Abstract
Allicin, a major ingredient of fresh garlic extract that is produced during the crushing of garlic cloves, exerts various beneficial biological effects, including a broad spectrum of antimicrobial activity, antihyperlipidaemic and antihypertensive effects. However, how allicin affects the immune system is less well known, and its effect on human T cells has never been studied. Here, we examined the in-vitro effects of allicin on the functioning of T cells related to their entry to inflamed extravascular sites. We found that allicin (20–100 µm) inhibits the SDF-1α (CXCL12)-induced T cell migration through fibronectin (FN), and that this inhibition is mediated by the down-regulation of (i) the reorganization of cortical actin and the subsequent T cell polarization, and (ii) T cell adhesion to FN. Moreover, allicin also inhibited T cell adhesion to endothelial cells and transendothelial migration. The mechanisms underlying these inhibitory effects of allicin are associated with its ability to down-regulate the phosphorylation of Pyk2, an intracellular member of the focal adhesion kinases, and to reduce the expression of the VCAM-1- and FN-specific α4β1-integrin (VLA-4). The ability of allicin to down-regulate these chemokine-induced and VLA-4-mediated T cell functions explains its beneficial biological effects in processes where T cells play an important role and suggests that allicin may be used therapeutically with chronic inflammatory diseases.
Introduction
The diverse beneficial health-related biological effects of garlic, Allium sativum, are attributed to its characteristic organosulphor compounds.1–4 Allicin (diallyl-thiosulphinate), the best-known and most extensively studied of these compounds, is a major ingredient of fresh garlic extract. Allicin is produced during the crushing of garlic cloves by the chemical interaction between the non-protein amino acid alliin and the enzyme alliinase, which is also present in the cloves.5
Allicin has a variety of therapeutic properties. For example, it exhibits antibacterial activity against a wide range of Gram-negative and Gram-positive bacteria and even acid fast.6,7 Moreover, allicin possesses a strong antifungal activity, particularly against Candida albicans8,9 as well as antiparasitic activity,10 and it is active against certain viruses.8 In addition to its effect on pathogens, allicin reduces serum cholesterol and lowers the LDL level.11 It suppresses the oxidation of LDL,12 thereby reducing the risk of atherosclerosis and the formation of fatty streaks (atherosclerosis) in hyperlipidaemic mice.13 In addition, allicin inhibits platelet aggregation,14 acts as a systemic vasodilator with an antihypertensive effect15 and inhibits the proliferation of human tumour cells.16
However, despite the fact that its beneficial effects regarding pathogens have been well established, little is known about whether allicin affects immune cell activation and behaviour. Interestingly, garlic extract was found to reduce the migration of neutrophils through endothelial cells.17 However, the effects of allicin on human T cells has never been studied. Here, we examined the in-vitro effects of allicin on the functioning of T lymphocytes within extravascular inflamed sites. T cell migration from the vascular compartment, across tissue barriers and through the extracellular matrix (ECM), is a key event during inflammation that is mediated primarily by β1-integrins.18 In these extravascular sites of inflammation, inflammatory mediators, including cytokines and chemokines, activate T cells and affect their recognition of ligands and binding of integrins.18–22
Our results demonstrate that allicin inhibits T cell migration through fibronectin (FN), a major cell adhesion glycoprotein of the ECM, by down-regulating the reorganization of cortical actin with subsequent T cell polarization independent of the interaction with FN, and also by inhibition of T cell adhesion to FN. The down-regulation of Pyk2 phosphorylation and very late antigen-4 (VLA-4) expression may account for these inhibitory effects of allicin. These mechanisms are probably also responsible for the observed allicin-induced inhibition of T cell adhesion to human umbilical vein endothelial cells (HUVEC) and the inhibition of transendothelial migration. Thus, allicin may be used therapeutically to down-regulate inflammatory reactions, such as these associated with autoimmunity and allergy.
Materials and methods
Reagents
Allicin was prepared by applying synthetic allicin onto an immobilized alliinase column, as described previously.23 Allicin concentration was determined according to Miron et al.24 The following reagents and chemicals were purchased from the following sources: RPMI-1640 (Gibco BRL), fetal calf serum (FCS), HEPES buffer, antibiotics, sodium pyruvate (Kibbutz Beit-Haemek, Israel), fibronectin (FN; Chemicon, Temecula, CA) and recombinant human SDF-1α (R&D Systems, Minneapolis, MN). Tissue culture plates were purchased from Becton Dickinson Labware (Franklin Lakes, NJ). Monoclonal antibodies (mAbs) directed against the α4, α5 and α6 subunits of the human β1-integrin, clones HP2/1 and JBSS, respectively, and antihuman LFA-1 (clone DF1524) as well as the anti-CXCR-4 mAb were obtained from Serotec (Oxford, UK); mAb antiphosphorylated Pyk2 (clone py881) was obtained from Biosource (Camarillo, CA) and antitotal Pyk2 (clone N-19) from Santa Cruz Biotech (Santa Cruz, CA). 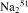 [Cr]O4 was purchased from Amersham Pharmacia Biotech (Little Chalfont, UK).
[Cr]O4 was purchased from Amersham Pharmacia Biotech (Little Chalfont, UK).
