Abstract
Dendritic cells (DCs) are important in the regulation of immune responses and it has been proposed that these cells play an important role in asthma; however, their role in food allergy is still largely unknown. Our aim was to study specific immunoglobulin E (IgE) and immunoglobulin G (IgG) responses in naïve recipients following adoptive transfer of myeloid DCs from allergic and control mice. The phenotypic features and lymphokine production of DCs were also investigated. CD11c+/hi B220– DCs isolated from spleen and Peyer's patches (PP) of cow's milk (CM) allergic and control mice were transferred intravenously (i.v.) into naïve syngeneic recipients, and IgE- and IgG-specific responses were evaluated. Experiments were also carried out to determine the levels of interferon-γ (IFN-γ) and interleukin (IL)-4 produced by splenocytes from naïve recipients following the adoptive transfer, and CD40 ligand (CD40L)-mediated IL-10 production by DCs from allergic and control mice. DCs isolated from spleen and PP of allergic mice, but not control groups, induced CM-specific IgG and IgE antibody production in naïve recipients in the absence of previous immunization, but did not modify the T helper 1 (Th1) and T helper 2 (Th2) balance. Furthermore, although no difference was observed in the expression of canonical DC surface markers, PP DCs from allergic mice produced less IL-10 than DCs from controls. We interpret these data as showing that DCs play a pivotal role in allergen-specific IgE responses and that a Th2-skewed response may not be involved in the early phase of allergic responses. The identification of the mechanisms underlying these events may help to design novel strategies of therapeutic intervention in food allergy.
Keywords: adoptive transfer, dendritic cell, food allergy, IgE
Introduction
Immunoglobulin E (IgE)-mediated allergic responses to food components are serious and life-threatening conditions and, according to a recent survey, are undergoing a rapid increase throughout the world.1,2 In the past few years it has become evident that allergen-specific T helper 2 (Th2) cells play a central role in the genesis and maintenance of allergic inflammatory reactions in both humans and mice.3,4 CD4+ T helper cells from atopic individuals and sensitized laboratory animals belong predominantly to the Th2 phenotype, characterized by production of relatively high levels of interleukin (IL)-4, IL-5 and IL-13, and a low level of interferon-γ (IFN-γ).5 Although the aetiology of IgE-mediated allergies is far from being completely understood, it is generally believed that the shifted balance in the Th1/Th2 response is directly correlated to an overproduction of allergen-specific IgE (reviewed in ref. 6). One of the most convincing pieces of evidence for the role of Th2 cells has come from recent work which demonstrates that peanut-allergic individuals show a Th2 polarization of cytokine production by antigen-specific T cells.4 In contrast, non-allergic donors, and donors who have outgrown their allergy, show a T helper 1 (Th1) response to peanut. However, the exact role of Th2 lymphokines on the various phases of allergic reactions, from sensitisation to establishing and maintaining chronic allergic reactions, is still unknown. Factors responsible for the polarization of the specific immune response into a predominant Th2 response in atopic patients remain largely undefined. It is well known that Th1 and Th2 are not derived from a distinct precursor, but rather develop from a common precursor under the influence of both environmental and genetic factors acting at the level of antigen presentation.7,8 Dendritic cells (DCs) are now recognized as the key player in antigen presentation, for their ability to internalize antigens at an extremely low concentration and for the highly efficient presentation of these, in the context of the major histocompatibility complex (MHC) class II molecule, to naïve T cells. Their ability to orchestrate Th1/Th2 responses is well documented and several studies have suggested a role for DCs in the pathogenesis of allergic diseases. Functional and phenotypic differences in DCs from allergic and non-allergic donors have been reported. DCs from allergic donors were found to display differential expression of human leucocyte antigen (HLA)-DR, CD11b, FcεRI9,10 and CD80.11 In addition, allergen-challenged DCs from atopic donors displayed an increased capability to induce the production of Th2 cytokines from autologous naïve, as well as memory, T cells and IgE antibody,12,13 compared to DCs from non-atopic donors. It has also been proposed that DCs in atopic donors may contribute to the allergic status by a reduced ability to produce IL-1214 and IL-10,15,16 a cytokine which is believed to be a key regulator of the balance between tolerance and allergy. A specific subset of these cells also seems to be involved in maintaining the chronic Th2 inflammation that is typical of airway hyper-responsiveness.17 This DC subset is capable of capturing airborne antigens and remains able to activate T cells for a long time after the initial exposure. The role of DCs in the IgE-mediated immune reactions is further highlighted by results obtained in severe combined immunodeficient (SCID) mice. Adoptive transfer, into SCID mice, of in vitro-generated DCs from patients sensitive to Dermatophagoides pteronyssinus (Dpt), induced a marked increase in the production of specific IgE antibody15 when challenged with the antigen Dpt.
However, all these data focused on allergic reactions of the respiratory tract, and nothing is known about the role of DCs in the generation, progression and maintenance of IgE-mediated allergic reactions to food. This prompted us to investigate several aspects of the biology and function of DCs in a well-established mouse model of type I hypersensitivity reactions to cow's milk (CM), which mimics human responses.3,18 Here we report that the adoptive transfer of splenic and Peyer's patch (PP)-derived DCs into naïve syngeneic recipients induced both IgG- and, more importantly, IgE-specific responses, even in the absence of antigen challenge. Furthermore, we observed that allergen-specific IgE production, following the adoptive transfer of DCs from allergic mice, may not be linked to a Th2-skewed response.
