to the site of inflammation.1,2 PMN activation results in increased phagocytosis, bacterial killing, release of lysosomal enzymes and superoxide anion generation. These actions explain the central role that PMNs play in the acute inflammatory response and are closely associated with tissue injury.1,2
Among chemotactic factors generated at the site of inflammation, interleukin-8 (CXCL8/IL-8) is a key mediator of PMN recruitment. CXCL8 belongs to the C-X-C branch of the chemokine family, which also includes CXCL1/GRO, CXCL7/NAP-2, CXCL5/ENA-78 and CXCL6/GCP-2.3,4 CXCL8 receptors (CXCR1 and CXCR2) are seven-transmembrane-domain receptors known to couple intracellular signal-transduction pathways involving the activation of Bordetella pertussis toxin-sensitive GTP-binding proteins, activation of phospholipase Cβ, formation of the second messenger inositol 1,4,5-triphosphate and the subsequent increase of intracellular calcium concentration.5–7 Phosphatidylinositol 3-kinase γ (PI3Kγ) activity has also been shown to increase in response to CXCL8; and the extracellular signal-regulated kinases (ERKs), belonging to the mitogen-activated protein kinase (MAPK) family, become activated in response to CXCL8.8,9 Although the intracellular mediators of CXCL8-induced cellular response have been extensively described, the signalling pathways required for CXCL8-induced PMN migration have not been fully characterized.
Proline-rich tyrosine kinase 2 (Pyk2) is a non-receptor protein tyrosine kinase (PTK) that is closely related to the p125 focal adhesion kinase (FAK). Pyk2 is expressed in different cell types, including brain cells, fibroblasts and haemopoietic cells.10–13 Human leucocytes express both p125 FAK and Pyk2, but only Pyk2 seems important for PMN cell functions.14,15 Pyk2 can interact with several signalling or cytoskeletal molecules, such as Src family PTKs, the Grb2 and p130Cas adaptors, paxillin and the Rho guanine nucleotide-exchange factor, Graf. Pyk2 couples several receptors, including integrin and chemokine receptors, with a variety of downstream effectors, including small G protein belonging to the Ras and Rho families, MAPKs, protein kinase C and inositol phosphate metabolism, thus regulating cell adhesion, migration, proliferation and survival.10,13,16,17
The present study demonstrates that CXCL8 induces Pyk2 tyrosine phosphorylation and enzymatic activity in human PMNs. By using a highly specific inhibitor of Src PTKs, namely PP1, we also report that Src PTKs are involved in CXCL8-induced Pyk2 phosphorylation. On the contrary, inhibition of PI3K activation by using the specific inhibitor, LY294002, does not affect CXCL8-induced Pyk2 phosphorylation. Inhibition of Pyk2 activation by PP1 is paralleled by the inhibition of CXCL8-mediated neutrophil chemotaxis. Moreover, overexpression of the kinase-dead mutant of Pyk2 (PykM), known to prevent Pyk2 enzymatic activity,10 blocked CXCL8-induced chemotaxis of HL-60-derived PMN-like cells. These data indicate that Pyk2 activation is a key event in mediating CXCL8-induced PMN migration.
Materials and methods
Materials
Recombinant CXCL8 was from PeproTech (Rocky Hill, NJ). RPMI-1640 and Opti MEM media were from Life Technologies (Paisley, UK). Fetal bovine serum (FBS) was from Hyclone (Sterile System; Logan, UT). Diff-Quik was from Harleco (Gibbstown, NJ). Micro Boyden chambers and polycarbonate filters were from Neuroprobe Inc. (Pleasanton, CA).
Anti-β2 integrin hybridoma was a kind gift of Dr F. Sanchez-Madrid (La Princesa Hospital, University of Madrid, Madrid, Spain). Anti-phosphotyrosine (anti-pTyr) immunoglobulin was from Upstate Biotechnology Inc., Laboratories (Lake Placid, NY); fluorescein-conjugated F(ab′)2 goat anti-mouse immunoglobulin was from Techno Genetics (Torino, Italy). Anti-CXCR1, anti-CXCR2, neutralizing anti-CXCR1, neutralizing anti-CXCR2 and anti-phospho-Pyk2 immunoglobulins were from BioSource International (Camarillo, CA). Rabbit antiserum anti-Pyk2 (600) and pcDNA encoding PykM were kindly provided by Dr J. Schlessinger (Department of Pharmacology, Yale University School of Medicine, New Haven, CT); goat polyclonal anti-Pyk2 (N19) was purchased from Santa Cruz Biotech. (Santa Cruz, CA). PP1 was from DBA Italia (Milano, Italy). Endotoxin [lipopolysaccharide LPS)] and LY294002 were from Sigma (St Louis, MO). [32P]ATP was from Amersham International (Bucks., UK).
Cells
PMNs were obtained from the buffy-coat of heparinized human peripheral blood from healthy volunteers through the courtesy of Centro Trasfusionale, Ospedale S. Salvatore (L'Aquila, Italy). PMNs were prepared to 99% purity by centrifugation through a Ficoll–Hypaque gradient.18
The human promyelocytic cell line, HL-60 (ATCC, Manassas, VA), was cultured in RPMI-1640 supplemented with 2 mm l-glutamine, 50 mg/ml gentamicin sulphate and 10% heat-inactivated FBS. HL-60 cells were induced to differentiate to PMN-like cells by exposure to 1·2% (v/v) dimethylsulphoxide (DMSO) for 5 days.19 Membrane expression of CXCR1 and CXCR2 was evaluated by using a fluorescence-activated cell sorter (FACScan; Becton Dickinson Immunocytometry System, San Jose, CA), as previously described.20
Differentiated HL-60 cells were transiently transfected by electroporation by the addition of 20 µg of pcDNA-derived plasmid (expressing PykM or the empty pcDNA3) to 400 µl of cell suspension, as described previously.21 Cell viability was >95% in all experiments, as measured by Trypan blue dye exclusion.
Migration assay
Cell migration was evaluated using a 48-well microchemotaxis chamber, as previously described.22 The chamber was incubated for 45 min (PMNs) or 2 hr (HL-60), at 37° in air with 5% CO2. At the end of incubation, filters were removed, fixed, stained with Diff-Quik and five oil-immersion fields at high magnification (100×) were counted after sample coding.