Human T cells
T cells were obtained from the peripheral blood of healthy human donors, and isolated on Ficoll-Histopaque prepacked columns and washed. B cells were depleted by adherence to nylon wool (Novamed, Jerusalem, Israel) in a humidified atmosphere (45 min, 37°, 7·5% CO2) as described previously.25,26 The non-adherent cells were eluted and washed. To remove monocytes, we incubated (2 hr, 37°, 7·5% CO2 humidified atmosphere) the cells on tissue culture grade Petri dishes. The non-adherent cells were collected and CD3+ T cells were isolated by negative selection with a mouse antihuman antibody cocktail (Pan T cell kit, Miltenyi Biotec, Germany) containing mAbs against CD11b, CD16, CD19, CD36, and CD56. The cells were then passed through separation columns (MiniMACS columns, Miltenyi Biotec).
Human umbilical vein endothelial cells
HUVEC were isolated from umbilical cord veins and cultured as described.27
Chemotaxis assays
The migration of human T cells was examined in a 24-well chemotaxis microchamber (6·5-mm diameter; Corning, NY). The two compartments of the microchambers were separated by a FN-coated (1·25 µg/well) polycarbonate filter (5 µm pore size; Osmonics Proteins Products, Livemore, CA). To induce T cell chemotaxis, SDF-1α (100 ng/ml) was added to the lower wells, and the 51[Cr]-radiolabelled T cells (2 × 105 cells in a 100 µl/well) in RPMI-1640 [containing 0·1% bovine serum albumin (BSA)] were placed in the upper chambers. Various concentrations of allicin were added, together with the cells to the upper chamber. After completing the assay (3 hr, 37° in a humidified, 7·5% CO2 atmosphere), the filters were removed and the number of migrating T cells was determined by the level of radioactivity present in the lower wells. Control wells contained radioactivity of the cells that passed without a chemoattractant. The results (± SD) are expressed as the mean percentage of migrating T cells from duplicate wells.
For migration experiments on tumour necrosis factor (TNF)-α-activated endothelial cells, primary HUVEC (passages 2 or 3) were left intact or stimulated on the filters for 18 hr with heparin-free culture media supplemented with TNF-α (2 ng/ml, 50 units/ml; R&D, Minneapolis, USA).
T cell adhesion assay
T cell adhesion to FN was analysed as described previously.25 Briefly, flat-bottomed microtitre well plates were precoated with FN (10 ng/ml; Sigma, St Louis, MO) and the remaining binding sites were blocked with 1% BSA. Next,51[Cr]-labelled T cells were resuspended in RPMI-1640 medium supplemented with 1% HEPES buffer and 0·1% BSA (adhesion medium), and were then added to the wells with different concentrations of allicin. The plates were incubated (60 min, 37° in a 7·5% CO2 humidified atmosphere) and then washed gently three times. The adherent cells were lysed (1 m NaOH, 0·1% Triton X-100 in H2O), removed and counted by a g-counter (Packard, Downers Grove, IL). The results (± SD) are expressed as the mean percentage of bound T cells from quadruplicate wells.
For adhesion experiments on resting or TNF-α HUVEC, primary HUVEC (passages 2 or 3) were left intact or stimulated for 18 hr with heparin-free culture media supplemented with TNF-α (2 ng/ml, 50 units/ml; R&D, Minneapolis, USA).
Actin polymerization
Human T cells (3 × 106 cells per ml) were preincubated with various concentrations of allicin (1 hr, 37° in a 7·5% CO2 humidified atmosphere) and treated with 100 ng/ml of SDF-1α for 15 seconds at 37° and then fixed by the addition of a threefold volume of 3·7% PFA for 10 min at 22°. Next, the cells were washed extensively and the membranes were permeabilized (2 min) in a solution containing HEPES (20 mm), sucrose (300 mm), NaCl (50 mm), MgCl2 (3 mm) and TritonX-100 (0·1%). Thereafter, the T cells were stained (30 min) with fluorescein isothyocyanate (FITC)-phalloidin (2 µg/ml), washed with phosphate buffered saline (PBS) and analysed by FACScan (Beckton Dickinson; Mountain View, CA) at 525 nm, using CellQuest software.
Western blot analysis of T cell lysates
Purified human T cells were incubated in starvation medium (RPMI-1640 medium without serum) for at least 24 hr before the experiments. The cells (5 × 106 per sample) were activated with SDF-1α (100 ng/ml) alone or with various concentrations of allicin (5 min, 37° in a 7·5% CO2 humidified atmosphere). The reaction was terminated by freezing the plates at −70° for 10 min. The thawed cells were incubated (60 min, 4°) in lysis buffer containing ethylenediaminetetraacetic acid (EDTA) (0·5 mm), NaCl (150 mm), NaF (10 nm), Tris (pH 7·5, 25 mm), Triton X-100 (1%), PMSF (200 µg/ml) and phosphatase inhibitor cocktail (1%; Sigma), cleared by centrifugation (30 min, 14 × 103 rpm), and the supernatants were analysed for protein content. Sample buffer was then added and after boiling, the samples were separated on 10% sodium deodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gel and transferred to nitrocellulose membranes. The membranes were blocked [TBST buffer containing low-fat milk (5%), Tris (pH 7·5, 20 mm), NaCl (135 mm) and Tween-20 (0·1%)], and probed with antibodies. The types of antibodies used were antiphosphorylated Pyk2 (pPyk2) and antitotal Pyk2 (tPyk2): 1·5 and 0·2 µg/ml, respectively. Immunoreactive protein bands were visualized using horseradish peroxidase-conjugated secondary antibody and the enhanced chemiluminescence (ECL) system.
