Abstract
Mycobacterium leprae (ML) GroES has been shown to induce strong T cell responses in tuberculoid as well as in exposed healthy contacts of leprosy patients, and therefore this antigen has been the focus of study as a potential vaccine candidate. Paradoxically, we have shown that ML GroES also induces extremely high titres of IgG1 antibody in leprosy patients across the disease spectrum, a response associated with disease progression. IgG1 antibodies in leprosy also show a negative association with interferon-γ, a critical T cell cytokine responsible for macrophage activation and intracellular killing of mycobacteria. We therefore queried if antibody and T cell responses were being evoked by different epitopes in ML GroES proteins. To address the issue of epitope recognition in mycobacterial diseases, we have analysed 16 peptides (15-mer peptides) spanning the entire ML and M. tuberculosis GroES protein in leprosy (n = 19) and tuberculosis (n = 9) patients and healthy endemic controls (n = 8). Our analysis demonstrates clearly that the dominant peptides evokingT cell and IgG subclass antibodies were different. The target of both T and B cell responses were cross-reactive epitopes in all groups. Differences in disease and healthy states related to the strength (mean intensity) of the T cell and antibody response. IgG1 and IgG3 antibodies were associated with disseminated disease and IgG 2 and IgG4 with disease limitation. Such comprehensive immune profiling of antigen-specific responses is critical to understanding the disease pathogenesis and also if these reagents are to be exploited for either diagnostic or vaccine purposes.
Introduction
The introduction of a multidrug regimen for leprosy has resulted in a tremendous decrease in the world burden of leprosy. However, with its long incubation period, leprosy would require vigilance over several decades. To keep the disease under control and work towards a long-term leprosy elimination goal, it is imperative that reagents for early diagnosis and vaccine candidates for high-risk groups be developed.
Mycobacteria not only survive but multiply within the professional phagocytes by their ability to evade the microbicidal activities and interfere with the antigen-presenting functions of macrophages, resulting in deviation of the adaptive immune system and down-regulation of the immune parameters that are critical to protective immunity.1,2 The clinical spectrum of leprosy is related directly to the strength of activation of various arms of the immune system. Leprosy patients with self-limiting tuberculoid leprosy show strong T cell reactivity, while patients with the disseminated or lepromatous form of the disease show low to absent T cell reactivity3 and augmented antibody responses.4,5 Therefore, the strength of T cell responses is considered to be critical to protection in leprosy.
Mycobacterium leprae, the causative agent of leprosy, remains one of the few bacterial pathogens of humans which cannot be cultivated in vitro and therefore development of a successful vaccine depends on the identification of antigens and epitopes that induce protective responses across the leprosy disease spectrum. Several biochemical, immunological and molecular approaches have been used recently for the identification and characterization of protein antigens of the leprosy bacillus.6–8 Of the 10 or more M. leprae antigens that have been characterized and cloned,9 heat shock proteins (hsps) have been shown to be strong targets of T cell responses in leprosy patients with tuberculoid or self-limiting disease.10 ML GroES, a homologue of the GroES gene product of Escherichia coli, and the human chaperonin 1011 and have been shown to participate in protein refolding.12 Because of the important physiological role that these hsps play, extensive structural analyses have been carried out on these proteins.11 The amino acid composition and sequence has been determined and the crystal structure of the ML GroES has been elucidated.13M. leprae GroES has been shown to induce strong T cell responses in tuberculoid14,15 as well as in exposed healthy contacts of leprosy patients,16 and therefore this antigen has been the focus of study as a potential vaccine candidate. Paradoxically, we have shown that ML GroES also induces extremely high titres of IgG1 antibody in leprosy patients,17 a response associated with disease progression. In leprosy, IgG1 antibodies also show significant negative association with interferon-γ,18 a critical T cell cytokine responsible for macrophage activation and intracellular killing of mycobacteria. Such responses in a vaccine candidate would be undesirable. However, this difficulty could be overcome if B and T cell responses were directed to different epitopes in the same protein. To address this issue we analysed both T and B cell responses to various peptides of ML GroES in an attempt to identify epitopes which may be differentially associated with T cell and IgG antibody subclass responses. Our results demonstrate clearly that the dominant targets of T and B cell responses in ML GroES were different and the nature of IgG subclass antibodies was different in disease and healthy individuals. Such comprehensive immune profiling of antigen-specific responses is critical to understanding the disease pathogenesis and also if these reagents are to be exploited for either diagnostic or vaccine purposes.
Materials and methods
Patients and controls
Newly diagnosed leprosy patients presenting at the Marie Adelaide Leprosy Center (MALC) were recruited to our studies and have been described in detail elsewhere.5 Leprosy patients with lepromatous (L = 9) or tuberculoid (T = 10) disease and who had not been treated for leprosy previously were diagnosed clinically as well as histologically on a 4 mm punch biopsy taken from the edge of an active lesion. Newly diagnosed sputum-positive pulmonary tuberculosis patients (P = 9) were recruited at the Masoomeen Trust Hospital. Eight healthy controls (EC = 8), who were employees of the Aga Khan University (AKU) and had no previous history of exposure to leprosy, were included in the study. Of these 50% were PPD positive (≥10 mm induration). Ethical approval was obtained from both the AKU ethical committee and MALC Human Rights Protection Committee. Written/oral consent as appropriate was obtained from both patients and control groups.