Materials and methods
Mouse model of food allergy
Female C3H/HeJ mice, 3 weeks old, were purchased from Charles River (Margate, UK) and maintained in a clean, access-restricted room, under conventional conditions, throughout the experiments. Animal experiments were conducted according to guidelines of the Animal Act 1986 (Scientific procedures) and the number of animals used was kept to a minimum. Mice were slightly anaesthetized with isofluorane and then intragastric feeding was performed using a stainless steel blunt feeding needle. Following an established procedure,3,18 mice were immunized using a mixture of homogenized CM and cholera toxin (CT) (Calbiochem, San Diego, CA) that contained 1·0 mg/g of body weight of CM together with 0·3 µg/g of body weight of CT. The CM + CT mixture was administered in phosphate-buffered saline (PBS) (final volume of 0·03 ml/g of body weight). Control groups were administered with the same dose of CM and CT alone or PBS (naïve). Mice were challenged five times at weekly intervals and finally challenged at week 6 with a double dose of CM administered 30 min apart. Given the fact that a small amount of CM products are commonly present in the mouse diet, we kept a small group of in-house bred mice under a controlled diet, as a control. For experiments of adoptive transfer, phenotypic analysis and lymphokine production, DCs were isolated from spleen and PP of sensitized and control mice 24 hr after giving the fifth dose of the sensitizing (or control) mixture. Additional groups of mice were challenged on week 6 with CM in order to check the percentage of mice that developed a type I hypersensitivity reaction to CM, as previously described.3 Our experiments showed that as many as 75% of C3H/HeJ mice sensitized with the CM + CT mixture displayed a strong allergic reaction, as established by a scoring system previously described.3
Preparation of DCs
Isolation and purification of DCs from spleen and intestinal PP from allergic and control mice was performed following a slightly modified procedure, as previously described.19 First, PP tissues were treated with media containing dithiothreitol, Hepes, 10% fetal calf serum (FCS) and 5 mm EDTA in Hanks' balanced salt solution (HBSS) for 90 min at room temperature (all chemicals were from Sigma Chemical Co., St Louis, MO) to remove epithelial cells and then washed extensively with HBSS. Spleen and PP were then treated with collagenase D (400 U/ml, Roche, Mannheim, Germany) and incubated at 37° for 10 min in the presence of EDTA. Single-cell suspensions were then prepared, and the cells were stained with anti CD11c–phycoerythrin (PE)-labelled antibody (BD Biosciences, San Diego, CA) and anti B220–allophycocyanin (APC) (Ebioscience, San Diego, CA). CD11c+/hi B220– cells were isolated by a Coulter Epics Altra (Coulter Becham, Beckman, High Wycombe, UK) flow cytometer. Sorted populations were screened for B- (CD19) and T- (CD3) cell contamination using flow cytometry. Sorting of DCs was carried out under very stringent conditions to exclude CD11c+/lo macrophages.20 Phenotypic analysis of DCs was carried out by flow cytometry using fluorescein isothiocyanate (FITC)-labelled anti-MHC class II (I-Ab), CD80, CD40 (BD Biosciences), CD86 (AMS Biotechnology, Abingdon, UK), and CD8α (Ebioscience) immunoglobulin. When appropriate, the blocking antibodies CD16 and CD32 (AMS Biotechnology) were employed.
Adoptive transfer of DCs and antibody responses
Naïve C3H/HeJ mice (of the same sex and age as DC donors) were injected intravenously (i.v.) (tail vein) with 1 × 105 CD11c+/hi B220– DCs suspended in saline solution. Five to seven mice per group were injected with splenic DCs, while a smaller number of mice in each group (n = 3 or 4) was used to determine the effect of PP DCs on the CM-specific IgE response. This was because of ethical considerations regarding the number of animals required to harvest the necessary number of PP DCs (1 × 105 per mouse). Mice were then caged, as described above, and antibody responses were evaluated by enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) at various time-points. The ELISPOT was carried out according to a protocol established in our laboratory.21 Briefly, nitrocellulose filter discs were coated overnight at 4°, in a humidified chamber, with cow's milk protein (CMP), unrelated antigen, phosphorylcholine–keyhole limpet haemocyanin (KLH) (both at 100 µg/ml) or PBS, as a background control. After blocking and extensive washing, filters were incubated, in triplicate, with various concentrations of splenocytes from allergic and control mice for 5 hr at 37°/5% CO2. Filters were then rinsed with PBS/EDTA (10 mm) for 10 min, rinsed with PBS and incubated with horseradish peroxidase (HRP)-labelled antibody against mouse IgG and IgE (Southern Biotech., Birmingham, AL), overnight at 4°. After careful washing, the reaction was developed using HRP colour-development reagents (Sigma). The reaction was then stopped with deionized water and spots were scored. Filters containing between 10 and 102 spots were used to calculate the number of antibody-forming cells (AFC)/107 splenocytes.
