Abstract
Although it is well established that CD4+ T cells generally recognize major histocompatibility complex (MHC) class II molecules, MHC class I-reactive CD4+ T cells have occasionally been reported. Here we describe the isolation and characterization of six MHC class I-reactive CD4+ T-cell lines, obtained by co-culture of CD4+ peripheral blood T cells with the MHC class II-negative, transporter associated with antigen processing (TAP)-negative cell line, T2, transfected with human leucocyte antigen (HLA)-B27. Responses were inhibited by the MHC class I-specific monoclonal antibody (mAb), W6/32, demonstrating the direct recognition of MHC class I molecules. In four cases, the restriction element was positively identified as HLA-A2, as responses by these clones were completely inhibited by MA2.1, an HLA-A2-specific mAb. Interestingly, three of the CD4+ T-cell lines only responded to cells expressing HLA-B27, irrespective of their restricting allele, implicating HLA-B27 as a possible source of peptides presented by the stimulatory MHC class I alleles. In addition, these CD4+ MHC class I alloreactive T-cell lines could recognize TAP-deficient cells and therefore may have particular clinical relevance to situations where the expression of TAP molecules is decreased, such as viral infection and transformation of cells.
Keywords: antigen presentation, CD4+ T cells, human studies, MHC class I
Introduction
It is well established that major histocompatibility complex (MHC) class I and class II molecules are recognized by CD8+ and CD4+ T lymphocytes, respectively. This association is a result of positive selection in the thymus; however, it appears that thymic selection may not be absolute, as many exceptions to this general rule have been reported, with both MHC class II-reactive CD8+ T cells1–5 and MHC class I-restricted CD4+ T cells6–11 being identified. Although the biological significance of T cells breaking the general rules of recognition remains unknown, it is intriguing that MHC class I-restricted CD4+ T cells have mainly been identified amongst tumour-infiltrating lymphocytes, or isolated following culture with tumour cells,12–19 perhaps suggesting a protective anti-tumour role for these cells in vivo. Indeed, MHC class I-reactive CD4+ T cells are capable of inducing inflammatory responses20,21 and thus these minor populations of CD4+ T cells, which exhibit unconventional recognition, may play a role in immune responses, either protective (in the case of immune responses to viruses8,9 or tumours) or detrimental (in the case of alloreactive responses that can result in the rejection of transplanted organs or in autoimmune responses).
We have investigated whether the occurrence of T cells, capable of interacting with the ‘wrong’ restriction element, potentially has a role in one group of inflammatory diseases – the spondyloarthropathies (SpA) – isolating CD4+ T cells that interacted directly with the MHC class I molecule human leucocyte antigen (HLA)-B27.22 Although there are a variety of hypotheses to explain the association of HLA-B27 with SpA, the mechanism underlying the role of HLA-B27 in disease is still undefined, even after 30 years of research.23,24 Current hypotheses include HLA-B27 functioning as a restriction element for CD8+ T cells,25–28 the expression of unusual forms of HLA-B27 (such as homodimers) causing disease,29,30 either by generating endoplasmic reticulum (ER) stress or through recognition by immune receptors other than the T-cell receptor (TCR), such as natural killer (NK) receptors.31,32 Evidence from HLA-B27 transgenic animals has also raised the possibility that CD4+ T cells are important in disease.33,34 Recently, HLA-B27-restricted CD4+ T cells have been characterized in mice transgenic for both HLA-B27 and a human HLA-B27-restricted TCR.35 This, together with our characterization of HLA-B27-reactive CD4+ T cells from a patient with ankylosing spondylitis, supports the possible involvement of MHC class I-reactive CD4+ T cells in spondyloarthropathy.
We isolated such cells by co-culturing highly purified CD4+ T cells from humans with the MHC class II-negative, TAP-negative cell line, T2, transfected with HLA-B27 (T2-B27). This cell line also expresses very low levels of HLA-A2, -B51 and -Cw1 on its surface, and here we describe the characterization of HLA-A2- and HLA-B51-reactive CD4+ T cells isolated from this co-culture system. Although CD4+ T cells, reactive to HLA-A2 expressed on T2 cells, have previously been reported,11 four of the T-cell lines characterized here only recognized HLA-B27+ cells, suggesting that HLA-B27 acts as a source of peptides presented by HLA-A2 or -B51. We have also investigated whether these T cells only respond to TAP-deficient cells, but find that whereas some of the CD4+ T cells only recognized TAP-deficient cells, others showed enhanced responses to HLA-A2 and HLA-B51 in the presence of TAP. These T cells represent a further unusual reactivity by CD4+ T cells, and have implications for our understanding of the interaction of the TCR with MHC. In clinical contexts this unusual alloreactivity may also have clinical relevance.
Materials and methods
Isolation of CD4+ T lymphocytes
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of two healthy individuals, LB (HLA-A2, -A29, -B27, -B44, -Cw1, -Cw16, -DR7) and SB (HLA-A3, -B27, -B39, -Cw12, -Cw16, -DR7, -DR16), and from two patients with ankylosing spondylitis, CC (HLA-B27, -Cw1, -DR1) and MM (HLA-A2, -A32, -B7, -B27, -Cw2, -Cw7, -DR15, -DR11), by Ficoll–Hypaque (Amersham Pharmacia Biotech, Bucks., UK)-gradient centrifugation. Monocytes were removed from PBMCs by adherence. Non-adherent cells were incubated at 4° with the following monoclonal antibodies (mAbs) at titrated concentrations: the CD8-specific mAb, UCHT4, the CD11b-specific mAb, OKMI, the CD19-specific mAb, BU12 (all kind gifts from P. Beverley, Jenner Institute, Oxford), the CD16-specific mAb, DJ130c (Dako, Ely, UK), and the CD56-specific mAb, MEM-188 (a gift from V. Horejsi, Academy of Sciences of the Czech Republic). After removal of excess mAb, sheep anti-mouse immunoglobulin G (IgG)-coated magnetic beads (Dynal AS, Oslo, Norway) were added to the cells. Cells labelled with beads were subsequently removed using a MACS magnet (Miltenyi Biotec, Bisley, UK). Three rounds of negative selection resulted in the isolation of CD4+ T-cell populations of 95% purity.