Antigens
M. leprae GroES (batch ML 10–2) and M. tuberculosis GroES (batch MT10-2) antigens were obtained from the WHO reference reagent bank through the courtesy of Dr Jan van Embden.
Antisera
Five millilitres of blood collected from leprosy patients was allowed to separate overnight at 4°. Serum was removed and centrifuged at 400 g for 15 min; the clear supernate was distributed in small aliquots and frozen at −70° before use.
Reagents, monoclonal antibodies and conjugates
Monoclonal antibodies specific for human IgG subclasses, HP 6001 (anti-IgG1) and HP 6002 (anti-IgG2), prepared at the Center for Disease Control, Atlanta, were a gift from the late Dr Charles Reimer. The specificity evaluation and performance characteristics of these antibodies are described in detail elsewhere17 and antimouse IgG (H + L chain-specific) antibodies conjugated to alkaline phosphatase was obtained commercially (Jackson Laboratories, NJ, USA) and diluted according to the manufacturer's recommendations.
Synthesis of overlapping 15-mer peptides of ML and MT GroES
Overlapping peptides spanning the whole length of the GroES protein were synthesized by manual solid-phase synthesis (Ramps, Du Pont) using Fmoc chemistry on Rink resin (4-2′, 4′-dimethoxyphenyl-Fmoc-aminomethyl)-phenoxy resin; Calbiochem Nottingham, UK) Fmoc-protected amino acids (Beachem, Switzerland) were converted to the hydroxybenzotriazole activated esters by treatment with hydroxybenzotriazole and N, N′-diisopropylcarbodiimide in dimethyl formamide (DMF). Subsequent coupling reactions were performed in DMF and the Fmoc groups were removed with 50% piperidine in DMF followed by a series of washes in DMF. After synthesis, side-chain protecting groups were removed and the peptides were cleaved off the resin in trifluoroacetic acid in the presence of appropriate scavengers. After cleavage, peptides were precipitated with diethylether and their purity assessed by analytical HPLC. The peptides were 15-mers overlapping by five amino acids with the exception of the C-terminal peptide (amino acids 91–100). Homologous cross-reactive peptides from MT GroES were also synthesized to span the entire MT GroES antigen. This comprised an additional six peptides with substitutions of 1–4 amino acids per peptide.
IgG subclass antibodies to GroES and its 15-mer peptides
IgG subclass enzyme-linked immunosorbent assays (ELISAs) to ML GroES and its peptides have been described in detail previously.17 Briefly, immulon 4 plates were coated with 1 µg/well of M. leprae GroES or 20 µg/well of the individual peptides from ML or MTGroES in carbonate buffer pH 9·6 for 2 h at 37° and then overnight at 4°. The plates were blocked with 200 µl PBS containing 5% BSA for 2 hr at 37° and washed subsequently in phosphate buffere saline (PBS) containing 0·05% Tween 20 (PBS-Tween) three times. Sera (100 µl) diluted in PBS-Tween containing 1·0% bovine serum albumin (BSA) were added to both peptide-coated and uncoated blocked wells (serum blank) at a single dilution of 1/20 and incubated for a further 2 hr at 37° and then overnight at 4°. For detection of IgG subclass-specific antibodies, wells were incubated with monoclonal antibodies specific for IgG1 (HP 6069) or IgG2 (HP 6002) subclasses for 2 hr at 37° and then overnight at 4°. The final incubation was with antimouse IgG conjugated to alkaline phosphatase (Fc-specific; Jackson Laboratories, USA). Results were expressed as optical density units (OD × dilution of the serum) after deducting background binding in the absence of antigen.
Lymphocyte transformation test (LTT) to assess T cell responses
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood (30 ml) by density sedimentation over Ficoll-Hypaque. Cells were washed three times with medium (RPMI-1640; BioWhittaker, Walkersville, MD, USA). Cells were counted and suspended in complete medium (RPMI-1640 with 2 mm l-glutamine, 100 mg/ml gentamicin, 15 mm HEPES and 20% autologous human plasma). Two hundred thousand cells per well were placed in round-bottomed microtitre tissue culture plates (Flow Laboratories, Irvine, UK) and M. leprae GroES (5 µg/ml) or M. tuberculosis GroES (5 µg/ml) were added to triplicate wells for each donor. Control wells received medium alone. The cultures were incubated for 5 days in 5% CO2 at 37°. One micro curie of [3H]-thymidine [specific activity 6·7 curies/m mole (Amersham, UK)] was added to each culture well for the final 24 hr. Cells were harvested after 18 hr with a PHD harvester (Cambridge Technology, Cambridge, MA, USA) and [3H]-thymidine incorporation was measured in a scintillation counter. Spontaneous incorporation of [3H]-thymidine in cultured cells ranged between 500 and 1000 counts per minute (cpm). To determine positive signals for LTT, a stimulation index of ≥2 (2 SI) and a minimum incorporation of ≥1500 δ cpm was considered positive.
Assessment of cytokines
Supernatants were collected from stimulated cells after 5 days for determination of cytokines interferon (IFN)-γ and interleukin (IL)-10. All cytokines were detected by ELISA-based assays. All reagents for the IFN-γ assay were obtained from PharMingen (San Diego, CA, USA). Reagents for IL-10 were obtained from Predicta (Cambridge, MA, USA). The assays were carried out according to the manufacturer's recommendation. Supernatants were diluted appropriately where necessary to obtain values within the detection range.