Immunoprecipitation and Western blotting
PMNs were resuspended in serum-free RPMI-1640 (5 × 107 cells per 300 µl tube), pretreated with 5 mm diisopropyl fluorophosphate (30 min at 37°) and then stimulated with vehicle, CXCL8 or anti-β2 monoclonal antibody (mAb).23 Stimulation was stopped by the addition of ice-cold wash buffer [0·4 mm Na2EDTA, 10 mm Na4P2O7, 10 mm NaF, 0·1 mm Na3VO4 in phosphate-buffered saline (PBS), pH 7·4], the cells were pelleted by centrifugation for 5 min at 500 g and then lysed for 30 min at 4° in ice-cold lysis buffer [1% (v/v) Triton-X-100, 1 mm CaCl2, 1 mm MgCl2, 0·1% NaN3, 1 mm phenylmethylsulphonyl fluoride (PMSF), 2 µg/ml aprotinin, 2 µg/ml leupeptin, 10 mm NaF, 10 mm iodoacetamide]. Lysates were clarified by centrifugation at 15 000 g for 15 min at 4° and the supernatants were subjected to immunoprecipitation with anti-Pyk2 (600) immunoglobulin, or immunoblotting. The immune complexes were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) (7·5% gel), transferred to nitrocellulose and immunoblotted with anti-pTyr or with anti-Pyk2 (N19) immunoglobulin.23 Pyk2 phosphorylation (HL-60) and PykM (HL-60) expression were evaluated in cell lysates by immunoblotting, as previously described.24
Immune complex kinase assay
PMNs were lysed in modified RIPA buffer (50 mm Tris–HCl, pH 7·5, 150 mm NaCl, 0·3% sodium deoxycholate, 0·1% Nonidet P-40, 1·5 mm MgCl2, 1 mm EDTA, 0·2 mm EGTA, 1 mm Na3VO4, 20 mm NaF, 25 µm ZnCl2, 1 mm PMSF, 10 µg/ml aprotinin and 10 µg/ml leupeptin), and equal amounts of cell lysates were immunoprecipitated with anti-Pyk2 (600) immunoglobulin. The immunoprecipitates were incubated, for 12 min at 23°, with 50 µl of Tris buffer containing 5 µCi [32P]ATP and 30 µg/ml poly(Glu-Tyr). The reaction was stopped by adding 2× SDS sample buffer, and 32P-labelled immunocomplexes were resolved on 7·5% SDS–PAGE. The gel was dried and the 32P-labelled proteins were made visible by autoradiography.
Results
CXCL8 induces tyrosine phosphorylation and tyrosine kinase activity of Pyk2 in human PMNs
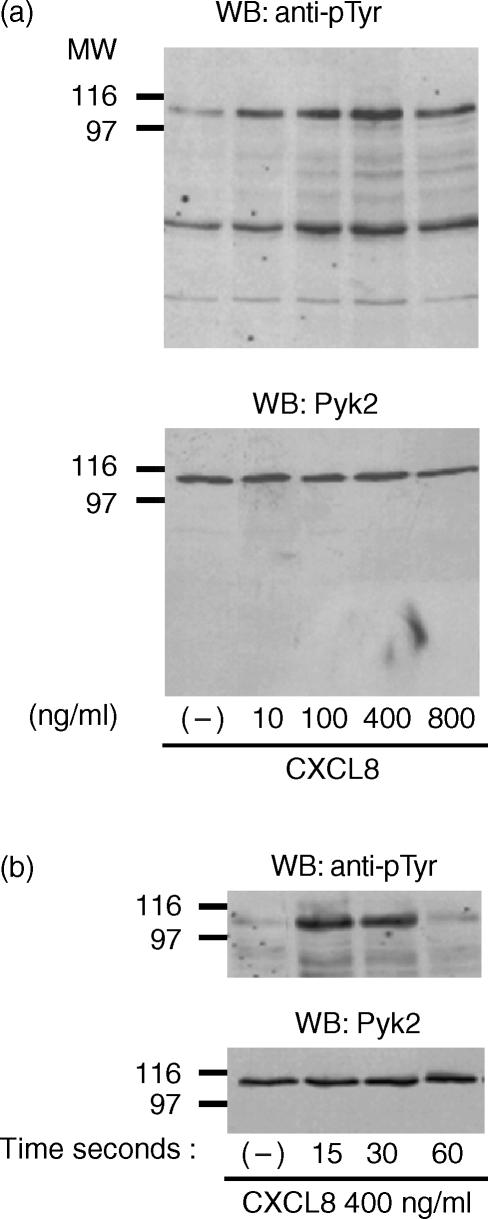
Treatment of PMNs with CXCL8 stimulated the tyrosine phosphorylation of an intracellular protein with an apparent molecular weight (MW) of 115 000 (Fig. 1). The effect of CXCL8 was concentration-dependent, starting at 10 ng/ml and reaching maximal levels of stimulation at 400 ng/ml (Fig. 1a, upper panel), and time-dependent, the 115 000-MW protein undergoing rapid phosphorylation after 15 and 30 seconds, and declining thereafter (Fig. 1b, upper panel). By reblotting the membranes with anti-Pyk2 immunoglobulin, we showed that the 115 000-MW band corresponds to Pyk2. Comparable amounts of Pyk2 protein were detected in each lane (Fig. 1a, 1b, lower panels).
Figure 1.
Interleukin-8 (CXCL8) induces tyrosine phosphorylation of an intracellular protein that corresponds to proline-rich tyrosine kinase 2 (Pyk2) in human polymorphonuclear neutrophils (PMNs). (a) PMNs were stimulated with vehicle or increasing concentrations of CXCL8 for 15 seconds at 37°. (b) PMNs were stimulated for different periods of time with vehicle or CXCL8 (400 ng/ml). The lysates were analysed by immunoblotting with antibody to phosphotyrosine (anti-pTyr) (upper panels) immunoglobulin. The same filters were probed with anti-Pyk2 (N19) immunoglobulin (lower panels). Data shown in the figure represent one of five independent experiments, with similar results obtained in each. MW, molecular weight; WB, Western blot.