Serum levels of CM-specific IgE and IgG were also determined in all groups of mice that underwent adoptive transfer of DCs. Reference curves were constructed by coating microtitration plates with albumin–dinitrophenyl (DNP) at 1 µg/ml, for IgE, and with glutenin for IgG. Dilutions of mouse anti-DNP IgE monoclonal antibody (mAb) (Sigma) or mouse anti-glutenin IgG mAb (clone IFR065) were then added. The plates were then incubated for 90 min at 37° and then washed with PBS. The plates were probed with either rat anti-mouse IgE (Southern Biotechnology Associates, Birmingham, AL) or goat anti-mouse IgG, both HRP-conjugated. Following a further 90-min incubation, substrate was added and the reaction stopped after 15 min. Absorbances at 450 nm were determined in a Dynatech mr5000 plate reader. Levels of specific serum IgE and IgG were subsequently determined following the same protocol, using CMP as coating protein and various dilutions of mouse sera as primary antibody.
Lymphokines production
First, CD40L-mediated production of IL-10 was assessed in DCs from CM-sensitized and control mice. CD11c+/hi B220– DCs were cultured overnight in the presence of CD40L (1–10 µg/ml) in a 96-well microtitre plate. Supernatants were then collected and IL-10 and IL-12 p40/p70 levels analysed by enzyme-linked immunosorbent assay (ELISA). Second, Th1 and Th2 lymphokines were determined in all groups of naïve recipients, following the adoptive transfer of DCs from sensitized and control groups, by evaluating the levels of IFN-γ and IL-4 following an in vitro T-cell recall response. In this case, spleen cells from allergic mice were placed in serum-free medium (RPMI-1640 containing 0·5% penicillin/streptomycin and 1% glutamine) and challenged with CMP or concanavalin A (Con A; a positive control) for 48–72 hr. The culture supernatants were then assayed for levels of IFN-γ and IL-4 using commercially available kits, according to the manufacturer's instructions. In addition, the number of individual T cells secreting IL-4 and IFN-γ was assessed by ELISPOT. In these experiments, T cells were purified, through a nylon wool column, from splenocytes of all groups of mice following the in vitro T-cell recall challenge. The ELISPOT was carried out as described above. In this case, anti-mouse IFN-γ or IL-4 (R4-6A2 or BVD4-1D11) were used as coating antibody, while anti-IFN-γ XMB1.2 or anti-IL-4 BVD6-24G2 (all antibodies were from Pharmingen, San Diego, CA) were used as detection antibody.
Statistics
Statistical analysis was performed by the Student's t-test and values were considered significant at a P-value of ≤0·05.
Results
Isolation and phenotypic analysis of splenic- and PP-derived DCs
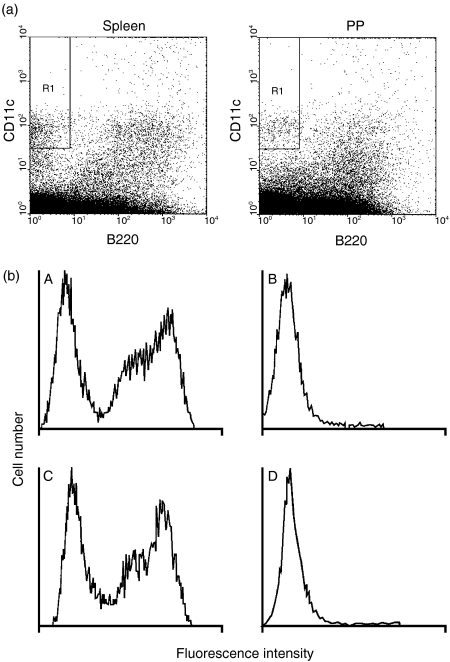
DCs from spleen and PP from allergic and control mice were isolated and their phenotype analysed by flow cytometry. It has been reported that manipulation of DCs by means of isolation from tissue followed by overnight culture can induce maturation and differentiation.19 Thus, DCs isolated in this way may not be entirely representative of the DC function in vivo. In order to circumvent this problem, we isolated DCs expressing CD11c+ (a well-established marker for murine myeloid DCs) from PP and spleen of allergic and control mice, using flow cytometry, without further manipulation, immediately after preparation of the cell suspension. CD11c+/hi B220– DCs19 were identified and sorted as shown in Fig. 1(a). The absence of T- and B-cell contamination was determined by flow cytometry using anti-CD3 and anti-CD19 immunoglobulin, respectively (Fig. 1b). Sorting of CD11c+/hi B220–cells was carried out under very stringent experimental conditions and this enabled us to rule out contamination with macrophages, which are characterized by the expression of CD11c+/lo.20
Figure 1.