Determination of positive signals for cytokine and IgG antibody subclass responses
Mean spontaneous release of cytokines (IFN-γ and IL-10) in unstimulated PBMC cultures and serum background in the absence of antigens in ELISA assays is shown for the four study groups in Table 1. There was very little spontaneous secretion of IFN-γ but some spontaneous secretion of IL-10 was observed which varied among different groups (range 11–34 pg/ml). In the case of IFN-γ and IL-10 the sensitivity of detection was 10 pg/ml and therefore a minimum concentration of 20 pg/ml was considered positive if it was 2 standard deviations (SD) above the mean background. In the case of the IgG subclass antibody responses, there was again considerable variation in background binding, both among groups and within individual donors. Because of this variability we deducted backgrounds for each donor and the results were expressed after deducting background values. For determination of positive signals, 2 SD above the mean background values (Table 1) were considered positive for IgG1-3 subclasses. In the case of IgG4 very low optical densities were observed for all groups and therefore a cut-off 3 SD above the mean background for the group was considered positive.
Table 1.
Background values detected in the absence of antigen; results are given as mean ± 1 SD for each parameter and in each individual group
| Cytokines | L = 9 (mean ± 1SD) | T = 10 (mean ± 1SD) | P = 9 (mean ± 1SD) | EC = 8 (mean ± 1SD) |
|---|---|---|---|---|
| IFN-γ | 0 ± 0 | 1·8 ± 5·7 | 0 ± 0 | 0 ± 0 |
| IL-10 | 13·9 ± 7·2 | 10·8 ± 3·7 | 33·8 ± 10·9 | 27·1 ± 11·6 |
| IgG1 | 10·19 ± 5·88 | 8·87 ± 9·73 | 4·79 ± 2·64 | 5·98 ± 6·03 |
| IgG2 | 2·7 ± 1·39 | 5·06 ± 3·81 | 3·3 ± 2·06 | 4·4 ± 4·23 |
| IgG3 | 2·73 ± 2·32 | 3·7 ± 6·1 | 1·78 ± 1·67 | 1·81 ± 1·55 |
| IgG4 | 0·19 ± 0·1 | 0·14 ± 0·11 | 0·17 ± 0·1 | 0·16 ± 0·1 |
L = leprosy patients with lepromatous disease; T = leprosy patients with tuberculoid disease; P = pulmonary tuberculosis patients; EC = healthy endemic controls. The number in each is indicated next to the letter for each group.
Results
Figure 1 shows the amino acids composition of the M. leprae (ML) and M. tuberculosis (MT) GroES proteins. The N-terminal end has several amino acid differences in MT and ML GroES and consequently has the potential to evoke leprosy-specific responses; therefore, we will refer to these as L peptides, while the C-terminal end is the shared region between ML and MTGroES and will be referred to as T/l peptides. These peptides were tested for T cell (LTT, IFN-γ and IL-10) and B cell (IgG 1–4) responses in 28 patients and eight healthy endemic controls to identify epitopes evoking T and B cell responses in ML GroES in disease and healthy states.
Figure 1.
Amino acid sequence of MT and ML GroES proteins.11
Immune profile of leprosy and tuberculosis patients to M. leprae GroES peptides
In order to compare the immune profile of all the immune parameters we first analysed the positive responders to ML GroES peptides, as shown in Fig. 2a in the four study groups. All peptides were immunoreactive in that they were able to stimulate at least one immune parameter. In general, the number in each group showing positive T cells responses was much lower than those giving positive signals in antibody responses. The differential signals in T and B cell responses may be due to the nature and stringency of determinants being recognized by the two arms of the immune system as well as by HLA-DR restrictions. We will address first the recognition of T cell epitopes in ML GroES.
Figure 2.
T cell (LTT, IFN-γ and IL-10) and B cell (IgG1–IgG4) antibody responses to the overlapping 15-mer peptides of ML GroES. Results are expressed as percentage giving a positive signal (determined as described in Materials and methods section). Solid lines indicate T cell responses and broken lines indicate B cell responses. Responses for leprosy patients [lepromatous (a) and tuberculoid leprosy (b)], healthy endemic controls (c) and pulmonary tuberculosis patients (d) are compared. The number in each group is shown in brackets next to the group. The symbol for each parameter is identical in the four panels. The x-axis shows the stimulating peptides. Prefix T/l before the peptides indicates that the peptide is shared with the M. tuberculosis (MT) GroES homologue. Prefix L indicates that this peptide has amino acid interchanges in the MT homologue.
T cell responses
The N-terminal end of the ML and MT GroES proteins has several amino acid interchanges (indicated as L peptides). This region has been shown to be recognized by tuberculoid patients in a species-specific manner.14,19,20 When T cell responses (solid lines) were analysed to this region, leprosy patients with disseminated lepromatous disease showed a higher number of positive responders to L1–15, while those with localized tuberculoid disease and EC groups showed a greater number of positive responders to L11–25. L11–25 also showed a concomitant secretion of IFN-γ in the T and EC groups but no IFN-γ responses were detected to either L1–15 or L11–25 in patients with L disease, suggesting that this peptide may be associated with protection in leprosy, as IFN-γ plays a key role in limiting infections in leprosy. Interestingly, there was a complete absence of T cell response in tuberculosis patients to this entire region (aa1–25). A low number of positive IL-10 responses was observed to several peptides in all patient groups as well as endemic controls.