Cell lines
The following cell lines were used: T2 (negative for TAP1 and TAP2, LMP2 and LMP7, and MHC class II);36 721.220(220) (negative for HLA-A and -B, and tapasin);37 and C1R (hemizygous for the HLA complex).38,39 These cell lines were also transfected with HLA-B*2705. In addition, Epstein–Barr virus-transformed lymphoblastoid cell lines (EBV-LCLs) were produced from PBMCs, as previously described,40 and T2-B27 cells reconstituted with TAP1 and TAP2 (T2-B27-TAP) were also used (a gift from R. Colbert, Children's Hospital Medical Center, Cincinnati, OH). Non-transfected cell lines were maintained in standard culture medium [RPMI 1640 (Gibco Life Technologies, Paisley, UK) containing 5% heat-inactivated fetal calf serum (Sigma, Poole, Dorset, UK), 20 mm HEPES (Sigma), 2 mm glutamine (Sigma), 100 µg/ml streptomycin (Gibco Life Technologies) and 100 U/ml penicillin (Gibco Life Technologies)] and transfected cell lines were maintained in standard culture medium supplemented with 0·5 mg/ml Geneticin G418 sulphate (Gibco Life Technologies) to maintain stable transfection.
Co-culture of CD4+ T cells with T2-B27
A total of 5 × 103 irradiated (60 gray) T2-B27 cells were added, to each 0·2-ml well of a 96-well plate, in T-cell culture medium [RMPI 1640 containing 5% heat-inactivated human AB serum (First link, Wolverhampton, West Midlands, UK), 20 mm HEPES, 100 µg/ml sodium pyruvate (Sigma), 1 × MEM non-essential amino acids (Sigma), 2 mm glutamine, 100 µg/ml streptomycin and 100 U/ml penicillin). Between 1 × 103 and 3 × 104 purified CD4+ T lymphocytes were added, per well, to T2-B27 cells. The cells were incubated for 6 days at 37° and subsequently maintained in T-cell culture medium supplemented with 10 U/ml recombinant interleukin-2 (rIL-2) (Chiron, Harefield, Middlesex, UK) for 6 weeks. Wells with proliferating cells were detected using light microscopy. T-cell lines were restimulated with irradiated T2-B27, as required.
T-cell proliferation assays
Proliferation assays were performed at 37° by culturing 2 × 104 T cells with 5 × 103−2·5 × 104 irradiated stimulator cells per well. Plates were cultured for 3 days, then pulsed with 1 µCi/well methyl-[3H]thymidine (methyl-[3H]TdR) (Amersham Pharmacia Biotech). After 6 hr, cells were transferred onto printed glass-fibre filter mats (Wallace-Perkin-Elmer, Warrington, UK). [3H]TdR incorporation was analysed using a β-plate counter (Wallace-Perkin-Elmer). T-cell proliferation, displayed as Δ counts per minute (c.p.m.), was determined by subtracting background [3H]TdR incorporation of stimulator cell lines from total [3H]TdR incorporation.
mAb inhibition studies
The following mAbs were used in inhibition studies at a final concentration of 10 or 20 µg/ml; the HLA-A, -B, and -C-specific mAb, W6/32, the HLA-B7 and -B27-specific mAb, ME1, the HLA-DR-specific mAb, L243, the HLA-A2-specific mAb, MA2.1, the free MHC class I heavy chain-specific mAb, HC10 (a kind gift from H. Ploegh, Harvard University, Boston, MA), and the CD4-specific mAb, Campath-9H (a gift from J. Issacs, University of Leeds, UK). The MHC-specific mAbs were incubated with stimulator cell lines for 1 hr at 37° before the addition of T cells. The CD4-specific mAb was incubated with T cells for 1 hr at 37° before the addition of stimulator cell lines. T-cell proliferation was determined by measuring [3H]TdR incorporation, as described above.
TCR expression
TCR-β and -α chain variable-region DNA was amplified by polymerase chain reaction (PCR) from cDNA using methods previously described.41,42 The PCR-amplified TCR was sequenced using a constant-region specific oligonucleotide primer and big dye biochemistry (Perkin-Elmer, Wokingham, UK) on an ABI automated sequencer.
Flow cytometry
Fluorescence-conjugated antibody specific for CD3 (Dako), CD4 (P. Beverley), CD8 (Dako), CD16 (Dako), CD56 (Dako), TCR-αβ (Pharmingen), TCR-γδ (Pharmingen) and mouse IgG (Dako) were used to determine the purity of CD4+ T-cell separation and the phenotype of isolated CD4+ T-cell lines. MHC expression on stimulator cell lines was determined using non-conjugated mAbs L243, W6/32, ME1 and HC10, followed by detection using anti-mouse IgG–fluorescein isothiocyanate (FITC) mAb. Flow cytometry data were collected on a FACSort (BD Biosciences) and analysed using WinMDI.
Results
Isolation of T2-B27-reactive CD4+ T cells
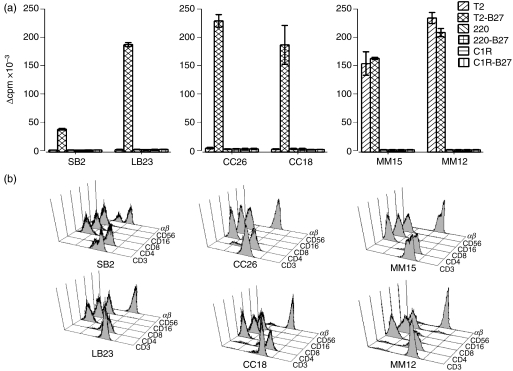
To investigate whether an unusual interaction can occur between human CD4+ T cells and MHC class I molecules, we developed a limiting-dilution co-culture system using T2-B27. T2-B27 does not express any MHC class II molecules; therefore the stimulation of MHC class II-restricted CD4+ T cells is prevented. Owing to its deficiency in the peptide transporter, TAP, T2-B27 expresses a low level of MHC class I molecules, although HLA-B27 expression is relatively preserved in the absence of TAP. We have previously reported the isolation of HLA-B27-specific CD4+ T-cell lines that proliferated in response to three HLA-B27 transfected cell lines T2-B27, 220-B27 (721.220 cell line transfected with HLA-B*2705) and C1R-B27 (C1R cell line transfected with B*2705), but not to T2, 220 or C1R.22 However, in addition, co-cultures with T2-B27 resulted in the isolation, from all B27+ individuals tested, of numerous CD4+ T-cell lines that proliferated in response to T2-B27 and/or T2, but to none of the other HLA-B27-transfected stimulator cell lines.
