Overview of t-cell signalling
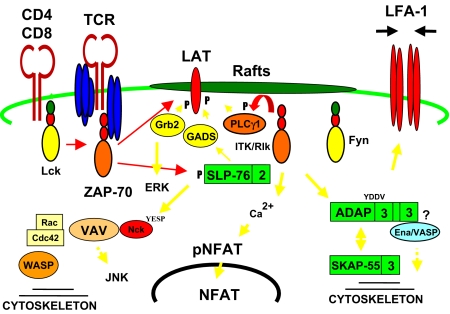
T-cell receptor (TCR) engagement results in the initiation of intracellular signalling cascades that lead to the initiation, amplification and/or inhibition of specific functions. These functions include the production of different cytokines and chemokines, the induction of anergy, the differentiation of T-cell subsets (i.e. Th1/Th2/TRegs) and the generation of cytotoxic T cells (CTLs). T-cell signalling is initiated by the Scr kinase p56lck and its ability to interact with the coreceptors CD4 and CD8.1,2 The concordant binding of the antigen–receptor complex and the coreceptors to major histocompatibility complex (MHC) antigens brings p56lck to the TCRζ/CD3 chains, leading to the phosphorylation of immunoreceptor-based tyrosine activation motifs (ITAMs) as defined by the sequence YXX(L/I)X6−8YXX(L/I).3,4 The phosphorylated ITAMs then recruit the syk family tyrosine kinase zeta associated protein-70 (ZAP-70), or the related protein SYK, via tandem binding to Scr homology 2 (SH2) domains.3,4 Phosphorylation leads to the activation of phospholipase Cγ (PLCγ1) which in turn hydrolyses phosphoatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and phosphoinositol 1,4,5-trisphosphosphate (IP3). This is mediated by the combined action of adaptors and the Tec kinases, inducible T-cell kinase (ITK) and resting lymphocyte kinase (RLK).5 DAG activates protein kinase C (PKC), while IP3 induces the release of intracellular Ca2+ for activation of the serine/threonine phosphatase calcineurin. Calcineurin in turn dephosphorylates the transcription factor nuclear factor in activated T cells (NFAT), allowing its entry into the nucleus.6 Proximal events also activate small GTP-binding proteins p21ras, p21rac and Cdc42, leading to the remodelling of the cytoskeleton and the activation of mitogen-activated protein kinases. These include the extracellular signal regulated kinase (ERKs), c-Jun N-terminal kinase (JNK) and p38, which in turn activate a variety of transcription factors such as NFkB and activator protein-1 (AP-1). It is the orchestration of this array of intracellular pathways that leads to multiple functions attributed to T cells in the immune response.
Adaptor proteins in t cells
Recent advances in T-cell signalling have been made with the identification of haematopoietic specific adaptor proteins, or molecular scaffolds.7–9 These adaptors lack enzymatic activity, and instead possess an array of binding sites and modules that bind to other proteins. A single protein therefore has the potential to regulate multiple discrete events and group proteins in the integration of signalling pathways. Adaptor molecules can function as both positive and negative regulators of T-cell function. Examples of adaptors with a positive effect include growth factor receptor-bound protein-2 (GRB-2), the linker for activation of T cell (LAT), GRB-2-related adaptor downstream of Shc (GADS) and the SH2-domain-containing leukocyte protein of 76 kDa (SLP-76), while others such as the phosphoprotein associated with (glycospingolipid enriched microdomains) GEMs (PAG), the SH2-interacting transmembrane adaptor protein (SIT) and downstream of tyrosine kinases (DOK) have a negative regulatory function. In addition, a number of other adaptors such as the adhesion and degranulation promoting adaptor protein (ADAP), the Src kinase-associated phosphoprotein of 55 kDa (SKAP-55) and the Wiskott–Aldrich syndrome protein (WASP) have regulatory effects on the cytoskeleton, on adhesion and on the ability of T cells to form conjugates with antigen-presenting cells (APCs).