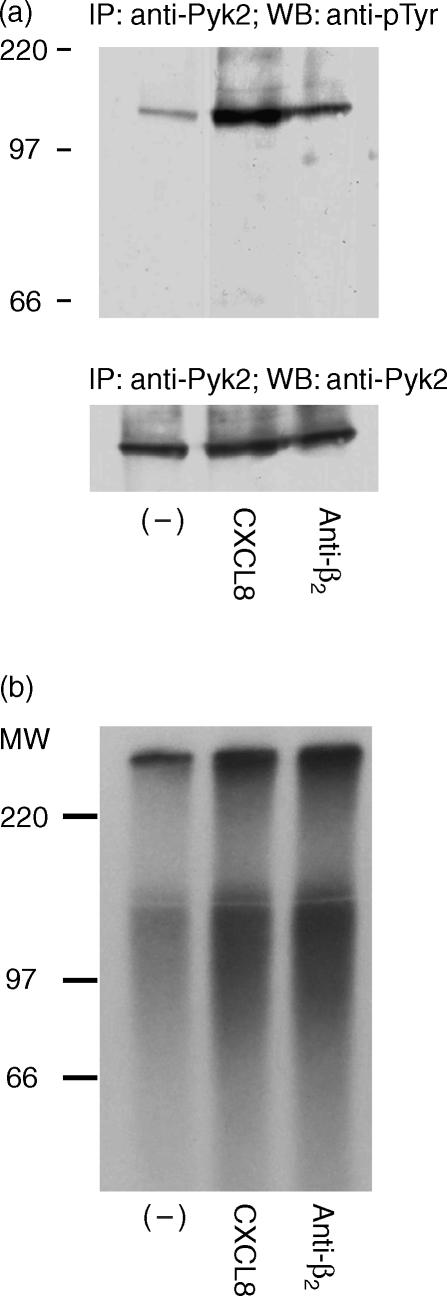
Induction of Pyk2 by CXCL8 was also confirmed by immunoprecipitation experiments. When suspended PMNs were stimulated with CXCL8 (400 ng/ml for 15 seconds), immunoprecipitation with anti-Pyk2 followed by immunoblotting with anti-pTyr demonstrated tyrosine phosphorylation of Pyk2 in response to CXCL8 (Fig. 2a, upper panel). Similar amounts of Pyk2 were immunoprecipitated in all samples, as indicated by the anti-Pyk2 antibody (Fig. 2a, lower panel).
Figure 2.
Interleukin-8 (CXCL8) induces tyrosine phosphorylation and tyrosine kinase activity of proline-rich tyrosine kinase 2 (Pyk2) in human polymorphonuclear neutrophils (PMNs). PMNs were stimulated with vehicle, CXCL8 (400 ng/ml; 15 seconds at 37°) or saturating concentrations of anti-β2 (30 min at 4°). The lysate was immunoprecipitated using antibody to proline-rich tyrosine kinase 2 (anti-Pyk2) (600). (a) The immunoprecipitate was immunoblotted by antibody to phosphotyrosine (anti-pTyr) (upper panel). The same filter was probed with anti-Pyk2 (N19; lower panel). (b) Tyrosine kinase activity was evaluated in the immunoprecipitate, as described in the Materials and methods. Data shown in the figure represent one of three independent experiments, with similar results obtained in each. IP, immunoprecipitate; MW, molecular weight.
To assess whether the increase of Pyk2 tyrosine phosphorylation led to the induction of Pyk2 enzymatic activity, Pyk2 immunoprecipitates from control or CXCL8-stimulated PMNs were analysed in an in vitro kinase assay. As shown in Fig. 2(b), Pyk2 immunoprecipitates from CXCL8-stimulated PMNs contained tyrosine kinase activity, evaluated as phosphorylation of the exogenous substrate polyGlu-Tyr (2·3-fold increase versus vehicle-stimulated group, determined by densitometric analysis). As a positive control, Pyk2 tyrosine phosphorylation (Fig. 2a) and its enzymatic activity were stimulated by a saturating concentration of anti-β2 antibody (Fig. 2b; 1·6-fold increase versus vehicle-stimulated group, determined by densitometric analysis), as previously described.23
Src PTKs are involved in CXCL8-induced Pyk2 phosphorylation and chemotaxis of human PMNs
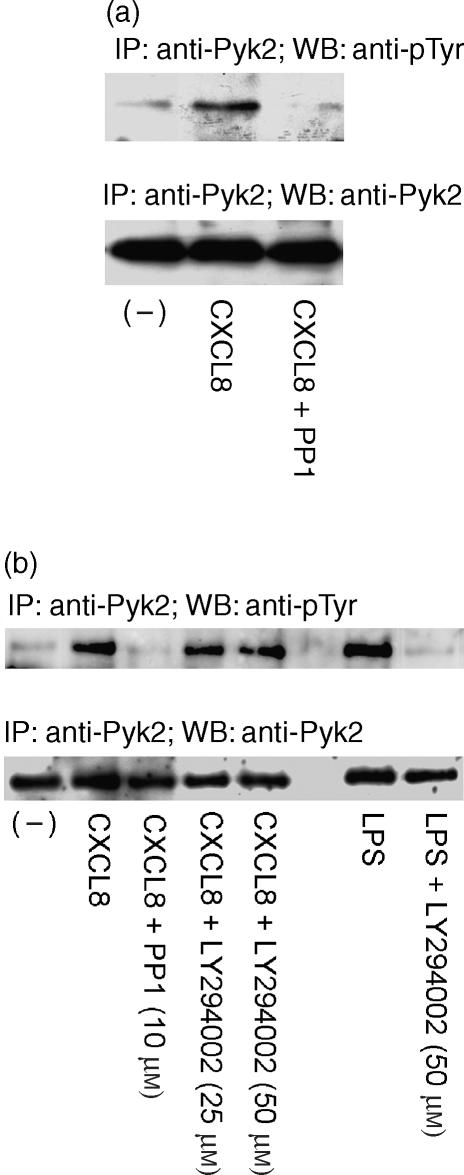
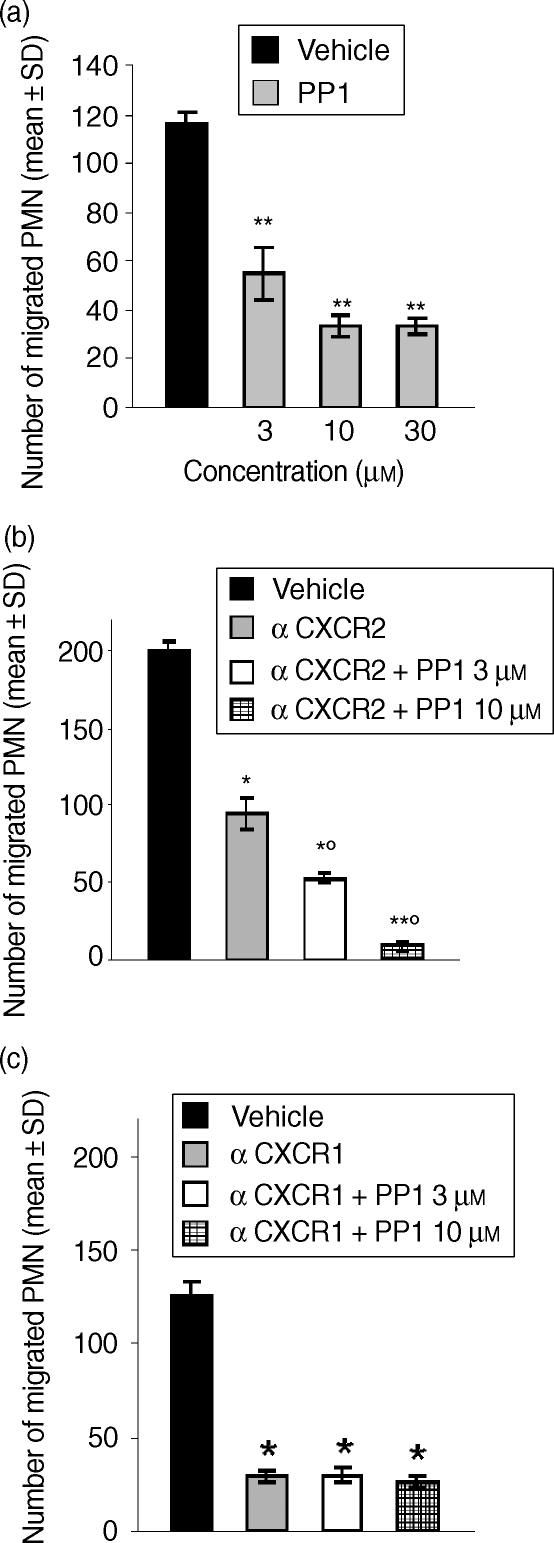
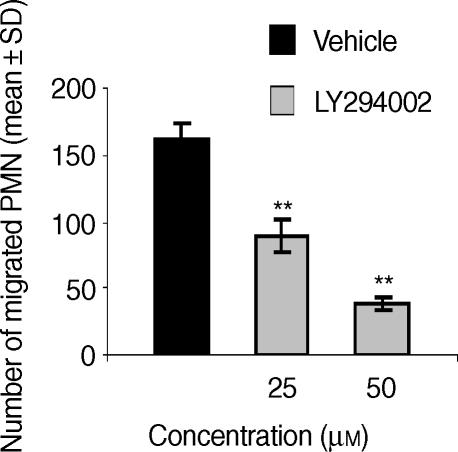