The second region with several amino acid interchanges is between aa51–70 and may have species-specific epitopes. Surprisingly, only the EC group showed a high number of positive LTT responders in this region with concomitant IFN-γ responses, while leprosy and tuberculosis patient groups likely to be carrying high bacterial loads showed no T cell responses to aa 51–70.
When responses were compared in diseased and healthy individuals to the shared C-terminal region (peptides T/L71–85, T/L81–95 and T/l 91–100), the most significant finding was the high number of positive responders in healthy controls (EC) by T cells (LTT, IFN-γ). This was not surprising, because healthy controls were BCG-vaccinated and living in a region highly endemic for tuberculosis. The absence of T cell responses in the patient group is intriguing, as we would expect these responses to be boosted during infection, while these results suggest that the T cell responses to this region may be down-modulated during disease.
B cell responses
We next analysed the four IgG subclass antibody responses to MLGroES peptides (Fig. 2; broken lines). IgG1 and IgG3 are opsonizing and complement-fixing antibodies and are considered to be markers of the proinflammatory response, while IgG4 has been shown to be associated with IgE and is considered to be a marker of Th2 response and has been shown to be associated with disease dissemination in intracellular infections.1
There was a clear association of IgG1 and IgG3 responses and IgG2 and IgG4 responses to different peptides. The most striking results were the differential recognition of peptides by the two sets of IgG subclasses. The N-terminal region (aa1–50) was the predominant target of IgG1 and IgG3 antibodies while the C-terminal end was the dominant target of IgG2 and IgG4.
The most dominant target of opsonizing antibodies was a shared peptide T/l 41–55. When healthy and diseased individuals were compared, a high number of positive responders to IgG1 antibody was associated only with leprosy disease (lepromatous = 89%; tuberculoid = 40%). The most surprising finding was that despite the fact that T/l 41–55 was a shared peptide of ML and MT GroES, tuberculosis patients did not show an IgG1 response to this peptide. A low level of IgG1 positive responses (11%) was also observed in healthy endemic controls. Thus differences in IgG1 antibody responses to peptide T/L41–55 between the groups were related to a higher number of positive responders rather than differential recognition of peptides among groups. IgG3 antibodies were also detected to this peptide (T/L41–55) but unlike IgG1, IgG3 was equally present in all groups including healthy controls. IgG1 also recognized a second N-terminal peptide L11–25, but there was no leprosy disease association as this response was present in low numbers in all groups. IL-10 has been shown to be an important factor in the switching of IgG3 to IgG1 antibodies in humans, but no consistent relationship was detected with either IgG1 or IgG3 responses with IL-10.
IgG2 and IgG4 picked up a high number of positive responders (>60%) to the C-terminal region peptides (T/L71–85, T/L81–95 and T/L91–100) but unlike T cell responses which were detected only in healthy groups to this region, IgG2 and IgG4 responses were detected in all groups, indicating an absence of association with either T cell responses or disease spectrum.
Intensity of response to shared and cross-reactive peptides of MLGroES
Low numbers of T cell-positive responses to small peptides is expected due to HLA-DR restriction, but this should not affect the intensity of response in positive donors. Because of the low number of positive responders, we have shown mean responses in only positive responders. Comparative intensity of response in the four groups is shown in Fig. 3 for T cell responses (LTT, IFN-γ and IL-10), with each group being represented by a different symbol.
Figure 3.
Mean intensity of T cell responses (LTT, IFN-γ and IL-10) in donors giving a positive signal to the 15-mer peptides of ML GroES in the four study groups described in Figure 2. Results are expressed after deducting background cpm incorporation or spontaneous secretion of cytokines in the absence of stimulus. Different groups are shown with different symbols. All other details including the donors in each group are as described in Figure 2.
Patients with lepromatous disease showed the highest intensity of LTT response to the N-terminal peptide L1–15, followed by weak responses to two other peptides in the region of aa1–55 (Fig. 3a). Tuberculoid leprosy patients and EC recognized a different set of peptides (L11–25 and L61–75) to leprosy patients with lepromatous disease. The highest intensity of LTT response was observed in the T group of patients to peptide L11–25. EC who had shown a high number of positive responders to the C-terminal end showed a much lower intensity of responses to these peptides.
When IFN-γ secretion was analysed in the four groups, only one peptide (L21–35) induced a strong IFN-γ response in L patients, which is not the peptide which stimulated LTT. In leprosy patients with lepromatous disease there was very little correlation of T cell proliferation with IFN-γ secretion. The C-terminal peptides showing a high number of positive responders to IFN-γ in EC did not show a strong intensity of response. The low intensity of response in endemic controls to shared peptides of mycobacteria is not surprising, as these may be baseline responses due to BCG vaccination or due to exposure to other environmental mycobacteria. This region has also been shown previously to be HLA-DR unrestricted.15 However, these results highlight the importance of both sets of analysis in evaluating immune recognition to peptides.
IL-10 is a down-regulatory cytokine and not surprisingly therefore the highest response was observed in the leprosy patients, both in terms of the number of peptides being recognized and the intensity of response, whereas that of the tuberculosis and control group is limited to a smaller number of peptides.