FACS assay
T cells (106 cells per sample) were incubated (1 hr) with various concentrations of allicin and then washed, centrifuged and incubated (30 min, 4°) with mouse monoclonal antibody (mAb) antihuman VLA-4, VLA-5, VLA-6, and CXCR4 (20 µg per 106 cells). The cells were then washed and incubated further (30 min, 4°) with FITC-conjugated rabbit antimouse antibody (Dako, Denmark), washed and resuspended in PBS (containing 1% FCS and 0·01% sodium azide; final concentration of 5 × 105 cells in 0·5 ml), and analysed by FACSort (Beckton Dickinson Labware, Franklin Lakes, NJ).
Results
Allicin inhibits the SDF-1α-induced T cell chemotaxis through FN
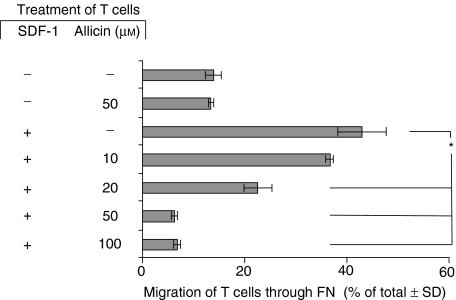
We used the Transwell microchamber system in which51[Cr]-labelled T cells were placed in the upper chamber with various concentrations of allicin, and SDF-1α (100 ng/ml) was added to the lower chamber. T cell migration through FN-coated filters was measured after 3 hr. As shown in Fig. 1, allicin alone (50 µm) did not affect T cell migration, whereas SDF-1α induced a marked (42%) T-cell chemotaxis. However, when both SDF-1α and allicin were present in the microchamber, a dose-dependent allicin-induced inhibition of T cell chemotaxis occurred; significant inhibition of T cell migration was already evident at a concentration of 20 µm allicin, and a maximal inhibitory effect was achieved at 50 µm. In another experiment we pretreated the cells with allicin (1 hr), and then washed the cells. Next, the allicin-treated (and control cells) were exposed to SDF-1α, and their subsequent chemotaxis was examined as described above. The results we obtained (data not shown) were identical to those described in Fig. 1. This excludes the possibility that allicin exerts its regulatory effect via a direct interaction with SDF-1α.
Figure 1.
Allicin inhibits SDF-1α-induced T cell chemotaxis through FN. Labelled T cells were placed in the upper well of the Transwell microchamber system with various concentrations of allicin. SDF-1α (100 ng/ml) was added to the lower chambers. T cell migration through the FN-coated filters was measured after 3 hr, and the percentage of the total cells migrating to the lower chamber was calculated after 3 hr. One experiment representative of five; *P < 0·05.
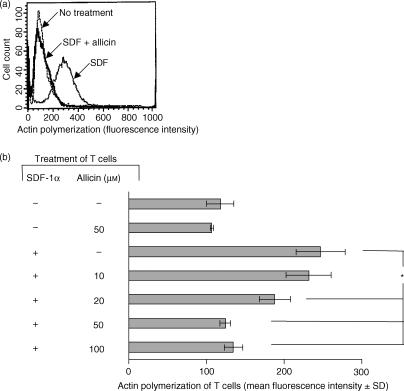
Allicin inhibits SDF-1α-induced T cell actin polymerization and polarization
T cell locomotion is associated with the generation of a leading lamella and this is driven mainly by rapid rearrangement and polymerization of cortical actin in response to activation by chemoattractants.28 Because allicin inhibited T cell migration through FN, we studied the effect of allicin on the polymerization of actin within human T cells. To this end, the cells were treated with allicin (1 hr) and then activated with SDF-1α (100 ng/ml) for 15 seconds. The redistribution of polymerized actin was determined by the intracellular staining of the cells with FITC-conjugated phalloidin, which binds actin only in its polymerized form. Allicin strongly inhibited, in a dose-dependent manner, the actin polymerization triggered by SDF-1α (Fig. 2a,b); a significant inhibition of actin polymerization was already found at a dose of 20 µm of allicin, and at 50 µm and higher allicin reduced the actin polymerization to the level of the non-treated cells. Thus, allicin-induced inhibition of T cell migration through FN is achieved by the inhibition of the reorganization of cortical actin.
Figure 2.
Allicin inhibits actin polymerization of T cells. T cells were treated with allicin (1 hr), and then activated with SDF-1α (100 ng/ml) for 15 seconds. The redistribution of polymerized actin was determined by the intracellular staining of the cells with FITC-conjugated phalloidin. Changes in fluorescence intensity with 50 µm allicin (a) and with various doses (b) are shown. One experiment representative of four; *P < 0·05.