To investigate the upstream events regulating CXCL8-induced Pyk2 activation in human PMNs, and in particular the role of PTKs belonging to the Src PTK family, we evaluated the effect of PP1, the specific inhibitor of Src PTKs. The effect of PP1 was evaluated at a concentration (10 µm) reported to block Src PTK activity.25 PMN pretreatment with PP1 abrogated CXCL8-induced Pyk2 phosphorylation (Fig. 3a). As Pyk2 tyrosine phosphorylation has been reported to be downstream of PI3K activation in human PMNs,26 we next evaluated the effect of the specific inhibitor of PI3K LY294002 on CXCL8-induced Pyk2 tyrosine phosphorylation. The effect of LY294002 was evaluated at a range of concentrations reported to inhibit β2 integrin-mediated Pyk2 activation in human PMNs.27 PMN pretreatment with LY294002 (25 µm or 50 µm) did not affect CXCL8-induced Pyk2 tyrosine phosphorylation (Fig. 3b). On the contrary, PMN preincubation with LY294002 (50 µm) abrogated LPS-induced Pyk2 tyrosine phosphorylation (Fig. 3b). We then evaluated whether Src PTKs could also be functionally involved in CXCL8-mediated PMN chemotaxis. The results shown in Fig. 4(a) demonstrate that PP1, at concentrations of 3, 10 and 30 µm, significantly reduced PMN migration induced by an optimal concentration (10 ng/ml)24 of CXCL8. Reduction of PMN migration was concentration-dependent, reaching a maximal inhibitory effect at 10 µm (71% inhibition). PP1 alone did not significantly affect PMN viability at any concentration tested (data not shown). As PP1 pretreatment did not completely block CXCL8-induced PMN chemotaxis, we then evaluated whether CXCR1- or CXCR2-mediated chemotaxis could be Src PTK independent. To achieve this, we evaluated the effect of PP1 on CXCL8 chemotaxis in the presence of a neutralizing concentration of anti-CXCR1 (3 µg/ml) or anti-CXCR2 (3 µg/ml) immunoglobulin. As shown in Fig. 4(b), pretreatment of PMNs with anti-CXCR2 significantly reduced CXCL8 chemotaxis (52% inhibition) and PP1 (3 µm or 10 µm) further reduced PMN migration, with the inhibition being almost complete at 10 µm (90% inhibition versus the groups treated with anti-CXCR2 alone). On the other hand, pretreatment of PMNs with a neutralizing concentration of anti-CXCR1 dramatically inhibited CXCL8 chemotaxis (76% inhibition) and PP1 did not further reduce PMN migration, suggesting that CXCR2-mediated chemotaxis could be Src PTK independent (Fig. 4c). Finally, we evaluated the effect of LY294002 (25 µm or 50 µm) on CXCL8-induced PMN chemotaxis. The data presented in Fig. 5 show that LY294002 significantly reduces CXCL8 chemotaxis (76% inhibition at 50 µm). LY294002 alone does not affect PMN viability (data not shown).
Figure 3.