Isolation and purification of dendritic cells (DCs) (CD11c+/hi B220–). DCs were isolated from both spleen and Peyer's patch (PP) of allergic and control mice using flow cytometry (a). DCs were isolated immediately after preparation of the cell suspension in order to minimize manipulation and culture-induced functional and phenotypic changes. Cells were gated using stringent conditions in order to avoid contamination with macrophages, characterized by expression of CD11c+/lo. CD11c+/hiB220– DCs were routinely checked, using flow cytometry, for contamination with T and B cells (b). CD3 (T cell) and CD19 (B cell) cells that formed a large proportion of the whole spleen suspension (A and C, respectively) were absent in the CD11c+/hiB220– DC sorted population (B and D, respectively).
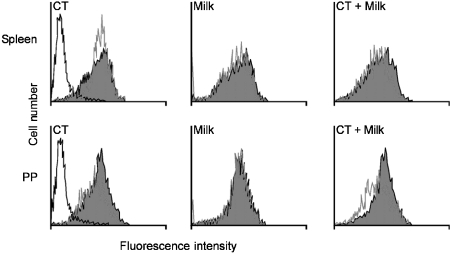
DCs from spleen and PP were then analysed for the expression of canonical DC markers, such as MHC class II (I-Ab), CD86, CD8α, CD80 and CD40. With the exception of MHC class II molecules, all the markers we tested were expressed at a very low level in both splenic- and PP-derived DCs, and no differences were observed in the expression of these markers in allergic mice compared to mice from control groups. In Fig. 2 we show the expression of MHC class II molecules in naïve mice compared to mice that had been treated with CT, CM and the combination of CT + CM (allergic group). A minor differential expression of MHC II was observed in the splenic DCs of mice treated with CT compared to naïve mice, but no difference in the expression of MHC class II antigen was observed between splenic and PP DCs, or between allergic and control non-allergic mice.
Figure 2.
Phenotypic analysis of dendritic cells (DCs) from allergic and control mice. Flow cytometry analysis revealed a low expression of all canonical DC markers in splenic and Peyer's patch (PP) DCs from both allergic and non-allergic donors. Histograms show the expression of major histocompatibility complex (MHC) class II molecules in cholera toxin (CT)-, cow's milk (CM)- and CM + CT-treated mice (white) versus naïve mice (dark).
IgE response in naive recipients following passive transfer of DC
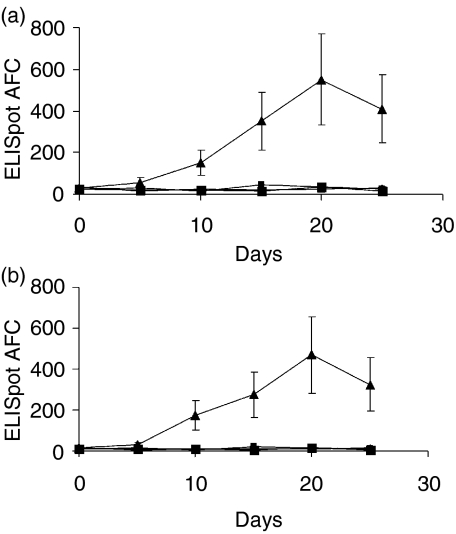
CD11c+/hi B220– DC from allergic (treated with CM + CT) and all control mice (naïve, CM- and CT-treated) were transferred i.v. into naïve syngeneic mice. A single injection was carried out in the tail vein and the specific antibody response was evaluated at various time-points by ELISPOT. As shown in Fig. 3(a), 3(b), splenic DCs from allergic mice induced a significant antigen-specific IgE response, while this was not observed when DCs from the control groups were used. The response became evident on day 10 after the passive transfer and peaked on day 20. Despite interindividual variability, the response was significantly different in mice that had received DCs from allergic mice compared to those that had received DCs isolated from all control groups (naïve, CM- and CT-treated). DCs from the control groups did not induce any detectable level of IgE; on the other hand, all mice that received DCs from allergic donors displayed a significant level of IgE-specific AFC.
Figure 3.
Cow's milk (CM)-specific immunoglobulin E (IgE) antibody response in naïve recipients following adoptive transfer of dendritic cells (DCs). Groups of naïve C3H/HeJ mice were injected intravenously (i.v.) with 1 × 105 DCs from CM-allergic mice [(treated with CM + cholera toxin (CT)] or control mice (naïve, CM and CT-treated mice), and anti-cow's milk protein (anti-CMP) IgE responses were evaluated by enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) at various time-points. Mice that were injected with splenic (a) and Peyer's patch (b) DCs from allergic donors showed a significant response, even in the absence of previous immunization. Each point represents the mean value (± standard deviation) from groups of five to seven (a) or three to four (b) mice. AFC, antibody-forming cells.
The same pattern of the kinetics of IgE response was observed when a smaller number of mice/group (n = 3−4) was injected with PP-derived DCs from allergic and control mice (Fig. 3b). However, the magnitude of the IgE-specific response appeared to be greater in mice transferred with PP-derived DCs (940 ± 340 AFC/106 spleen cells on day 20) compared to mice treated with splenic DCs (540 ± 225 AFC/106 spleen cells on day 20). A similar pattern was observed when levels of specific IgE and IgG were evaluated in the sera of mice following the adoptive transfer of DCs (Table 1). In this case, serum antibody levels of both isotypes were raised in naïve mice injected with DCs from allergic mice. Noteworthy, DCs from CM-immunized, but not allergic, mice induced the production of IgG, but not of IgE. Thus, in allergic mice, myeloid DCs have acquired the ability to initiate the production of allergen-specific IgE.