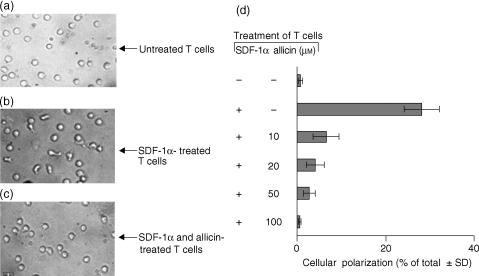
In order to explore whether the inhibition of actin polymerization by allicin influences the subsequent cellular polarization, we seeded T cells under tissue culture conditions in the presence or absence of SDF-1α, with or without allicin. After 1 hr of incubation, the morphology of the cells was examined using light microscopy. In each field, the percentage of polarized cells of the total cells added was calculated. At 20–100 µm, allicin significantly reduced the SDF-1α-induced T cell polarization (Fig. 3a–d). Thus, the ability of allicin to inhibit T cell migration through FN is mediated by inhibition of the cytoskeleton rearrangement and the cellular morphological changes that are associated with such T cell activation by SDF-1α.
Figure 3.
Allicin inhibits T cell polarization induced by SDF-1α. T cells were maintained (1 hr) under tissue culture conditions in the presence of SDF-1α (100 ng/ml). T cell morphology was examined using light microscopy. In each field, the percentage of polarized cells of the total cells added was calculated. One experiment representative of five.
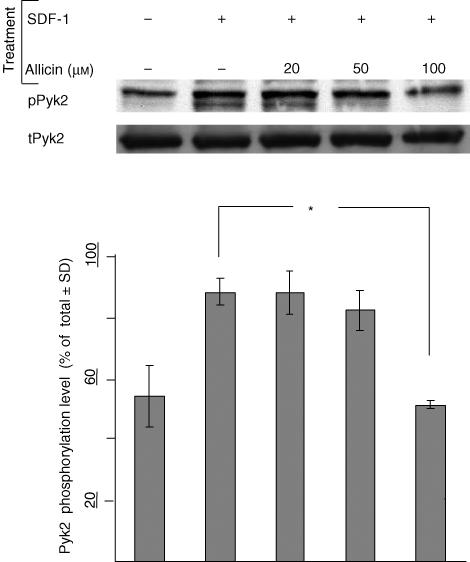
Allicin inhibits the SDF-1α-induced Pyk-2 phosphorylation
The previous experiments indicated that allicin is an inhibitor of SDF-1α-induced T cell migration through FN. Inhibition of SDF-1α-induced actin polymerization by allicin and the subsequent T cell polarization probably accounts for the inhibition of T cell migration. This inhibition was independent of any contact with FN. Pyk2 is a member of the family of intracellular focal adhesion kinases and plays an important role in the formation of focal adhesion contacts.29 Tyrosine phosphorylation of Pyk2 increases the activity of this cytoplasmatic kinase and mediates multiple signalling pathways regulating cell migration.30–32 Treatment with SDF-1α results in rapid phosphorylation of Pyk-2.33 Therefore, we examined the effect of allicin on the SDF-1α-induced phosphorylation of Pyk2. To this end, T cells were incubated with SDF-1α (100 ng/ml), with or without allicin, and the extent of Pyk2 phosphorylation was examined in their lysates. Allicin, at 100 µm, significantly reduced the phosphorylation of Pyk2 (Fig. 4). As shown in this Fig. 4, the anti-Pyk-2 mAb used by us appears to precipitate two adjacent protein bands; we chose to analyse the amount of phosphorylation of the upper band, as its molecular weight was identical to that of the phosphorylated form Pyk-2. Interestingly, the apparent inhibitory concentration of allicin on Pyk-2 phosphorylation was as high as 100 µm. This may be explained by the relatively high amount of cells present in the wells in this specific assay (e.g. 5 × 106 cells per 300 µl). Note that this inhibition of the SDF-1α-induced Pyk2 phosphorylation was achieved independently of FN.
Figure 4.
Allicin inhibits the SDF-1α-induced Pyk-2 phosphorylation. T cells were incubated (5 min) with SDF-1α (100 ng/ml) and with various concentrations of allicin, and the extent of Pyk2 phosphorylation was examined in the lysates. One experiment representative of three; *P < 0·05.
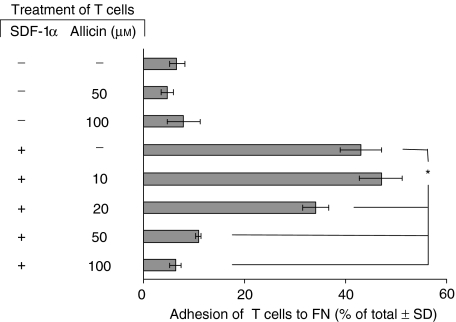
Allicin inhibits SDF-1α-induced T cell adhesion to FN
T cell migration requires its adhesion to the ECM and the formation of focal adhesions. In order to study the effect of allicin on T cell adhesion,51[Cr]-labelled T cells were preincubated (1 hr) with various concentrations of allicin, then cultured with SDF-1α (100 ng/ml) in microtitre wells that were precoated with FN. T cell adhesion was measured 60 min later. The results, shown in Fig. 5, indicate that SDF-1α alone induced a marked (48%) T cell adhesion to the immobilized FN. However, allicin, which had no proadhesive effect when used alone, inhibited the SDF-1α-induced T cell adhesion to FN. Inhibition was achieved already with 20 µm of allicin and the maximal inhibitory effect was achieved with a concentration of 50 µm. Thus, in addition to its ability to inhibit the SDF-1α-induced T cell chemotaxis, allicin appears to attenuate the ability of T cells bind to immobilized FN.
Figure 5.