Src protein tyrosine kinases (PTKs), but not phosphatidylinositol 3-kinase (PI3K), is involved in interleukin-8 (CXCL8)-induced proline-rich tyrosine kinase 2 (Pyk2) phosphorylation. (a) Human polymorphonuclear neutrophils (PMNs) were pretreated with vehicle or PP1 (10 µm) for 15 min at 37°. PMNs were then stimulated with vehicle or CXCL8 (400 ng/ml) for 15 seconds at 37° in serum-free RPMI-1640. (b) PMNs were pretreated with vehicle, PP1 (10 µm) or LY294002 (25 µm or 50 µm) for 15 min at 37°. PMNs were then stimulated with vehicle, CXCL8 (400 ng/ml; 15 seconds at 37°) or endotoxin [lipopolysaccharide (LPS)] (100 ng/ml, 30 min at 37°) in RPMI supplemented with 1% fetal bovine serum (FBS). Cell lysates were immunoprecipitated using anti-Pyk2 (600) and analysed by Western blotting using anti-pTyr (upper panels). The same filter was probed with anti-Pyk2 (N19) (lower panels). Data shown in the figure represent one of three independent experiments, with similar results obtained in each. IP, immunoprecipitate; WB, Western blot.
Figure 4.
Src protein tyrosine kinase (PTK) activation is involved in interleukin-8 (CXCL8)-mediated CXCL8 chemotaxis. (a) Human polymorphonuclear neutrophils (PMNs) were preincubated with vehicle or PP1 (3 µm, 10 µm or 30 µm) for 15 min at 37°. (b) PMNs were preincubated with neutralizing anti-CXCR2 immunoglobulin (3 µg/ml), in the presence or absence of PP1 (3 µm or 10 µm), for 15 min at 37°. (c) PMNs were preincubated with neutralizing anti-CXCR1 (3 µg/ml) immunoglobulin, in the presence or absence of PP1 (3 µm or 10 µm), for 15 min at 37°. Cells were then tested for their ability to migrate in response to CXCL8 (10 ng/ml). Data are expressed as mean values ± standard deviation (SD) of three independent experiments. Spontaneous PMN migration was 13 ± 4. *P < 0·05 versus CXCL8 alone (Mann–Whitney U-test); **P < 0·01 versus CXCL8 alone (Mann–Whitney U-test); ‡P < 0·05 versus anti-CXCR2 alone pretreated group (Mann–Whitney U-test).
Figure 5.
Phosphatidylinositol 3-kinase (PI3K) activation is involved in interleukin-8 (CXCL8)-induced human polymorphonuclear neutrophil (PMN) chemotaxis. PMNs were preincubated with vehicle or LY294002 (25 µm or 50 µm) for 15 min at 37°. Cells were then tested for their ability to migrate in response to CXCL8 (10 ng/ml). Data are expressed as mean values ± standard deviation (SD) of three independent experiments. Spontaneous PMN migration: 23 ± 5 **P < 0·01 versus CXCL8 alone (Mann–Whitney U-test).
CXCL8-induced Pyk2 kinase activity controls the chemotaxis of HL-60-derived PMN-like cells
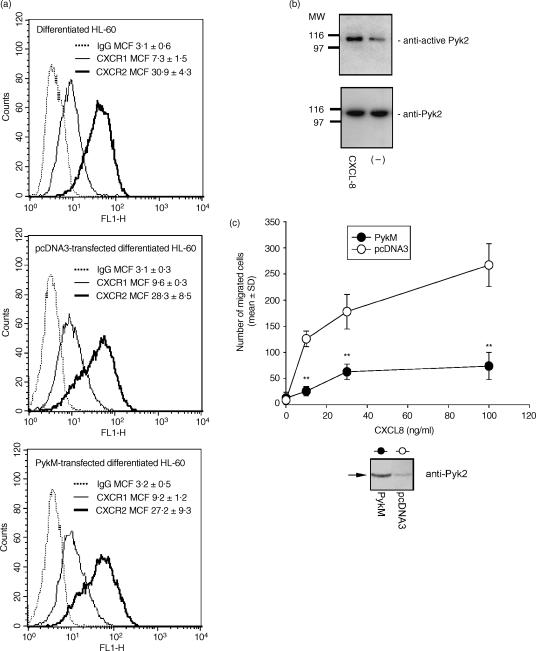
To provide direct evidence on the role of Pyk2 in PMN chemotaxis, we used PykM, a mutant with the ability to prevent Pyk2 enzymatic activity.10,23 HL-60 cells were induced to differentiate to PMN-like cells by exposure to DMSO for 5 days.19 Differentiated cells expressed CXCR1 and CXCR2 (Fig. 6a) and migrated in response to CXCL8 (data not shown). As shown for PMNs, CXCL8 (400 ng/ml for 15 seconds) significantly induced Pyk2 tyrosine phosphorylation in HL-60-derived PMN-like cells (Fig. 6b).
Figure 6.
Overexpression of PykM (the kinase-dead mutant of proline-rich tyrosine kinase 2)-blocked interleukin-8 (CXCL8) chemotaxis in HL-60-derived human polymorphonuclear neutrophil (PMN)-like cells. (a) Membrane expression of CXCR1 and CXCR2 receptors in differentiated HL-60 cells, or differentiated HL-60 cells transiently transfected with pcDNA3-derived plasmid expressing PykM or empty pcDNA3, was determined by flow cytometry analysis. Cells were labelled with anti-CXCR1, anti-CXCR2 or isotype-matched control monoclonal antibodies (mAbs) followed by incubation with fluorescein isothiocyanate (FITC) goat anti-mouse Ab. Dotted, solid and heavy type lines represent isotype-control Ab, anti-CXCR1 Ab and anti-CXCR2 Ab, respectively. Mean channel fluorescence (MCF) is reported. (b) Differentiated HL-60 (5 × 107/ml) were stimulated with CXCL8 (400 ng/ml) for 15 seconds at 37°. The lysate was analysed by immunoblotting with anti-active proline-rich tyrosine kinase 2 (Pyk2) (upper panel). The same filter was probed with anti-total-Pyk2 (N19; lower panel). (c) Differentiated HL-60 cells were transfected with pcDNA-derived plasmid expressing PykM or with the empty pcDNA3. Overexpression of PykM was demonstrated by Western blotting with anti-Pyk2 (N19), 40 hr after electroporation (low). Transfected cells were then tested for their ability to migrate in response to increasing concentrations of CXCL8. Data are expressed as mean values ± standard deviation (SD) of three independent experiments. **P < 0·01 versus empty pcDNA3-transfected cells [analysis of variance (anova)]. MW, molecular weight.