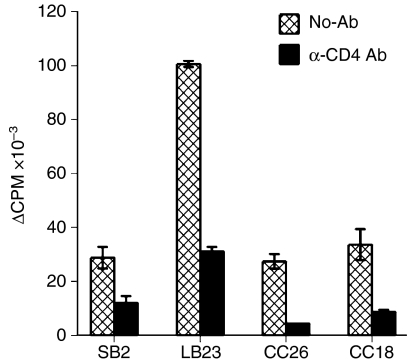
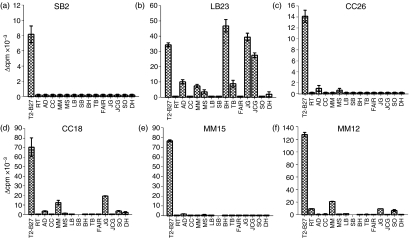
To identify the molecules recognized by these CD4+ T cells, analysis was performed on four T2-B27-reactive CD4+ T-cell lines (SB2, LB23, CC26, CC18), from three separate donors, which showed proliferation in response to T2-B27, but not to non-transfected T2, and, in addition, two CD4+ T-cell lines (MM15 and MM12) from a fourth donor that showed proliferative responses to both T2 and T2-B27, but not to the other stimulator cell lines (Fig. 1). Analysis of TCR expression by reverse transcription (RT)–PCR showed each of the lines to express one or two TCR-A and one or two TCR-B chains (data not shown), consistent with their being clonal or perhaps, in some cases, biclonal. As T2-B27 does not express MHC class II molecules, these CD4+ T-cell lines are not conventional MHC class II-restricted CD4+ T cells. Nevertheless, the responses to T2-B27 were shown to be dependent on the CD4 co-receptor, as inhibition of the responses was observed in the presence of a blocking CD4-specific mAb (Fig. 2).
Figure 1.
CD4+ T-cell lines proliferate in response to T2-B27 (a T2-cell line transfected with HLA-B*2705). (a) Proliferation is expressed as Δ counts per minute (c.p.m.) of CD4+ T-cell lines to a panel of B27+ and B27− stimulator cell lines. Data shown are the means of duplicate wells ± standard deviation (SD). (b) Flow cytometric analysis of the phenotype of the six T2-B27-reactive T-cell lines.
Figure 2.
Responses to T2-B27 (a T2-cell line transfected with HLA-B*2705) are CD4 dependent. Proliferative responses are displayed as Δ counts per minute (c.p.m.) to T2-B27 in the presence or absence of a blocking monoclonal antibody (mAb) specific for CD4. Data shown are the means of triplicate results ± standard deviation (SD).
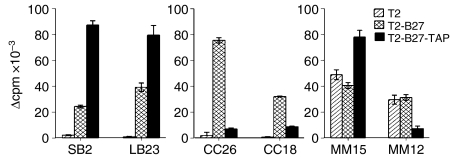
Differential responses to T2-B27 reconstituted with TAP molecules
To determine if the molecules recognized by the panel of T2-B27- or T2-reactive CD4+ T-cell lines were dependent on the deficiency of TAP in T2, the ability of the CD4+ T-cell lines to proliferate in response to T2-B27 reconstituted with TAP1 and TAP2 (T2-B27-TAP) was investigated. Two patterns of reactivity towards T2-B27-TAP were observed (Fig. 3); three of the CD4+ T-cell lines showed increased proliferative responses to T2-B27-TAP compared to T2-B27 (SB2, LB23, MM15), while three of the lines exhibited decreased responses to T2-B27-TAP compared to T2-B27 (CC26, CC18, MM12). Therefore, the T2-B27- and T2-reactive CD4+ T-cell lines differ in the structure they recognize, with some recognizing antigenic peptides (or HLA conformations) whose availability is enhanced by the presence TAP, and others whose presence is favoured by the absence of TAP.
Figure 3.
Differential responses to T2-B27 (a T2-cell line transfected with HLA-B*2705) on reconstitution of TAP molecules. Proliferative responses, displayed as Δ counts per minute (c.p.m.) of the CD4+ T-cell lines to T2, T2-B27 and T2-B27-TAP (T2-B27 reconstituted with TAP1 and TAP2). Data shown represent the mean values of triplicate wells ± standard deviation (SD).