Positive regulators of t-cell signalling
Linker for activation of T cell (LAT)
LAT is the first in an array of adaptors that is needed for the successful transmission of signals from the TCR/CD3 complex (Fig. 1). It is a transmembrane adaptor with a short extracellular region and a tyrosine-rich cytoplasmic tail.10,11 The nature of the binding sites on LAT and the properties of LAT-deficient T cells have provided insights into the manner in which the adaptor is connected to signalling. There are binding sites for PLCγ1, GRB-2 and GADS. Tyrosine residue 132 binds the SH2 domain of PLCγ1, residues at 171, 191 and 226 bind GRB-2, and residues 171 and 191 bind to GADS.12 PLCγ1 binding could couple LAT to Ca2+ mobilization, GRB-2 binding to the activation of ERKs, and GADS to the downstream adaptor SLP-76. LAT-deficient Jurkat cells are defective in tyrosine phosphorylation of several proteins, including PLCγ1, Ca2+ mobilization and NFAT gene transcription.13,14 LAT-deficient mice also show a block at the pre-TCR stage of thymic differentiation,15 while knock-in mice expressing the LAT-PLCγ1 binding mutant have confirmed the importance of this interaction for PLCγ1 activation, calcium mobilization and NFAT function.16 Interestingly, the loss of binding did not affect activation of the ERK pathway in normal cells, although an ERK activation defect has been observed in Jurkat cells.12,17,18 LAT function requires acylation, which is needed for localization to lipid rafts (otherwise known as GEMs or microdomains).13,14 These microdomains are enriched in relatively ordered cholesterol-rich domains that possess key proteins needed for signalling.19 Other LAT-related functions include cytoskeletal-dependent spreading20 and Th1/Th2 differentiation, where deficient mice show defects in Th1 development.21
Figure 1.
Structure and functional domains of adaptor proteins. The structures of immune cell adaptors discussed in this review are shown, including linker for activation of T cell (LAT), SH2-domain-containing leukocyte protein of 76 kDa (SLP-76), Grb2-related adaptor downstream of Shc (GADS), phosphoprotein associated with GEMS (PAG), SH2-interacting transmembrane adaptor protein (SIT), adhesion and degranulation promoting adaptor protein (ADAP), Vav and Src kinase-associated phosphoprotein of 55 kDa (SKAP-55). Adaptor proteins, by definition, lack enzymatic activity or transcriptional domains, but instead express a variety of modular domains or binding sites, which mediate interactions with other proteins. In this way they act as scaffolds, facilitating the formation of multiprotein complexes and the integration of diverse signalling cascades. YYY, tyrosine phosphorylation sites; Pro, proline-rich region; Sam, XXX; SH2, Scr homology 2 domain; SH3, Scr homology 3 domain; PH, pleckstrin-homology domain; NLS, nuclear localization sequence; DH, Dbl-homology domain; CH, calponin-homology domain.
Grb2-related adaptor downstream of Shc (GADS)
GADS is a member of the family of SH2- and SH3-domain-containing adaptor proteins. GADS plays a critical role in TCR-mediated signalling by linking LAT with another T-cell adaptor protein, SLP-76, coupling membrane-proximal events to downstream signalling pathways. The GADS protein consists of a central SH2 domain flanked by a proline-rich region and two SH3 domains (Fig. 1).22 Following TCR stimulation, GADS binds to phosphorylated LAT via its SH2 domain, whilst the SH3 domains bind to polyproline residues (residues 224–244) on SLP-76, promoting the formation of a complex between SLP-76 and LAT (Fig. 2). In this way, GADS allows SLP-76 and its associated proteins to be recruited to the membrane. Analysis of GADS-deficient mice revealed impaired T-cell development, with specific defects in both positive and negative selection of thymocytes.23 Expression library screening has revealed that, in addition to a connection with SLP-76, GADS associates with the serine/threonine kinase haematopoietic progenitor kinase-1 (HPK1) which has been implicated in the activation of the JNK pathway.22,24,25
Figure 2.
Role of adaptors in T-cell signalling. Ligation of the T-cell receptor (TCR) leads to the activation of Scr family protein tyrosine kinases (PTKs), such as Lck, which phosphorylate key tyrosine residues within immunoreceptor-based tyrosine activation motifs (ITAMs) on CD3 and TCRζ chains. This results in the phosphorylation and activation of the syk family PTK zeta associated protein-70 (ZAP-70), which subsequently phosphorylates the linker for activation of T cell (LAT) and SH2-domain-containing leukocyte protein of 76 kDa (SLP-76), allowing them to interact with SH2-containing proteins. SLP-76 and its associated molecules are recruited to LAT in the plasma membrane via an interaction with the Grb2-related adaptor downstream of Shc (GADS). This leads to the SLP-76-dependent activation of phospholipase Cγ (PLCγ1), which results in intracellular Ca2+ mobilization and protein kinase C (PKC) activation. Phosphorylation of SLP-76 allows SH2-mediated binding to the adaptors Vav and Nck, leading to cytoskeletal reorganization. SLP-76 also binds to adhesion and degranulation promoting adaptor protein (ADAP) [previously termed Fyn T-binding protein (FYB)/SLP-76-associated protein (SLAP)] in a pathway that involves Fyn-T and binding to Src kinase-associated phosphoprotein of 55 kDa (SKAP-55) and SKAP-55R.