Table 1.
Levels of cow's milk protein (CMP)-specific immunoglobulin E (IgE) and immunoglobulin G (IgG) in sera (ng/ml)
| Group | Day 0 | Day 10 | Day 20 | Day 30 |
|---|---|---|---|---|
| IgE | ||||
| AT-Naïve* | <4 | <4 | <4 | <4 |
| AT-CM | <4 | <4 | <4 | <4 |
| AT-CT | <4 | <4 | <4 | <4 |
| AT-CM + CT | <0 | <4 | 210 ± 22† | 165 ± 25 |
| IgG | ||||
| AT_Naïve | 20 ± 10 | 19 ± 8 | 16 ± 5 | 20 ± 10 |
| AT-CM | 19 ± 8 | 45 ± 20 | 80 ± 29 | 190 ± 50 |
| AT-CT | 15 ± 6 | 20 ± 8 | 25 ± 10 | 20 ± 10 |
| AT-CM + CT | 15 ± 8 | 70 ± 19 | 350 ± 40 | 920 ± 200 |
Naïve mice received adoptive transfer (AT) of splenic dendritic cells (DCs) from sensitized (AT-CM + CT) or control mice (AT-Naïve, AT-CM, AT-CT). CM, cow's milk; CT cholera toxin.
Mean±standard error (SE) (n = 5−7 mice/group). Values up to 4 ng/ml were considered to be below the level of sensitivity of the enzyme-linked immunosorbent assay (ELISA).
Adoptive transfer of DCs from allergic donors does not alter the balance of the Th1 and Th2 response in the spleen of IgE-producing mice
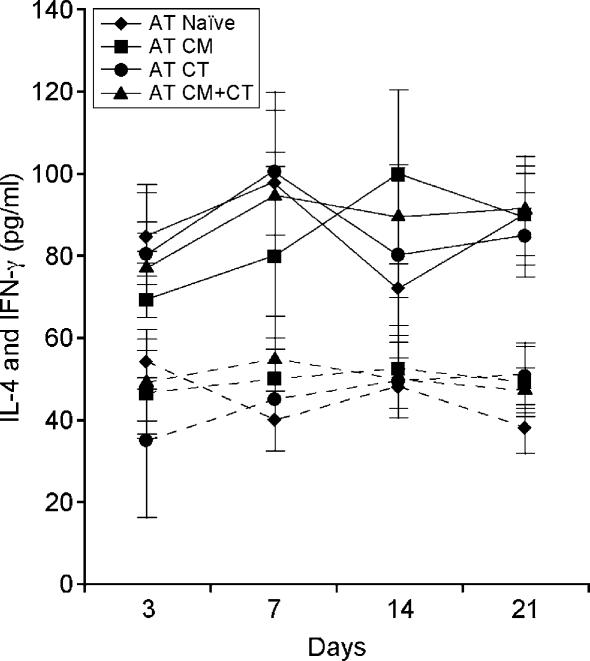
Th2 lymphokines are thought to play a critical role in IgE-mediated allergic reactions. If this was true, one would expect to observe an increase in the production of IL-4 in the spleen of mice that produced IgE following adoptive transfer of DCs from allergic mice. With this in mind, we determined the production of IFN-γ and IL-4 by splenocytes of all groups of mice that underwent adoptive transfer following an in vitro T-cell recall response. In these experiments, the levels of IFN-γ and IL-4 were determined, at various time-points after the adoptive transfer, in splenocytes following in vitro challenge with CMP, or Con A as a positive control, for 48–72 hr. We found that IFN-γ and IL-4 levels did not differ significantly in spleen cell cultures from mice that developed an IgE antibody response compared to those that did not (Fig. 4). The absence of an increased Th2 response showed that the splenic immunological microenvironment of mice that displayed a DC-mediated IgE production did not differ, in regard to Th1 and Th2 responses, from that of mice which did not show a DC-mediated IgE production. Although IFN-γ and IL-4 are the prototype of the Th1 and Th2 response, respectively, their production is not restricted to T cells alone. Indeed, a wide variety of other cell types secrete these lymphokines. We therefore used ELISPOT to determine the number of individual IFN-γ- and IL-4-secreting T cells following the in vitro T-cell recall challenge. In Table 2 we show that although an increase in the number of both IFN-γ- and IL-4-producing T cells was observed in mice following the adoptive transfer of DCs from CM (AT-CM)- and CM + CT (AT-CM + CT)-treated mice, the IL-4/IFN-γ ratio did not change in comparison to mice reconstituted with DCs from other groups (AT-Naïve; AT-CT). These results show that a Th2-skewed response may not be required in the early phase of allergic reaction, which is characterized by the initial production of allergen-specific IgE.
Figure 4.