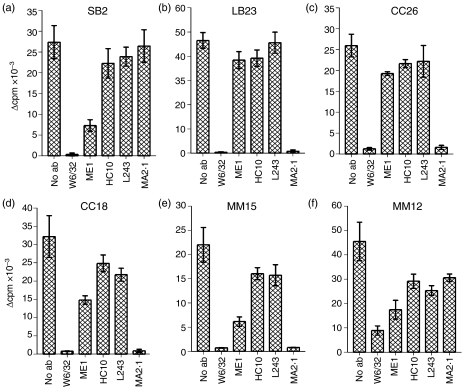
An MHC class I-specific mAb inhibits the proliferative responses of the CD4+ T-cell lines to T2-B27
To identify the molecules recognized by the CD4+ T-cell lines, the ability of a panel of mAbs, specific for MHC molecules, to inhibit the proliferative responses to T2-B27, was tested (Fig. 4). As expected, no significant inhibition was observed using the HLA-DR-specific mAb, L243, as T2-B27 is MHC class II negative (although slightly lower responses were observed by CC18, MM15 and MM12 in the presence of L243, the responses were similar to those found in the presence of HC10, which has the same isotype as L243; therefore, this slight decrease was not considered to be significant). In contrast, complete inhibition of responses by all of the CD4+ T-cell lines to T2-B27 was observed with W6/32, a mAb that recognizes MHC class I heavy chains associated with β2m. This suggests that these CD4+ T-cell lines indeed recognize an MHC class I molecule. As T2-B27 expresses HLA-A2, HLA-B51 and HLA-Cw1,43 together with transfected HLA-B27, any one of these MHC class I molecules could be recognized on the surface of T2-B27. Direct recognition of conventional HLA-B27/β2m heterodimers was unlikely in view of the lack of complete inhibition with the HLA-B27-specific mAb, ME1, in marked contrast to the HLA-B27-reactive CD4+ T cells that we have previously reported. However, for four of the six CD4+ T-cell lines (LB23, CC26, CC18, MM15), complete inhibition of responses was observed using the HLA-A2-specific mAb MA2.1, identifying HLA-A2 as the MHC class I molecule recognized by these T cells. As three of the lines responded to T2-B27, but not to T2, these results are consistent with the recognition of an HLA-B27-derived peptide presented by HLA-A2; in one case (LB23), the peptide's availability was enhanced when TAP was present, whereas for the other two (CC18 and CC26), the peptide was only presented efficiently when TAP was absent. In contrast, MM15 recognized HLA-A2 presenting a peptide that is available in both T2 and T2-B27, and preferentially transported by TAP. As for the other two CD4+ T-cell lines, SB2 and MM12, as they were inhibited by W6/32 but not with ME1 or MA2.1, it is probable that they recognize either HLA-B51 or HLA-Cw1.
Figure 4.
Inhibition of responses to T2-B27 (a T2-cell line transfected with HLA-B*2705) by major histocompatibility complex (MHC) class I-specific monoclonal antibodies (mAbs). Proliferative responses, displayed as Δ counts per minute (c.p.m.), to T2-B27 in the presence or absence of 10 µg/ml of the MHC class I-specific mAb, W6/32, the human leucocyte antigen (HLA)-B27-specific mAb, ME1, the free heavy chain-specific mAb, HC10, the HLA-DR-specific mAb, L243, and the HLA-A2-specific mAb, MA2.1. Data shown are the means of triplicate wells ± standard deviation (SD).
Some MHC class I-reactive CD4+ T-cell lines proliferate to EBV-LCL
To determine further the nature of MHC class I recognition by these CD4+ T-cell lines, we tested their ability to respond to a panel of HLA-typed EBV-LCLs (Table 1). Three of the CD4+ T-cell lines (SB2, CC26, MM15) did not proliferate to any of the EBV-LCL lines tested (Fig. 5). For CC26 this is expected as there was no response to T2-B27-TAP, and all the LCL express TAP normally. The results for MM15 are consistent with recognition of a TAP-dependent peptide present in the cell line T2, but not generally present in LCL. However, although SB2 did not recognize any LCL, it showed significant proliferative responses to the EBV-negative, MHC class II-negative myeloid cell line, U937 (data not shown), which, like T2, expresses HLA-B51 and HLA-Cw1. As no HLA-Cw1 LCL were recognized, HLA-B51 seems the most probable restriction element, unless U937 and T2 share an antigenic peptide lacking in all the HLA-Cw1+ LCL tested, in which case HLA-Cw1 could still be the restricting element. The ability of SB2 to recognize T2-B27, rather than T2, in the absence of TAP, suggests that HLA-B27 may be a source of a peptide which increases HLA-B51 expression in the absence of TAP, but in TAP-expressing cells, such as U937, this is not required. However, these suggestions are speculative because we have been unable to measure directly the surface expression of HLA-B51.
Table 1.
Human leucocyte antigen (HLA) molecules expressed by the panel of cell lines
| HLA alleles expressed | ||||
|---|---|---|---|---|
| Cell line | A | B | C | DR |
| T2 | A2* | B51* | Cw1* | – |
| T2-B27 | A2* | B27 B51* | Cw1* | – |
| T2-B27-TAP | A2 | B27, B51 | Cw1 | – |
| 220 | – | – | Cw1 | DR1 |
| 220-B27 | – | B27 | Cw1 | DR1 |
| C1R | – | – | Cw4 | DR12 |
| C1R-B27 | – | B27 | Cw4 | DR12 |
| U937 | A3,A26 | B18, B51 | Cw1,Cw3 | – |
| TB | A1, A2 | B27, B37 | Cw1, Cw6 | DR1, DR4 |
| MM | A2, A32 | B7, B27 | Cw2, Cw7 | DR15,DR11 |
| RT | A1, A25 | B27, B56 | Cw1, Cw2 | DR1, DR4 |
| CC | – | B27 | Cw1 | DR1 |
| DH | A2 | B8, B27 | Cw1, Cw7 | DR1, DR17 |
| AD | A2 | B44, B27 | Cw5, Cw2 | DR4 |
| BH | A2, A24 | B27, B57 | Cw1, Cw6 | DR7 |
| LB | A3, A29 | B44, B27 | Cw1,Cw16 | DR7 |
| SB | A3 | B39, B27 | Cw12,Cw16 | DR7, DR16 |
| JCG | A1, A2 | B8, B62 | ND | DR3, DR7 |
| SO | A2 | ND | ND | ND |
| JG | A1, A2 | B8, B44 | Cw5, Cw7 | DR1, DR4 |
Indicates lower expression of HLA alleles owing to a defect in TAP expression.
ND, not determined; T2-B27, T2 cell line transfected with HLA-B*2705; T2-B27-TAP, T2-B27 reconstituted with TAP1 and TAP2.
Figure 5.
Proliferative responses are observed to the Epstein–Barr virus-transformed lymphoblastoid cell line (EBV-LCL) by three of the major histocompatibility complex (MHC) class I-reactive CD4+ T-cell lines. Proliferative responses are expressed as Δ counts per minute (c.p.m.) to a panel of EBV-LCL. Proliferative responses to T2-B27 (a T2-cell line transfected with HLA-B*2705) are shown as a control. Data shown represent the mean values of triplicate results.