SH2-domain-containing leukocyte protein of 76 kDa (SLP-76)
SLP-76 is another multidomain adaptor protein, linked to LAT-GADS, which plays an important role in the activation of PLCγ1 and other downstream signalling pathways. SLP-76 has several distinct domains: an N-terminal SAM domain, an acidic region with key tyrosine motifs (YESP, YESP and YEPP), a central proline-rich region and a carboxy-terminal SH2 domain26 (Fig. 1). As in the case of LAT and GADS, studies involving SLP-76-deficient T cells and SLP-76 knockout and mutant mice have provided insights into the role of this adaptor in T-cell development and function.27,28 Phosphorylation of PLCγ1 in SLP-76-deficient Jurkat T cells is severely impaired, resulting in defective Ca2+ mobilization and inositol 1,4,5-triphosphate and interleukin (IL)-2 production.27 Interestingly, this loss of phosphorylation is more selective than that seen in LAT-deficient T cells, where a reduction in the phosphorylation of multiple substrates has been observed. This is consistent with the notion that SLP-76 operates downstream of LAT. The mechanism by which SLP-76 is coupled to PLCγ phosphorylation is unclear, but involves Tec family kinases such as ITKs and RLKs. Both kinases phosphorylate SLP-76, but ITK has also been shown to bind to the adaptor.29,30 Studies involving SLP-76–/– mice have revealed that SLP-76 is required for pre-TCR signalling, with a developmental block at the double negative (DN) stage.31,32 Examination of mice expressing different mutations of SLP-76 have demonstrated a differential requirement for specific domains of SLP-76 in the regulation of T-cell development, proliferation and effector function, providing an insight into the specific molecular mechanisms underlying SLP-76 function.30
SLP-76 has also been implicated in the regulation of cytoskeletal changes in activated T cells though its association with the adaptor proteins Vav, Nck and ADAP [previously termed Fyn T-binding protein (FYB)/SLP-76-associated protein (SLAP)].7–9 TCR ligation is accompanied by the remodelling of the actin cytoskeleton, an event needed for signalling. The N-terminus of SLP-76 has two key tyrosine residues which directly associate with the SH2 domain of Vav.33–35 Phosphorylation of these residues and binding is regulated by ZAP-70 and SYK.35,36 SLP-76 can cooperate with Vav in the up-regulation of IL-2 gene transcription,33,34 although Vav-SLP-76 binding per se is not essential.35 Vav is a multidomain protein comprising a calponin homology (CH) domain followed by an acidic (Ac) motif, a DBL-homology (DH) domain, a pleckstrin-homology (PH) domain, a zinc finger (ZF)-like domain, two SH3 domains and an SH2 domain (Fig. 1). The presence of so many domains within Vav suggests that it may serve to interact with, or bring together, many signal transduction pathways. The DH domain is central to Vav function as it possesses guanine nucleotide exchange (GEF) activity for members of the Rho/Rac family of GTPases, which include the proteins Rac and Cdc42. GTP-bound Cdc42 subsequently associates with WASP. This protein, when activated by Cdc42 and PIP2, can stimulate actin polymerization through the Arp2/3 complex.37 SLP-76 also binds the adaptor Nck, another WASP-binding protein, via its YESP domain,38,39 and a model has been proposed whereby SLP-76 acts as a scaffold bringing Nck and WASP into proximity with Vav and Cdc42-GTP.40 Together, these findings suggest that SLP-76 is connected to the regulation of the actin cytoskeleton, although evidence directly demonstrating this link has yet to be published.
Negative regulators of t-cell signalling
Adaptors were originally described as molecules involved in the positive regulation of T-cell signalling events. However, there are increasingly more negative regulatory adaptor proteins being described, which are important in the control of T-cell receptor signalling. Such proteins are important in terminating TCR signalling and preventing the inappropriate activation of T cells. Two recently identified transmembrane adaptors with negative regulatory function, PAG and SIT, are discussed in more depth below.
Phosphoprotein associated with GEMS (PAG)
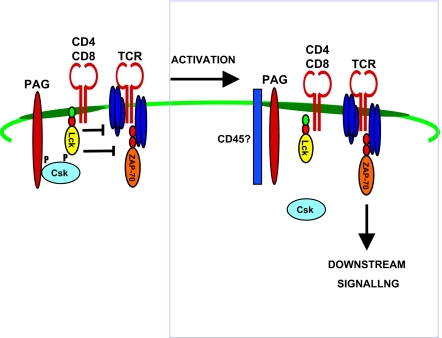
PAG, also known as Csk-binding protein (Cbp), is a transmembrane adaptor protein found in lipid rafts.41,42 PAG comprises a short 16-amino acid extracellular domain and a 397-amino acid cytoplasmic domain containing a dicysteine motif (CSSC), a palmitolylation site for targeting PAG to GEMS43 and 10 tyrosine residues (Fig. 1).41,42 In resting T cells, PAG is constitutively tyrosine-phosphorylated and binds the major negative regulator of Src kinases, the tyrosine kinase c-terminal Src kinase (Csk) (Fig. 3). Csk phosphorylates the C-terminal tyrosine of Scr kinases such as Lck, causing it to bind to its internal SH2 domain, leading to kinase domain inactivation. Csk SH2 domain binding to at least one of the tyrosine-based signalling motifs in the cytoplasmic tail of PAG leads to its localization to the rafts and activation.44–46 Once in the rafts, Csk inhibits the activity of Src family kinases. Following TCR stimulation, PAG becomes transiently dephosphorylated by the phosphatase CD45, which results in the release of Csk from the GEMs, allowing Src kinases to initiate downstream signalling events47 (Fig. 3). In this way, the PAG–Csk complex transmits negative regulatory signals, helping to keep resting T cells in a quiescent state.