Allicin inhibits SDF-1α-induced T cell adhesion to FN. Labelled T cells were preincubated (1 hr) with various concentrations of allicin and then cultured in microtitre wells that were precoated with FN. The wells were washed 60 min later and adhesion of the remaining FN-bound T cells was then measured. One experiment representative of four; *P < 0·05.
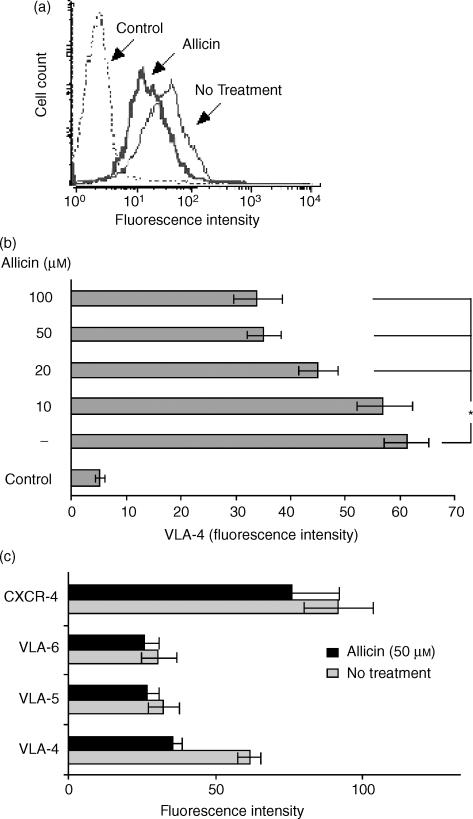
Allicin down-regulates the expression of VLA-4 on T cells
Members of the β1-integrin subfamily, including VLA-4 and VLA-5, are involved in lymphocyte adhesion to and migration through FN.34 Therefore, we investigated whether allicin changes the level of expression of these receptors, as well as the expression of the SDF-1α receptor CXCR-4. To this end, T cells were preincubated with allicin (1 hr) and then with the indicated mAb; mAb anti-VLA-6 was used as a control. Allicin specifically reduced the expression of VLA-4 (Fig. 6a); this reduction was significant at 20 µm (Fig. 6b) and reached its maximal effect at 50 µm. No significant reduction was observed in the expression of VLA-5, VLA-6 and CXCR4. We also assessed the effect of allicin on T-cell LFA-1 expression and found that, under identical condition, allicin did not affect the expression of this integrin (data not shown).
Figure 6.
Allicin down-regulates cell surface expression of VLA-4 on T cells. T cells were preincubated with allicin (1 hr) and then with the indicated mAb. Allicin reduced the expression of VLA-4 (a); this reduction was significant at 20 µm (b) and reached its maximal effect at 50 µm. No significant reduction was inspected in the expression of VLA-5, 6 and CXCR-4 (c). One experiment representative of five; *P < 0·05.
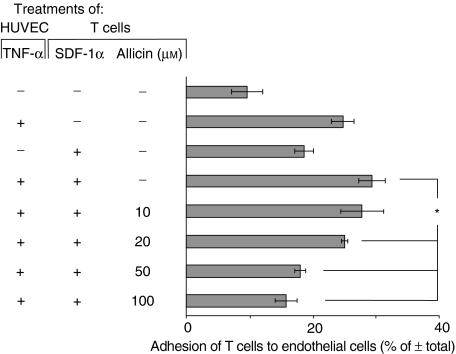
Allicin inhibits SDF-1α-induced T cell adhesion to endothelial cells
In addition to being a receptor for FN, VLA-4 also serves as a receptor for VCAM-1 on endothelial cells, and consequently regulates T cell transmigration.35 Therefore, we investigated whether the allicin-induced down-regulation in VLA-4 expression also influenced T cell adhesion to HUVEC expressing VCAM-1. To this end, T cells were incubated on wells that were precoated with TNF-α-treated HUVEC. As shown in Fig. 7, allicin inhibited the SDF-1α-induced T cell adhesion to endothelial cells; significant inhibition was achieved already with 20 µm of allicin and the maximal effect was obtained with 100 µm.
Figure 7.
Allicin inhibits SDF-1α-induced T cell adhesion to endothelial cells. Labelled T cells were incubated on wells that were precoated with TNF-α-treated HUVEC. Significant inhibition was already achieved at a concentration of 20 µm of allicin, and the maximal inhibitory effect was achieved at 100 µm. This is one experiment representative of three; *P < 0·05.
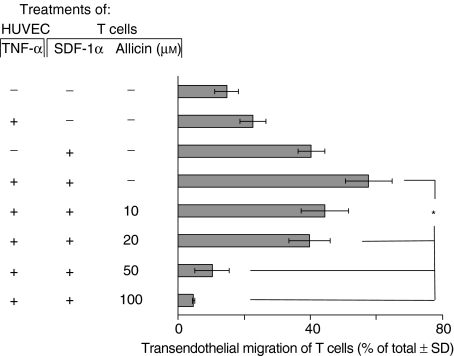
Allicin inhibits SDF-1α-induced T cell transendothelial migration
In order to investigate whether the inhibition of SDF-1α-induced T cell adhesion to endothelial cells by allicin influences T cell transendothelial migration we used the Transwell microchambers, which were precoated with TNF-α-pretreated HUVEC, and we then measured T cell migration to SDF-1α. As shown in Fig. 8, allicin inhibited the SDF-1α-induced T cell migration through endothelial cells in a dose-dependent manner. The maximal inhibitory effect was achieved with a concentration of 100 µm; here the migration rate was even lower than the rate of spontaneous T cell migration. Thus, in addition to its ability to inhibit T cell chemotaxis through FN, allicin down-regulates SDF-1α-induced T cell transendothelial migration.