The other three T-cell lines showed proliferative responses to selected EBV-LCLs. For LB23 and CC18, the pattern of recognition of EBV-LCL correlated with the LCL expression of HLA-A2 and -B27; LB23 recognized all HLA-A2/B27-positive cell lines tested, whereas CC18 showed a more restricted pattern of recognition of HLA-A2/B27-positive cell lines; the responses by CC18 were at a low level compared with recognition of T2-B27, consistent with the inhibition of response by the presence of TAP. The mAb, MA2.1, completely inhibited recognition of these EBV-LCLs (data not shown), suggesting that the CD4+ T cells can also recognize HLA-A2 on MHC class II-positive cells, and not merely on MHC class II-negative cell lines. For LB23, responses to HLA-A2/B8+ LCL were observed in addition to those to HLA-A2/B27, suggesting that both HLA-B8 and -B27 may contribute similar peptides that can bind to HLA-A2 and be recognized by LB23. By aligning the two recognized HLA-B alleles – HLA-B27 and HLA-B8 – with one not recognized, HLA-B51, we identified amino acids that were shared by the two recognized alleles, but absent in HLA-B51. Using the eptiope-prediction programme, syfpeithi (http://syfpeithi.bmi-heidelberg.com), three peptide epitopes (QSEAGSHTL, TLQSMYGCD, QRKWEAARV), from the peptide-binding groove of HLA-B27/B8, were predicted to be HLA-A*0201, with significant scores. Peptides from the corresponding sequence in HLA-B51 had negative or significantly lower scores. Similar peptides may also be recognized by CC18, which also exhibited recognition of both B27- and B8-positive cell lines. For CC26, the peptide appears to be specific to HLA-B27 and hence is likely to include unique residues around the peptide-binding groove.
MM12 also responded to four EBV-LCLs, albeit at a low level, again consistent with the inhibitory effects of TAP expression by the LCL. Interestingly, the stimulatory EBV-LCL included the autologous cell line (Fig. 5); all the other clones failed to respond to the autologous EBV-LCL, suggesting that they were alloreactive. As responses of MM12 to T2 were not HLA-A2 restricted (but were inhibited by W6/32), and the EBV-LCL that were recognized all share expression of HLA-A2, it is possible that an HLA-A2-derived peptide is being recognized. However, as HLA-A2 is the only HLA class I allele common to T2 and the autologous LCL, MM12 must recognize a peptide presented by more than one class I allele (e.g. HLA-Cw1 on T2 or HLA-Cw2 on the LCL). The deduced specificities of the six clones are summarized in Table 2.
Table 2.
Summary of specificity of cell lines
| Recognition of T2 cell lines | ||||||
|---|---|---|---|---|---|---|
| T2 | T2-B27 | T2-B27-TAP | Inhibition | Responses to EBV-LCL | Molecule recognized | |
| SB2 | – | + | +++ | W6/32 | No | HLA-B51. In the absence of TAP recognizes a B27-derived peptide presented by B51 |
| LB23 | – | + | +++ | W6/32, MA2.1 | Yes A2+, B27+ LCL A2+ B8+ LCL | HLA-A2 presenting a B27-derived peptide whose expression is enhanced by TAP |
| CC26 | – | + | – | W6/32, MA2.1 | No | HLA-A2 presenting a B27-derived peptide only present on TAP-deficient cells |
| CC18 | – | + | – | W6/32, MA2.1 | Yes. Weak responsesto a limited number of A2+, B27+ or A2+ B8+ LCL | HLA-A2 presenting a B27-derived peptide preferentially expressed on TAP-deficient cells |
| MM15 | + | + | ++ | W6/32, MA2.1 | No | HLA-A2 on T2 cells |
| MM12 | + | + | – | W6/32 | Yes. No conserved pattern of recognition | Possible A2-derived peptide presented by more than one HLA class I allele |
EBV-LCL, Epstein–Barr virus-transformed lymphoblastoid cell line; HLA, human leucocyte antigen; T2-B27, T2 cell line transfected with HLA-B*2705; T2-B27-TAP, T2-B27 reconstituted with TAP1 and TAP2.
Discussion
To determine whether CD4+ T cells could interact with MHC class I molecules, we co-cultured highly purified CD4+ T cells with the MHC class II-negative stimulator cell line, T2, transfected with HLA-B27. Although we sought initially to determine whether CD4+ T lymphocytes could interact directly with HLA-B27, the co-culture system we developed resulted in the isolation of a panel of CD4+ T cells that interacted with other MHC class I molecules.
Four of the CD4+ T-cell lines (LB23, CC26, CC18 and MM15), characterized here, clearly recognized HLA-A2, as responses to T2-B27 were inhibited by both the MHC class I-specific mAb, W6/32, and the HLA-A2-specific mAb, MA2.1. Recognition of HLA-A210,15 (and more specifically recognition of HLA-A2 expressed on T2) by CD4+ T cells has previously been described.11 However, unlike these previously described CD4+ T cells, three of the HLA-A2-restricted CD4+ T cells (LB23, CC26 and CC18) only responded to T2-B27, and not to T2, suggesting that HLA-B27 can act as a source of peptide bound to HLA-A2, and that the specificity of the clones is for this combination. The HLA-A2-restricted response of CC26 and CC18 to T2-B27 was influenced by the defect in TAP expression in the T2-cell line, as their responses to T2-B27 were significantly decreased when T2-B27 was transfected with TAP1 and TAP2 to reconstitute an active peptide transporter. As CC26 failed to respond to any cells of a panel of EBV-LCL, many of which express HLA-A2 and HLA-B27, it appears that CC26 recognizes HLA-A2 presenting a peptide, derived from HLA-B27, that does not require transportation by TAP. When TAP is expressed, conventional MHC class I-binding peptides may out-compete the TAP-independent peptide. Although CC18 also exhibited impaired recognition of HLA-A2 on T2-B27 in the presence of TAP, this cell line showed a low level of response to some HLA-A2+ EBV-LCLs, suggesting that it could recognize both TAP-independent and -dependent peptides; alternatively, HLA-A2 molecules bearing the necessary TAP-independent peptides may be present at a higher level on EBV-LCL than on T2-B27-TAP. LB23 similarly showed recognition of HLA-A2 expressed on T2-B27 and EBV-LCLs, except that the response to T2-B27 was enhanced by the reconstitution of TAP molecules. Taken together, these results point to HLA-B27 being a source for both TAP-dependent and -independent peptides that can be bound by MHC class I molecules. Previous studies have identified peptides from MHC antigens amongst those that can be eluted from cell-surface class I MHC molecules.44 HLA-B27 contains a large number of peptides predicted to bind to HLA-A2, which could be tested for recognition by the CD4+ T-cell lines.