Figure 3.
Negative regulation of T-cell signalling by phosphoprotein associated with GEMS (PAG). PAG is constitutively tyrosine-phosphorylated in resting T cells, and associates with Csk, a negative regulator of Scr kinases, via its SH2 domain. Following T-cell receptor (TCR) engagement, PAG is dephosphorylated by a PTPase, most likely CD45. This leads to the dissociation of Csk, which relieves Src kinases of inhibition, enabling them to transmit signals downstream.
PAG has also been shown to bind the cytoplasmic adaptor protein EBP50 [ezrin/radixin/moesin (ERM)-binding phosphoprotein of 50 kDa], which is also known as NHERF (Na+/H+ exchange regulatory factor). The EBP50 protein interacts with ERM family proteins which are known to bind the actin cytoskeleton, and the PAG–EBP50–ERM complex has been shown to regulate the process of synapse formation and activation in T cells.48,49
SH2-interacting transmembrane adaptor protein (SIT)
SIT is a glycosylated transmembrane adaptor protein, expressed by lymphocytes, which is involved in the negative regulation of antigen receptor signalling.50–52 This adaptor has an extracellular domain of 18 amino acids, a 20-amino acid transmembrane region and a 136-amino acid cytoplasmic tail containing five potential sites of tyrosine phosphorylation, which mediate binding to the Grb2 and Src family kinases (Fig. 1).50,52 Amongst these five phosphorylation sites is an immunoreceptor tyrosine-based inhibition motif (ITIM) which mediates the association of SIT with the tyrosine phosphatase SH2 domain-containing protein tyrosine phosphatase 2 (SHP-2).50 Mutation of the tyrosine residue within this ITIM almost completely abrogates the interaction between SIT and SHP-2. However, surprisingly, this mutation has no effect on SIT-mediated inhibition of NFAT activity, indicating that the interaction between SIT and SHP-2 is not required for this effect.50 SIT has also been shown to interact with Grb-2 via two consensus YxN motifs, but the mutation of both of these binding sites has no effect on the inhibitory function of SIT.52 Co-precipitation experiments suggest that Csk could represent the effector molecule which mediates the negative regulatory function of SIT on TCR-mediated signalling.52 However, further studies are required to formally demonstrate this and to elucidate the precise mechanism by which SIT mediates inhibition of TCR signalling events.
Adaptor proteins and the cytoskeleton
Modification of the actin cytoskeleton has been shown to be important in antigen recognition and T-cell activation. TCR-mediated cytoskeletal changes not only are required for cell motility, but are important in the regulation of cellular adhesion. Recently, there has been much attention focused on the signalling pathways involved in TCR-mediated regulation of the cytoskeleton. Indeed, several adaptors have now been described which have been implicated in the regulation of adhesion and cytoskeletal changes in activated T cells. Two such adaptor proteins, ADAP and SKAP-55, are discussed below.