Levels of interferon-γ (IFN-γ) and interleukin-4 (IL-4) in mice following the adoptive transfer (AT) of dendritic cells (DCs). All groups of mice that underwent AT of DCs were tested for the production of IFN-γ (dotted lines) and IL-4 (solid lines) at various time-points by enzyme-linked immunosorbent assay (ELISA). Splenocytes were cultured for 48–72 hr in the presence of cow's milk protein (CMP) or concanavalin A (Con A), as a positive control. No differences were observed among the various groups of mice. The expected increase in IL-4 production was not observed in mice (AT-CM + CT) that did produce CM-specific antibody following the AT of DCs from allergic donors. Thus, it appears that a T helper 2 (Th2)-skewed response is not required, at least initially, to trigger production of an allergen-specific immunoglobulin E (IgE) response. CM, cow's milk; CT, cholera toxin.
Table 2.
Number of interleukin-4 (IL-4) and interferon-γ (IFN-γ)-producing T cells: enzyme-linked immunosorbent spot-forming cell assay (ELISPOT)
| Day 0 | Day 7 | Day 21 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T cells * | IL-4 | IFN-γ | r | IL-4 | IFN-γ | r | IL-4 | IFN-γ | r |
| AT-Naïve | 230±30† | 82±12 | 2·8‡ | 200±21 | 90±15 | 2·2 | 235±41 | 97±19 | 2·4 |
| AT-CM | 220±28 | 75±15 | 2·9 | 402±90 | 174±55 | 2·3 | 275±40 | 119±25 | 2·3 |
| AT-CT | 200±25 | 76±20 | 2·6 | 270±40 | 128±31 | 2·1 | 225±35 | 92±33 | 2·4 |
| AT-CM + CT | 240±38 | 85±29 | 2·8 | 450±112 | 187±20 | 2·4 | 304±50 | 132±28 | 2·3 |
Splenocytes from naïve mice that had received adoptive transfer (AT) of splenic dendritic cells (DCs) from sensitized mice (AT-CM+ CT) or control mice (AT-Naïve, AT-CM, AT-CT), were cultured in vitro at various time-points after the adoptive transfer (days 0, 7, 21) in the presence of cow's milk protein (CMP). Following their isolation, T cells from all groups were used to determine the number of interleukin-4 (IL-4)- and interferon-γ (IFN-γ)-producing T cells by ELISPOT. CM, cow's milk; CT cholera toxin.
ELISPOT was carried out in triplicate, and values represent the mean±standard error (SE) (n = 5−7 mice/group).
Means were used to calculate the IL-4/IFN-γ ratio, and no significant differences were observed at any time-point.
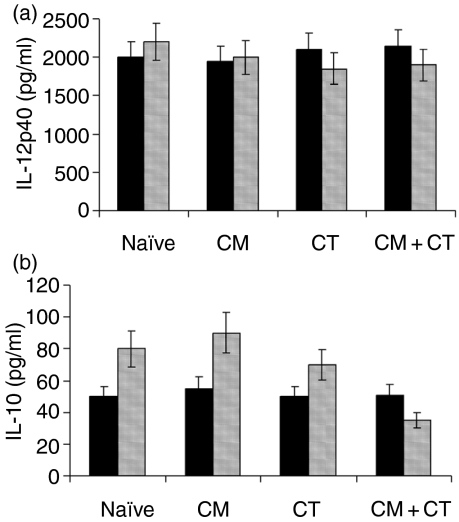
CD40-mediated production of IL-10, but not IL-12, is impaired in PP DCs from allergic mice
DCs produce cytokines in response to a variety of stimuli, including interaction with T cells. We have determined the production of IL-12 and IL-10 in both splenic and PP-derived DCs from allergic and control groups upon challenge with CD40L, a molecule which is expressed by activated T cells. We observed that DCs from all groups displayed the same ability to produce IL-12 in response to CD40L, and that no significant difference was detectable between splenic and PP DCs (Fig. 5a). On the other hand, a significant reduction (P < 0·01) in the production of IL-10 was seen in PP DCs from allergic mice compared to control groups (Fig. 5b). Interestingly, an impaired IL-10 production was detected in PP DCs, but not in splenic DCs. Although the level of CD40-mediated production of IL-10 by myeloid DCs is usually low,19 the differences between allergic and non-allergic mice were significant. The production of IL-5 was not observed in either spleen- or PP-derived DCs from any group of mice.
Figure 5.
Interleukin (IL)-10 production by dendritic cells (DCs) from allergic and control mice. CD40-mediated production of IL-12p40 (a) and IL-10 (b) from splenic (black bars) and Peyer's patch (PP)-derived (grey bars) DCs. While no significant changes were observed in regard to either IL-12p40 or -p70 (data not shown), a significant reduction in IL-10 production (P < 0·01) was detected in PP DCs from allergic mice (CM + CT). CM, cow's milk; CT, cholera toxin.
Discussion
An overproduction of allergen-specific IgE is considered to be the most important event linked to a type I allergic reaction to food components. Here we described evidence to show that the systemic administration of DCs isolated from CM-allergic mice induced the production of CM-specific IgE and IgG when passively transferred into naïve syngeneic mice, even in the absence of antigen exposure. Naïve mice that were reconstituted with allergic DCs did not develop a type I hypersensitivity reaction similar to that observed in mice sensitized with allergen and CT, even following the delivery of a single or double dose of CM (C. Nicoletti et al., unpublished), although levels of CM-specific IgE were raised significantly. The absence of the allergic status in the latter cohort, despite the presence of significant amounts of allergen-specific IgE, is not fully understood, and it could be related to the number of DCs transferred. It might be that by increasing the number of DCs, the critical level of IgE required to trigger a full type-I reaction, following administration of the specific food component, could be reached.