Unlike the other HLA-A2-specific CD4+ T-cell lines described above, MM15 was not dependent on HLA-B27, as both T2 and T2-B27 were recognized to a similar extent. In addition, recognition of HLA-A2 by MM15 is not an allo-reactive response to MHC, as subject MM is HLA-A2 positive; however, an HLA-A2-restricted alloresponse to a peptide unique to the T2-cell line, possibly a minor histocompatibility antigen, is probable because MM15 did not respond to the autologous LCL. In this case, the peptide is more abundant in the presence of TAP, as responses to T2-B27-TAP were significantly greater than those to T2-B27.
Thus, the HLA-A2-reactive CD4+ T-cell lines contain examples of the recognition of HLA-A2 as an alloantigen (binding peptides derived from HLA-B27) and HLA-A2 as a restriction element for a ‘foreign’ peptide, such as a minor histocompatibility antigen. The other two CD4+ T-cell lines characterized showed that additional MHC class I molecules can be recognized by CD4+ T cells. Thus, SB2 had properties consistent with the recognition of HLA-B51 as an alloantigen, together with a peptide from HLA-B27 (when T2-B27 was recognized), or an equivalent peptide available in the cell line U937, which is HLA-B27 negative. The precise specificity of MM12 is difficult to define; its response to T2 was inhibited by W6/32, but not by MA2.1, suggesting recognition of HLA-Cw1 or HLA-B51. However, responses were seen to certain EBV-LCL that do not express HLA-Cw1 or HLA-B51. It is possible therefore that this T cell recognizes an epitope common to several MHC class I alleles but, interestingly, this ability extends to the recognition of an epitope expressed by the autologous EBV-LCL. This activity is similar to that shown by many self-restricted CD8+ T-cell clones that have concomitant alloreactivity.3
In all the cases we have described, CD4+ T cells show unconventional interactions with MHC class I molecules, but interestingly, these responses were still dependent on the CD4 co-receptor, as inhibition was observed with a blocking mAb to CD4. It has been shown, following TCR triggering, that CD4 is still downregulated in the absence of co-receptor interaction with MHC, allowing the association of p56Lck with ZAP-70 and the CD3 ζ chain.45 Therefore, although the inhibition observed with anti-CD4 may imply that the CD4 co-receptor can bind to MHC class I molecules, the inhibitory effect could be a result of interference with the recruitment of this signalling tyrosine kinase p56Lck to the immunological synapse.
What is the biological significance of MHC class I-reactive CD4+ T cells? Although we isolated such cells by using a non-physiological stimulus in vitro (an MHC class II-negative cell line with a defect in antigen processing), the results clearly indicate an ability of CD4+ T cells to engage with class I MHC antigens, both as alloantigens and as presenters of allogeneic, and possibly syngeneic, peptides. In transplantation biology, CD4+ T cells could clearly be involved in MHC class I-restricted allogeneic responses to both major and minor histocompatibility antigens, and this may be relevant clinically. In the case of HLA-B27-associated diseases, where rodent models implicate an involvement of CD4+ T cells, our results, together with those previously reported, indicate that interactions between CD4+ T cells and HLA-B27 can occur. In the present study, the involvement of HLA-B27 was shown to act as a source of both TAP-dependent and -independent peptides that can be presented to CD4+ T cells by other MHC class I alleles. Whether this property is more evident for HLA-B27 than other alleles is as yet unclear, but the possibility that CD4+ T cells, with these anomalous specificities, might be involved in the pathogenesis of spondyloarthropathy merits further investigation. Finally, our use of a TAP-deficient cell line to isolate the CD4+ T cells may be relevant physiologically, as defects in the expression of TAP molecules commonly occur in vivo, particularly during viral infection46–49 and transformation of cells.50–54 Inhibition of TAP by viruses or neoplasia may allow the presentation of TAP-independent self-peptides. As these will not be expressed in the thymus (where TAP is active), the T-cell repertoire will not have been purged of these autoreactive cells. Our demonstration of the recognition of TAP-independent peptides, albeit by CD4+ T cells, indicates that inhibition of TAP might be a mechanism linking virus infection and the breaking of self-tolerance. However, the existence of a CD4+ T-cell repertoire for MHC class I alleles, which do not require TAP-transported peptides, could also provide a ‘back-up’ immune response in the context of viral or tumour immunity, which might be boosted therapeutically.
Acknowledgments
This work was funded by the Arthritis Research Campaign and GlaxoSmithKline.