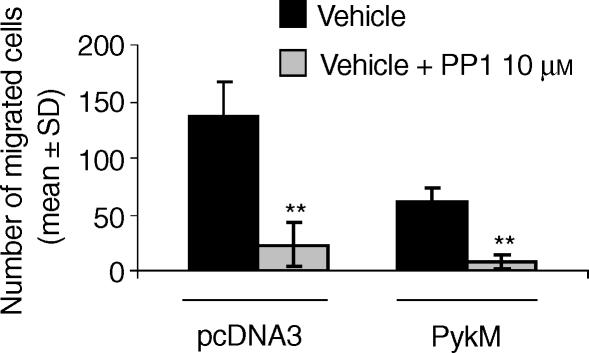
PMN-like cells derived from the HL-60 cell line were next transiently transfected with a pcDNA3-derived plasmid expressing PykM, or with the empty pcDNA3 plasmid, and then assayed for CXCL8 migration. As shown in Fig. 6(c), the overexpression of PykM dramatically reduced CXCL8-induced HL-60 cell migration. The inhibitory effect was present over the whole CXCL8 concentration range tested (10–100 ng/ml), with maximal inhibition (80%) being observed at 100 ng/ml CXCL8. Overexpression of the PykM construct was demonstrated by Western blotting of whole-cell lysates (Fig. 6c, bottom). Transfected HL-60-derived PMN-like cells did not show any change of CXCR1 and CXCR2 expression (Fig. 6a). Finally, we evaluated whether PP1 could also inhibit CXCL8-induced HL-60 cell migration. To achieve this, PMN-like cells derived from the HL-60 cell line, transiently transfected with the empty pcDNA3 plasmid, were pretreated with PP1 (10 µm) and then assayed for CXCL8 (100 ng/ml) chemotaxis. As shown in Fig. 7, PP1 significantly reduced the CXCL8-induced HL-60 migration. Similarly, pretreatment with PP1 (10 µm) significantly reduced CXCL8 chemotaxis on HL-60 derived PMN-like cells transfected with PykM, suggesting that Src PTK may be directly involved in the control of CXCL8-mediated chemotaxis besides regulating Pyk2 activation.
Figure 7.
Src protein tyrosine kinase (PTK) activation is involved in interleukin-8 (CXCL8)-induced HL-60-derived PMN-like cell migration. HL-60-derived human polymorphonuclear neutrophil (PMN)-like cells were transiently transfected with pcDNA-derived plasmid expressing PykM, or with empty pcDNA3. Cells were preincubated with vehicle or PP1 (10 µm) for 15 min at 37° and then tested for their ability to migrate in response to CXCL8 (100 ng/ml). Data are expressed as mean values ± standard deviation (SD) of three independent experiments. **P < 0·01 versus the corresponding vehicle-treated group (Mann–Whitney U-test).
Discussion
We show, in this study, that Pyk2 activation is a key event in mediating the CXCL8-induced chemotaxis of human PMNs. CXCL8 induces Pyk2 tyrosine phosphorylation as well as Pyk2 enzymatic activity in human PMNs. Inhibition of Src PTK activation inhibited both Pyk2 activation and PMN migration induced by CXCL8. Inhibition of Pyk2 enzymatic activity in HL-60-derived PMN-like cells inhibited CXCL8-induced cell migration.
Previous data have reported that CXCL8 increases protein tyrosine phosphorylation in human PMNs, this effect being mostly represented by phosphorylation of a protein of ≈ 115 000–116 000 MW.28 Our data demonstrate that this protein is Pyk2 and that CXCL8 induces Pyk2 tyrosine phosphorylation in a concentration- and time-dependent manner in human PMNs. To the best of our knowledge, no previous studies have reported the activation of Pyk2 in CXCL8-stimulated PMNs. Among the family of FAKs, human leucocytes express both p125 FAK and Pyk2, but only Pyk2 plays a functional role in activated PMNs,14,15 suggesting that FAK-mediated adhesion/cytoskeletal reorganization of PMNs could be essentially mediated by Pyk2 activation. In agreement with this hypothesis, Pyk2 localizes to podosomes and focal adhesion sites in stimulated PMNs, and specific inhibition of Pyk2 activation is associated with the inhibition of tumour necrosis factor-induced PMN spreading.26
Tyrosine phosphorylation of Pyk2 in human PMNs was reported to be dependent on β2 integrin-mediated cell adhesion.29 Our results demonstrate that CXCL8-induced Pyk2 activation may also occur in suspended PMNs. Previous reports have indicated that several chemokines, including CCL5/RANTES (regulated on activation, normal, T-cell expressed, and secreted), CXCL12/SDF-1 (stromal-cell-derived factor 1) and CCL2/MCP-1 (monocyte chemoattractant protein-1), induce a rapid increase of Pyk2 tyrosine phosphorylation in suspended cells.30–32 Moreover, LPS can induce Pyk2 tyrosine phosphorylation in suspension as well as in adherent monocytes,33 demonstrating that Pyk2 can undergo tyrosine phosphorylation in the absence of signals provided by adhesion. The finding that Pyk2 could be activated by mechanisms other than cell adhesion is consistent with the hypothesis that both soluble agonists and extracellular matrix adhesion receptors have to synergize to fully activate a cellular response.34 Indeed, it was found that Pyk2 is able to localize to focal contact sites via its C-terminal domain where it propagates integrin-initiated signals, and is also able to associate with growth factor receptors via its N-terminus.34 In addition, stimulation of Jurkat T cells with anti-T-cell receptor immunoglobulin results in Pyk2 tyrosine phosphorylation. Activation of T cells with the α4β1 integrin ligand [vascular cell adhesion molecule-1 (VCAM-1)] and anti-T-cell receptor immunoglobulin induced rapid and sustained synergistic Pyk2 phosphorylation,35 further supporting the indication that Pyk2 may represent a molecular link between integrin and soluble agonists.