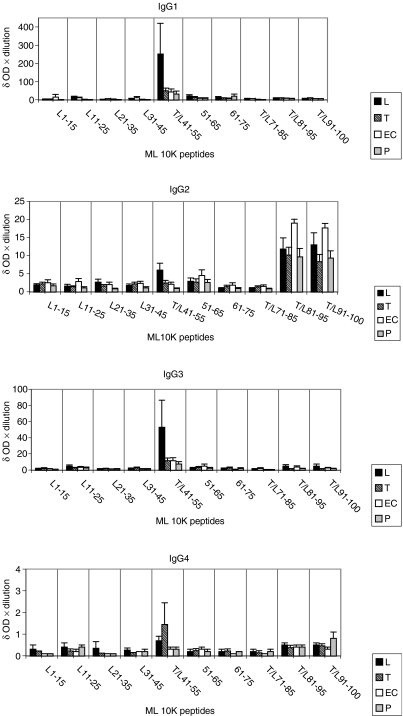
We next examined the intensity of IgG antibody subclass signal in patients and control groups. The results are given as group mean ± 1 SEM after deducting background binding of serum in the absence of antigen (Fig. 4). The highest intensity of IgG1 and IgG3 antibodies to the peptides was seen in patients with lepromatous disease, while the intensity of IgG2 and IgG4 was similar or higher in patients with tuberculoid disease and endemic controls. In the case of IgG2 the dominant target was the shared peptide T/L80–100, while in the case of IgG4 the dominant target was T/L41–55. The absence of Th2 activation in lepromatous patients is interesting, but the explanation may lie in the suppression of both Th1 and Th2 responses by the higher IL-10 responses in patients with lepromatous disease. However, these relationships need to be examined much more critically and in larger groups for firm conclusions to be drawn.
Figure 4.
Mean intensity of IgG subclass antibody responses to the 15-mer peptides in each group. Results are given as mean of the four groups described after deducting background binding in the absence of antigen. Different groups are shown as boxes with different fillings, while the vertical line indicates 1 SE around the mean (1 SEM). All other details including the donors in each group are as described in Figure 2.
In summary, our results suggest that T and B cells responses are directed overwhelmingly to the N terminal regions of GroES in leprosy patients while healthy endemic controls recognize predominantly the C-terminal region of MLGroES. N-terminal regions have amino acid interchanges with MTGroES and therefore are likely to contain species-specific M. leprae determinants. To evaluate if any of the immune responses being detected to the N-terminal regions were species- or disease-specific in leprosy patients, we compared immune responses to corresponding peptides in the ML and MT GroES proteins.
Comparison of the immune responses to ML and MT GroES corresponding peptides
Six peptides in ML and MT GroES differed by 1–4 amino acids. The corresponding peptide homologues in MT GroES antigen with 1–4 amino acid interchanges were tested in parallel to detect if any of the response were directed towards species-specific epitopes in leprosy patients. Table 2 shows T cell responses to the homologues of ML and MT GroES peptides in the four study groups. The grey areas show concordant responses to ML and MT GroES peptides, suggesting recognition of shared epitopes, while the boxed areas show responses to L peptides in only the leprosy disease groups (lepromatous and tuberculoid leprosy), and may be a disease-specific response. It was clear that in disease groups as well as in the healthy EC group responses were overwhelmingly to shared epitopes. The only peptide which showed a response in a disease-specific as well as a species-specific manner was LTT to peptide L1–15, which was detected in L patients only. This peptide differed by only a single amino acid and truncation or amino acid substitution analysis will have to be carried to determine if this is indeed species-specific recognition. IFN-γ and IL-10 also show similar concordant responses to corresponding peptides.
Table 2.
T-cell responses to the corresponding peptides of ML and MT GroES proteins; results are given as percentage of donors giving a positive signal (cut-off shown in the table below each parameter)
| Immune parameters | L | T | EC | P | L | T | EC | P | L | T | EC | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTT | LTT | LTT | LTT | IFN-γ | IFN-γ | IFN-γ | IFN-γ | IL-10 | IL-10 | IL-10 | IL-10 | ||
| Amino acid no. | Cut-off values for positivity Amino acid sequence | 2 SI > 1500 δ cpm | >χ + 2 SD > δ 20 pg/ml | >χ + 2 SD > δ 20 pg/ml | |||||||||
| L1-15 | VAKVKIKPLEDKILV (ML) | 22 | |||||||||||
| T1-15 | -----N--------- (MT) | 11 | |||||||||||
| L11-25 | DKILVQAGEAETMTP (ML) | 11 | 10 | 25 | 20 | 25 | 11 | ||||||
| T11-25 | -------N----T-A (MT) | 11 | 10 | 25 | 30 | 25 | 11 | ||||||
| L21-35 | ETMTPSGLVIPENAK (ML) | 10 | 11 | 11 | 11 | 20 | 25 | ||||||
| T21-35 | --T-A------DT-- (MT) | 10 | 11 | ||||||||||
| L31-45 | PENAKEKPQEGTVVA (ML) | 11 | 10 | ||||||||||
| T31-45 | -DT------------ (MT) | 11 | 11 | 22 | |||||||||
| L51-65 | WDEDGAKRIPVDVSE (ML) | 10 | 11 | ||||||||||
| T51-65 | ---------E-----L-A- (MT) | 11 | 10 | 11 | 11 | 10 | 22 | ||||||
| L61-75 | VDVSEGDIVIYSKYG (ML) | 38 | 11 | 25 | 11 | 11 | 10 | ||||||
| T61-75 | L-A----T------- (MT) | 13 | 11 | 10 | 25 | 11 | 11 | 11 | |||||
| ML10K | >M. leprae | 33 | 40 | 50 | 11 | 44 | 50 | 63 | 33 | 78 | 90 | 88 | 67 |
| MT10K | M. tuberculosis | 22 | 30 | 63 | 11 | 33 | 60 | 50 | 11 | 67 | 70 | 13 | 79 |
Italic data indicate concurrent recognition of ML and MT GroES peptides. Bold data indicate that responses were present only in leprosy patients to ML peptide only. All other details including the donors in each group are as described in Fig. 2.