Adhesion and degranulation promoting adaptor protein (ADAP)
Upon activation, the SH2 domain of SLP-76 interacts with the adaptor ADAP.53,54 It is expressed as two isoforms of 120 and 130 kDa (ADAP-120/130), with the 130-kDa isoform being preferentially expressed in peripheral T cells.55 ADAP contains several proline-rich regions and an SH3 domain which mediate binding to SKAP-55 (see below), 16 putative tyrosine phosphorylation sites which allow binding to SH2 domains of SLP-76 and Fyn, two putative nuclear localization sequences (NLS), and an Ena (enabled)/VASP (vasodilator-stimulated phosphoprotein) homology 1 domain binding site (EVH1) (E/DFPPPPXD/E)7,53,56–58 (Fig. 1). Mapping studies have identified the Y595 and Y651-YDDV motifs as binding sites for SLP-76, while the Y625-YDGI motif binds the SH2 domain of the Src family kinase Fyn.56,57 Following TCR stimulation, Fyn phosphorylates these signalling motifs and regulates binding of SLP-76 and Fyn to ADAP (Fig. 2).53,54,57,59 Tyrosine phosphorylation of ADAP following TCR ligation is diminished in Fyn-deficient T cells, supporting a link between this kinase and the ADAP signalling pathway.53
The role of ADAP as a positive or negative regulator of T-cell function was initially uncertain. Transfection studies with ADAP have yielded conflicting results on the role of this adaptor in the regulation of T-cell activation. One study found that ADAP attenuated IL-2 production,53 whereas another report suggested a negative regulatory role for this protein.54 However, cotransfection of ADAP with Fyn and SLP-76 was shown to up-regulate TCR-driven IL-2 transcription.56,57 Furthermore, ADAP-deficient peripheral T cells exhibit defects in proliferation, cytokine production and lymphocyte functional antigen-1 (LFA-1) clustering following TCR stimulation.60,61 Similarly, ADAP was shown to enhance β1 integrin clustering in mast/basophilic cells; however, clustering of the high-affinity immunoglobulin E (IgE) receptor FcεRI was unaffected.62,63 The effect of ADAP on integrin clustering has been reported to facilitate stromal cell-derived factor 1 alpha (SDF1α)-mediated T-cell migration64 and enhance conjugate formation between T cells and antigen-presenting cells (APCs).65 Together, these findings suggest that ADAP is important in coupling TCR-mediated actin cytoskeletal changes to the activation of integrin function, but exactly how this is achieved is unclear. One possible mechanism by which ADAP may regulate integrin adhesion is through an association with proteins of the Ena/VASP family. These proteins are important in the regulation of actin dynamics, controlling processes such as fibroblast migration, axon guidance, and T-cell polarization, and in the actin-based motility of the intracellular pathogen Listeria monocytogenes.66 ADAP was recently shown to co-localize with Ena/VASP proteins and WASP, Vav and F-actin at the interface between T cells and anti-CD3-coated beads.58 In activated T cells, ADAP associates with Ena/VASP family proteins and is found within multiprotein complexes containing WASP, Nck, and SLP-76. Inhibition of binding between ADAP and Ena/VASP proteins or between WASP and the Arp2/3 complex inhibits TCR-mediated actin rearrangement, suggesting that these interactions are important in linking TCR signalling to cytoskeletal remodelling.58 Clearly, further studies are required to determine the precise role of ADAP in the regulation of the actin cytoskeleton.
Src kinase-associated phosphoprotein of 55 kDa (SKAP-55)
ADAP constitutively associates with another adaptor protein, SKAP-55 or Scap1 (NCBI assignment).25,67 SKAP-55 is a T-cell-specific adaptor protein which comprises a unique N-terminal region followed by a pleckstrin-homology (PH) domain and a carboxy-terminal SH3 domain (Fig. 1). The SH3 domain of SKAP-55 associates with the proline-rich region of ADAP25,68 and, at the same time, the tyrosine-based RKxxYxxY motif in SKAP-55 mediates ADAP SH3 domain binding.69 Significant progress has been made in identifying the role of this adaptor in T-cell function. A recent study demonstrated that SKAP-55 potently increases T-cell/APC conjugate formation and enhances integrin-mediated adhesion.65 SKAP-55 co-localizes with F-actin at the T-cell/APC synapse, enhances cellular adhesion via binding of LFA-1 to fibronectin and intercellular adhesion molecule-1 (ICAM-1), and promotes clustering of LFA-1.65 SKAP-55-induced integrin clustering, in turn, mediates high-avidity integrin binding. T-cell/APC conjugate formation induces the translocation of SKAP-55 to lipid rafts, a process which is regulated by LFA-1 and TCR engagement. The translocation of SKAP-55 to the membrane brings it into close proximity with other signalling components which include the kinase Fyn, which has the capacity to phosphorylate SKAP-55, linking this protein to downstream signalling events such as the enhancement of ERK activity.69 During T-cell/APC interactions, engaged receptors and their associated tyrosine kinases assemble at the area of cell contact into spatially organized supramolecular activation clusters (SMACs), which comprise an inner central SMAC (cSMAC) enriched with TCR, CD2 and CD28 surrounded by a peripheral SMAC (pSMAC) enriched with LFA-1.70,71 Adhesive interactions play a central role in the formation of SMACs, and since ADAP and SKAP-55 have been shown to enhance LFA-1-mediated adhesion during T-cell/APC interactions, it is likely that these adaptors control the formation of SMACs. However, future studies will help to clarify the role of these adaptors in the formation of the immunological synapse.