The induction of Pyk2 by CXCL8 seems to be regulated by the activation of Src PTKs, inasmuch as PP1 (the inhibitor of Src PTKs) prevents both Pyk2 activation and cell migration induced by CXCL8. These data are in keeping with previous reports that Pyk2 tyrosine phosphorylation is dependent on the activation of Src PTKs in human PMNs.29 Although, among Src PTKs, PP1 is known to be a specific inhibitor for Lyn in human PMN,36 thus suggesting that Pyk2 tyrosine phosphorylation might be downstream to Lyn activation, further experiments are needed to elucidate this hypothesis in our experimental model. Src PTK activation seems to selectively regulate CXCR1-mediated chemotaxis, with CXCR1 being the predominant receptor in mediating CXCL8 chemotaxis,37 whereas CXCR2-mediated chemotaxis could be Src PTK independent. Induction of Pyk2 by CXCL8 seems to be PI3K independent, inasmuch as the inhibitor of PI3K LY294002 does not affect CXCL8-induced Pyk2 tyrosine phosphorylation. LY294002 was tested at a range of concentrations reported to inhibit β2 integrin-mediated Pyk2 activation in human PMNs,27 and LY294002 effectiveness was also confirmed in our experimental conditions by inhibition of LPS-induced Pyk2 tyrosine phosphorylation.33 On the other hand, PMN pretreatment with LY294002 significantly reduced CXCL8-chemotaxis. These data are in keeping with previous reports that PI3K activation is crucial in CXCL8-mediated chemotaxis38,39 and suggest that PI3K activation could be either independent or downstream to Pyk2 action in CXCL8-stimulated PMNs.
Overexpression of PykM in differentiated HL-60 cells dramatically reduced CXCL8-mediated chemotaxis, strongly suggesting that Pyk2 activation plays a key role in CXCL8-induced chemotaxis. Several lines of evidence suggest that Pyk2 tyrosine phosphorylation is closely linked to chemotaxis and cell migration in multiple cell types. It has recently been demonstrated that Pyk-2 acts as a receptor-proximal link between integrin and chemokine receptor signalling, and a Pyk-2/Rac pathway plays a pivotal role in the control of natural killer (NK) cell transendothelial migration.40 Indeed, Pyk2 localization to membrane ruffles and lamellipodia suggests that its site of action is in these structures, where new adhesion sites are formed during cell spreading and migration.26,29,34 Additional support for FAKs in mediating cell migration comes from studies showing that p125 FAK-deficient cells are refractory to motility signals from platelet-derived and epidermal growth factors.41 Finally, it has been recently reported that the expression of a dominant negative mutant of p125 FAK perturbs the migratory response to C-X-C chemokines in cell transfectants expressing CXCR1 and CXCR2 receptors.42 Similarly to human PMNs, CXCL8 chemotaxis seems to be regulated by Src PTK activation in differentiated HL-60 cells, where cellular migration is strongly reduced by PP1. In addition, PP1 pretreatment also significantly reduced CXCL8 chemotaxis in differentiated HL-60 cells overexpressing PykM, suggesting that Src PTK may be directly involved in the control of CXCL8-mediated chemotaxis besides regulating Pyk2 activation.
In summary, we found that CXCL8 induces Pyk2 activation in human PMNs. Pyk2 activation, but not PI3K, is downstream to Src PTK activation. Specific inhibition of Pyk2 activity was paralleled by a strong reduction of PMNs chemotaxis, pinpointing the key role of Pyk2 in CXCL8-mediated chemotaxis. Among CXCL8 receptors, Src PTK activation selectively regulates CXCR1-mediated chemotaxis in human PMNs, with CXCR1 being the predominant receptor mediating CXCL8 chemotaxis. Consistent with the pathophysiological role played by CXCL8 in several inflammatory diseases, our results indicate that Pyk2 may represent a suitable target for the development of new anti-inflammatory drugs.
Acknowledgments
We thank Dr Angela Gismondi for discussion and criticism of the manuscript.
Abbreviations
- CXCL/IL-8
interleukin-8
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- MAPK
mitogen-activated protein kinase
- LPS
lipopolysaccharide (endotoxin)
- PMN
polymorphonuclear neutrophil
- PI3K
phosphatidylinositol 3-kinase
- anti-pTyr
anti-phosphotyrosine
- PTK
protein tyrosine kinase
- Pyk2
proline-rich tyrosine kinase 2
- PykM
kinase-dead mutant of Pyk2
References
- 1.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 2.Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–20. doi: 10.1016/s0167-5699(00)01672-8. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–7. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 4.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–6. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 6.Thelen M, Peveri P, Kernen P, von Tscharner V, Waltz A, Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988;2:2702–6. [PubMed] [Google Scholar]
- 7.Smith RJ, Sam LM, Leach KL, Justen JM. Postreceptor events associated with human neutrophil activation by interleukin-8. J Leukoc Biol. 1992;52:17–26. doi: 10.1002/jlb.52.1.17. [DOI] [PubMed] [Google Scholar]
- 8.Naccache PH, Levasseur S, Lachance G, Chakravarti S, Bourgoin SG, McColl SR. Stimulation of human neutrophils by chemotactic factors is associated with the activation of phosphatidylinositol 3-kinase γ. J Biol Chem. 2000;275:23636–41. doi: 10.1074/jbc.M001780200. [DOI] [PubMed] [Google Scholar]
- 9.Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–8. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 10.Lev S, Moreno H, Martinez R, et al. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–45. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 11.Avraham S, London R, Fu Y, et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–51. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 12.Astier A, Avraham H, Manie SN, Groopman J, Canty T, Avraham S, Freedman AS. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after β1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–32. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 13.Gismondi A, Bisogno L, Mainiero F, Palmieri G, Piccoli M, Frati L, Santoni A. Proline-rich tyrosine kinase-2 activation by β1 integrin fibronectin receptor cross-linking and association with paxilin in human natural killer cells. J Immunol. 1997;159:4729–36. [PubMed] [Google Scholar]