Figure 8.
Allicin inhibits SDF-1α-induced T cell transendothelial migration. The filters of the Transwell microchamber were precoated with TNF-α-treated HUVEC. The maximal inhibitory effect of allicin was achieved at a concentration of 100 µm. One experiment representative of three; *P = 0·05.
Discussion
In this study we found that allicin, the major ingredient of fresh garlic extract, down- regulates T cell interactions with FN (Table 1 summarizes these findings). Specifically, we found that allicin, when used in concentrations of 20–100 µm, inhibits T cell migration through FN, and that this inhibition is mediated by down-regulation of two different steps in immune cell locomotion into and within inflamed sites. First, allicin inhibited the reorganization of cortical actin through inhibition of its polymerization and of the subsequent T cell polarization. This inhibition occurred in the absence of FN (and therefore was β1-integrin-independent). Secondly, allicin inhibited the adhesion of T cells to FN. We have shown two possible mechanisms for these inhibitory effects of allicin on T cells. Allicin down-regulated the phosphorylation of Pyk2, an intracellular member of the focal adhesion kinases, which is phosphorylated upon SDF-1α treatment, and also mediates β1-integrins to multiple signalling pathways that regulate adhesion and migration. In addition, allicin down-regulated the expression of the cell-surface integrin VLA-4. These mechanisms are probably also responsible for the allicin-induced inhibition of T cell adhesion to endothelial cells and inhibition of transendothelial migration. Our findings that allicin down-regulates T cell interactions with FN and HUVEC suggest that this ability of allicin is of major importance as T cell locomotion through endothelial cells and the ECM is a key event during inflammation. This locomotive ability of T cells is associated with cytoskeleton-related functions within the cells. Among them are the reorganization of cortical actin by its polymerization, the subsequent cell polarization and the formation of pseudopodia36,37 which enable the cells to adhere to and migrate through the ECM and endothelial cells. Both these steps of T cell locomotion, e.g. the reorganization of cortical actin with subsequent cell polarization and the adhesion to FN and HUVEC, were inhibited by allicin.
Table 1.
Summary of allicin-induced inhibition of SDF-1α-mediated T-cell functions; shown are percentage inhibition by allicin used at 50 µm
| Functions | % Inhibition |
|---|---|
| I. Cell activities | |
| 1. Migration | |
| A. Through HUVEC | 18 |
| B. Through FN | 14 |
| 2. Adhesion | |
| A. Through HUVEC | 61 |
| B. Through FN | 22 |
| II. Intra- and extracellular effects | |
| 1. Actin polymerization | 50 |
| 2. Cellular polarization | 10 |
| 3. Pyk-2 phosphorylation* | 58 |
| 4. VLA expression (fluoresence intensity) | 57 |
Allicin at 100 µm.
The apparent inhibitory properties of allicin regarding T cell functions are not related to any cytotoxic effect of this organic compound, because even after 5 hr of incubation with the maximal concentration of allicin used in this study (100 µm), we did not find significant cell death. Moreover, after the incubation time with allicin, the cells were washed and 24 hr later showed complete rehabilitation of the SDF-1α-induced polarization (data not shown).
What are the mechanisms whereby allicin affects T cell locomotion and interactions with FN and endothelial cells? We have shown two possible explanations for the apparent down-regulatory effects of allicin on T cells. The first is the inhibition of the phosphorylation of Pyk-2. Treatment with SDF-1α results in rapid phosphorylation of Pyk-2.28 Because there was no significant reduction in the expression of CXCR-4 by allicin, it is reasonable to assume that this compound inhibits one or more steps in the signalling pathway between the ligated CXCR-4 and Pyk-2. Once phosphorylated, Pyk-2 activates MAP kinases31 which participate in cell migration38 and its down-regulation by allicin explains the inhibitory effect of this compound in this step of cytoskeleton-related functioning of T cells and therefore on their migration. This signalling pathway, from CXCR-4 to PYK-2, is independent of the interaction of T cells with FN and therefore is β1-integrin-independent. We assume that this may explain the inhibition of actin polymerization in T cells exposed to allicin alone, and therefore the inhibition of subsequent cell polarization that was found in the absence of FN. Whether or not Pyk2 phosphorylation leads to or is linked to actin rearrangement in T cells, our results imply that allicin blocks both of these intracellular processes associated with T-cell adhesion to and migration through ECM. The second mechanism of action of allicin is the down-regulation in the expression of VLA-4, a cell surface receptor that mediates the adhesion to and migration through endothelial cells and FN. Indeed, the allicin-induced down-regulation of VLA-4 expression on T cells was accompanied by the inhibition of T cell adhesion to endothelial cells, and consequently transendothelial migration. These two down-regulatory effects of allicin may be linked, as phosphorylation of Pyk2 is also regulated by β1-integrins and links these integrins to multiple signalling pathways that regulate adhesion and migration processes.30–32,39 Therefore, this allicin-induced VLA-4 down-regulation is responsible for the inhibition of T cell adhesion to and migration through FN both directly as an adhesion molecule by binding less to FN and indirectly as transducing molecules by regulating the phosphorylation of Pyk2.40 Future studies should be performed to indicate whether allicin, applied in different concentration, also affects the SDF-1α-induced T-cell Ca+2 signalling and G protein activation.