Future directions in adaptor protein research
The recent identification of adaptor proteins has greatly contributed to our understanding of the way in which membrane proximal signalling events lead to the initiation and integration of downstream signalling pathways in T cells. By acting as scaffolds for the assembly of proteins, these proteins act as integrators of T-cell signalling. Although many adaptor proteins have been characterized, the biological functions of a host of others need to be elucidated. Understanding the functions of the different signalling domains of adaptors will require some complex studies in which adaptors with defined mutations in their signalling domains are introduced back into genetically modified mice or deficient cell lines. Further to this, information on the assembly, spatial dynamics and intracellular location of adaptor complexes will help to elucidate the precise mechanisms by which adaptors integrate diverse signalling networks. New imaging technologies, coupled with the development of new fluorescent labels and sensors and the use of more sophisticated computer software for image acquisition and analysis, may provide insights into the dynamics of adaptor proteins and their interactions with other signalling components in living cells.72–74. Hopefully, such studies will lead to a better understanding of TCR signalling in the future.
Acknowledgments
This work was supported by NIH grant A139021 and a grant from the Wellcome Trust, London (CER is a Principal Research Fellow of the Wellcome Trust).
References
- 1.Rudd CE. CD4, CD8 and the TcR/CD3 complex: a novel class of protein tyrosine kinase receptor. Immunol Today. 1990;11:400–4. doi: 10.1016/0167-5699(90)90159-7. [DOI] [PubMed] [Google Scholar]
- 2.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–8. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 3.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 4.Wange RL, Samelson LE. Complex complexes: signaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 5.Lewis CM, Broussard C, Czar MJ, Schwartzberg PL. Tec kinases: modulators of lymphocyte signaling and development. Curr Opin Immunol. 2001;13:317–25. doi: 10.1016/s0952-7915(00)00221-1. [DOI] [PubMed] [Google Scholar]
- 6.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Ann Rev Immunol. 1977;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 7.Rudd CE. Adaptors and molecular scaffolds in immune cell signaling. Cell. 1999;96:5–8. doi: 10.1016/s0092-8674(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 8.Samelson LE. Adaptor proteins and T-cell antigen receptor signaling. Prog Biophys Mol Biol. 1999;71:393–403. doi: 10.1016/s0079-6107(98)00050-9. [DOI] [PubMed] [Google Scholar]
- 9.Peterson EJ, Clements JL, Fang N, Koretzky GA. Adaptor proteins in lymphocyte antigen-receptor signaling. Curr Opin Immunol. 1998;10:337–44. doi: 10.1016/s0952-7915(98)80173-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang WJ, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 11.Weber JR, Orstavik S, Torgersen KM, et al. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J Exp Med. 1998;187:1157–61. doi: 10.1084/jem.187.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–61. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Trible RP, Samelson LE. LAT palmitoyation: its essential role in membrane microdomain targetgin and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:1–20. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 14.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCγ and the Ras pathway. Immunity. 1998;9:617–26. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–50. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 16.Sommers CL, Park CS, Lee J, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–3. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J Biol Chem. 2001;276:29588–95. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 18.Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem J. 2001;356:461–71. doi: 10.1042/0264-6021:3560461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything. Lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–81. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 20.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading. A role for the signal transduction adaptor LAT. Immunity. 2001;14:315–29. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 21.Aguado E, Richelme S, Nunez-Cruz S, et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296:2036–40. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 22.Liu SK, Fang N, Bouchard D, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein, Gads, functions in T-cell receptor signaling through interactions with SLP-76 and LAT. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 23.Yoder J, Pham C, Iizuka YM, Kanagawa O, Liu SK, McGlade J, Cheng AM. Requirement for the SLP-76 adaptor GADS in T cell development. Science. 2001;291:1987–91. doi: 10.1126/science.1057176. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Nature. 1996;383:716–9. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Kang H, Raab M, da Silva A, Kraeft S-K, Rudd CE. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc Natl Acad Sci USA. 1998;95:8779–84. doi: 10.1073/pnas.95.