Abbreviations
- C1R-B27
C1R cell line transfected with B*2705
- EBV
Epstein–Barr virus
- EBV-LCL
EBV-transformed lymphoblastoid cell line
- HLA
human leucocyte antigen
- MHC
major histocompatibility complex
- PBMC
peripheral blood mononuclear cells
- T2-B27
T2 cell line transfected with HLA-B*2705
- T2-B27-TAP
T2-B27 reconstituted with TAP1 and TAP2
- 220-B27
721.220 cell line transfected with HLA-B*2705
- TCR
T-cell receptor
References
- 1.Spits H, Ijssel H, Thompson A, de Vries JE. Human T4+ and T8+ cytotoxic T lymphocyte clones directed at products of different class II major histocompatibility complex loci. J Immunol. 1983;131:678–83. [PubMed] [Google Scholar]
- 2.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki H, Eshima K, Takagaki Y, et al. Origin of a T cell clone with a mismatched combination of MHC restriction and coreceptor expression. J Immunol. 1994;153:4496–507. [PubMed] [Google Scholar]
- 4.Heemskerk MH, Schilham MW, Schoemaker HM, Spierenburg G, Spaan WJ, Boog CJ. Activation of virus-specific major histocompatibility complex class II-restricted CD8+ cytotoxic T cells in CD4-deficient mice. Eur J Immunol. 1995;25:1109–12. doi: 10.1002/eji.1830250438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bot A, Casares S, Bot S, von Boehmer H, Bona C. Cellular mechanisms involved in protection against influenza virus infection in transgenic mice expressing a TCR receptor specific for class II hemagglutinin peptide in CD4+ and CD8+ T cells. J Immunol. 1998;160:4500–7. [PubMed] [Google Scholar]
- 6.Flomenberg N, Russo C, Ferrone S, Dupont B. HLA class I specific T lymphocyte clones with dual alloreactive functions. Immunogenetics. 1984;19:39–51. doi: 10.1007/BF00364474. [DOI] [PubMed] [Google Scholar]
- 7.Strassman G, Bach FH. OKT4+ cytotoxic T cells can lyse targets via class I molecules and can be blocked by monoclonal antibody against T4 molecules. J Immunol. 1984;133:1705–9. [PubMed] [Google Scholar]
- 8.Moore RL, Fox BS. CD4+ class I-restricted T cells specific for HIV gp160 315–329. Cell Immunol. 1994;154:43–53. doi: 10.1006/cimm.1994.1055. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Wilson JM. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol. 1995;155:2564–70. [PubMed] [Google Scholar]
- 10.De Bueger M, Bakker A, Goulmy E. Existence of mature human CD4+ T cells with genuine class I restriction. Eur J Immunol. 1992;22:875–8. doi: 10.1002/eji.1830220338. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Kimura S, Aoki N, Sato K, Celis E, Katagiri M. Existence of MHC class I-restricted alloreactive CD4+ T cells reacting with peptide transporter-deficient cells. Immunogenetics. 2001;53:626–33. doi: 10.1007/s00251-001-0379-7. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi Y, Hoon DS, Park MS, Terasaki PI, Foshag LJ, Morton DL. Induction of CD4+ cytotoxic T cells by sensitization with allogeneic melanomas bearing shared or cross-reactive HLA-A. Cell Immunol. 1992;139:411–25. doi: 10.1016/0008-8749(92)90082-z. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Vanky F, Klein E. MHC class-I-restricted auto-tumor-specific CD4+ CD8− T-cell clones established from autologous mixed lymphocyte-tumor-cell culture (MLTC) Int J Cancer. 1992;51:962–7. doi: 10.1002/ijc.2910510621. [DOI] [PubMed] [Google Scholar]
- 14.LeMay LG, Kan-Mitchell J, Goedegebuure P, Harel W, Mitchell MS. Detection of melanoma-reactive CD4+ HLA-class I-restricted cytotoxic T cell clones with long-term assay and pretreatment of targets with interferon-gamma. Cancer Immunol Immunother. 1993;37:187–94. doi: 10.1007/BF01525434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darrow TL, Abdel-Wahab Z, Quinn-Allen MA, Seigler HF. Recognition and lysis of human melanoma by a CD3+, CD4+, CD8− T-cell clone restricted by HLA-A2. Cell Immunol. 1996;172:52–9. doi: 10.1006/cimm.1996.0214. [DOI] [PubMed] [Google Scholar]
- 16.Jacob L, Somasundaram R, Smith W, Monos D, Basak S, Marincola F, Pereira S, Herlyn D. Cytotoxic T-cell clone against rectal carcinoma induced by stimulation of a patient's peripheral blood mononuclear cells with autologous cultured tumor cells. Int J Cancer. 1997;71:325–32. doi: 10.1002/(sici)1097-0215(19970502)71:3<325::aid-ijc3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Bagot M, Echchakir H, Mami-Chouaib F, et al. Isolation of tumor-specific cytotoxic CD4+ and CD4+ CD8dim+ T-cell clones infiltrating a cutaneous T-cell lymphoma. Blood. 1998;91:4331–41. [PubMed] [Google Scholar]
- 18.Nishimura MI, Avichezer D, Custer MC, et al. MHC class I-restricted recognition of a melanoma antigen by a human CD4+ tumor infiltrating lymphocyte. Cancer Res. 1999;59:6230–8. [PubMed] [Google Scholar]
- 19.Somasundaram R, Robbins P, Moonka D, Loh E, Marincola F, Patel A, Guerry D, Herlyn D. CD4(+), HLA class I-restricted, cytolytic T-lymphocyte clone against primary malignant melanoma cells. Int J Cancer. 2000;85:253–9. doi: 10.1002/(sici)1097-0215(20000115)85:2<253::aid-ijc17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–8. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 21.Trobonjaca Z, Leithauser F, Moller P, Bluethmann H, Koezuka Y, MacDonald HR, Reimann J. MHC-II-independent CD4(+) T cells induce colitis in immunodeficient RAG(−/−) hosts. J Immunol. 2001;166:3804–12. doi: 10.4049/jimmunol.166.6.3804. [DOI] [PubMed] [Google Scholar]
- 22.Boyle LH, Goodall JC, Opat SS, Gaston JS. The recognition of HLA-B27 by human CD4(+) T lymphocytes. J Immunol. 2001;167:2619–24. doi: 10.4049/jimmunol.167.5.2619. [DOI] [PubMed] [Google Scholar]
- 23.Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704–6. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- 24.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HLA-27. Lancet. 1973;1:904–7. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 25.Hermann E, Yu DT, Meyer zum Buschenfelde KH, Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–50. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 26.Kuon W, Lauster R, Bottcher U, et al. Recognition of chlamydial antigen by HLA-B27-restricted cytotoxic T cells in HLA-B*2705 transgenic CBA (H-2k) mice. Arthritis Rheum. 1997;40:945–54. doi: 10.1002/art.1780400524. [DOI] [PubMed] [Google Scholar]