What are the possible mechanisms for the down-regulation of VLA-4 expression? We propose three possible mechanisms: (i) direct binding of allicin to VLA-4 and therefore blockage of the binding of the mAb anti-VLA-4 (ii); internalization of the receptor; or (iii) its shedding. Blocking the receptor can be conducted directly by the interaction of allicin with the receptor binding site or indirectly by interaction with other sites of the receptor, with subsequent conformational changes in the active binding site, which prevent its binding. The major biological effect of allicin is attributed to its rapid interaction with thiol-containing proteins41 in the cell, resulting in the inactivation of these proteins. This bond with the thiol groups can be reversed by using thiol-containing compounds, such as the intracellular SH-regulator, glutathione or more reactive reagents such as dithiothreitol (DTT) and 2-mercaptoethanol (2-ME). Using these three compounds, we could not reverse the effect of allicin on actin polymerization or cell polarization (data not shown). Thus, we concluded that the mechanism governing the down-regulation in VLA-4 expression may not be through direct or indirect binding of allicin to this receptor. Hence, the other possible mechanisms of VLA-4 down-regulation, e.g. internalization or shedding of the receptor, should be studied in the future.
The ability of allicin to down-regulate T cell functions related to their adhesion and migration may explain some of the beneficial health-related biological effects of allicin, for example, reducing the formation of fatty streaks (atherosclerosis) in blood vessels13—a process in which inflammation and T cells play an important role.42 Overall, our results indicate that by virtue of its ability to down-regulate T cell-blood vessel walls and T cell–ECM interactions, allicin may be used clinically to inhibit T cell-mediated diseases. Additional studies should therefore be performed to verify our hypothesis in vivo.
References
- 1.Adetumbi M, Javor GT, Lau BH. Allium sativum (garlic) inhibits lipid synthesis by Candida albicans. Antimicrob Agents Chemother. 1986;30:499–501. doi: 10.1128/aac.30.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch HP, Lawson LD, editors. Garlic: the Science and therapeutic application of Allium sativum Land related species. 2. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- 3.Miron T, Rabinkov A, Mirelman D, Wilchek M, Weiner L. The mode of action of allicin: its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim Biophys Acta. 2000;1463:20–30. doi: 10.1016/s0005-2736(99)00174-1. [DOI] [PubMed] [Google Scholar]
- 4.Lawson LD. Garlic: a review of its medicinal effects and indicated active compounds. In: Lawson LD, Bauer R, editors. Phytomedicines of Europe: their Chemistry and Biological Activity. Washington: American Chemical Society; 1998. pp. 176–209. [Google Scholar]
- 5.Stoll A, Seebeck E. Chemical investigation on alliin, the specific principle of garlic. Adv Enzymol. 1951;11:377–400. doi: 10.1002/9780470122563.ch8. [DOI] [PubMed] [Google Scholar]
- 6.Uchida Y, Takahashi T, Sato N. The characteristics of the antimicrobial activity of garlic. Jpn J Antibiotics. 1975;28:638–42. [PubMed] [Google Scholar]
- 7.Harris JC, Cottrell SL, Plummer S, Lloyd D. Antimicrobial properties of Allium sativum (garlic) Appl Microbiol Biotechnol. 2001;57:282–6. doi: 10.1007/s002530100722. [DOI] [PubMed] [Google Scholar]
- 8.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microb Infect. 1999;1:125–9. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Azuma K. Evaluation of the in vitro antifungal activity of allicin. Antimicrob Agents Chemother. 1977;13:743–9. doi: 10.1128/aac.11.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirelman D, Monheit D, Varon S. Inhibition of growth of Entamoeba histolytica by allicin, the active principle of garlic extract (Allium sativum) J Infect Dis. 1987;156:243–4. doi: 10.1093/infdis/156.1.243. [DOI] [PubMed] [Google Scholar]
- 11.Eilat S, Oestraicher Y, Rabinkov A, Ohad D, Mirelman D, Battler A, Eldar M, Vered Z. Alteration of lipid profile in hyperlipidemic rabbits by allicin, an active constituent of garlic. Coron Artery Dis. 1995;6:985–90. [PubMed] [Google Scholar]
- 12.Lau BH. Suppression of LDL oxidation by garlic. J Nutr. 2001;131:S985–8. doi: 10.1093/jn/131.3.985S. [DOI] [PubMed] [Google Scholar]
- 13.Abramovitz D, Gavri S, Harats D, et al. Allicin-induced decrease in formation of fatty streaks (atherosclerosis) in mice fed a cholesterol-rich diet. Coron Artery Dis. 1999;10:515–9. doi: 10.1097/00019501-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Briggs WH, Xiao H, Parkin KL, Shen C, Goldman IL. Differential inhibition of human platelet aggregation by selected Allium thiosulfinates. J Agric Food Chem. 2000;48:5731–5. doi: 10.1021/jf0004412. [DOI] [PubMed] [Google Scholar]