Table 3 shows IgG subclass1–4 antibody responses in the four groups. Interestingly, with these set of peptides the predominant responses were observed with the IgG1 antibody subclass only. The only peptides to show an IgG1 response to the ML peptide in the absence of concurrent response to the MT peptides was L11–25 in leprosy patients. All other peptides showed concordant responses to both ML and MT peptides with IgG1, suggesting recognition of shared peptides. The other three IgG subclasses did not show strong responses and were detected mainly to both ML and MT homologues. These observations again support our hypothesis that in the leprosy disease state, immune responses are directed overwhelmingly to cross-reactive rather than species-specific epitopes.
Table 3.
B-cell responses to the corresponding peptides of ML and MT GroES proteins; results given as percentage in each group giving a positive signal (cut-off shown in the table below each parameter)
| Immune parameters | L | T | EC | P | L | T | EC | P | L | T | EC | P | L | T | EC | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG1 | IgG1 | IgG1 | IgG2 | IgG2 | IgG2 | IgG2 | IgG3 | IgG3 | IgG3 | IgG3 | IgG4 | IgG4 | IgG4 | IgG4 | ||
| Amino acid no. | Cut-off values for positivity Amino acid sequence | mean ± 2 SD | mean ± 2 SD | mean ± 2 SD | mean ± 3 SD | ||||||||||||
| L1-15 | VAKVKIKPLEDKILV (ML) | 13 | |||||||||||||||
| T1-15 | -----N---------------- (MT) | 11 | 11 | 10 | 38 | ||||||||||||
| L11-25 | DKILVQAGEAETMTP (ML) | 33 | 10 | 11 | 13 | 11 | 13 | 10 | 33 | ||||||||
| T11-25 | ------N-----T-A (MT) | 13 | 30 | ||||||||||||||
| L21-35 | ETMTPSGLVIPENAK (ML) | 11 | 10 | ||||||||||||||
| T21-35 | --T-A------DT-- (MT) | 22 | 11 | 11 | |||||||||||||
| L31-45 | PENAKEKPQEGTVVA (ML) | 20 | 11 | ||||||||||||||
| T31-45 | -DT------------ (MT) | 10 | 13 | 11 | |||||||||||||
| L51-65 | WDEDGAKRIPVDVSE (ML) | 33 | 10 | 12·5 | 22 | 11 | 12·5 | 11 | 10 | 25 | 11 | ||||||
| T51-65 | -----E-----L-A- (MT) | 33 | 30 | 12·5 | 11 | 11 | 11 | 11 | |||||||||
| L61-75 | VDVSEGDIVIYSKYG (ML) | 22 | 13 | 22 | 12·5 | 10 | |||||||||||
| T61-75 | L-A-----T------ (MT) | 11 | 13 | ||||||||||||||
| ML10K | M. leprae | 88 | 40 | 25 | 55 | 44 | 10 | 12·5 | 33 | 44 | 10 | 37·5 | 33 | 50 | 20 | 0 | 22 |
| MT10K | M. tuberculosis | 33 | 10 | 0 | 22 | 55 | 0 | 0 | 11 | 55·5 | 30 | 37·5 | 33 | 25 | 0 | 25 | 11 |
Italic data indicate concurrent recognition of ML and MT GroES peptides. Bold data indicate that responses were present only in leprosy patients to ML peptide only. All other details including the donors in each group are as described in Fig. 2.
Discussion
The most established tenet in mycobacterial disease is that strong T cell responses are associated with disease localization and protection,3 and therefore antigens which evoke strong T cell responses are potential vaccine candidates. Recently this concept gained further credence, when Pym et al.21 showed that the introduction of a genetic region (RD1) present in virulent M. tuberculosis but deleted in the BCG vaccine strain, which codes for transport of strong T cell immunogens (including ESAT 6 and culture filtrate protein 10), enhanced protection against tuberculosis in experimental murine models. Among the M. leprae antigens ML GroES has been shown to be the dominant target of strong T cells responses in both healthy household contacts of leprosy patients as well as in the more localized tuberculoid form of the disease.14–16 This antigen has therefore been the focus of study as a potential vaccine candidate. We have questioned this assumption on the basis that this antigen also evokes strong IgG1 antibody responses which are associated negatively with IFN-γ, a key cytokine required for intracellular bacterial killing. Our study was therefore an attempt to identify epitopes which evoke only T cell responses and do not evoke deleterious antibody responses simultaneously. Our results demonstrate clearly that the dominant targets of T and B cell responses to GroES proteins were directed to different epitopes. It was interesting to note that T cell responses in healthy controls were associated with IgG2 and IgG4 responses and were restricted to a defined region in GroES proteins, the C-terminal region (aa81–100), and could be a potential vaccine candidate. The most predominant target of IgG1 antibodies (T/L41–55) was a very poor T cell immunogen, thus encouraging the hope that B cell epitopes associated with pathogenic responses can be eliminated from T cell immunogens.