- 14.Fuortes M, Jin WW, Nathan C. Beta 2 integrin-dependent tyrosine phosphorylation of paxillin in human neutrophils treated with tumor necrosis factor. J Cell Biol. 1994;127:1477–83. doi: 10.1083/jcb.127.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez R, Boxer LA, Suchard SJ. Beta2 integrins are not required for tyrosine phosphorylation of paxillin in human neutrophils. J Immunol. 1997;159:5568–75. [PubMed] [Google Scholar]
- 16.Salgia R, Avraham S, Pisick E, et al. The related adhesion focal tyrosine kinase forms a complex with paxillin in hematopoietic cells. J Biol Chem. 1996;271:31222–6. doi: 10.1074/jbc.271.49.31222. [DOI] [PubMed] [Google Scholar]
- 17.Ganju RK, Hatch WC, Avraham H, Ona MA, Druker B, Avraham S, Groopman JE. RAFTK, a novel member of the focal adhesion kinase family is phosphorylated and associates with signaling molecules upon activation of mature T lymphocytes. J Exp Med. 1997;185:1055–63. doi: 10.1084/jem.185.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English D, Andersen BR. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradient of Ficoll–Hypaque. J Immunol Methods. 1974;5:249–52. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- 19.Rossbacher J, Wagner L, Pasternack MS. Inhibitory effect of haptoglobin on granulocyte chemotaxis, phagocytosis and bactericidal activity. Scand J Immunol. 1999;50:399–404. doi: 10.1046/j.1365-3083.1999.00609.x. [DOI] [PubMed] [Google Scholar]
- 20.Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587–94. [PubMed] [Google Scholar]
- 21.Hao LJ, Yang D, Fujii Y, Yamauchi A, Suzuki N, Kikuchi H, Kaneda Y, Nakamura M. Phorbol ester-potentiated liposomal transfection to monocytic PLB-985 cells. J Biochem. 2000;128:989–98. doi: 10.1093/oxfordjournals.jbchem.a022851. [DOI] [PubMed] [Google Scholar]
- 22.Falk W, Goodwin RH, Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–47. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 23.Gismondi A, Jacobelli J, Mainiero F, Paolini R, Piccoli M, Frati L, Santoni A. Cutting edge: functional role for proline-rich tyrosine kinase 2 in NK cell-mediated natural cytotoxicity. J Immunol. 2000;164:2272–6. doi: 10.4049/jimmunol.164.5.2272. [DOI] [PubMed] [Google Scholar]
- 24.Bizzarri C, Pagliei S, Brandolini L, Mascagni P, Caselli G, Transidico P, Sozzani S, Bertini R. Selective inhibition of interleukin-8-induced neutrophil chemotaxis by ketoprofen isomers. Biochem Pharmacol. 2001;61:1429–37. doi: 10.1016/s0006-2952(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 25.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brisette WH, Weringer EJ, Pollok BA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 26.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104:327–35. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dib K, Melander F, Axelsson L, Dagher MC, Aspenstrom P, Andersson T. Down-regulation of Rac activity during β2 integrin-mediated adhesion of human neutrophils. J Biol Chem. 2003;278:24181–8. doi: 10.1074/jbc.M302300200. [DOI] [PubMed] [Google Scholar]
- 28.L'Heureux GP, Bourgoin S, Jean N, McColl SR, Naccache PH. Diverging signal transduction pathways activated by interleukin-8 and related chemokines in human neutrophils: interleukin-8, but not NAP-2 or GROα, stimulates phospholipase D activity. Blood. 1995;85:522–31. [PubMed] [Google Scholar]
- 29.Yan SR, Novak MJ. β2 integrin-dependent phosphorylation of protein-tyrosine kinase Pyk2 stimulated by tumor necrosis factor α and fMLP in human neutrophils adherent to fibrinogen. FEBS Lett. 1999;451:33–8. doi: 10.1016/s0014-5793(99)00539-6. [DOI] [PubMed] [Google Scholar]
- 30.Davis CB, Dikic I, Unutmaz D, et al. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–8. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1α and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–8. [PubMed] [Google Scholar]
- 32.Yamasaki M, Arai H, Ashida N, Ishii K, Kita T. Monocyte chemoattractant protein 1 causes differential signalling mediated by proline-rich tyrosine kinase 2 in THP-1 cells. Biochem J. 2001;355:751–6. doi: 10.1042/bj3550751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams LM, Ridley AJ. Lipopolysaccharide induces actin reorganization and tyrosine phosphorylation of Pyk2 and paxillin in monocytes and macrophages. J Immunol. 2000;164:2028–36. doi: 10.4049/jimmunol.164.4.2028. [DOI] [PubMed] [Google Scholar]
- 34.Hauck CR, Klingbeil CK, Schlaepfer DD. Focal adhesion kinase functions as a receptor-proximal signaling component required for directed cell migration. Immunol Res. 2000;21:293–303. doi: 10.1385/IR:21:2-3:293. [DOI] [PubMed] [Google Scholar]
- 35.van Seventer GA, Mullen MM, van Seventer JM. Pyk2 is differentially regulated by β1 integrin- and CD28-mediated co-stimulation in human CD4+ T lymphocytes. Eur J Immunol. 1998;28:3867–77. doi: 10.1002/(SICI)1521-4141(199811)28:11<3867::AID-IMMU3867>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
-
36.Yan SR, Novak MJ. Src-family kinase-p53/Lyn p56 plays an important role in TNF-alpha-stimulated production of
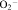 by human neutrophils adherent to fibrinogen. Inflammation. 1999;23:167–78. doi: 10.1023/a:1020245129632. [DOI] [PubMed] [Google Scholar]
by human neutrophils adherent to fibrinogen. Inflammation. 1999;23:167–78. doi: 10.1023/a:1020245129632. [DOI] [PubMed] [Google Scholar] - 37.Hammond ME, Lapointe GR, Feucht PH, et al. IL-8 induces neutrophil chemotaxis predominantly via type I receptors. J Immunol. 1995;155:1428–33. [PubMed] [Google Scholar]
- 38.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science. 2000;287:1049–53. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 39.Knall C, Worthen GS, Johnson GL. Interleukin-8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci USA. 1997;94:3052–7. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gismondi A, Jacobelli J, Strippoli R, et al. Proline-rich tyrosine kinase 2 and Rac activation by chemokine and integrin receptors controls NK cell transendothelial migration. J Immunol. 2003;170:3065–73. doi: 10.4049/jimmunol.170.6.3065. [DOI] [PubMed] [Google Scholar]
- 41.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–56. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 42.Feniger-Barish R, Yron I, Meshel T, Matityahu E, Ben-Baruch A. IL-8-induced migratory responses through CXCR1 and CXCR2: association with phosphorylation and cellular redistribution of focal adhesion kinase. Biochemistry. 2003;42:2874–86. doi: 10.1021/bi026783d. [DOI] [PubMed] [Google Scholar]