- 15.Elkayam A, Mirelman D, Peleg E, Wilchek M, Miron T, Rabinkov A, Sadetzki S, Rosenthal T. The effects of allicin and enalapril in fructose-induced hyperinsulinemic hyperlipidemic hypertensive rats. Am J Hypertens. 2001;14:377–81. doi: 10.1016/s0895-7061(00)01298-x. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch K, Danilenko M, Giat J, et al. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr Cancer. 2000;38:245–54. doi: 10.1207/S15327914NC382_14. [DOI] [PubMed] [Google Scholar]
- 17.Hobauer R, Frass M, Gmeiner B, Kaye AD, Frost EA. Garlic extract (Allium sativum) reduces migration of neutrophils through endothelial cell monolayers. Middle East J Anesthesiol. 2000;15:649–58. [PubMed] [Google Scholar]
- 18.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 19.Moser B, Loetscher M, Piali L, Loetscher P. Lymphocyte responses to chemokines. Int Rev Immunol. 1998;16:323–44. doi: 10.3109/08830189809043000. [DOI] [PubMed] [Google Scholar]
- 20.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–6. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–8. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 22.Gilat D, Hershkoviz R, Mekori YA, Vlodavsky I, Lider O. Regulation of adhesion of CD4+ T lymphocytes to intact or heparinase-treated subendothelial extracellular matrix by diffusible or anchored RANTES and MIP-1 beta. J Immunol. 1994;153:4899–906. [PubMed] [Google Scholar]
- 23.Tchernychev B, Rabinkov A, Miron T, Wilchek M. Natural antibodies against alliinase in human serum and polyclonal antibodies elicited in rabbit, share the same immunogenic determinants. Immunol Lett. 2000;71:43–7. doi: 10.1016/s0165-2478(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 24.Miron T, Rabinkov A, Mirelman D, Weiner L, Wilchek M. A spectrophotometric assay for allicin and alliinase (Alliin lyase) activity: reaction of 2-nitro-5-thiobenzoate with thiosulfinates. Anal Biochem. 1998;265:317–25. doi: 10.1006/abio.1998.2924. [DOI] [PubMed] [Google Scholar]
- 25.Ariel A, Yavin EJ, Hershkoviz R, et al. IL-2 induces T cell adherence to extracellular matrix: inhibition of adherence and migration by IL-2 peptides generated by leukocyte elastase. J Immunol. 1998;161:2465–72. [PubMed] [Google Scholar]
- 26.Franitza S, Alon R, Lider O. Real-time analysis of integrin-mediated chemotactic migration of T lymphocytes within 3-D extracellular matrix-like gels. J Immunol Meth. 1999;225:9–25. doi: 10.1016/s0022-1759(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 27.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrador JM, Nieto M, Sanchez-Madrid F. Cytoskeletal rearrangement during migration and activation of T lymphocytes. Trends Cell Biol. 1999;9:228–33. doi: 10.1016/s0962-8924(99)01553-6. [DOI] [PubMed] [Google Scholar]
- 29.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104:327–35. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zrihan-Licht S, Fu Y, Settleman J, Schinkmann K, Shaw L, Keydar I, Avraham S, Avraham H. RAFTK/Pyk2 tyrosine kinase mediates the association of p190 RhoGAP with RasGAP and is involved in breast cancer cell invasion. Oncogene. 2000;19:1318–28. doi: 10.1038/sj.onc.1203422. [DOI] [PubMed] [Google Scholar]
- 31.van Seventer GA, Mullen MM, van Seventer JM. Pyk2 is differentially regulated by beta1 integrin- and CD28-mediated co-stimulation in human CD4+ T lymphocytes. Eur J Immunol. 1998;28:3867–77. doi: 10.1002/(SICI)1521-4141(199811)28:11<3867::AID-IMMU3867>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–56. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 33.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors. a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–8. [PubMed] [Google Scholar]
- 34.Avdalovic M, Fong D, Formby B. Adhesion and costimulation of proliferative responses of human gamma delta T cells by interaction of VLA-4 and VLA-5 with fibronectin. Immunol Lett. 1993;35:101–8. doi: 10.1016/0165-2478(93)90077-f. [DOI] [PubMed] [Google Scholar]
- 35.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 36.Oliver T, Lee J, Jacobson K. Forces exerted by locomoting cells. Semin Cell Biol. 1994;5:139–47. doi: 10.1006/scel.1994.1018. [DOI] [PubMed] [Google Scholar]
- 37.Ratner S, Sherrod WS, Lichlyter D. Microtubule retraction into the uropod and its role in T cell polarization and motility. J Immunol. 1997;159:1063–7. [PubMed] [Google Scholar]
- 38.Cheresh DA, Leng J, Klemke RL. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J Cell Biol. 1999;146:1107–16. doi: 10.1083/jcb.146.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lev S, Moreno H, Martinez R, et al. Protein tyrosine kinase PYK2 involved in Ca (2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–45. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 40.van Seventer GA, Shimizu Y, Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991;3:294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]
- 41.Rabinkov A, Miron T, Konstantinovski L, Wilchek M, Mirelman D, Weiner L. The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins. Biochim Biophys Acta. 1998;1379:233–44. doi: 10.1016/s0304-4165(97)00104-9. [DOI] [PubMed] [Google Scholar]
- 42.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]