Concurrent analysis of several immune parameters in each donor resulted necessarily in restriction of the group sizes, which may in turn limit the number of epitopes being recognized in our study. The highest T cell responses were observed to the C-terminal end (aa70–100) in healthy EC. This region has been shown to be HLA unrestricted and has previously shown binding to several HLA types.15 T cell responses in leprosy patients were directed mainly to the N-terminal end, where several amino acids interchanges occur, and therefore this region is likely to have species-specific determinants. However, when MT GroES homologous peptides were tested in parallel it was observed that the overwhelming response was to shared rather than species-specific epitopes. This is not surprising, as GroES is a highly conserved protein among mycobacterial species as well as across several other pathogenic species.13 Recognition of cross-reactive epitopes may also be a reflection on the intensity of transmission with other mycobacterial species as well as wide coverage with BCG in our population. The baseline T and B cell memory responses to these conserved epitopes shared by the infecting pathogen in endemic populations may determine the expansion of T cells during disease states. Our observations may therefore provide a rational explanation for the paradoxical observation that mycobacterial GroES proteins, despite 40% homology with human GroES,13 have been shown to be targets of strong T and B cell responses.14,15,17 Such recognition of cross-reactive epitopes may actually afford protection (herd immunity?) and is consistent with the observation in Malawi where BCG provided higher protection for leprosy than tuberculosis.22
Not surprisingly, differences in disease and healthy states related more to the strength (mean intensity) of the T cell and antibody response. The highest intensity of IgG1 and IgG3 antibodies to the peptides was seen in patients with lepromatous disease, while the intensity of IgG2 and IgG4 was similar or higher in patients with tuberculoid disease and endemic controls. We observed both IgG1 and IgG3 antibody responses to the shared epitope, aa41–55 in leprosy patients, but healthy controls and tuberculosis patients showed only IgG3 antibodies to this epitope. It is therefore tempting to speculate that switching of IgG3 to IgG1 antibodies may be occurring to this epitope in leprosy patients only. IL-10 has been identified to be the switch factor for IgGI in humans.23 However, we could not find a correlation of IgG1 binding and secretion of IL-10 to individual epitopes. An alternative explanation could therefore be that IgG1 memory B cells were being expanded in patients with lepromatous disease, rather than an IgG switch event occurring which may require additional T cell factors which may be absent or down-regulated in lepromatous patients. IgG2 and IgG4 were directed predominantly towards the C-terminal end in all groups, irrespective of the disease status and the presence of T cell responses. The coordinate expression of IgG1 and IgG3, and IgG2 and IgG4, has been reported in several studies24,25 including our studies with both the GroES and 18 KD proteins of M. leprae.17,26 We now show that that this coordinate expression of IgG subclasses exists within the same disease to different epitopes. The overwhelming recognition by all IgG subclasses of shared epitopes further supports our hypothesis that expansion of baseline memory T and B cell responses to cross-reactive epitopes shared across several pathogens may be occurring in leprosy patients. Identification of cross-reactive epitopes that are able to induce protective immunity and are non-HLA-DR-restricted would provide useful vaccine candidates. Such comprehensive immune profiling of antigen-specific responses is critical not only to understanding the disease pathogenesis but also if these reagents are to be exploited for either diagnostic or vaccine purposes.
Acknowledgments
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). The authors would like to thank the Marie Adelaide Leprosy Centre, Karachi, Pakistan for patient material, Dr Sebastian Lucas, University College School of Medicine, London, UK (currently St Thomas's Hospital) for providing histopathology on all leprosy patients, and Dr Ghaffar Dawood for recruiting tuberculosis patients. We thank Philip Broadbent for peptide synthesis at LSHTM. We would also like to thank Miss Regina D'Souza for secretarial help.