- 27.Ugrinovic S, Mertz A, Wu P, Braun J, Sieper J. A single nonamer from the Yersinia 60-kDa heat shock protein is the target of HLA-B27-restricted CTL response in Yersinia-induced reactive arthritis. J Immunol. 1997;159:5715–23. [PubMed] [Google Scholar]
- 28.Fiorillo MT, Maragno M, Butler R, Dupuis ML, Sorrentino R. CD8(+) T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest. 2000;106:47–53. doi: 10.1172/JCI9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen RL, O'Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999;162:5045–8. [PubMed] [Google Scholar]
- 30.Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, Colbert RA. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002;277:23459–68. doi: 10.1074/jbc.M110336200. [DOI] [PubMed] [Google Scholar]
- 31.Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol. 2001;167:5543–7. doi: 10.4049/jimmunol.167.10.5543. [DOI] [PubMed] [Google Scholar]
- 32.Kollnberger S, Bird L, Sun MY, Retiere C, Braud VM, McMichael A, Bowness P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002;46:2972–82. doi: 10.1002/art.10605. [DOI] [PubMed] [Google Scholar]
- 33.Breban M, Fernandez-Sueiro JL, Richardson JA, Hadavand RR, Maika SD, Hammer RE, Taurog JD. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J Immunol. 1996;156:794–803. [PubMed] [Google Scholar]
- 34.May E, Dorris ML, Satumtira N, Iqbal I, Rehman MI, Lightfoot E, Taurog JD. CD8alphabeta T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J Immunol. 2003;170:1099–105. doi: 10.4049/jimmunol.170.2.1099. [DOI] [PubMed] [Google Scholar]
- 35.Roddis M, Carter RW, Sun MY, Weissensteiner T, McMichael AJ, Bowness P, Bodmer HC. Fully functional HLA B27-restricted CD4+ as well as CD8+ T cell responses in TCR transgenic mice. J Immunol. 2004;172:155–61. doi: 10.4049/jimmunol.172.1.155. [DOI] [PubMed] [Google Scholar]
- 36.Salter RD, Cresswell P. Impaired assembly and transport of HLA-A and -B antigens in a mutant T×B cell hybrid. EMBO J. 1986;5:943–9. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwood R, Shimizu Y, Sekhon GS, DeMars R. Novel allele-specific, post-translational reduction in HLA class I surface expression in a mutant human B cell line. J Immunol. 1994;153:5525–36. [PubMed] [Google Scholar]
- 38.Zemmour J, Little AM, Schendel DJ, Parham P. The HLA-A,B ‘negative’ mutant cell line C1R expresses a novel HLA-B35 allele, which also has a point mutation in the translation initiation codon. J Immunol. 1992;148:1941–8. [PubMed] [Google Scholar]
- 39.Edwards PA, Smith CM, Neville AM, O'Hare MJ. A human-hybridoma system based on a fast-growing mutant of the ARH-77 plasma cell leukemia-derived line. Eur J Immunol. 1982;12:641–8. doi: 10.1002/eji.1830120804. [DOI] [PubMed] [Google Scholar]
- 40.Young JL, Goodall JC, Beacock-Sharp H, Gaston JS. Human gamma delta T-cell recognition of Yersinia enterocolitica. Immunology. 1997;91:503–10. doi: 10.1046/j.1365-2567.1997.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henwood J, Loveridge J, Bell JI, Gaston JS. Restricted T cell receptor expression by human T cell clones specific for mycobacterial 65-kDa heat-shock protein: selective in vivo expansion of T cells bearing defined receptors. Eur J Immunol. 1993;23:1256–65. doi: 10.1002/eji.1830230610. [DOI] [PubMed] [Google Scholar]
- 42.Weekes MP, Wills MR, Mynard K, Carmichael AJ, Sissons JG. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J Virol. 1999;73:2099–108. doi: 10.1128/jvi.73.3.2099-2108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young NT, Mulder A, Cerundolo V, Claas FH, Welsh KI. Expression of HLA class I antigens in transporter associated with antigen processing (TAP)-deficient mutant cell lines. Tissue Antigens. 1998;52:368–73. doi: 10.1111/j.1399-0039.1998.tb03057.x. [DOI] [PubMed] [Google Scholar]
- 44.Parham P. Presentation of HLA class I-derived peptides: potential involvement in allorecognition and HLA-B27-associated arthritis. Immunol Rev. 1996;154:137–54. doi: 10.1111/j.1600-065x.1996.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 45.Viola A, Salio M, Tuosto L, Linkert S, Acuto O, Lanzavecchia A. Quantitative contribution of CD4 and CD8 to T cell antigen receptor serial triggering. J Exp Med. 1997;186:1775–9. doi: 10.1084/jem.186.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–5. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 47.Lehner PJ, Karttunen JT, Wilkinson GW, Cresswell P. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc Natl Acad Sci USA. 1997;94:6904–9. doi: 10.1073/pnas.94.13.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn K, Gruhler A, Galocha B, et al. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–21. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 49.Hengel H, Koopmann JO, Flohr T, Muranyi W, Goulmy E, Hammerling GJ, Koszinowski UH, Momburg F. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity. 1997;6:623–32. doi: 10.1016/s1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 50.Seliger B, Maeurer MJ, Ferrone S. TAP off – tumors on. Immunol Today. 1997;18:292–9. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 51.Chen HL, Gabrilovich D, Tampe R, Girgis KR, Nadaf S, Carbone DP. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet. 1996;13:210–3. doi: 10.1038/ng0696-210. [DOI] [PubMed] [Google Scholar]
- 52.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cromme FV, Airey J, Heemels MT, Ploegh HL, Keating PJ, Stern PL, Meijer CJ, Walboomers JM. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med. 1994;179:335–40. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singal DP, Ye M, Qiu X. Molecular basis for lack of expression of HLA class I antigens in human small-cell lung carcinoma cell lines. Int J Cancer. 1996;68:629–36. doi: 10.1002/(SICI)1097-0215(19961127)68:5<629::AID-IJC13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]