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackman JK, Motto DG, Sun Q, Tanemoto M, Turck CW, Peltz GA, Koretzky GA, Findell PR. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J Biol Chem. 1995;270:7029–32. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 27.Kumar L, Pivniouk V, de la Fuente MA, Laouini D, Geha RS. Differential role of SLP-76 domains in T cell development and function. Proc Natl Acad Sci USA. 2002;99:884–9. doi: 10.1073/pnas.022619199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-γ1 in a SLP-76-deficient T cell. Science. 1998;218:413–6. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 29.Schneider H, Guerette B, Guntermann C, Rudd CE. Resting lymphocyte kinase (Rlk/Txk) targets lymphoid adaptor SLP-76 in the cooperative activation of interleukin-2 transcription in T-cells. J Biol Chem. 2000;275:3835–40. doi: 10.1074/jbc.275.6.3835. [DOI] [PubMed] [Google Scholar]
- 30.Lucas JA, Miller AT, Atherly LO, Berg LJ. The role of Tec family kinases in T cell development and function. Immunol Rev. 2003;191:119–38. doi: 10.1034/j.1600-065x.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 31.Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hirstka RF, Williamson RA, Koretzky GA. Requirement for the leucocyte-specific adpater protein SLP-76 for normal T cell development. Science. 1998;281:416–9. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 32.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt FW, Geha RS. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–38. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 33.Onodera H, Motto DG, Koretzky GA, Rothstein DM. Differential regulation of activation-induced tyrosine phosphorylation and recruitment of SLP-76 to Vav by distinct isoforms of the CD45 protein-tyrosine phosphatase. J Biol Chem. 1996;271:22225–30. doi: 10.1074/jbc.271.36.22225. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Motto DG, Koretsky GA, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 35.Raab M, da Silva AJ, Findell PR, Rudd CE. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TcRζ/CD3 induction of interleukin 2. Immunity. 1997;6:1–11. doi: 10.1016/s1074-7613(00)80422-7. [DOI] [PubMed] [Google Scholar]
- 36.Wardenburg JB, Fu C, Jackman JK, et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–4. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 37.Rohatgi R, Ma L, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links CDc24-dependent signals to actin assembly. Cell. 1999;97:221–31. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 38.Bubeck Wardenburg J, Pappu R, Bu JY, Mayer B, Chernoff J, Straus D, Chan AC. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–16. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 39.Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J Cell Sci. 2001;114:4307–18. doi: 10.1242/jcs.114.23.4307. [DOI] [PubMed] [Google Scholar]
- 40.Zeng R, Cannon JL, Abraham RT, Way M, Billadeau DD, Bubeck-Wardenberg J, Burkhardt JK. SLP-76 coordinates Nck-dependent Wiskott–Aldrich syndrome protein recruitment with Vav-1/Cdc42-dependent Wiskott–Aldrich syndrome protein activation at the T cell-APC contact site. J Immunol. 2003;171:1360–8. doi: 10.4049/jimmunol.171.3.1360. [DOI] [PubMed] [Google Scholar]
- 41.Brdicka T, Pavlistova D, Leo A, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 43.Zhang WTR, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–46. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 44.Leo A, Wienands J, Baier G, Horejsi V, Schraven B. Adapters in lymphocyte signaling. J Clin Invest. 2002;109:301–9. doi: 10.1172/JCI14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeuchi S, Takayama Y, Ogawa A, Tamura K, Okada M. Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J Biol Chem. 2000;275:29183–6. doi: 10.1074/jbc.C000326200. [DOI] [PubMed] [Google Scholar]
- 46.Torgersen KM, Vang T, Abrahamsen H, et al. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J Biol Chem. 2001;276:29313–8. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 47.Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol Cell Biol. 2003;23:2017–78. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itoh K, Sakakibara M, Yamasaki S, et al. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J Immunol. 2002;168:541–4. doi: 10.4049/jimmunol.168.2.541. [DOI] [PubMed] [Google Scholar]
- 49.Brdickova N, Brdicka T, Andera L, Spicka J, Angelisova P, Milgram SL, Horejsi V. Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton. FEBS Lett. 2001;507:133–6. doi: 10.1016/s0014-5793(01)02955-6. [DOI] [PubMed] [Google Scholar]
- 50.Marie-Cardine A, Kirchgessner H, Bruyns E, et al. SHP2-interacting transmembrane adaptor protein (SIT), a novel disulfide-linked dimer regulating human T cell activation. J Exp Med. 1999;189:1181–94. doi: 10.1084/jem.189.8.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hubener C, Mincheva A, Lichter P, Schraven B, Bruyns E. Complete sequence, genomic organization, and chromosomal localization of the human gene encoding the SHP2-interacting transmembrane adaptor protein (SIT) Immunogenetics. 2001;53:337–41. doi: 10.1007/s002510100328. [DOI] [PubMed] [Google Scholar]