The mechanism by which DCs alone induced a specific IgE response is not clear. DCs are professional antigen-presenting cells (APC) and, as such, they have the ability to process and present antigen to naïve T cells. This step is crucial in determining the nature of T helper cell responses. The ability of CD11c+ DCs to induce an allergen-specific IgE response has been shown in SCID mice reconstituted with DCs from patients sensitized to dust mite upon antigen challenge.13 It was hypothesized that DCs can stimulate the production of IL-4 (which is required to mediate the switch towards IgE) by specific T cells and by delivering the necessary second signals to B cells via CD40/CD40L. However, this does not seem to be the case in our model. Indeed, we have observed that adoptive transfer of myeloid DCs from sensitized mice induced a significant allergen-specific IgE response without altering the profile of Th1 and Th2 responses. Although a vast amount of data support the notion of a pivotal role played by Th2 lymphokines in allergic reactions, the pattern and extent of the involvement of Th2 lymphokines on the genesis of adverse reactions to food remains to be determined. In this context, our results would indicate that Th2-skewed responses may be important in the progression and maintenance of allergic status, rather than in the early stages characterized by the production of allergen-specific IgE.
Despite the differences in the regulation of antibody response between DCs from allergic and all the other control groups, the phenotypic characterization of DCs from both PP and spleen from all groups of mice (allergic and non-allergic) revealed a low expression of regulatory molecules, such as CD40, CD80, CD86 and CD8α, indicating a low degree of maturation of the DC population analysed. Furthermore, although higher compared with the expression of other surface markers, the level of MHC II molecules did not differ significantly between splenic and PP-derived DC and was not affected by the allergic status. A remarkably higher expression of MHC II molecules in PP DCs compared to splenic DCs has been reported in BALB/c mice.19 Our results suggest that this is not always the case and that the expression of regulatory molecules on DCs from various lymphoid organs varies according to the genetic make-up of the host.
We next observed that CD40-mediated IL-10 production is reduced in PP DCs from allergic mice, while no changes were observed in splenic DCs; on the other hand, production of IL-12 was not affected in allergic mice. It is important to bear in mind that IL-10 is a lymphokine which is believed to play a central role in controlling the balance between tolerance and allergy. The role of IL-10 in shaping the outcome of immune responses is further highlighted by the notion that this lymphokine has the ability to convert in vitro immature DCs into tolerizing APCs.22 Our finding, that IL-10 production is impaired in DCs during sensitization, also lends support to the notion that the long-term reduction of IgE levels and the resolution of allergy, following specific immunotherapy, is linked to a marked increase in the levels of IL-10 in cutaneous biopsies.23 We carried out analysis of IL-10 production in DCs from allergic and non-allergic mice following challenge with CD40, a molecule normally expressed on the surface of T cells. It may be that deficiency in IL-10 production by DCs during antigen presentation to T cells plays an important role in the onset of the allergic status. Also, IL-10 production is specifically reduced in PP DCs, a cell population that is considered to play a central role in the sensitization process to food components in the gut.24 However, allergic reactions are not always linked to a reduced production of IL-10. Recently, a marked increase in the amount of IL-10 produced in vitro by splenocytes isolated from C3H/HeJ mice undergoing the same sensitization procedure described here, has been reported.3 The discrepancy with our results can be explained, in the first instance, by the fact that in the latter case the whole spleen-cell population was used as source of this lymphokine. IL-10 is a lymphokine that is produced by cell types other than DCs, including T cells and macrophages,25,26 and it may be that the decline in IL-10 production by CD11c+/hi B220– DCs reflects allergy-related changes taking place in that specific cell population. Second, in our experiment, DCs were isolated 24 hr after delivery of the last sensitizing dose, while, in the other study, splenocytes were isolated from mice immediately after the elicitation of the type I response. Thus, the production of IL-10 was analysed in cells at a different stage of activation.
Taken together, our data demonstrate that myeloid DCs have the potential to initiate an IgE-mediated immune response, even in the absence of antigen challenge, thereby pointing to a very important role exerted by this type of DC in host sensitisation. The identification of the cytokines and costimulatory molecules underlying these events may help to design new strategies for therapeutic intervention
Acknowledgments
The authors are indebted to Profs T. T. MacDonald, I. Kimber and X. M. Li for critical review of the manuscript. This work was supported by a Competitive Strategic Grant from The Biotechnology and Biological Sciences Research Council (to C.N.) and intramural funds of the University of Siena (to E.B.).