Abbreviations
- L
= leprosy patients with lepromatous disease
- T
= leprosy patients with tuberculoid disease
- P
= pulmonary tuberculosis patients
- EC
= healthy endemic controls
- ML
= Mycobacterium leprae
- MT
= Mycobacterium tuberculosis
- hsps
= heat shock proteins
- prefix
T/l before the peptides indicates that this peptides is shared with M. tuberculosis (MT) GroES homologue
- prefix
L indicates that this peptide has amino acid interchanges in the MT homologue
References
- 1.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of IFN gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine Candidiasis: Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993;150:925–31. [PubMed] [Google Scholar]
- 3.Myvang B, Godal T, Ridley DS, Froland SS, Song YK. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973;14:541–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Melsom R, Harboe M, Myrvang B, Godal T, Belehu A. Immunoglobulin class specific antibodies to M. leprae in leprosy patients, including the intermediate group and healthy contacts as a step in the development of methods for serodiagnosis of leprosy. Clin Exp Immunol. 1982;47:225–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain R, Kifayet A, Chiang T. Immunoglobulin G1 (IgG1) and IgG3 antibodies are markers of progressive disease in leprosy. Infect Immun. 1995;63:410–5. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter SW, Rivoire B, Mehra V, Bloom BR, Brennan PJ. The major native proteins of the leprosy bacillus. J Biol Chem. 1990;265:14065–8. [PubMed] [Google Scholar]
- 7.Pessolani MCV, Brennan PJ. Molecular definition and identification of new proteins of Mycobacterium Leprae. Infect Immun. 1996;64:5425–7. doi: 10.1128/iai.64.12.5425-5427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivoire B, Pessolani MCV, Bozic CM, Hunter SW, Hefta SA, Mehra V, Brennan P. Chemical definition, cloning, and expression of the major protein of the leprosy bacillus. Infect Immun. 1994;62:2417–25. doi: 10.1128/iai.62.6.2417-2425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young RA, Sweetser D, Mehra V, Buchanan T, Davis RW, Bloom BR. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985;316:450–2. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]
- 10.Young DB, Lathigra R, Hendrix R, Sweetser D, Young RA. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci USA. 1988;85:4267–70. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta RS. Evolution of the chaperonin families (Hsp60, Hsp10 and Tcp-1) of protein and the origin of eukaryotic cells. Mol Microbiol. 1995;15:1–11. doi: 10.1111/j.1365-2958.1995.tb02216.x. [DOI] [PubMed] [Google Scholar]
- 12.Mayhew M, da Silva ACR, Martin J, Erdjument-Bromage H, Tempst P, Hartl FU. Protein folding in the central cavity of the GroEL–GroES chaperonin complex. Nature. 1996;379:420–6. doi: 10.1038/379420a0. [DOI] [PubMed] [Google Scholar]
- 13.Mande SC, Bloom BR, Hol WGJ. Structure of the heat shock protein chaperonin-10 of Mycobacterium leprae. Science. 1997;271:203–7. doi: 10.1126/science.271.5246.203. [DOI] [PubMed] [Google Scholar]
- 14.Mehra V, Bloom BR, Bajardi AC, et al. A major T cell antigen of Mycobacterium leprae is a 10 kD heat shock cognate protein. J Exp Med. 1992;175:275–84. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Sette A, Rodda S, et al. Determinants of T cell reactivity to the Mycobacterium leprae GroES homologue. J Immunol. 1997;159:335–43. [PubMed] [Google Scholar]
- 16.Launois P, N'Diaye MN, Cartel JL, Mane I, Drowart A, Van Vooren JP, Sarthou JL, Huygen K. Fibronectin binding antigen 85 and the 10-kilodalton GroES-related heat shock protein are the predominant TH-1 responses inducers in leprosy contacts. Infect Immun. 1995;63:88–93. doi: 10.1128/iai.63.1.88-93.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain R, Dockrell HM, Chiang TJ. Dominant recognition of a cross-reactive B cell epitope in Mycobacterium leprae 10 K antigen by immunoglobulin G1 antibodies across the disease spectrum in leprosy. Immunology. 1999;96:620–7. doi: 10.1046/j.1365-2567.1999.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain RA, Kifayet M, Dojki and Dockrell HM. Selective correlation of interferon-γ tumour necrosis factor α and granulocyte macrophage colony-stimulating factor with immunoglobulin G1 and Immunoglobulin G3 subclass antibody in leprosy. Immunology. 1999;98:238–43. doi: 10.1046/j.1365-2567.1999.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain R, Dockrell HM, Shahid F, Zafar S, Chiang TJ. Leprosy patients with lepromatous disease recognizes cross reactive T cell epitopes in the M. leprae GroES antigen. Clin Exp Immunol. 1998;114:204–9. doi: 10.1046/j.1365-2249.1998.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chua-Intra B, Peerapakorn S, Davey N, et al. T cell recognition of mycobacterial GroES peptides in Thai leprosy patients and contacts. Infect Immun. 1988;66:4903–9. doi: 10.1128/iai.66.10.4903-4909.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pym AS, Brodin P, Majelessi L, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9:533–9. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 22.Fine PEM, Maine N, Ponnighaus JM, Clarkson JA. Protective efficacy of BCG against leprosy in Northern Malawi. Lancet. 1986;2:499–502. doi: 10.1016/s0140-6736(86)90367-3. [DOI] [PubMed] [Google Scholar]
- 23.Fujieda S, Saxon A, Zhang K. Direct evidence that gamma 1 and gamma 3 switching in human B cell is interleukin-10 dependent. Mol Immunol. 1996;33:1335–43. doi: 10.1016/s0161-5890(96)00092-2. [DOI] [PubMed] [Google Scholar]
- 24.Skvaril F, Schilt U. Characterization of the subclasses and light chain types of IgG antibodies to rubella. Clin Exp Immunol. 1984;55:671–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Riesen WF, Skvaril F, Braun DG. Natural infection of man with group A Streptococci. Levels, restriction in class, subclass and type: and clonal appearance of polysaccaride group-specific antibodies. Scand J Immunol. 1976;5:383–90. doi: 10.1111/j.1365-3083.1976.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 26.Hussain R, Menz B, Dockrell HM, Chiang TJ. Recognition of Mycobacterium leprae recombinant 18000 MW epitopes by IgG subclasses in leprosy. Immunology. 1995;84:290–7. [PMC free article] [PubMed] [Google Scholar]