- 52.Pfrepper KI, Marie-Cardine A, Simeoni L, et al. Structural and functional dissection of the cytoplasmic domain of the transmembrane adaptor protein SIT (SHP2-interacting transmembrane adaptor protein) Eur J Immunol. 2001;31:1825–36. doi: 10.1002/1521-4141(200106)31:6<1825::aid-immu1825>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 53.da Silva AJ, Li Z, de Vera C, Canto E, Findell P, Rudd CE. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulate interleukin 2 production. Proc Natl Acad Sci USA. 1997;94:7493–8. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musci MA, Hendricks-Taylor LR, Motto DG, Paskind M, Kamens J, Turck CW, Koretzky GA. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J Biol Chem. 1997;272:11674–7. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- 55.Veale M, Raab M, Li Z, da Silva A, Kraeft S-K, Weremowicz S, Morton CC, Rudd CE. Novel isoform of lymphoid adaptor FYB (FYB-130) interacts with SLP-76 and regulates interleukin 2 production. J Biol Chem. 1999;274:28427–35. doi: 10.1074/jbc.274.40.28427. [DOI] [PubMed] [Google Scholar]
- 56.Geng L, Raab M, Rudd CE. Cutting edge. SLP-76 cooperativity with FYB/FYN-T in the up-regulation of TCR-driven IL-2 transcription requires SLP-76 binding to FYB at Tyr595 and Tyr651. J Immunol. 1999;163:5753–7. [PubMed] [Google Scholar]
- 57.Raab M, Kang H, da Silva A, Zhu X, Rudd CE. FYN-FYB-SLP-76 interactions define a TcRζ/CD3 mediated tyrosine phosphorylation pathway that regulates interleukin 2 transcription in T-cells. J Biol Chem. 1999;274:21170–9. doi: 10.1074/jbc.274.30.21170. [DOI] [PubMed] [Google Scholar]
- 58.Krause M, Sechi MS, Konradt M, Monner D, Gertler FB, Wehland J. Fyn-binding protein (Fyb)/SLP-76-associated protein, vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link TCR signaling to the actin cytoskeleton. J Cell Biol. 2000;149:181–94. doi: 10.1083/jcb.149.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.da Silva AJ, Rosenfield JM, Mueller I, Bouton A, Hirai H, Rudd CE. Biochemical analysis of p120/130: a protein-tyrosine kinase substrate restricted to T and myeloid cells. J Immunol. 1997;158:2007–16. [PubMed] [Google Scholar]
- 60.Griffiths EK, Krawczyk C, Kong Y-Y, et al. Positive regulation of T cells activation and integrin adhesion by the adapter Fyb/Slap. Science. 2001;293:2260–3. doi: 10.1126/science.1063397. [DOI] [PubMed] [Google Scholar]
- 61.Peterson EJ, Woods ML, Dmowski SA, et al. Coupling of the TCR to integrin activation by SLAP-130/Fyb. Science. 2001;293:2263–5. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 62.Geng L, Rudd CE. Adaptor ADAP (adhesion and degranulation promoting adaptor protein) regulates β-1 integrin clustering on mast cells. Biochem Biophys Res Comm. 2001;289:2042–50. doi: 10.1006/bbrc.2001.6117. [DOI] [PubMed] [Google Scholar]
- 63.Geng L, Pfister S, Kraeft SK, Rudd CE. Adaptor FYB (Fyn-binding protein) regulates integrin-mediated adhesion and mediator release: differential involvement of the FYB SH3 domain. Proc Natl Acad Sci USA. 2001;98:11527–32. doi: 10.1073/pnas.191378198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hunter AJ, Ottoson N, Boerth N, Koretzky GA, Shimizu Y. A novel function for the Slap-130/Fyb adapter protein in Beta-1 integrin signalling and T cell migration. J Immunol. 2000;164:1143–7. doi: 10.4049/jimmunol.164.3.1143. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Moon EY, Azouz A, Wu X, Smith A, Schneider H, Hogg N, Rudd CE. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat Immunol. 2003;4:366–74. doi: 10.1038/ni913. [DOI] [PubMed] [Google Scholar]
- 66.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. ENA/VASP proteins. Regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–64. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 67.Marie-Cardine A, Bruyns E, Eckerskorn C, Kirchgessner H, Meuer S, Schraven B. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J Biol Chem. 1997;272:16077–80. doi: 10.1074/jbc.272.26.16077. [DOI] [PubMed] [Google Scholar]
- 68.Kang H, Freund C, Duke-Cohan JS, Musacchio A, Wagner G, Rudd CE. SH3 domain recognition of a proline-independent tyrosine-based RKxxYxxY motif in immune cell adaptor SKAP55. EMBO J. 2000;19:2889–99. doi: 10.1093/emboj/19.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu L, Yu Z, Shen SH. SKAP55 recruits to lipid rafts and positively mediates the MAPK pathway upon T cell receptor activation. J Biol Chem. 2002;277:40420–7. doi: 10.1074/jbc.M206023200. [DOI] [PubMed] [Google Scholar]
- 70.Dustin ML, Shaw AS. Costimulation: building an immunological synapse. Science. 1999;283:649–50. doi: 10.1126/science.283.5402.649. [DOI] [PubMed] [Google Scholar]
- 71.Monks CR, Freiburg BA, Kupher H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 72.Lippincott-Schwartz J, Patterson GH. Development and use of fluorescent protein markers in living cells. Science. 2003;300:87–91. doi: 10.1126/science.1082520. [DOI] [PubMed] [Google Scholar]
- 73.Eils R, Athale C. Computational imaging in cell biology. J Cell Biol. 2003;161:477–81. doi: 10.1083/jcb.200302097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmermann T, Rietdorf J, Pepperkok R. Spectral imaging and its applications in live cell microscopy. FEBS Lett. 2003;546:87–92. doi: 10.1016/s0014-5793(03)00521-0. [DOI] [PubMed] [Google Scholar]