References
- 1.Sampson HA. Food allergy. J Allergy Clin Immunol. 2003;111:S540–7. doi: 10.1067/mai.2003.134. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH. Food Allergy. Lancet. 2002;360:701–10. doi: 10.1016/S0140-6736(02)09831-8. [DOI] [PubMed] [Google Scholar]
- 3.Morafo V, Srivastava K, Huang CK, Kleiner G, Lee SY, Sampson HA, Li AM. Genetic susceptibility to food allergy is linked to differential Th2-Th1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003;111:1122–8. doi: 10.1067/mai.2003.1463. [DOI] [PubMed] [Google Scholar]
- 4.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut allergic children and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–72. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagnani S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol. 2000;105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 6.Ebo DG, Stevens WJ. IgE-mediated food allergy. Extensive review of the literature. Acta Clin Belg. 2001;56:234–47. doi: 10.1179/acb.2001.035. [DOI] [PubMed] [Google Scholar]
- 7.Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol. 2001;13:275–82. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 8.Moser M, Murphy KM. Dendritic cell regulation of Th1-Th2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 9.van den Heuvel MM, Vanhee DDC, Postmus PE, Hoefsmit ECM, Beelen RHJ. Functional and phenotypic differences of monocyte-derived dendritic cells from allergic and nonallergic patients. J Allergy Clin Immunol. 1998;101:90–5. doi: 10.1016/S0091-6749(98)70198-8. [DOI] [PubMed] [Google Scholar]
- 10.Holloway JA, Holgate ST, Semper AE. Expression of the high affinity IgE receptor on peripheral blood dendritic cells: differential binding of IgE in atopic asthma. J Allergy Clin Immunol. 2001;107:1009–18. doi: 10.1067/mai.2001.115039. [DOI] [PubMed] [Google Scholar]
- 11.Yamasuga S, Masuda K, Ohno K, Tsujimoto M. Antigen-specific enhancement of CD80 mRNA expression in experimentally induced sensitized dog with Japanese cedar pollen. J Vet Med Sci. 2003;65:295–300. doi: 10.1292/jvms.65.295. [DOI] [PubMed] [Google Scholar]
- 12.Bellinghausen I, Brand U, Knop J, Saloga J. Comparison of allergen stimulated dendritic cells from atopic and non atopic donors dissecting their effect on autologous naïve and memory T helper cells of such donors. J Allergy Clin Immunol. 2000;105:988–96. doi: 10.1067/mai.2000.105526. [DOI] [PubMed] [Google Scholar]
- 13.Hammad H, Duez C, Fahy O, et al. Human dendritic cells in a severe combined immunodeficiency mouse model: their potentiating role in the allergic reaction. Lab Invest. 2000;80:605–14. doi: 10.1038/labinvest.3780065. [DOI] [PubMed] [Google Scholar]
- 14.Reider N, Reider D, Ebner S, Holzmann S, Herold M, Fritsch P, Romani N. Dendritic cells contribute to the development of atopy by an insufficiency in IL-12 production. J Allergy Clin Immunol. 2002;109:89–95. doi: 10.1067/mai.2002.120556. [DOI] [PubMed] [Google Scholar]
- 15.Charbonnier AS, Hammad H, Gosset P, Stewart GA, Alkan S, Tonnel AB, Pestel J. Der p 1-pulsed myeloid and plasmacytoid dendritic cells from house dust-mite sensitized allergic patients dysregulate the T cell response. J Leukoc Biol. 2003;73:91–9. doi: 10.1189/jlb.0602289. [DOI] [PubMed] [Google Scholar]
- 16.Muller G, Muller A, Tuting T, Steinbrink K, Saloga J, Szalma C, Knop J, Enk AH. IL-10 treated dendritic cells modulate immune response of naïve and sensitized T cells in vivo. J Invest Dermatol. 2002;119:836–41. doi: 10.1046/j.1523-1747.2002.00496.x. [DOI] [PubMed] [Google Scholar]
- 17.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;271:93–5. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 18.Li XM, Schofield JD, Huang CK, Kleiner GI, Sampson HA. A murine model of cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–14. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce IL-10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metlay JP, Witmer-Pack R, Agger MT, Crowley D, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells, as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–71. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicoletti C, Borghesi C. Detection of autologous anti-idiotypic antibody forming cells by a modified enzyme linked immunospot (ELISPOT) Res Immunol. 1992;143:919–25. doi: 10.1016/0923-2494(92)80115-2. [DOI] [PubMed] [Google Scholar]
- 22.Steinbrink K, Wolfl M, Jonuleit M, Knop J, Enk AH. Induction of tolerance by IL-10 treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 23.Nasser SM, Ying S, Meng Q, Kay AB, Ewan PW. Interleukin 10 levels increase in cutaneous biopsies of patient undergoing wasp venom immunotherapy. Eur J Immunol. 2001;31:3704–13. doi: 10.1002/1521-4141(200112)31:12<3704::aid-immu3704>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Brandtzaeg P. Nature and function of gastrointestinal antigen presenting cells. Allergy. 2001;56:16–20. doi: 10.1034/j.1398-9995.2001.00903.x. [DOI] [PubMed] [Google Scholar]
- 25.Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complex. J Endotoxin Res. 2002;8:477–81. doi: 10.1179/096805102125001118. [DOI] [PubMed] [Google Scholar]
- 26.Mosser DM. The many faces of macrophages activation. J Leukoc Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]